3.1.2. Structural Equation Modeling (SEM)
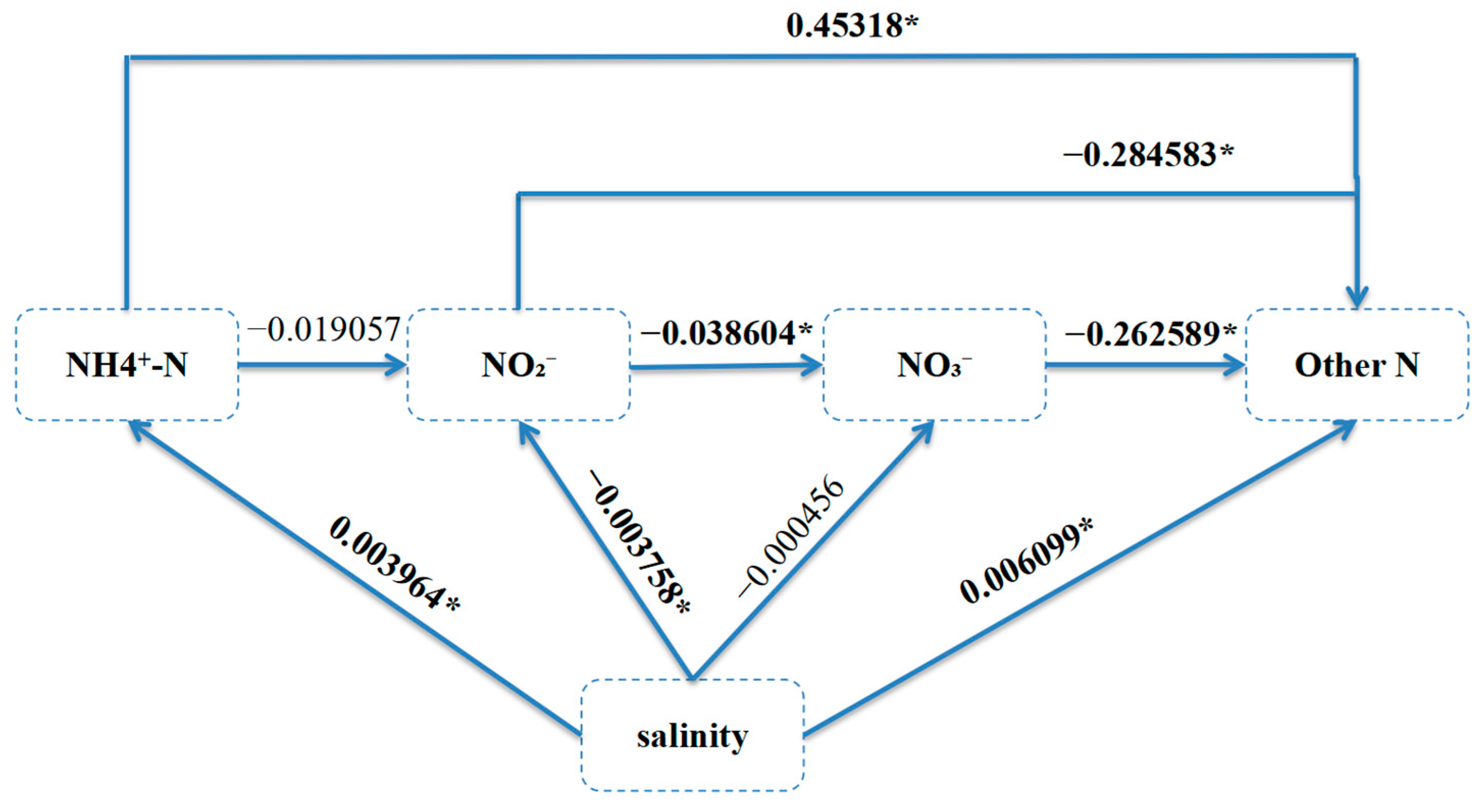
In this study, direct and varying effects among salinity, NH4+-N, NO2−-N, NO3−-N, and other forms of nitrogen (other_N) were analyzed using structural equation modeling (SEM). The variance estimates for all variables were statistically significant (p < 0.05), indicating there were significant interactions between these variables.
Salinity has different effects on the transport and transformation of different forms of nitrogen. The figure above shows statistically significant positive correlations between salinity and both NH4+-N and other forms of nitrogen (other_N). Specifically, for every 1 unit increase in salinity, the level of ammonia nitrogen increases by 0.004 units, while the increase in other_N was more significant (correlation coefficient of 0.006). In contrast, salinity had a significant inhibitory effect on NO2−-N with a correlation coefficient of −0.0038, suggesting that an increase in salinity may lead to a decrease in NO2−-N concentration.
In terms of variance effects, the variance estimates for all variables were statistically significant (p < 0.05). Salinity (salinity) had a slight positive but statistically significant effect on NH4+-N with a correlation coefficient of 0.003964, indicating that the concentration of NH4+-N increased slightly with increasing salinity. Salinity had a slightly positive effect on other_N with a correlation coefficient of 0.006099. Salinity had a significant negative effect on NO2−-N with a correlation coefficient of −0.003758, indicating that an increase in salinity may lead to a decrease in NO2−-N concentration. NH4+-N had a significant positive effect on other nitrogen (other_N), with a correlation coefficient of 0.45318, which is a relatively strong positive relationship, indicating that an increase in ammonia nitrogen significantly enhances the concentration of other forms of nitrogen. NO2−-N had a significant negative effect on other nitrogen (other_N) with a correlation coefficient of −0.284583, indicating that an increase in NO2−-N can lead to a decrease in other forms of nitrogen. NO3−-N also had a significant negative effect on other_N, with a correlation coefficient of −0.262589, which also indicates that an increase in NO3-N may lead to a decrease in other forms of nitrogen.
The direct and variance effects between salinity and different nitrogen forms were analyzed thoroughly using SEM (
Table 3,
Figure 3), which revealed the multi-scale regulation mechanism of salinity in the nitrogen cycle. The results showed that the direct effects of salinity on nitrogen forms were mainly reflected in the following: Firstly, for every 1 unit increase in salinity, the concentration of NH
4+-N increased significantly by about 0.004 units (correlation coefficient of 0.003964), which can be attributed to the fact that salinity inhibited the activity of nitrifying microorganisms, thus slowing down the conversion process of ammonia nitrogen into nitrite and NO
3-N. Secondly, the positive effect of salinity on other forms of nitrogen (other_N) was more significant (correlation coefficient of 0.006099), suggesting that salinity may reduce the loss of gaseous nitrogen by inhibiting denitrification. In addition, salinity significantly decreased NO
2−-N concentration (correlation coefficient of −0.003758), which may be due to the rapid oxidation (to NO
3−-N) or reduction (to N
2O/N
2) of nitrite under highly saline conditions. In terms of interactions between different nitrogen forms, ammoniacal nitrogen had a significant positive effect on other nitrogen forms, with a significant increase of 0.453 units for every 1 unit increase in ammoniacal nitrogen concentration (correlation coefficient of 0.45318), emphasizing the position of ammoniacal nitrogen as a ‘core node’ in the nitrogen cycle, possibly through nitrification (NH
4+→NO
2−→NO
3−), thus driving the transformation of nitrogen forms. In addition, there was a strong negative effect (correlation coefficient of −0.284583) between NO
2−-N and other forms of nitrogen, which may be due to the rapid oxidation of nitrite to NO
3-N or its participation in the denitrification process, resulting in the depletion of other forms of nitrogen. NO
3−-N exhibited a significant negative feedback effect on other nitrogen forms (correlation coefficient of −0.262589), potentially inhibiting upstream nitrogen transformation.
3.1.3. Analysis of the Effect of Salinity on the Conversion Rate of the Nitrogen Cycle
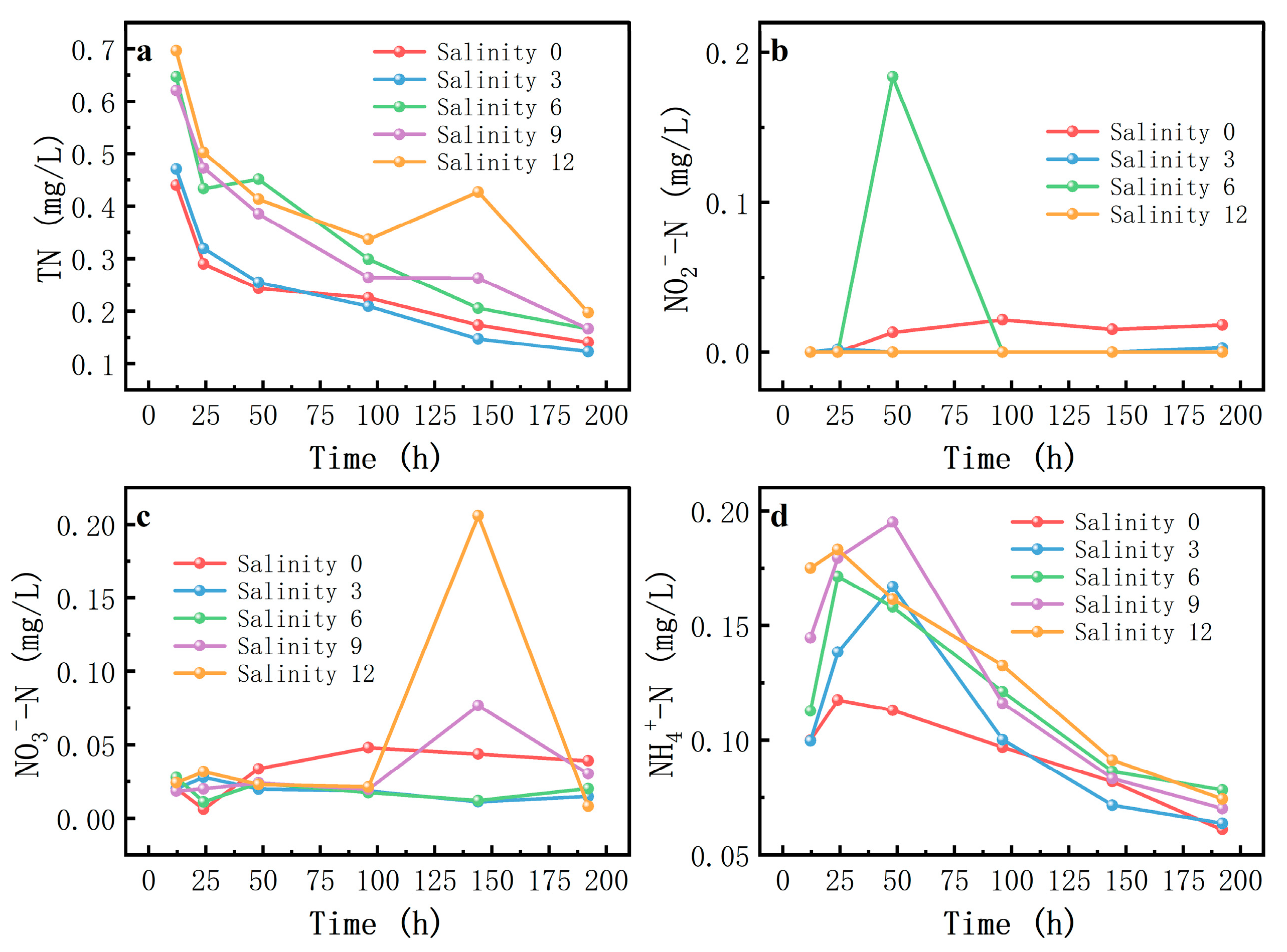
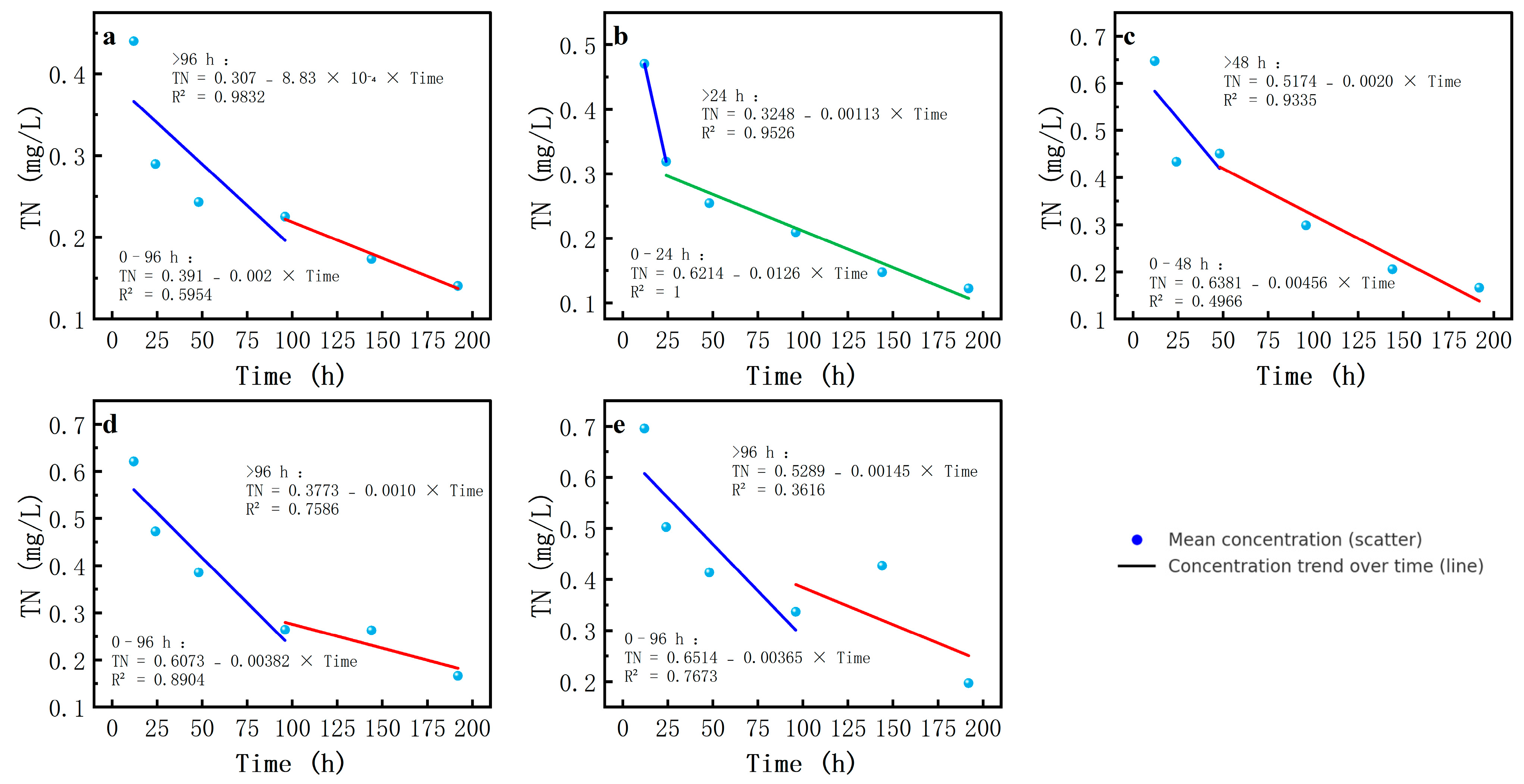
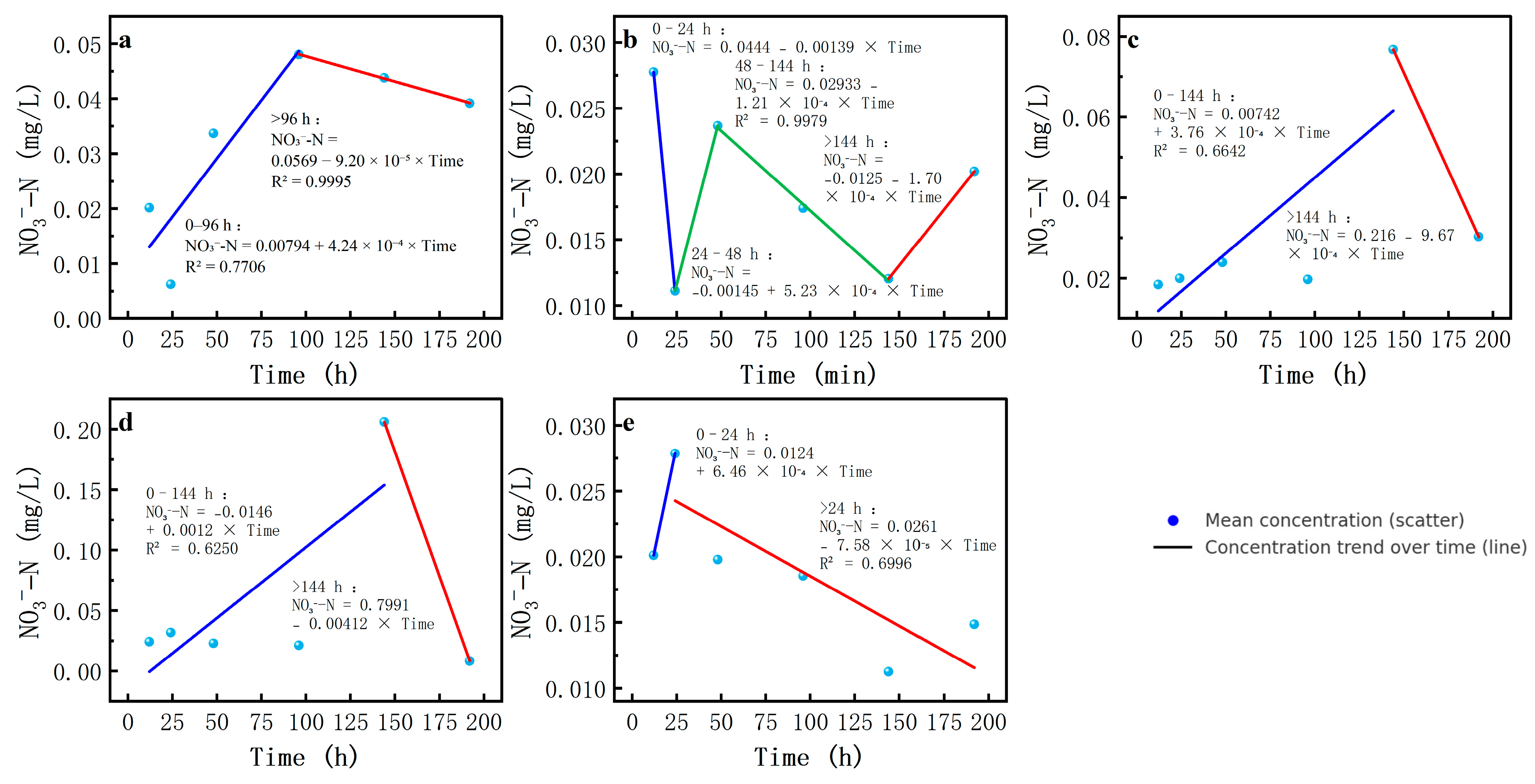
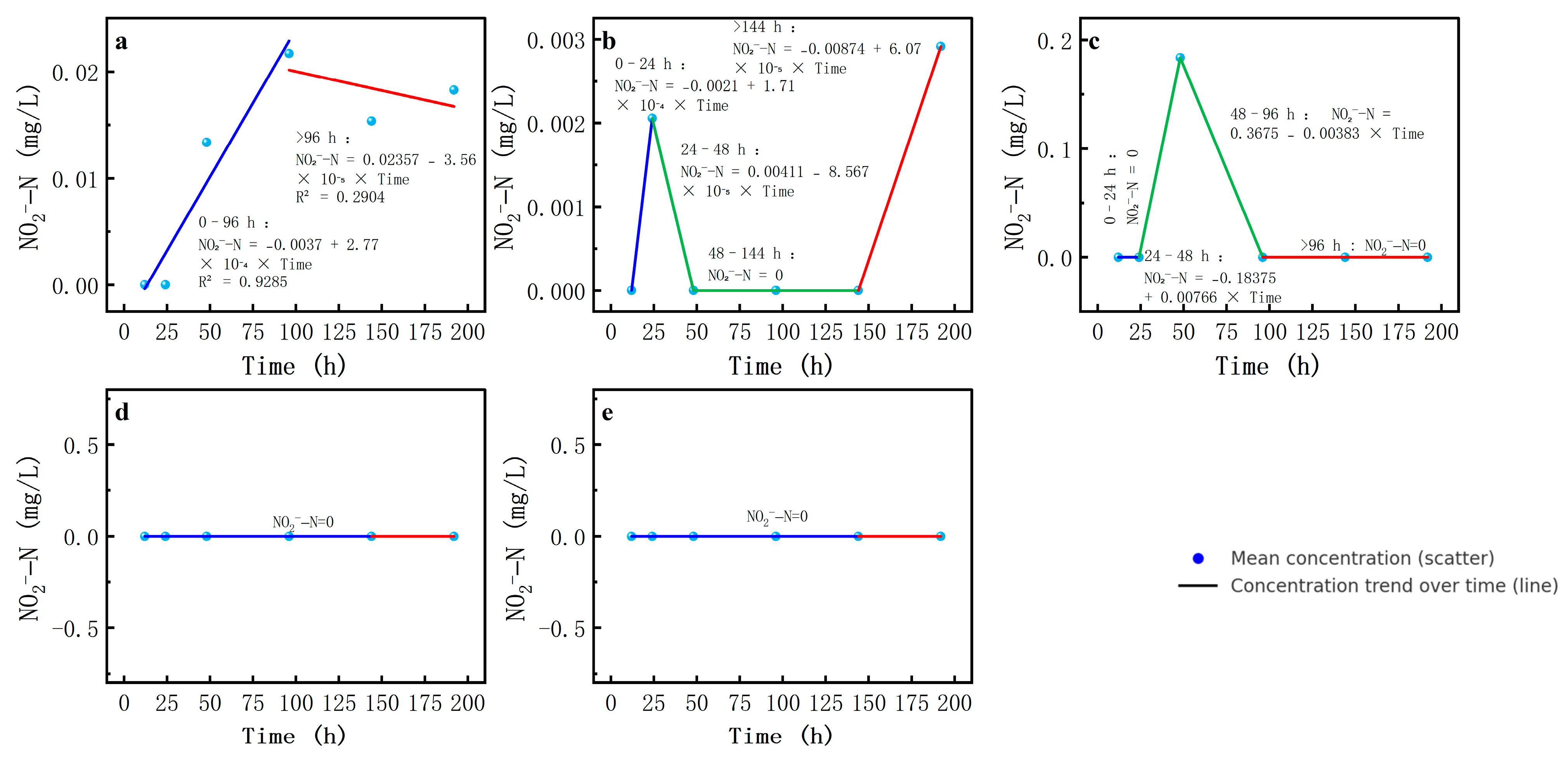
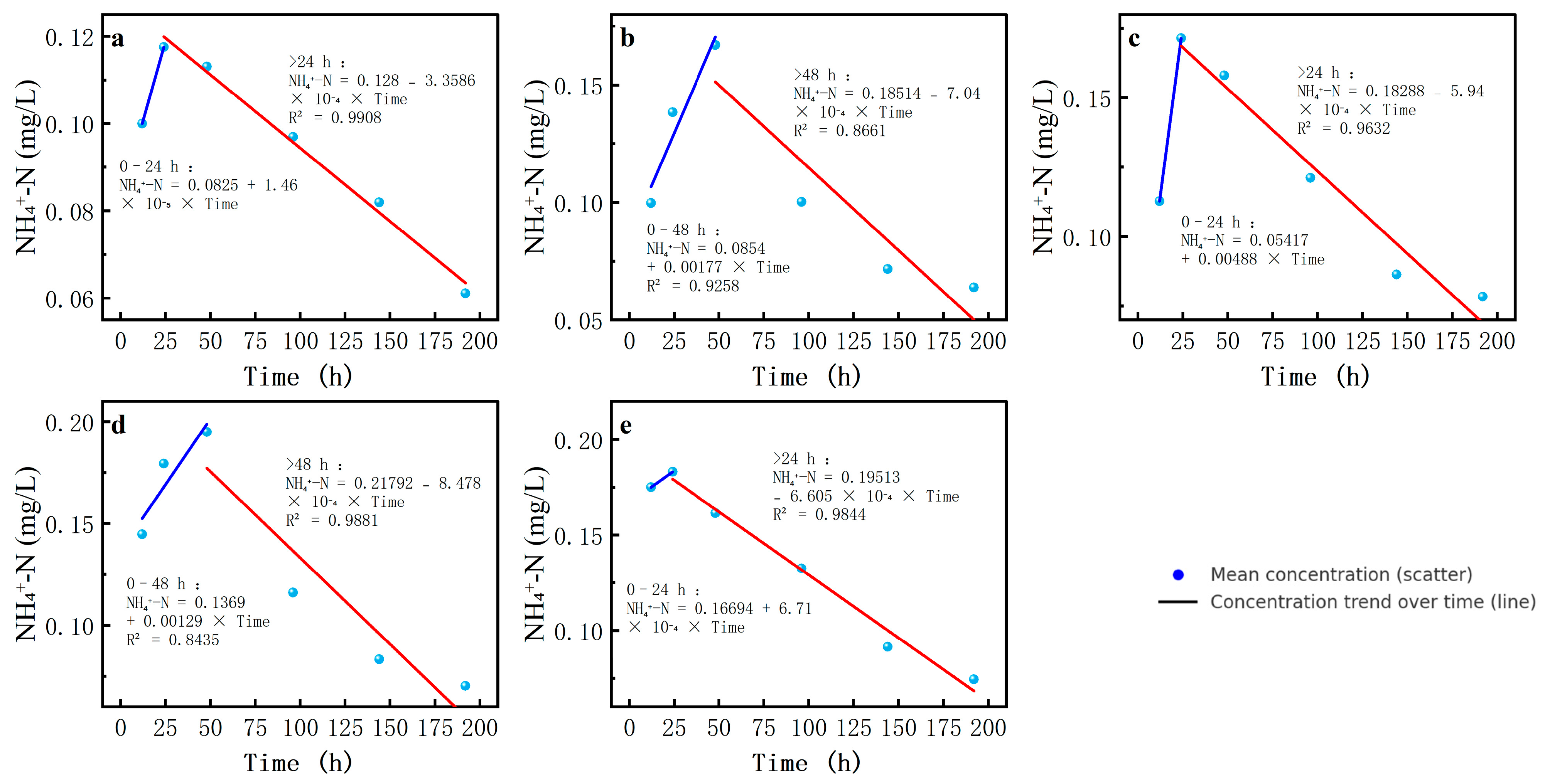
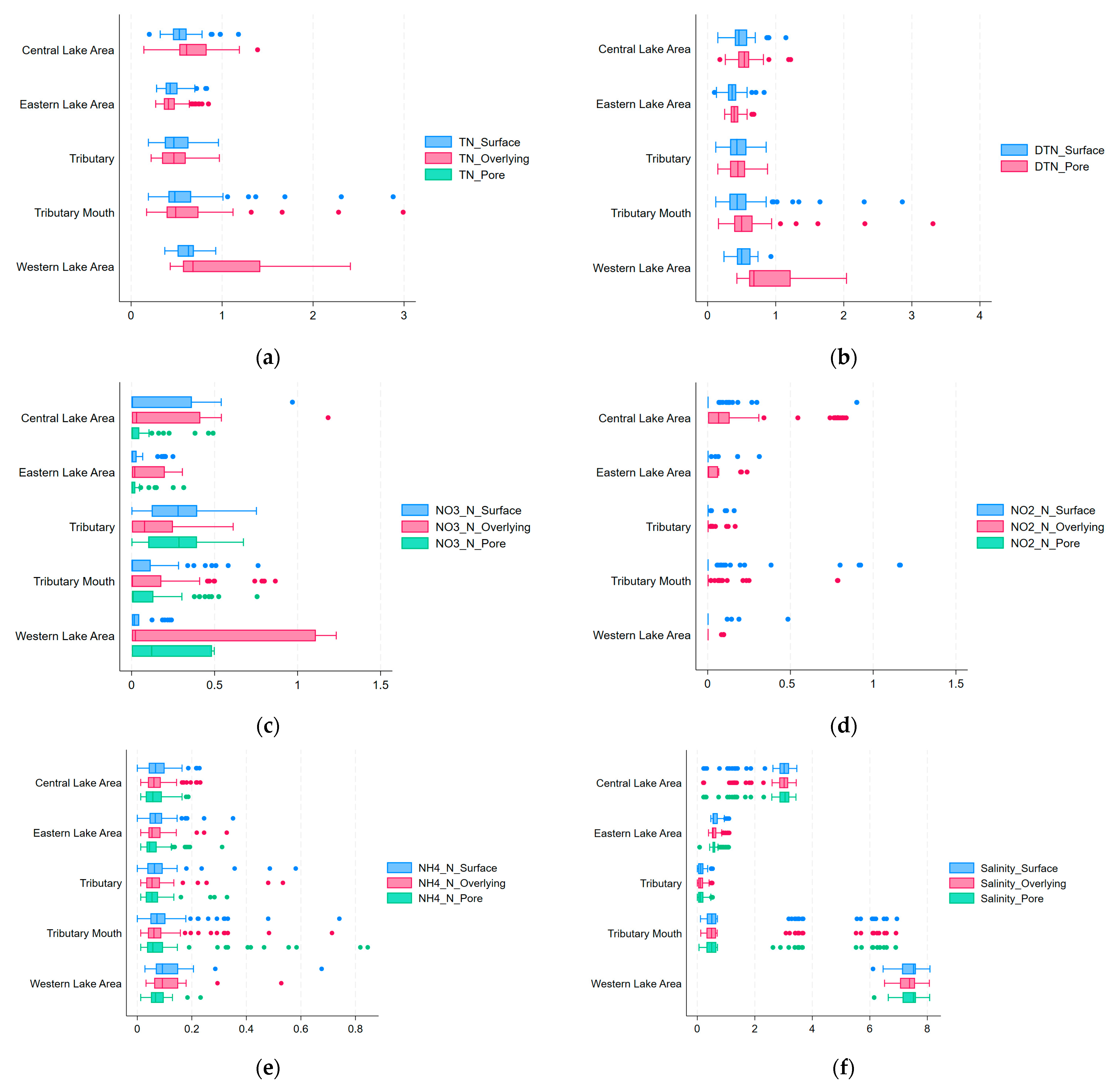
A visualization of different nitrogen forms obtained from the different salinity levels at different time points is shown in
Figure 4. The image shows various characteristics of the different nitrogen concentrations in the different forms.
Firstly, the TN concentration exhibited a consistent declining trend throughout the experiment, with salinity-dependent attenuation rates. Under freshwater conditions (0‰ salinity), the TN concentration decreased more slowly, whereas with increasing salinity, the decrease in the TN concentration accelerated, especially at a salinity of 12, where the TN concentration decreased more evidently in the late stage of the experiment.
Secondly, NO2−-N concentrations were low at the beginning of the experiment but peaked at some salinity levels and then gradually decreased. At a salinity of 6, the NO2−-N concentration increased rapidly to the highest value at the beginning of the experiment and then decreased sharply, while under other salinity scenarios, the concentration change was relatively smooth and the peak occurred at a lower time and magnitude.
Thirdly, the changes in NO3−-N revealed complex salinity-mediated modulations. At a salinity of 0, the NO3−-N concentration was relatively stable and low, while at a salinity of 12, the concentration showed a significant peak in the process of experiment, followed by a rapid decline. At other salinities, the magnitude of the change in NO3−-N concentration was relatively small.
Fourthly, the NH4+-N concentration was high at the beginning of the experiment and then gradually decreased, but the rate of decrease and the final stabilized concentration differed at different salinity levels. In the 0-salinity scenario, the ammonia nitrogen concentration decreased slowly and finally stabilized at a relatively high level, while as the salinity increased, the ammonia nitrogen concentration decreased faster, especially under conditions with salinities of 9 and 12. NH4+-N concentration decreased more significantly in the late stage of the experiment and finally stabilized at a lower concentration level.
In order to describe the cyclic transformation rate of the different forms of nitrogen, the forms of nitrogen were first grouped according to the type of pollutant. For those forms that showed clear inflection points, segmented regression analysis was performed, and inflection points were set accordingly. Regression analysis was used to obtain the regression coefficients of each group and then to calculate the release rate constants (k-values) of the nitrogen forms. Based on these rate constants, the cyclic transformation rates of nitrogen forms at different salinities were further analyzed to determine the salinity thresholds. The specific analysis results of the changes in concentrations under different haline gradients are given below.
TN concentrations decreased over time, while salinity significantly affected the rate of decline. At a salinity of 0, TN decreased slowly because the pure water was unsuitable for the growth of associated bacteria. The absolute k-value of the TN concentration was highest at a salinity of 3‰, indicating a greater nitrogen loss to the atmosphere (
Table 4,
Figure 5).
NO
3−-N and NO
2−-N were the two most critical intermediates. The change in NO
3−-N concentration consisted of an initial increase followed by a decrease at salinities of 0, 3, 9, and 12‰. The increase in NO
3−-N concentration at 12‰ salinity was characterized by the largest k-value. At this salinity, the higher NO
2−-N concentration in the water body facilitated the generation of NO
3−-N through the nitrification process. In the second phase at 12‰ salinity, the absolute value of k was the largest, indicating a faster reduction in NO
3−-N, with the highest NO
3−-N reduction rate in denitrification. At other salinities, the NO
3−-N concentration varied smoothly with lower peaks, suggesting that nitrite production and consumption reached equilibrium at low and intermediate salinities (
Table 5,
Figure 6).
At 6‰ salinity, the NO
3− concentration exhibited a distinct response, rapidly increasing to a peak at the start of the experiment, then sharply decreasing. This suggests a dynamic interplay between salinity inhibition and competition between NOB and ammonia-oxidizing bacteria (AOB). As the salinity increased to 12‰, AOB-dominated nitrification became predominant, while NOB-driven processes and anammox were inhibited. Instead, with fluctuations at 6‰ salinity and possibly more N
2O production at 12‰ salinity, the reaction shifted to the following:
The reaction that produced NO
2−-N is known as nitrification, where AOB oxidizes ammonia nitrogen to nitrite:
NOB further oxidizes nitrite to NO
3−-N:
NO
2−-N had the largest absolute k-value in the second segment at 3‰ salinity. The k-value in the first segment was the highest at 6‰ salinity, indicating the fastest increase in NO
2−-N concentration at this salinity. This observed peak in NO
2−-N concentration at around 6‰ salinity, followed by a sharp decline at higher salinities, may be explained by a trade-off between AOB tolerance and NOB inhibition. Specifically, AOB appear to maintain activity at moderate salinity levels, leading to nitrite accumulation, whereas elevated salinity levels increasingly inhibit NOB activity, reducing the oxidation of nitrite to nitrate and resulting in the observed pattern. At high salinities of 9‰ and 12‰, NO
2−-N concentrations were essentially below the detection limit. In contrast to other studies in wastewater treatment environments, where a significant decrease in AOB activity was found at salinities > 10‰, this experiment suggests that low salinities (9‰ and 12‰) may be sufficient to inactivate AOB and inhibit ammonia oxidation reactions in natural environments such as lake sediment discharges (
Table 6,
Figure 7).
The NH
4+-N concentration first showed an increasing and then a decreasing trend, which was significantly influenced by salinity. The NH
4+-N concentration increased most rapidly at 6‰ salinity, while the decrease was fastest at 9‰ salinity, primarily due to nitrification or anammox processes (
Table 7,
Figure 8).