Mangrove Habitat Health Assessment in the Sanya River: Multidimensional Analysis of Diatom Communities and Physicochemical Water Properties
Abstract
1. Introduction
2. Materials and Methods
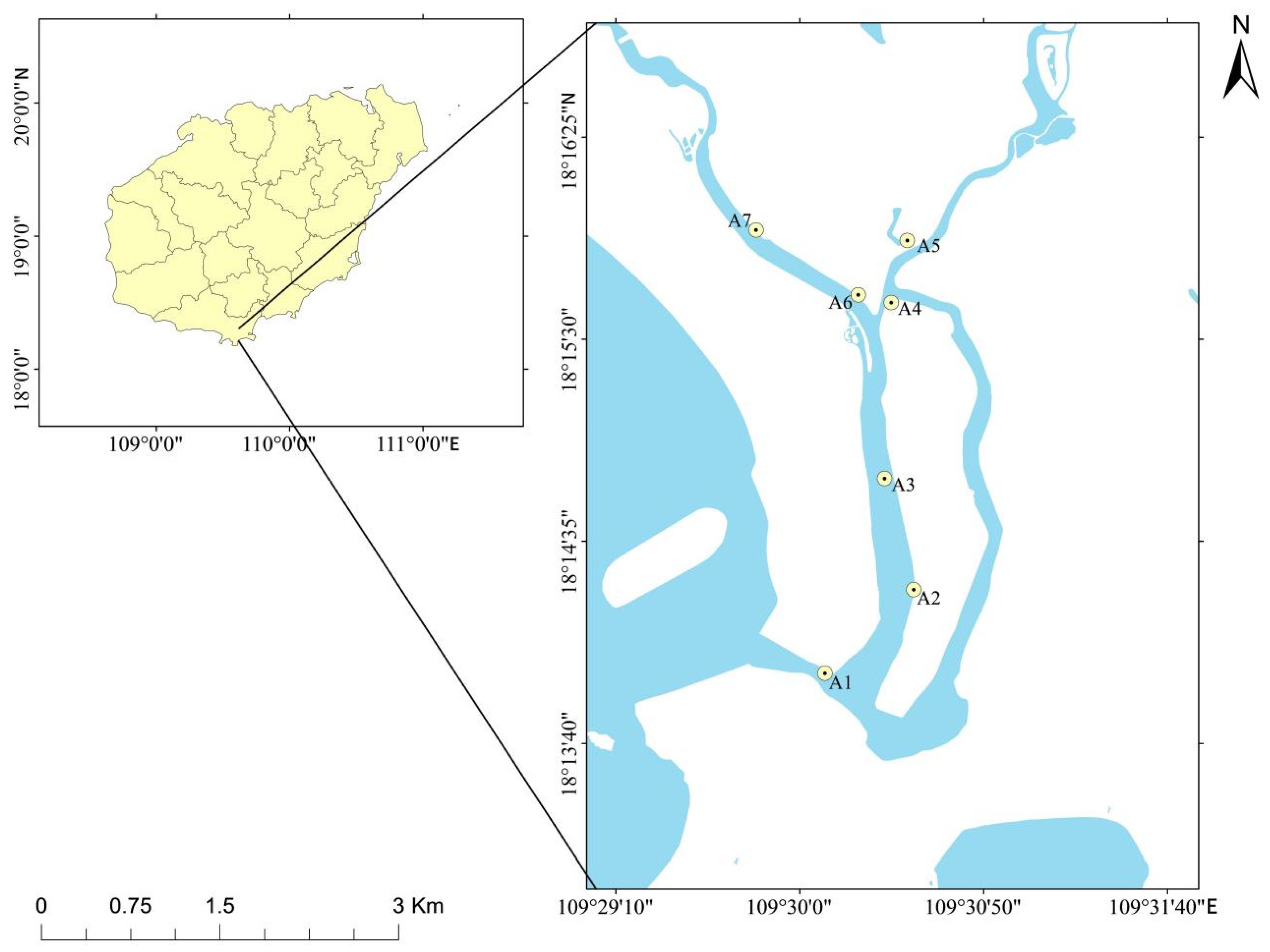
2.1. Description of Study Site
2.2. Phytoplankton Sample Collection and Processing
2.3. Water Quality Parameter Assessment
2.4. Data Processing and Analysis
3. Results
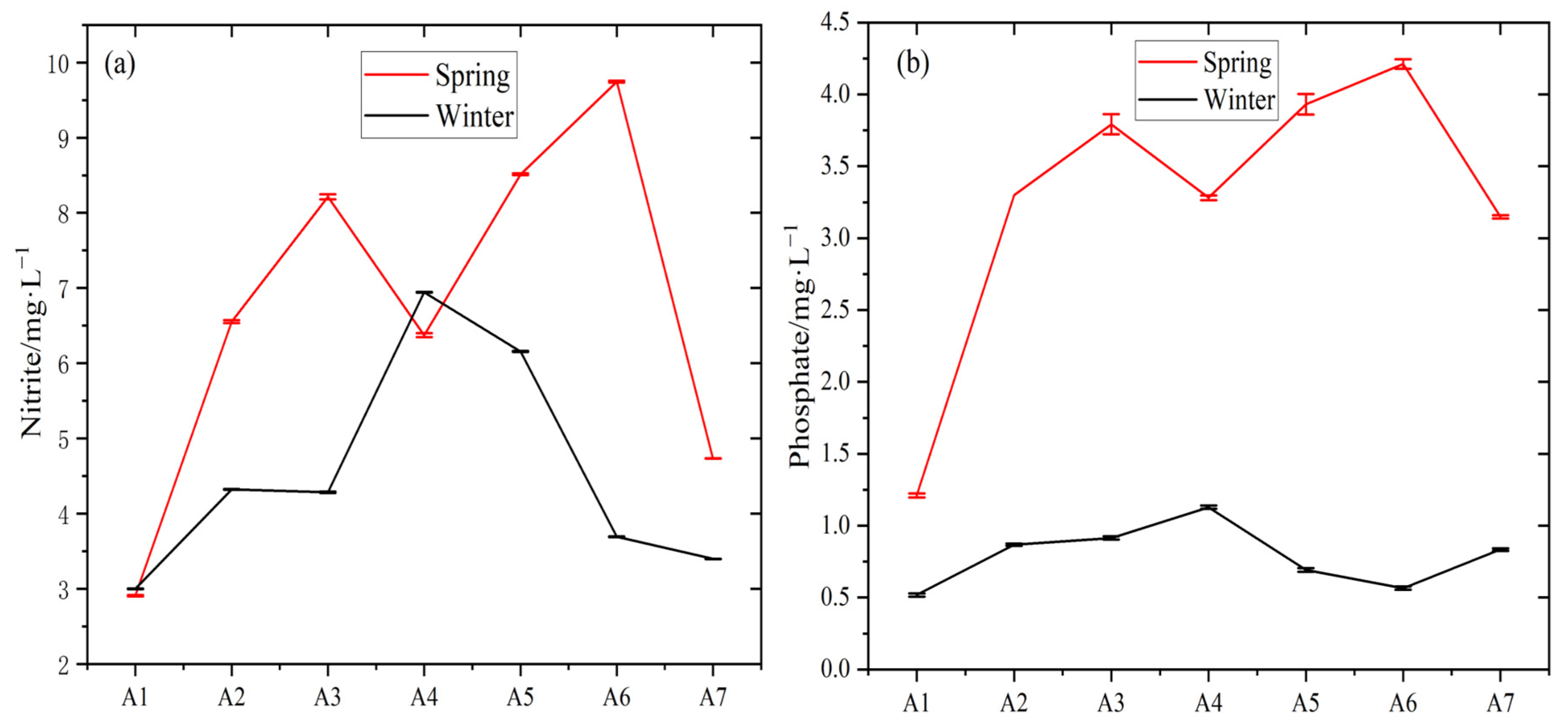
3.1. Physicochemical Water Properties
3.2. Diatom Community Structure in the Sanya River
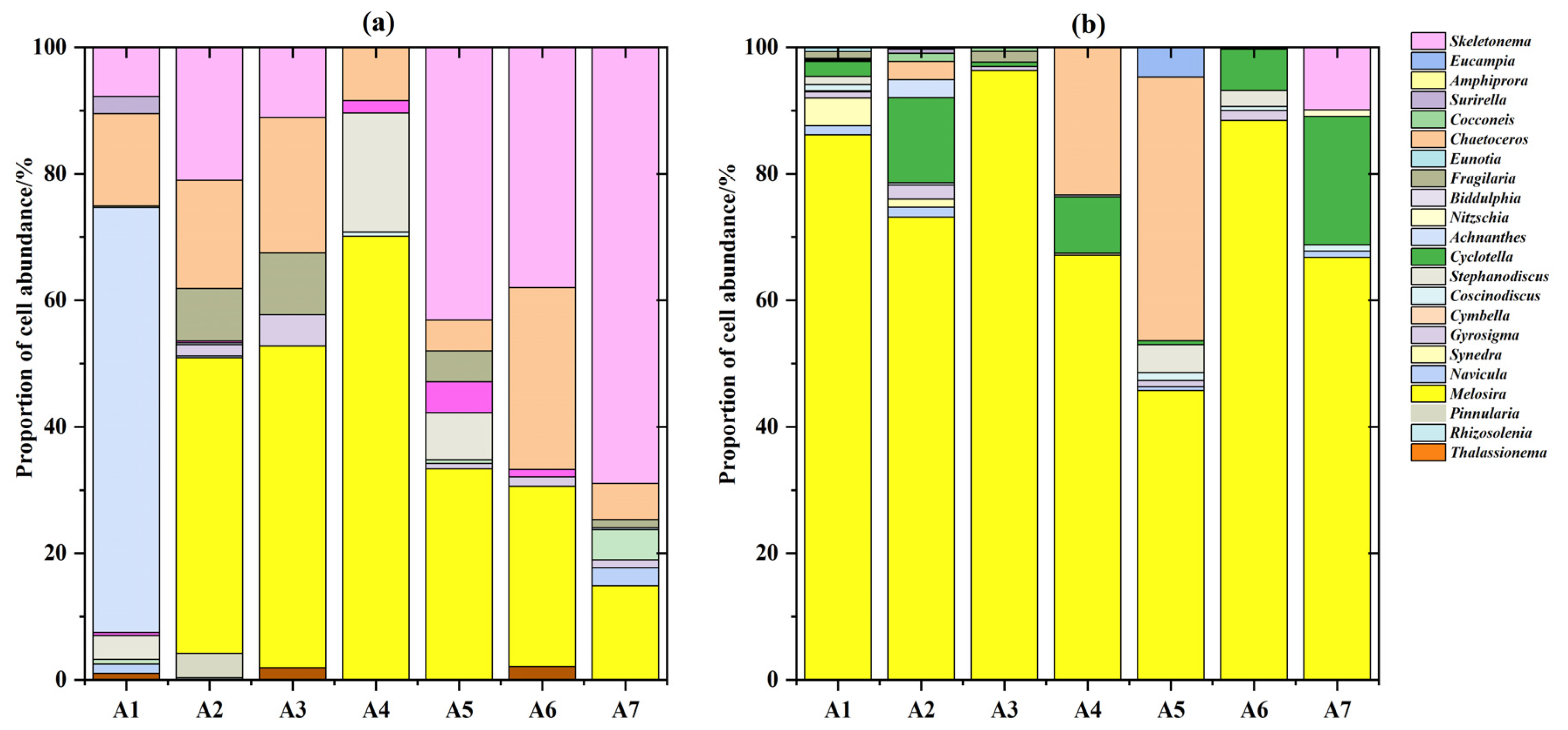
3.2.1. Diatom Species Composition
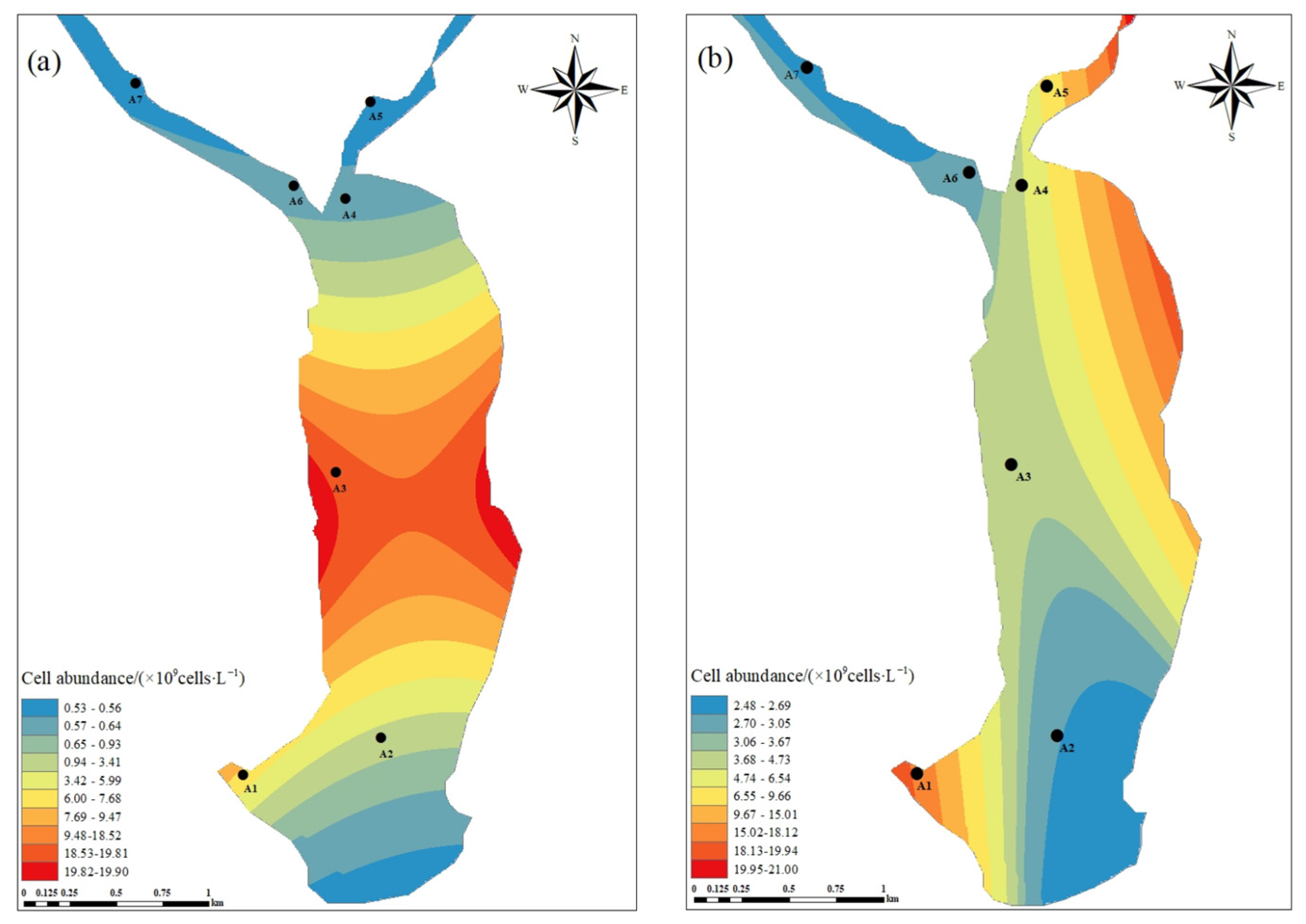
3.2.2. Cell Abundance of Phytoplankton
3.2.3. Dominant Diatom Species
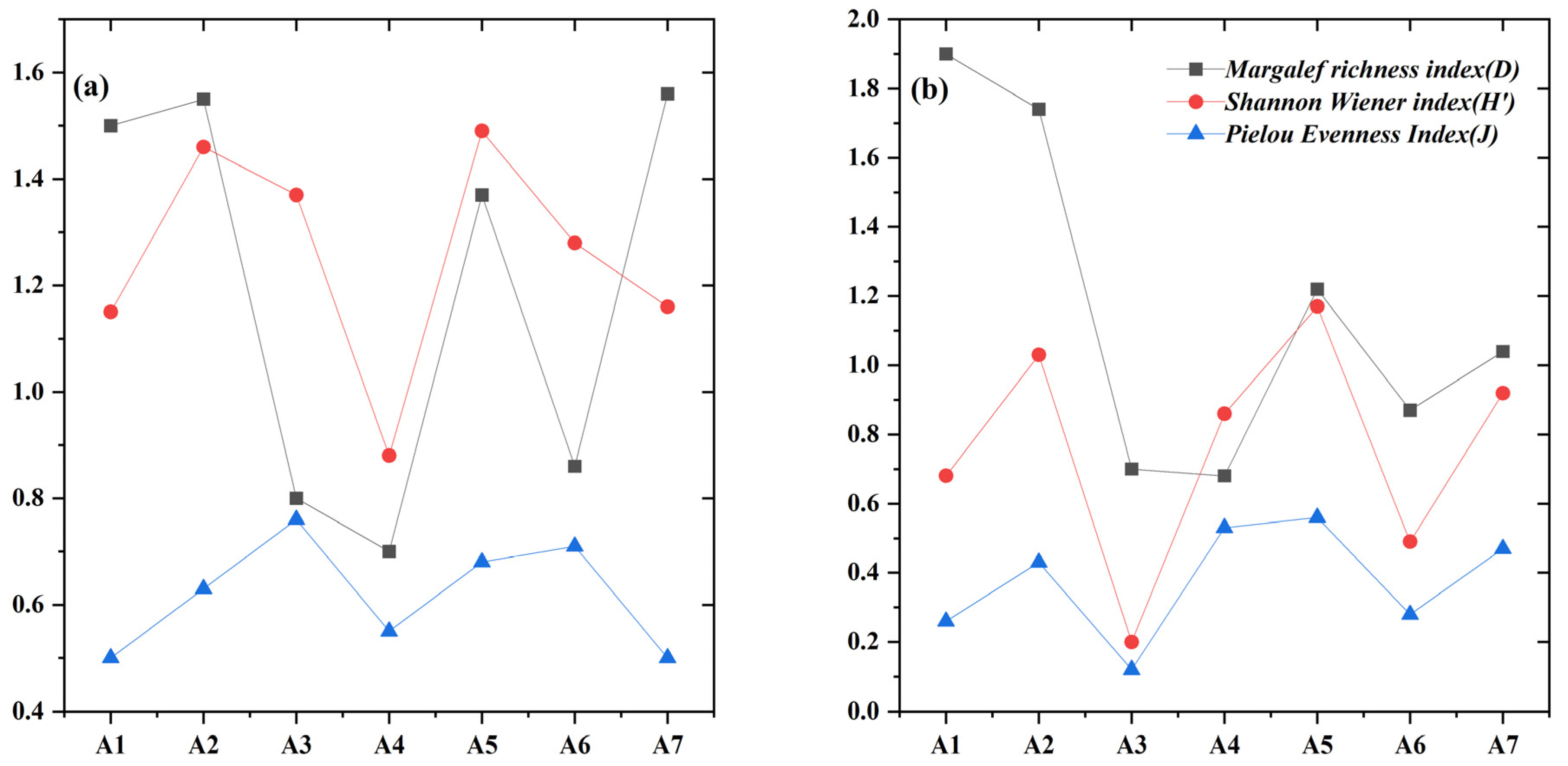
3.2.4. Phytoplankton Diversity Indices
4. Discussion
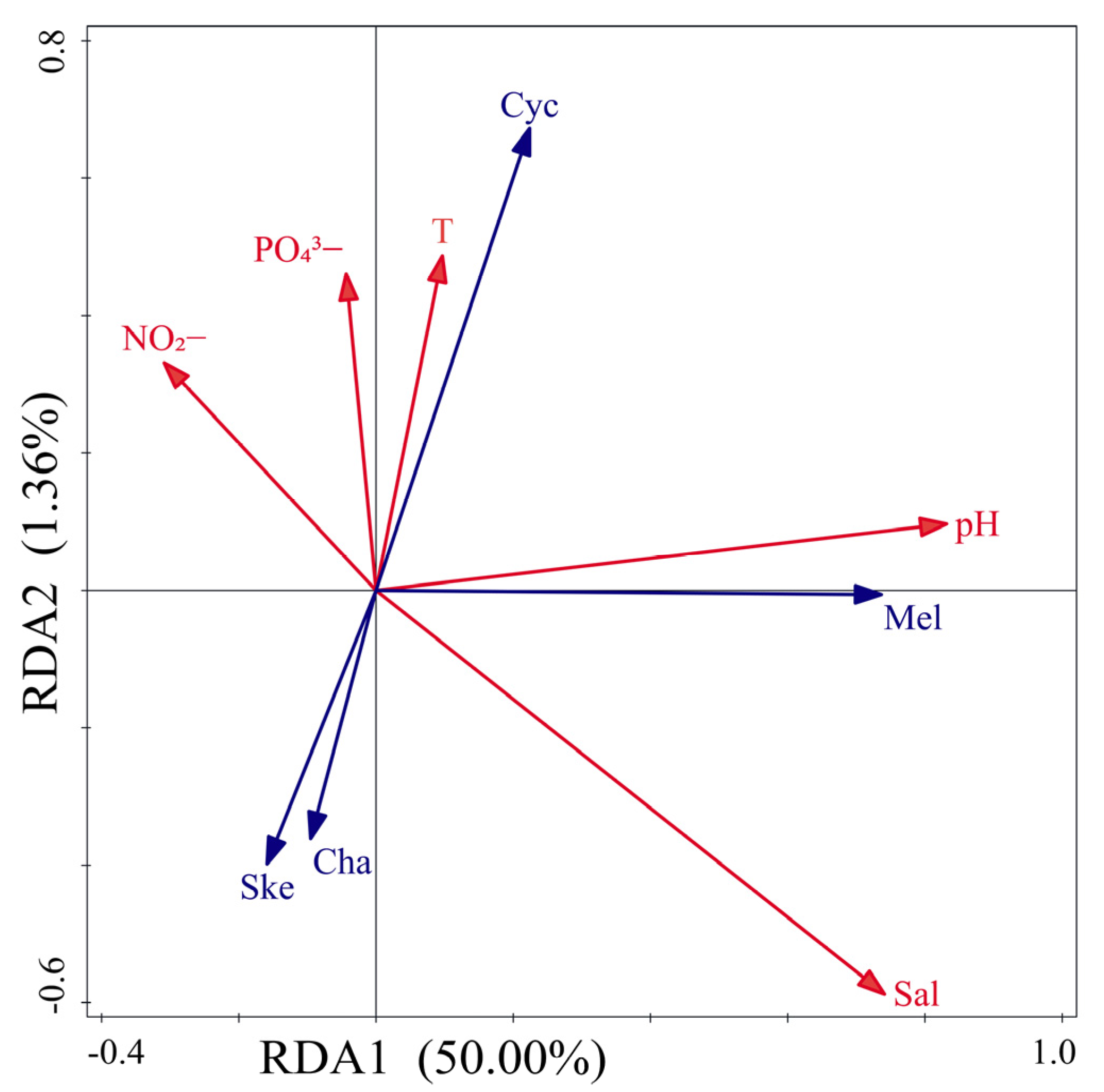
4.1. Relationships Between Diatom Communities and Environmental Factors
4.2. Effects of Seasonal Changes and Human Activities on the Structure of Diatom Communities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; He, Y.; Li, W.; Guo, X.; Xiao, C.; Zhao, T. High-throughput sequencing of diatom community, its spatial and temporal variation and interrelationships with physicochemical factors in danjiangkou reservoir, China. Water 2022, 14, 1609. [Google Scholar] [CrossRef]
- Luo, X.; Pan, K.; Wang, L.; Li, M.; Li, T.; Pang, B.; Kang, J.; Fu, J.; Lan, W. Anthropogenic inputs affect phytoplankton communities in a subtropical estuary. Water 2022, 14, 636. [Google Scholar] [CrossRef]
- Rana, A.S.; Verma, P.; Mittal, P.; Mahajan, M. Investigation of Diatom Communities in Highlands of Western Himalayas: Geographical and Ecological Patterns. Ann. Rom. Soc. Cell Biol. 2022, 26, 1347–1367. [Google Scholar]
- Marchetto, A.; Sforzi, T. Using benthic diatoms for estimating lake ecological quality: Comparing different taxonomic resolution. Adv. Oceanogr. Limnol. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, Q.; Burford, M.A.; Sheldon, F.; Bunn, S.E. Benthic diatom based indices for water quality assessment in two subtropical streams. Front. Microbiol. 2017, 8, 601. [Google Scholar] [CrossRef] [PubMed]
- Potapova, M.; Charles, D.F. Diatom metrics for monitoring eutrophication in rivers of the United States. Ecol. Indic. 2007, 7, 48–70. [Google Scholar] [CrossRef]
- Bennion, H.; Battarbee, R.W.; Sayer, C.D.; Simpson, G.L.; Davidson, T.A. Defining reference conditions and restoration targets for lake ecosystems using palaeolimnology: A synthesis. J. Paleolimnol. 2011, 45, 533–544. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Ralph, P.J. Microalgal bioremediation of emerging contaminants—Opportunities and challenges. Water Res. 2019, 164, 114921. [Google Scholar] [CrossRef]
- Xue, H.; Wang, L.; Zhang, L.; Wang, Y.; Meng, F.; Xu, M. Exploration of Applicability of Diatom Indices to Evaluate Water Ecosystem Quality in Tangwang River in Northeast China. Water 2023, 15, 3695. [Google Scholar] [CrossRef]
- Baird, M.E.; Middleton, J.H. On relating physical limits to the carbon: Nitrogen ratio of unicellular algae and benthic plants. J. Mar. Syst. 2004, 49, 169–175. [Google Scholar] [CrossRef]
- Sundbäck, K.; Linares, F.; Larson, F.; Wulff, A.; Engelsen, A. Benthic nitrogen fluxes along a depth gradient in a microtidal fjord: The role of denitrification and microphytobenthos. Limnol. Oceanogr. 2004, 49, 1095–1107. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef]
- Meyer, A.; Alric, B.; Dézerald, O.; Billoir, E.; Coulaud, R.; Larras, F.; Mondy, C.P.; Usseglio-Polatera, P. Linking Micropollutants to Trait Syndromes across Freshwater Diatom, Macroinvertebrate, and Fish Assemblages. Water 2022, 14, 1184. [Google Scholar] [CrossRef]
- Dembowska, E.A. The Use of Phytoplankton in the Assessment of Water Quality in the Lower Section of Poland’s Largest River. Water 2021, 13, 3471. [Google Scholar] [CrossRef]
- Im, J.-K.; Sim, Y.-B.; Hwang, S.-J.; Byeon, M.-S.; Kang, T.-G. Temporal and Seasonal Variations in a Phytoplankton Community Structure in Artificial Lake Uiam, South Korea. Water 2023, 15, 4118. [Google Scholar] [CrossRef]
- Fariman, A.G.; Abir, S.; Dolatabadi, F.; Naseri, A.; Abedi, E.; Sayareh, F. A comparative analysis of phytoplankton assemblages in mangrove estuaries of the Persian Gulf and the Sea of Oman. Reg. Stud. Mar. Sci. 2025, 81, 103968. [Google Scholar]
- Bohra, V.; Tam, F.Y.; Chen, L.; Lai, K.K.-Y.; Lam, W.; Xu, S.J.-L.; Zhou, H.-C.; Lang, T.; Lee, C.-L.; Lee, F.W.-F. Untangling Structural and Functional Diversity of Prokaryotic Microbial Assemblage on Mangrove Pneumatophores. J. Mar. Sci. Eng. 2024, 12, 802. [Google Scholar] [CrossRef]
- Wang, Q. Development of a Periphytic Diatom-Based Comprehensive Diatom Index for Assessing the Trophic Status of Lakes in the Lower Reaches of the Yangtze River, China. Water 2021, 13, 3570. [Google Scholar] [CrossRef]
- Nunes, M.; Lemley, D.A.; Machite, A.; Adams, J.B. Benthic diatom diversity in microtidal mangrove estuaries. Mar. Pollut. Bull. 2024, 206, 116706. [Google Scholar] [CrossRef]
- Alongi, D.M. The role of bacteria in nutrient recycling in tropical mangrove and other coastal benthic ecosystems. Hydrobiologia 1994, 285, 19–32. [Google Scholar] [CrossRef]
- Sullivan, M.J. Applied diatom studies in estuaries and shallow coastal environments. In The Diatoms: Application for the Environmental and Earth Sciences; Cambridge University Press: Cambridge, UK, 1999; pp. 334–351. [Google Scholar]
- Iftekhar. Functions and development of reforested mangrove areas: A review. Int. J. Biodivers. Sci. Manag. 2008, 4, 1–14. [Google Scholar] [CrossRef]
- Mizanur, M.R.; Martin, Z.; Imran, A.; Donato, D.; Kanzaki, M.; Xu, M. Co-benefits of protecting mangroves for biodiversity conservation and carbon storage. Nat. Commun. 2021, 12, 3875. [Google Scholar]
- Liao, B.W.; Zhang, Q.M. Distribution, area and species composition of mangroves in China. Wetl. Sci. 2014, 12, 435–440. [Google Scholar]
- Chen, X.; Wang, Y.; Jiang, L.; Huang, X.; Huang, D.; Dai, W.; Cai, Z.; Wang, D. Water quality status response to multiple anthropogenic activities in urban river. Environ. Sci. Pollut. Res. 2022, 30, 3440–3452. [Google Scholar] [CrossRef]
- Lin, J.; Zhu, H.; Liu, G.; Huang, M.; Xie, Z. Composite Scheme of Comprehensive Improvement for Urban Rivers. In Proceedings of the 6th International Conference on Advances in Energy, Environment and Chemical Engineering, Jinan, China, 19 June 2020. [Google Scholar]
- Ning, Y.F.; Zhang, J.T.; Huang, J.H.; Long, H.-F.; Huang, Q.-S. Systematic treatment of urban river pollution. In Proceedings of the 5th International Conference on Advances in Energy Resources and Environment Engineering, Chongqing, China, 6–8 December 2019. [Google Scholar]
- Zhou, W.H.; Li, T.; Cai, C.H.; Huang, L.; Wang, H.; Xu, J.; Dong, J.; Zhang, S. Spatial and temporal dynamics of phytoplankton and bacterioplankton biomass in Sanya Bay, northern South China Sea. J. Environ. Sci. 2009, 21, 595–603. [Google Scholar] [CrossRef]
- Salmo, S.G.; Lovelock, C.; Duke, N.C. Vegetation and soil characteristics as indicators of restoration trajectories in restored mangroves. Hydrobiologia 2013, 720, 1–18. [Google Scholar] [CrossRef]
- Lewis, R.R., III; Brown, B.M.; Flynn, L.L. Methods and criteria for successful mangrove forest rehabilitation. In Coastal Wetlands; Elsevier: Amsterdam, The Netherlands, 2019; pp. 863–887. [Google Scholar]
- Zhong, Y.; Cai, M.; Cui, J.; Chen, X.; Wang, S.; Chen, Z.; Zhang, S. Spatial Distribution Patterns of Phytoplankton and Their Relationship with Environmental Factors in the Jinjiang River, China. Water 2024, 16, 1497. [Google Scholar] [CrossRef]
- Im, K.J.; Sim, B.Y.; Byun, H.J.; Park, C.-H.; Hwang, S.-J.; Kang, T.-G. The Seasonal Environmental Factors and Phytoplankton Composition of Lake Paldang, the Largest Water Source in South Korea. Water 2024, 16, 3504. [Google Scholar] [CrossRef]
- Kim, K.H.; Cho, H.I.; Hwang, A.E.; Han, B.-H.; Kim, B.-H. Advancing River Health Assessments: Integrating Microscopy and Molecular Techniques through Diatom Indices. Water 2024, 16, 853. [Google Scholar] [CrossRef]
- Pawlik, M.M.; Ficek, D. Detection of Autumnal Concentration ofCoscinodiscus graniiin the Southern Baltic—A Method for In Situ Measurement of Marine Particles. Water 2024, 16, 1091. [Google Scholar] [CrossRef]
- Wairegi, K.S. Shannon Mathematical Theory of Communication; Nokia Bell Labs: Murray Hill, NJ, USA, 2020. [Google Scholar]
- Pielou, E.C. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Buzzati-Traversoa, A.; ProvasoliLuigi. Perspectives in Marine Biology; University of California Press: Berkeley, CA, USA, 1958. [Google Scholar]
- Ulanowicz, R.E. Information theory in ecology. Comput. Chem. 2001, 25, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Lampitt, R.S.; Wishner, K.F.; Turley, C.M.; Angel, M.V. Marine snow studies in the Northeast Atlantic Ocean: Distribution, composition and role as a food source for migrating plankton. Mar. Biol. 1993, 116, 689–702. [Google Scholar] [CrossRef]
- Rahaman, S.M.B.; Golder, J.; Ms, R.; Afm, H.; Huq, K.; Begum, S.; Islam, S.S.; Bir, J. Spatial and temporal variations in phytoplankton abundance and species diversity in the Sundarbans mangrove forest of Bangladesh. J. Mar. Sci. Res. Dev. 2013, 3, 1–9. [Google Scholar]
- Tanaka, K.; Choo, P.-S. Influences of nutrient outwelling from the mangrove swamp on the distribution of phytoplankton in the Matang Mangrove Estuary, Malaysia. J. Oceanogr. 2000, 56, 69–78. [Google Scholar] [CrossRef]
- Rajkumar, M.; Perumal, P.; Prabu, V.A.; Perumal, N.V.; Rajasekar, K.T. Phytoplankton diversity in Pichavaram mangrove waters from south-east coast of India. J. Environ. Biol. 2009, 30, 489–498. [Google Scholar]
- Wang, Y.K.; Stevenson, R.J.; Metzmeier, L. Development and evaluation of a diatom-based Index of Biotic Integrity for the Interior Plateau Ecoregion, USA. J. N. Am. Benthol. Soc. 2005, 24, 990–1008. [Google Scholar] [CrossRef]
- Wu, N.; Schmalz, B.; Fohrer, N. Study progress in riverine phytoplankton and its use as bio-indicator—A review. Austin J. Hydrol. 2014, 1, 9. [Google Scholar]
- Virta, L.; Soininen, J.; Norkko, A. Biodiversity loss threatens the current functional similarity of beta diversity in benthic diatom communities. Microb. Ecol. 2021, 81, 293–303. [Google Scholar] [CrossRef]

| Parameter | Spring | Winter | p Value | ||
|---|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | ||
| Temp./(°C) | 26.0~29.3 | 28.17 ± 1.87 | 23.50~24.00 | 23.81 ± 0.20 | 0.000 ** |
| Sal./(g·kg−1) | 3.00~18.00 | 11.43 ± 4.76 | 7.00~16.00 | 10.43 ± 3.10 | 0.650 |
| pH | 7.60~7.95 | 7.69 ± 0.15 | 7.31~7.65 | 7.43 ± 0.12 | 0.004 ** |
| Phosphate/(mg·L−1) | 1.21~4.21 | 3.27 ± 0.99 | 0.52~1.13 | 0.79 ± 0.21 | 0.000 ** |
| Nitrite/(mg·L−1) | 2.91~9.75 | 6.72 ± 2.35 | 3.00~6.95 | 4.54 ± 1.47 | 0.06 |
| Dominant Species | Spring | Winter |
|---|---|---|
| Melosira sp. | 0.70 | 0.30 |
| Skeletonema sp. | - | 0.22 |
| Chaetoceros sp. | 0.02 | 0.15 |
| Cyclotella sp. | 0.14 | - |
| Achnanthes sp. | - | 0.03 |
| Fragilaria sp. | - | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; He, S.; Mai, J.; Xu, R.; He, Y.; Zhu, W.; Peng, Z.; Wu, X.; Han, Y. Mangrove Habitat Health Assessment in the Sanya River: Multidimensional Analysis of Diatom Communities and Physicochemical Water Properties. Water 2025, 17, 1770. https://doi.org/10.3390/w17121770
Yan Y, He S, Mai J, Xu R, He Y, Zhu W, Peng Z, Wu X, Han Y. Mangrove Habitat Health Assessment in the Sanya River: Multidimensional Analysis of Diatom Communities and Physicochemical Water Properties. Water. 2025; 17(12):1770. https://doi.org/10.3390/w17121770
Chicago/Turabian StyleYan, Yiwei, Sijia He, Jiaqi Mai, Ruizhe Xu, Yueqin He, Wenda Zhu, Zirui Peng, Xiangen Wu, and Yu Han. 2025. "Mangrove Habitat Health Assessment in the Sanya River: Multidimensional Analysis of Diatom Communities and Physicochemical Water Properties" Water 17, no. 12: 1770. https://doi.org/10.3390/w17121770
APA StyleYan, Y., He, S., Mai, J., Xu, R., He, Y., Zhu, W., Peng, Z., Wu, X., & Han, Y. (2025). Mangrove Habitat Health Assessment in the Sanya River: Multidimensional Analysis of Diatom Communities and Physicochemical Water Properties. Water, 17(12), 1770. https://doi.org/10.3390/w17121770







