Development of Antifouling Polyvinylidene Fluoride and Cellulose Acetate Nanocomposite Membranes for Wastewater Treatment Using a Membrane Bioreactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrospun Nanofiber Membrane
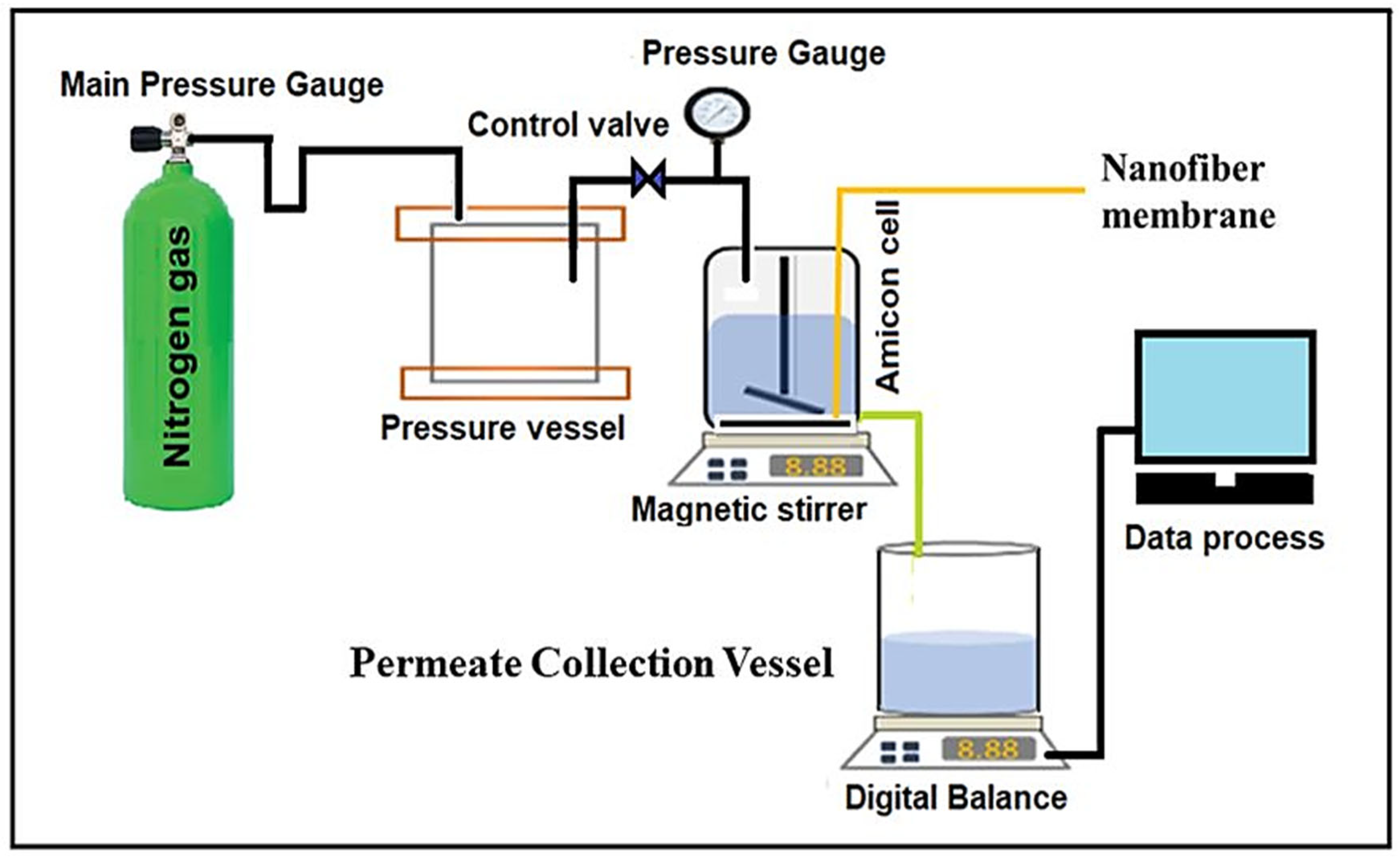
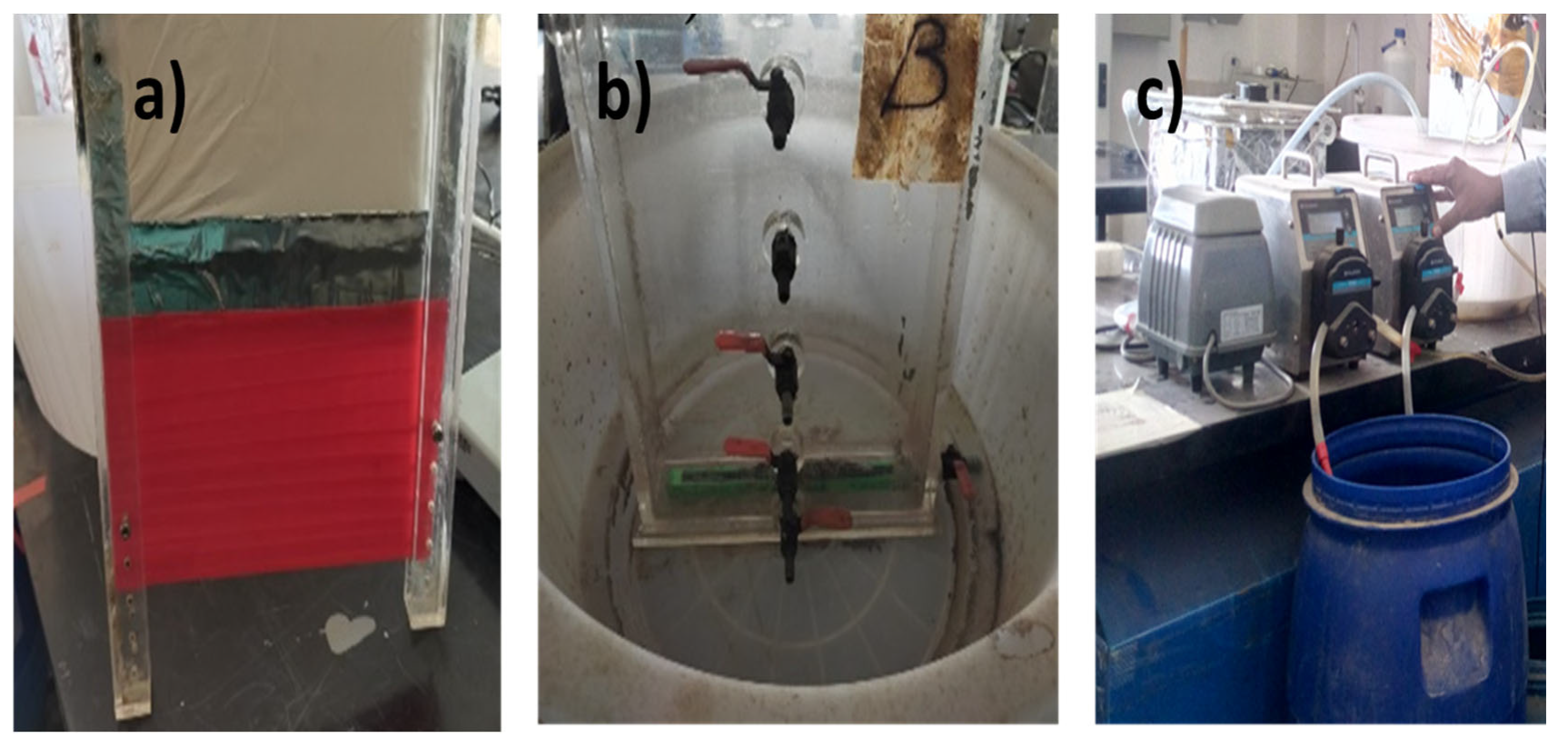
2.2. Dead-End Filtration Experimental Set-Up
2.3. Nanofiber Characterization
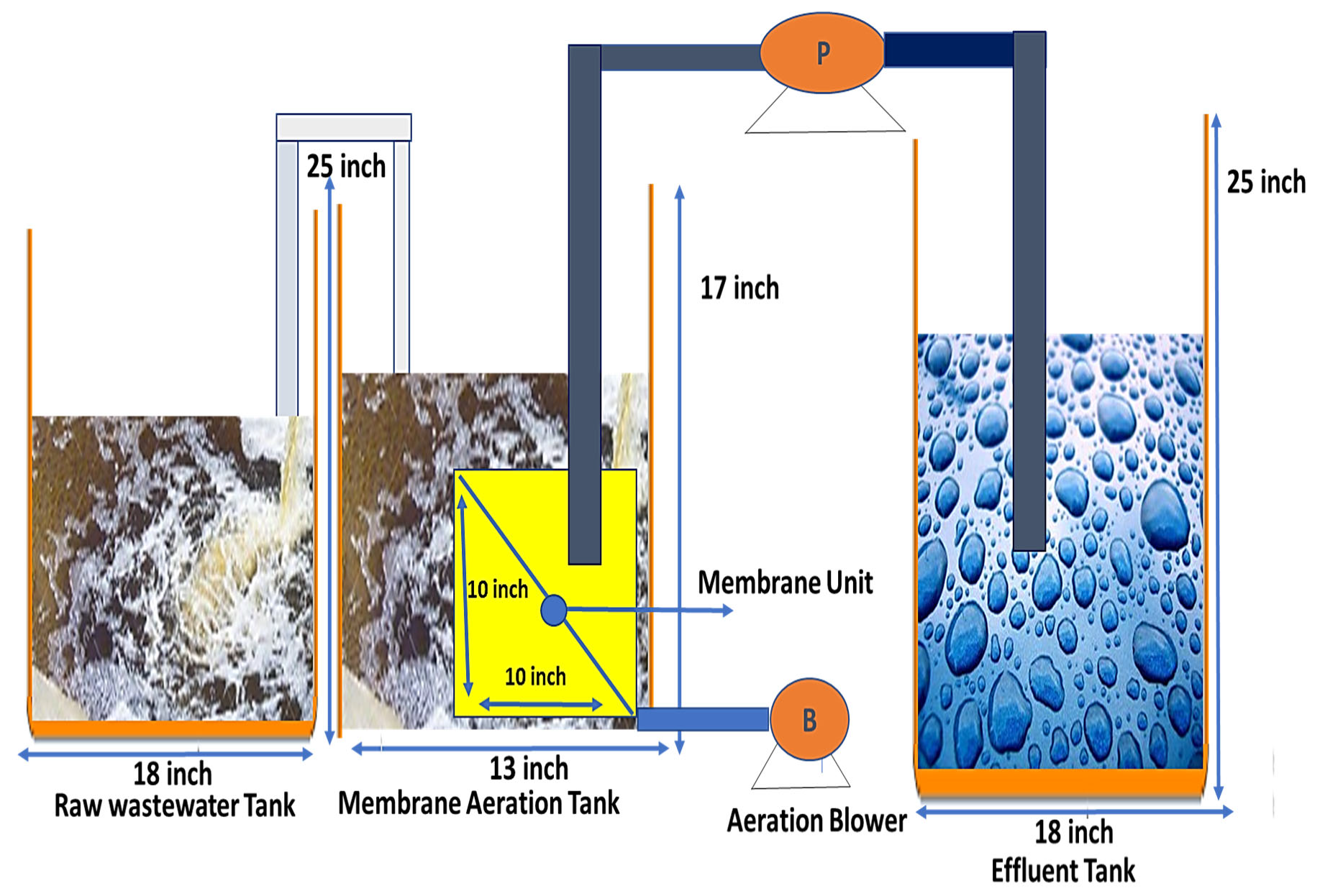
2.4. Submerged Membrane Use in Bioreactors for Wastewater Treatment
3. Results and Discussion
3.1. Surface Morphology of the Nanofiber Membranes
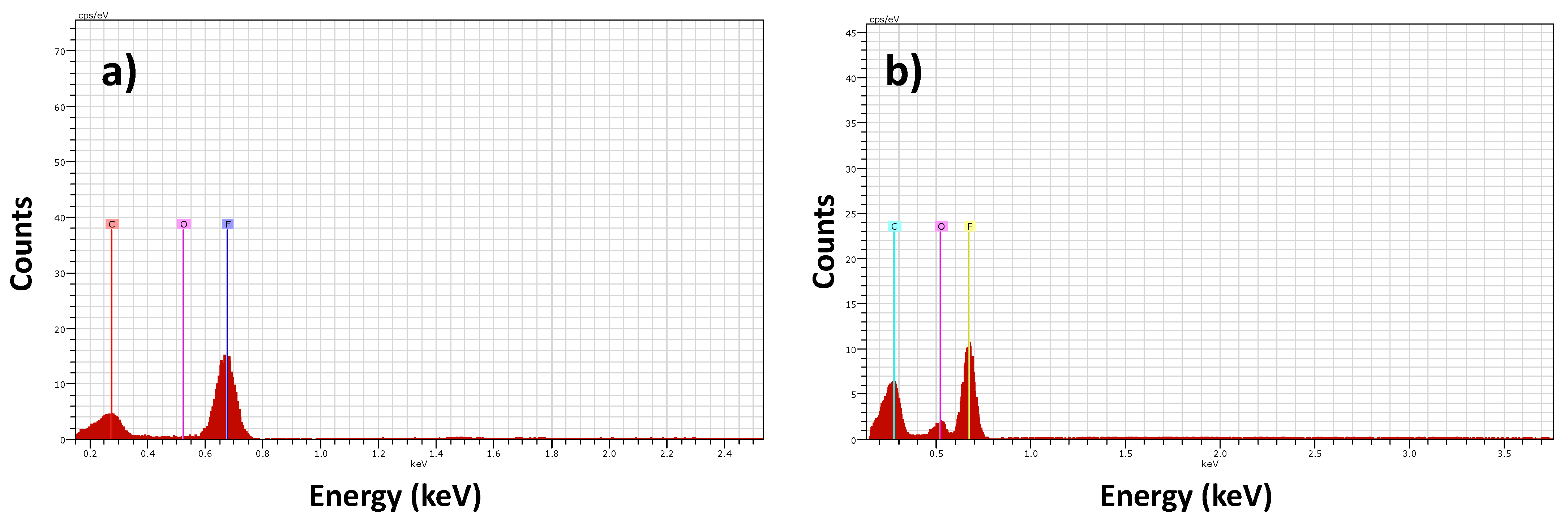
3.2. Energy Dispersive Spectroscopy (EDS)
3.3. FTIR Spectra of the Nanofiber Membranes
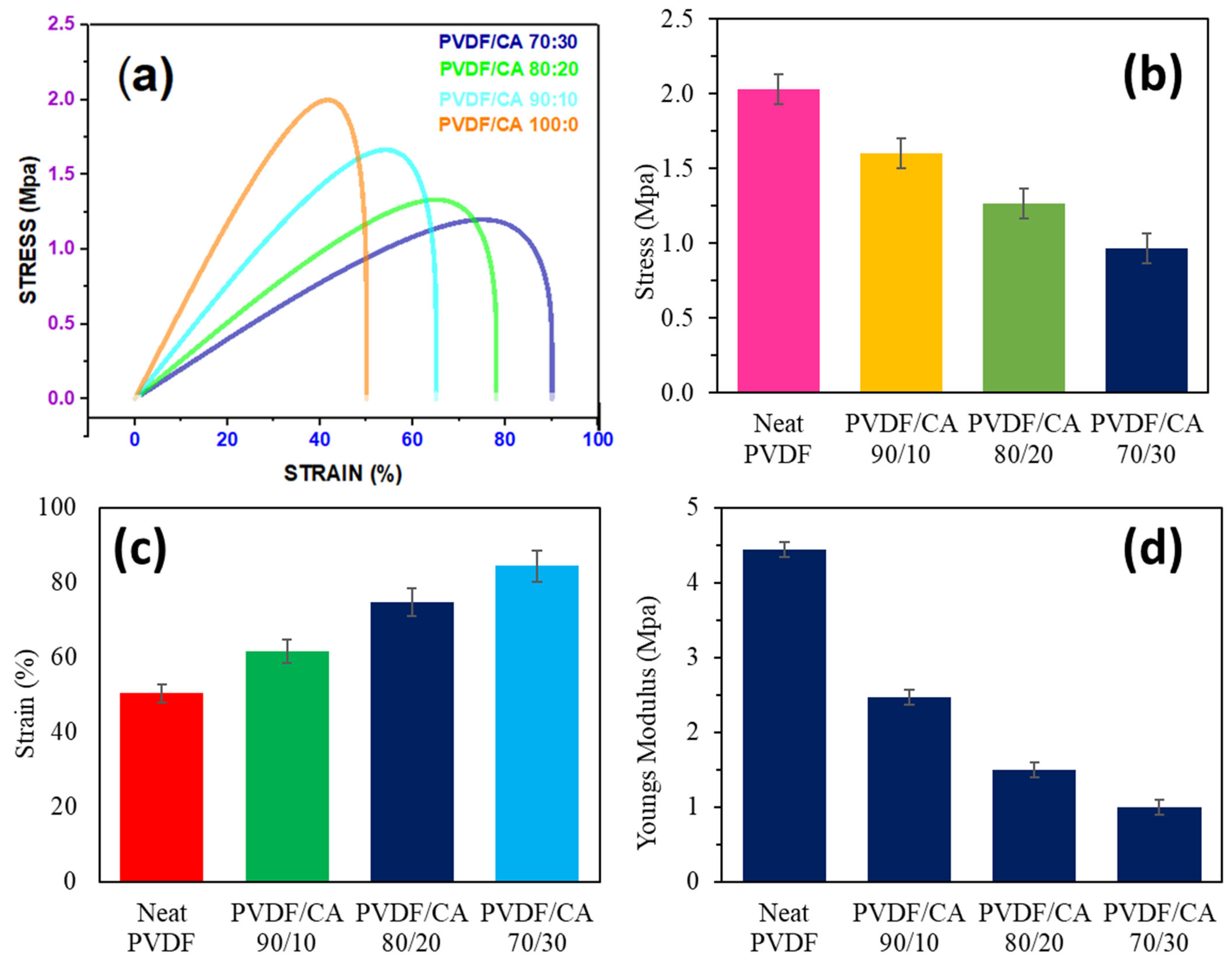
3.4. Membrane Tensile Strength
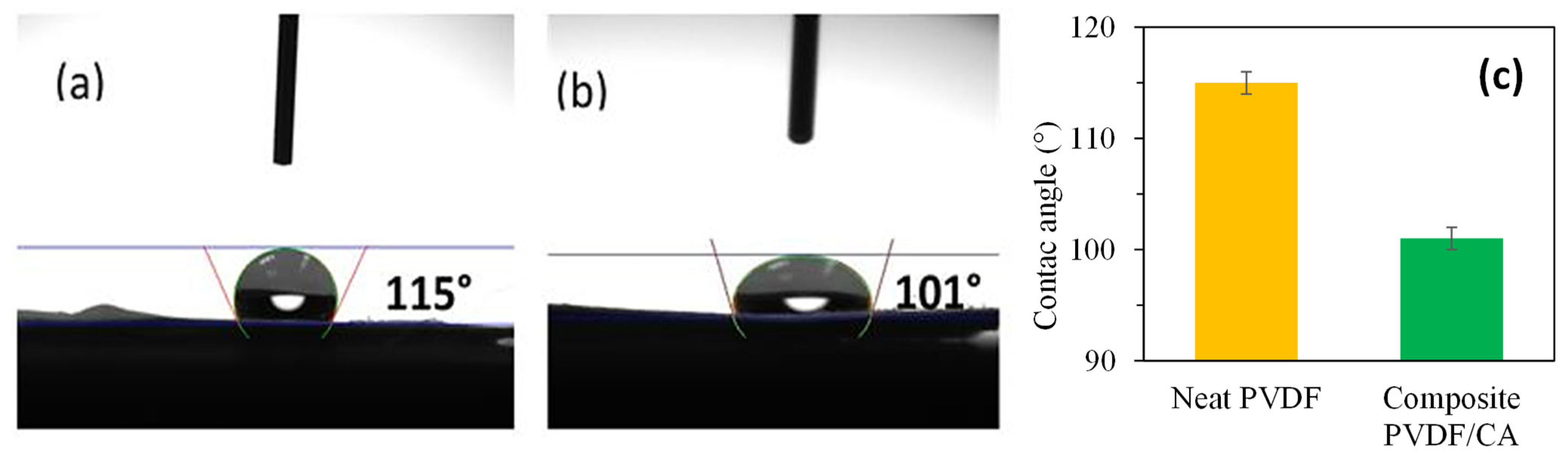
3.5. Membrane Hydrophobicity/Hydrophilicity
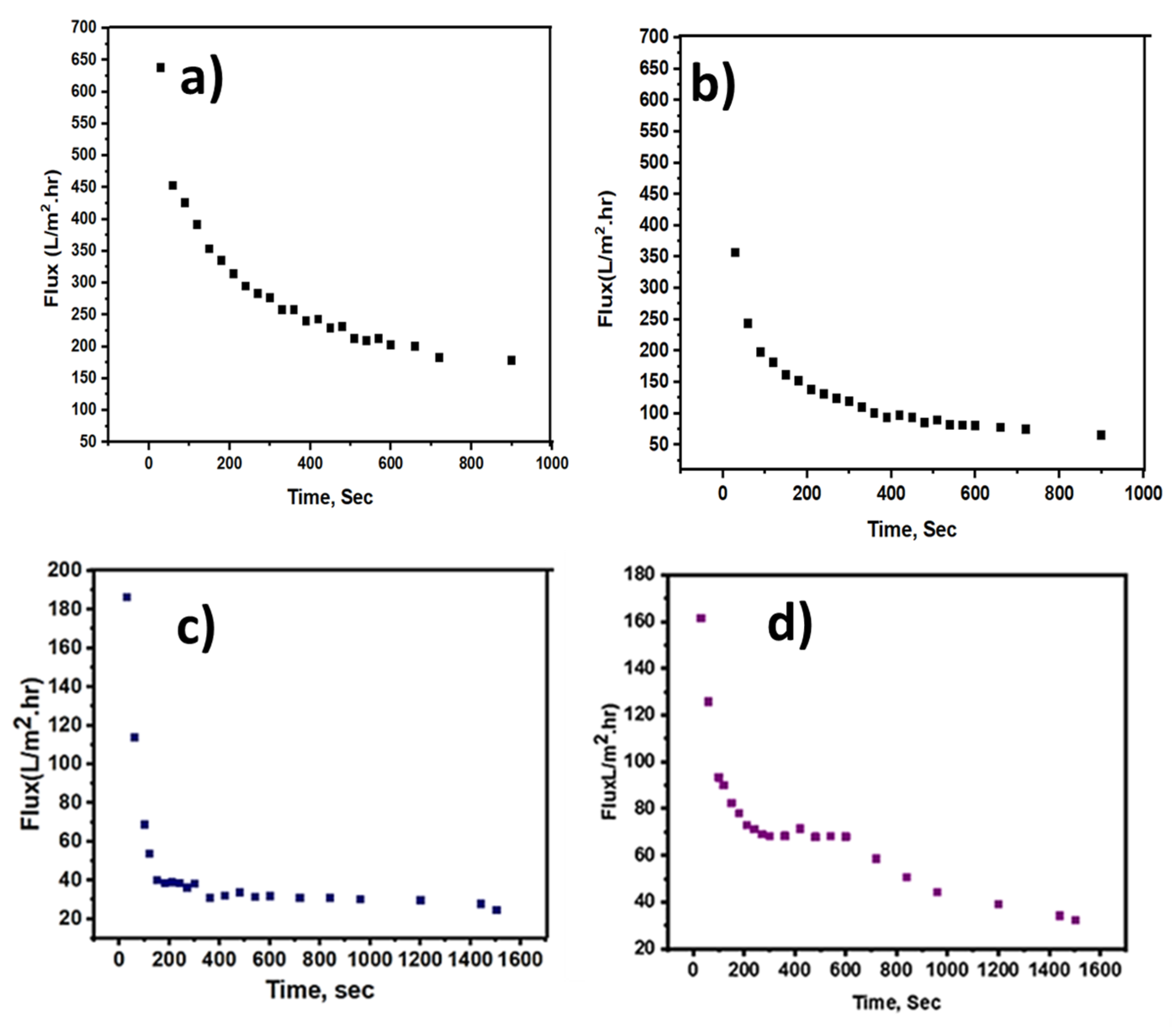
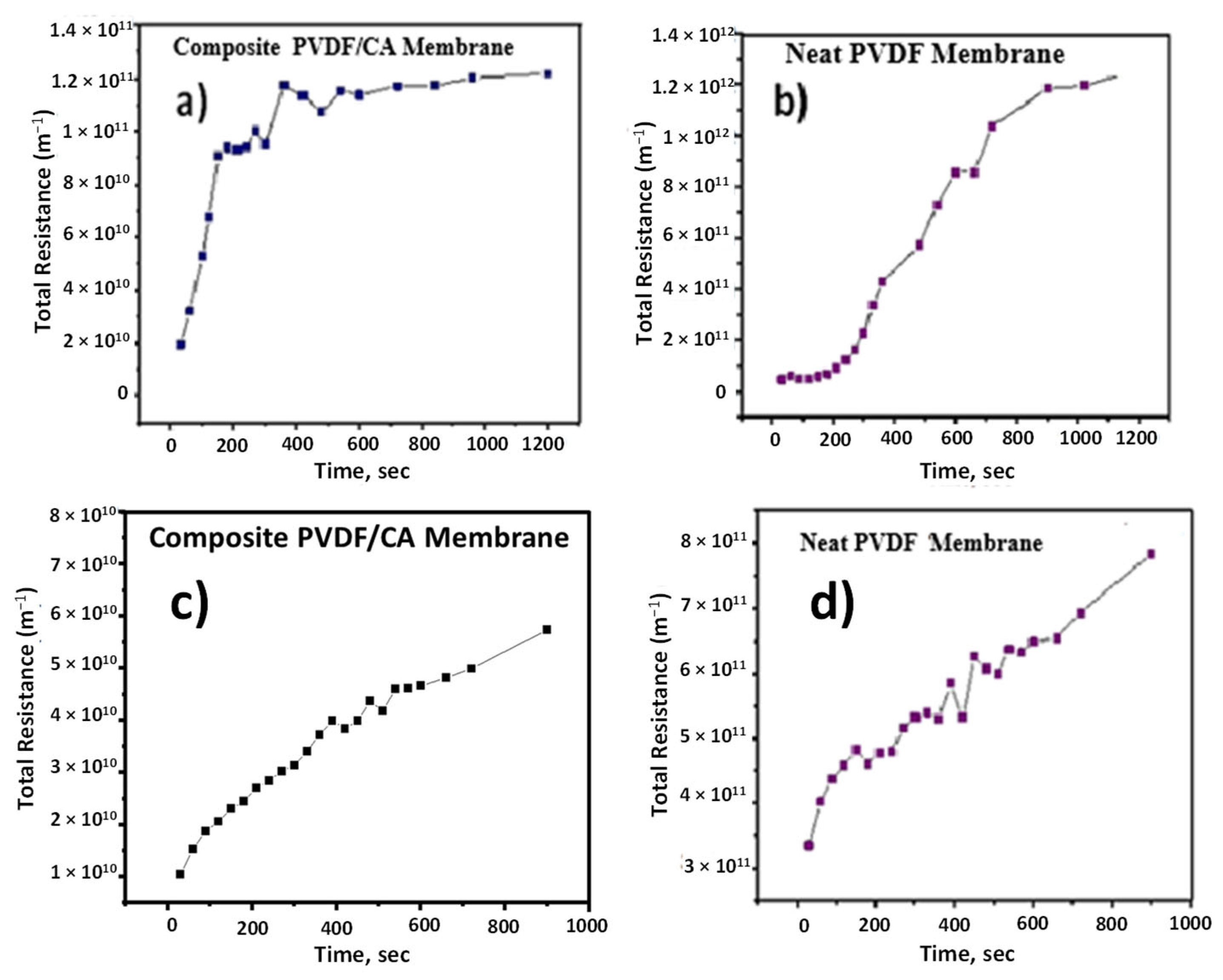
3.6. Water Flux and Filtration Resistance
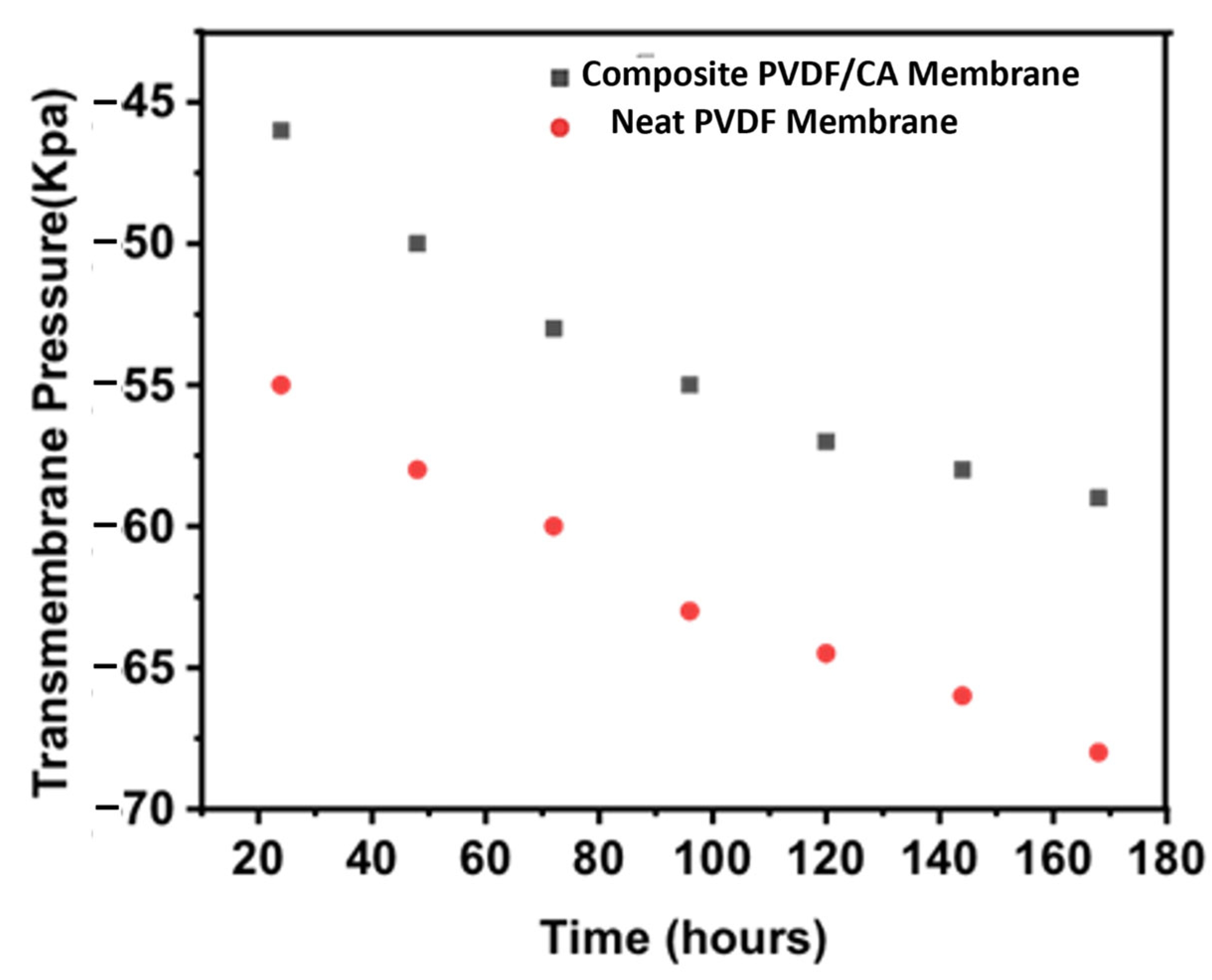
3.7. Submerged Membrane in a Membrane Bioreactor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khulbe, K.; Feng, C.; Matsuura, T.; Ismail, A. Progresses in membrane and advanced oxidation processes for water treatment. Membr. Water Treat. 2012, 3, 181–200. [Google Scholar] [CrossRef]
- Si, J.; Zhao, M.; Marcelle, S.S.A.; Wang, Q.; Cui, Z.; Liu, X. Design and modification of Janus polyvinylidene fluoride/deacetylated cellulose acetate nanofiber membrane and its multifunctionality. Adv. Mater. Interfaces 2023, 10, 2201550. [Google Scholar] [CrossRef]
- Kumarage, S.; Munaweera, I.; Kottegoda, N. A comprehensive review on electrospun nanohybrid membranes for wastewater treatment. Beilstein J. Nanotechnol. 2022, 13, 137–159. [Google Scholar] [CrossRef]
- Kamalesh, R.; Karishma, S.; Saravanan, A.; Yaashikaa, P. Emerging breakthroughs in membrane filtration techniques and their application in agricultural wastewater treatment: Reusability aspects. Sustain. Chem. Environ. 2024, 8, 100183. [Google Scholar] [CrossRef]
- Karki, S.; Hazarika, G.; Yadav, D.; Ingole, P.G. Polymeric membranes for industrial applications: Recent progress, challenges and perspectives. Desalination 2024, 573, 117200. [Google Scholar] [CrossRef]
- Wenten, I.; Bazant, M.Z.; Khoiruddin, K. Mitigating electrodialysis membrane fouling in seawater desalination. Sep. Purif. Technol. 2024, 345, 127228. [Google Scholar] [CrossRef]
- Du, G.; Tang, K.; Song, C.; Sun, J.; Sun, F.; Chen, C. The operating parameters optimization of LEP-N-MBR system for urban domestic wastewater treatment: Nitrogen and carbon removal, membrane fouling characteristics and microbial community. Sep. Purif. Technol. 2025, 363, 132290. [Google Scholar] [CrossRef]
- Ren, L.; Yu, S.; Li, J.; Li, L. Pilot study on the effects of operating parameters on membrane fouling during ultrafiltration of alkali/surfactant/polymer flooding wastewater: Optimization and modeling. RSC Adv. 2019, 9, 11111–11122. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of water treatment membranes: A review of the underlying causes, monitoring techniques and control measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef]
- Chen, R.; Nie, Y.; Hu, Y.; Miao, R.; Utashiro, T.; Li, Q.; Xu, M.; Li, Y.-Y. Fouling behaviour of soluble microbial products and extracellular polymeric substances in a submerged anaerobic membrane bioreactor treating low-strength wastewater at room temperature. J. Membr. Sci. 2017, 531, 1–9. [Google Scholar] [CrossRef]
- Janga, N.; Ren, X.; Kim, G.; Ahn, C.; Cho, J.; Kim, I.S. Characteristics of soluble microbial products and extracellular polymeric substances in the membrane bioreactor for water reuse. Desalination 2007, 202, 90–98. [Google Scholar] [CrossRef]
- Ramesh, A.; Lee, D.-J.; Hong, S. Soluble microbial products (SMP) and soluble extracellular polymeric substances (EPS) from wastewater sludge. Appl. Microbiol. Biotechnol. 2006, 73, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Ovez, S.; Turken, T.; Kose-Mutlu, B.; Okatan, S.; Durmaz, G.; Guclu, M.C.; Guclu, S.; Chellam, S.; Koyuncu, I. Manufacturing of antibiofouling polymeric membranes with bismuth-BAL chelate (BisBAL). Desalination Water Treat. 2016, 57, 12941–12955. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Kang, G.-D.; Cao, Y.-M. Application and modification of poly (vinylidene fluoride)(PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Gradys, A.; Kim, S.K.; Persano, L.; Marzec, M.; Kryshtal, A.; Busolo, T.; Toncelli, A.; Pisignano, D.; Bernasik, A. Enhanced piezoelectricity of electrospun polyvinylidene fluoride fibers for energy harvesting. ACS Appl. Mater. Interfaces 2020, 12, 13575–13583. [Google Scholar] [CrossRef]
- Cha, B.J.; Yang, J.M. Preparation of poly (vinylidene fluoride) hollow fiber membranes for microfiltration using modified TIPS process. J. Membr. Sci. 2007, 291, 191–198. [Google Scholar] [CrossRef]
- Cheng, L.-P. Effect of temperature on the formation of microporous PVDF membranes by precipitation from 1-octanol/DMF/PVDF and water/DMF/PVDF systems. Macromolecules 1999, 32, 6668–6674. [Google Scholar] [CrossRef]
- Kong, J.; Li, K. Oil removal from oil-in-water emulsions using PVDF membranes. Sep. Purif. Technol. 1999, 16, 83–93. [Google Scholar] [CrossRef]
- Benzinger, W.; Parekh, B.; Eichelberger, J. High temperature ultrafiltration with kynar® poly (vinylidene fluoride) membranes. Sep. Sci. Technol. 1980, 15, 1193–1204. [Google Scholar] [CrossRef]
- Chabot, S.; Roy, C.; Chowdhury, G.; Matsuura, T. Development of poly (vinylidene fluoride) hollow-fiber membranes for the treatment of water/organic vapor mixtures. J. Appl. Polym. Sci. 1997, 65, 1263–1270. [Google Scholar] [CrossRef]
- Khayet, M.; Khulbe, K.; Matsuura, T. Characterization of membranes for membrane distillation by atomic force microscopy and estimation of their water vapor transfer coefficients in vacuum membrane distillation process. J. Membr. Sci. 2004, 238, 199–211. [Google Scholar] [CrossRef]
- Sriyanti, I.; Ramadhani, R.F.; Almafie, M.R.; Ap Idjan, M.K.N.; Syafri, E.; Solihah, I.; Sanjaya, M.R.; Jauhari, J.; Fudholi, A. Physicochemical and mechanical properties of polyvinylidene fluoride nanofiber membranes. Case Study. Chem. Environ. Eng. 2024, 9, 100588. [Google Scholar] [CrossRef]
- Pramono, E.; Alfiansyah, R.; Ahdiat, M.; Wahyuningrum, D.; Radiman, C.L. Hydrophilic poly (vinylidene fluoride)/bentonite hybrid membranes for microfiltration of dyes. Mater. Res. Express 2019, 6, 105376. [Google Scholar] [CrossRef]
- Wei, D.; Ye, C.; Ahmed, A.; Xu, L. Batch preparation of nanofibers containing nanoparticles by an electrospinning device with multiple air inlets. Beilstein J. Nanotechnol. 2023, 14, 141–150. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Chen, S.; Zhang, J.; Wang, X. Hydrophilicity and crystallization behavior of PVDF/PMMA/TiO2 (SiO2) composites prepared by in situ polymerization. J. Polym. Res. 2012, 19, 1–9. [Google Scholar] [CrossRef]
- Wei, D.W.; Wei, H.; Gauthier, A.C.; Song, J.; Jin, Y.; Xiao, H. Superhydrophobic modification of cellulose and cotton textiles: Methodologies and applications. J. Bioresour. Bioprod. 2020, 5, 1–15. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Liu, T.; Liu, Z.; Li, N.; Zhang, Y.; Xiao, C.; Feng, X. Microporous CA/PVDF membranes based on electrospun nanofibers with controlled crosslinking induced by solvent vapor. J. Membr. Sci. 2016, 512, 1–12. [Google Scholar] [CrossRef]
- Koriem, O.A.; Showman, M.S.; El-Shazly, A.H.; Elkady, M.F. Cellulose acetate/polyvinylidene fluoride based mixed matrix membranes impregnated with UiO-66 nano-MOF for reverse osmosis desalination. Cellulose 2023, 30, 413–426. [Google Scholar] [CrossRef]
- Gurave, P.M.; Rastgar, M.; Mizan, M.M.H.; Srivastava, R.K.; Sadrzadeh, M. Superhydrophilic electrospun polyamide-imide membranes for efficient oil/water separation under gravity. ACS Appl. Eng. Mater. 2023, 1, 3134–3146. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Guo, H.; Liu, L.; Xu, W.; Duan, G. Structural design toward functional materials by electrospinning: A review. E-Polymers 2020, 20, 682–712. [Google Scholar] [CrossRef]
- Esmaeili, A. Wastewater treatment application of nanofibers and their composites. In Polymeric Nanofibers and Their Composites; Elsevier: Amsterdam, The Netherlands, 2025; pp. 227–253. [Google Scholar]
- Jiang, S.; Chen, Y.; Duan, G.; Mei, C.; Greiner, A.; Agarwal, S. Electrospun nanofiber reinforced composites: A review. Polym. Chem. 2018, 9, 2685–2720. [Google Scholar] [CrossRef]
- Duan, G.; Jin, M.; Wang, F.; Greiner, A.; Agarwal, S.; Jiang, S. Core effect on mechanical properties of one dimensional electrospun core-sheath composite fibers. Compos. Commun. 2021, 25, 100773. [Google Scholar] [CrossRef]
- Wang, L.; Dai, S.; Liu, X.; Wang, X.; Lu, H. A ternary system oleophilic–hydrophobic membrane prepared by electrospinning for efficient gravity-driven oil–water separation. SN Appl. Sci. 2019, 1, 797. [Google Scholar] [CrossRef]
- Ghanim, M.S.; Soydemir, G.; Yılmaz, F.; Perendeci, N.A.; Karagündüz, A.; Alazaiza, M.Y. Anaerobic Dynamic Membrane Bioreactors (AnDMBRs): Are They an Efficient Way to Treat High-Strength Wastewater? Water 2025, 17, 787. [Google Scholar] [CrossRef]
- Ghonimy, M.; Alharbi, A.; Saad, S.A.; Hussein, N.S. Improving Wastewater Quality Using Ultrafiltration Technology for Sustainable Irrigation Reuse. Water 2025, 17, 870. [Google Scholar] [CrossRef]
- Shim, J.K.; Na, H.S.; Lee, Y.M.; Huh, H.; Nho, Y.C. Surface modification of polypropylene membranes by γ-ray induced graft copolymerization and their solute permeation characteristics. J. Membr. Sci. 2001, 190, 215–226. [Google Scholar] [CrossRef]
- Amy, G. Fundamental understanding of organic matter fouling of membranes. Desalination 2008, 231, 44–51. [Google Scholar] [CrossRef]
- Rong, G.; Zhou, D.; Pang, J. Preparation of high-performance antifouling polyphenylsulfone ultrafiltration membrane by the addition of sulfonated polyaniline. J. Polym. Res. 2018, 25, 1–9. [Google Scholar] [CrossRef]
- He, W.; Ma, Z.; Yong, T.; Teo, W.E.; Ramakrishna, S. Fabrication of collagen-coated biodegradable polymer nanofiber mesh and its potential for endothelial cells growth. Biomaterials 2005, 26, 7606–7615. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded nanofibers formed during electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Moradi, G.; Zinadini, S.; Rajabi, L.; Dadari, S. Fabrication of high flux and antifouling mixed matrix fumarate-alumoxane/PAN membranes via electrospinning for application in membrane bioreactors. Appl. Surf. Sci. 2018, 427, 830–842. [Google Scholar] [CrossRef]
- Akhondi, E.; Zamani, F.; Law, A.W.; Krantz, W.B.; Fane, A.G.; Chew, J.W. Influence of backwashing on the pore size of hollow fiber ultrafiltration membranes. J. Membr. Sci. 2017, 521, 33–42. [Google Scholar] [CrossRef]
- Hou, Y.; Cheng, L.; Zhang, Y.; Yang, Y.; Deng, C.; Yang, Z.; Chen, Q.; Wang, P.; Zheng, L. Electrospinning of Fe/SiC hybrid fibers for highly efficient microwave absorption. ACS Appl. Mater. Interfaces 2017, 9, 7265–7271. [Google Scholar] [CrossRef]
- Zheng, W.; Hu, J.; Han, Z.; Wang, Z.; Zheng, Z.; Langer, J.; Economy, J. Synthesis of porous carbon fibers with strong anion exchange functional groups. Chem. Commun. 2015, 51, 9853–9856. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Liang, H.; Xiao, C. Preparation of polyurethane/poly (vinylidene fluoride) blend hollow fibre membrane using melt spinning and stretching. Mater. Sci. Technol. 2011, 27, 661–665. [Google Scholar] [CrossRef]
- Xu, Y.; Zou, L.; Lu, H.; Kang, T. Effect of different solvent systems on PHBV/PEO electrospun fibers. RSC Adv. 2017, 7, 4000–4010. [Google Scholar] [CrossRef]
- Wang, X.-M.; Waite, T.D. Impact of gel layer formation on colloid retention in membrane filtration processes. J. Membr. Sci. 2008, 325, 486–494. [Google Scholar] [CrossRef]
- Hong, J.; He, Y. Effects of nano sized zinc oxide on the performance of PVDF microfiltration membranes. Desalination 2012, 302, 71–79. [Google Scholar] [CrossRef]
- Sousa, M.R.S.; Lora-García, J.; López-Pérez, M.-F.; Heran, M. Identification of foulants on polyethersulfone membranes used to remove colloids and dissolved matter from paper mill treated effluent. Water 2020, 12, 365. [Google Scholar] [CrossRef]
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Elkony, Y.; Mansour, E.-S.; Elhusseiny, A.; Hassan, H.; Ebrahim, S. Novel grafted/crosslinked cellulose acetate membrane with N-isopropylacrylamide/N, N-methylenebisacrylamide for water desalination. Sci. Rep. 2020, 10, 9901. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H. Mass Transfer: Advances in Sustainable Energy and Environment Oriented Numerical Modelling; BoD–Books on Demand; InTech: Rijeka, Croatoa, 2013. [Google Scholar]
- Aussawasathien, D.; Ketkul, K.; Hrimchum, K.; Threepopnatkul, P. Effects of poly (lactic acid) and polybutylene succinate ratios on properties of composite foams containing activated carbons. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2019. [Google Scholar]
- Gao, S.-S.; Zhang, X.-H.; Geng, M.-Y.; Tian, J.-Y. Effect of membrane material and pore size on membrane fouling during filtration of algae-laden water. Water Sci. Eng. 2025, in press. [Google Scholar] [CrossRef]



| Parameters | Value |
|---|---|
| Distance from the tip of the needle to the collector | 128–150 mm |
| Voltage applied | 22–24 kV |
| Moisture | 40–50% |
| Speed of the metallic collector | 300 rpm |
| Speed of the pump filled with polymer solution | 1.6 mL/min |
| Needle tip diameter | 0.006 cm |
| Membrane drying time | 18–24 h |
| Angle of the syringe with the abscissa | 12 degrees |
| Solution | Ambient Conditions | Electrospinning Parameters | |||
|---|---|---|---|---|---|
| Polymer/Solution | Composition | Temperature | Humidity | Applied Voltage | Distance |
| PVDF | 16% | 80 °C | 40–45% | 20 kV | 10 cm |
| CA | 18% | 25 °C | 40–45% | 16 kV | 11 cm |
| PVDF/CA | 80:20 | 25 °C | 40–45% | 20 kV | 11 cm |
| Parameters | Average ± Std |
|---|---|
| Total suspended solids of sludge | 1700 ± 49 mg/L |
| Oxygen concentration | 2.5 ± 0.2 mg/L |
| Operation time | 25 days |
| Temperature | 28 ± 1 °C |
| Reactor flowrate | 14 ± 1 mL/min |
| Parameters | Average ± Std |
|---|---|
| Total suspended solids | 78 ± 1.88 mg/L |
| pH | 8.3 ± 0.28 |
| Chemical oxygen demand | 651 ± 22 mg/L |
| Turbidity | 37 ± 1.2 NTU |
| Membrane Type | Nanofiber Diameter (nm) | Pore Size (µm) | Porosity (%) |
|---|---|---|---|
| Neat PVDF | 530 ± 60 | 0.51 ± 3 | 83 ± 3 |
| Composite PVDF/CA | 860 ± 83 | 0.54 ± 2 | 91 ± 2 |
| Composite PVDF/CA | Neat PVDF | |||
|---|---|---|---|---|
| Element | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) |
| Carbon | 21.6 | 29.9 | 18.0 | 25.6 |
| Oxygen | 10.1 | 10.4 | 4.0 | 4.3 |
| Fluorine | 68.3 | 59.7 | 78.0 | 70.1 |
| Neat PVDF | Composite PVDF/CA | |
|---|---|---|
| Chemical oxygen demand (mg/L) | 105.0 ± 8 | 92.0 ± 5 |
| Total suspended solids (mg/L) | 0.00 | 0.00 |
| pH | 7.2 ± 0.1 | 6.8 ± 0.3 |
| Turbidity (NTU) | 1.3 ± 0.6 | 1.1 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallah, N.B.; Shah, A.A.; Pirzada, A.M.; Ali, I.; Ullman, J.L.; Mahar, R.B.; Khan, M.I. Development of Antifouling Polyvinylidene Fluoride and Cellulose Acetate Nanocomposite Membranes for Wastewater Treatment Using a Membrane Bioreactor. Water 2025, 17, 1767. https://doi.org/10.3390/w17121767
Mallah NB, Shah AA, Pirzada AM, Ali I, Ullman JL, Mahar RB, Khan MI. Development of Antifouling Polyvinylidene Fluoride and Cellulose Acetate Nanocomposite Membranes for Wastewater Treatment Using a Membrane Bioreactor. Water. 2025; 17(12):1767. https://doi.org/10.3390/w17121767
Chicago/Turabian StyleMallah, Nabi Bakhsh, Ayaz Ali Shah, Abdul Majeed Pirzada, Imran Ali, Jeffrey Layton Ullman, Rasool Bux Mahar, and Mohammad Ilyas Khan. 2025. "Development of Antifouling Polyvinylidene Fluoride and Cellulose Acetate Nanocomposite Membranes for Wastewater Treatment Using a Membrane Bioreactor" Water 17, no. 12: 1767. https://doi.org/10.3390/w17121767
APA StyleMallah, N. B., Shah, A. A., Pirzada, A. M., Ali, I., Ullman, J. L., Mahar, R. B., & Khan, M. I. (2025). Development of Antifouling Polyvinylidene Fluoride and Cellulose Acetate Nanocomposite Membranes for Wastewater Treatment Using a Membrane Bioreactor. Water, 17(12), 1767. https://doi.org/10.3390/w17121767








