Effects of BDE3 and the Co-Existence Copper on Photosynthesis and Antioxidative Enzymes in Salvinia natans (L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cultivation of Duckweed
2.3. Photosynthetic Pigment Content
2.4. Lipid Peroxidation
2.5. Enzyme Activity Measurement
2.6. Cu2+ Accumulation in Duckweed
2.7. BDE3 Quantification in Nutrient Solution
2.8. Bioconcentration Factor (BCF) and Translocation Factor (TLF) of Cu2+
2.9. Statistical Data Analysis
3. Results
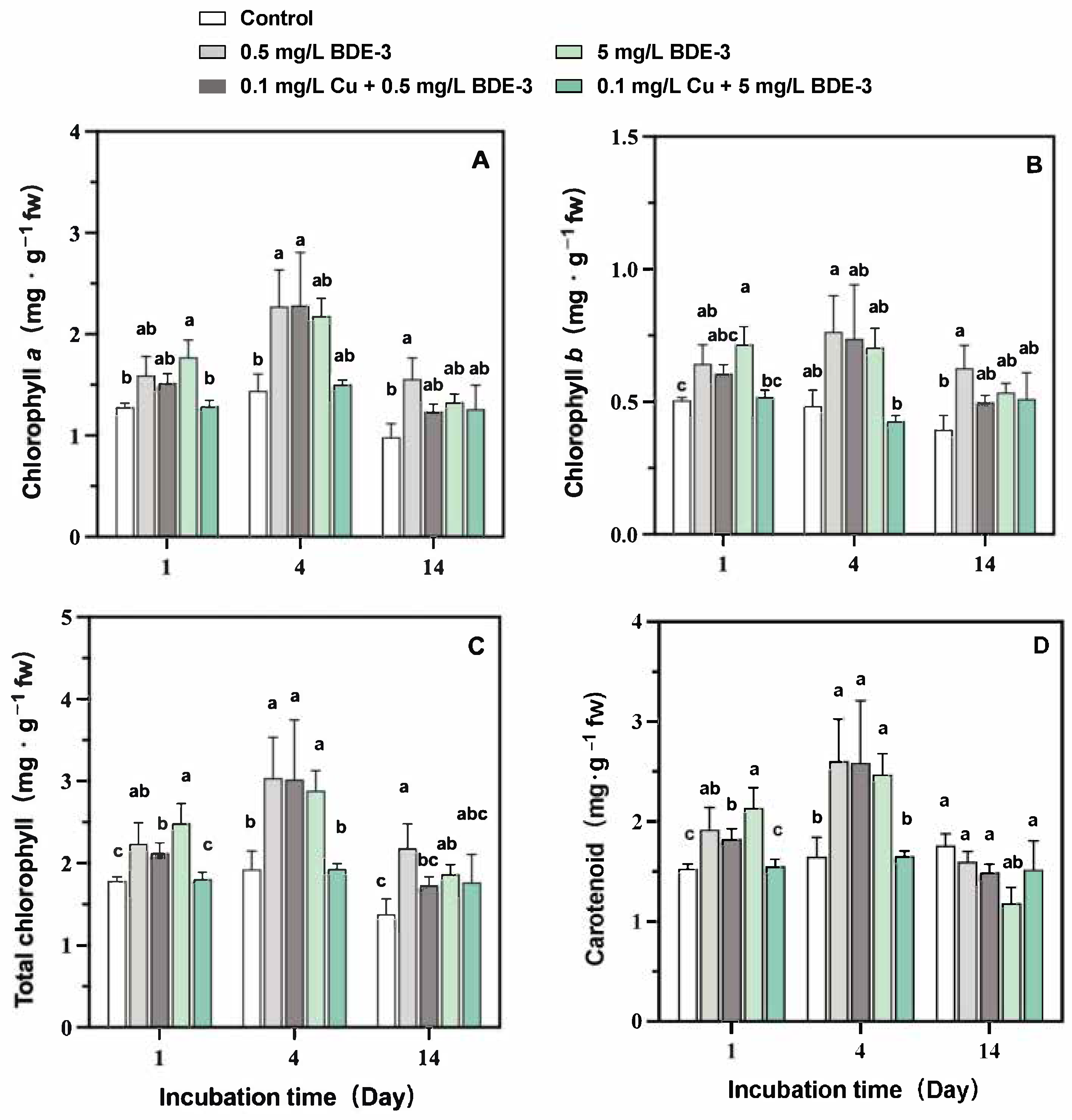
3.1. Effects on Photosynthetic Pigments
3.2. MDA
3.3. Antioxidative Enzyme Activities
3.4. Cu Accumulation in S. natans
3.5. Removal of BDE3 by S. natans
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meng, Y.T.; Zhao, Q.Y.; Wang, L.Y.; Xu, C.; Qiu, N.W.; Wang, R.J.; Zhou, F. Evaluation of the phytotoxicity of decabromodiphenyl ether (BDE-209) in Chinese cabbage. Biol. Plant. 2022, 66, 67–75. [Google Scholar] [CrossRef]
- Eguchi, A.; Isobe, T.; Ramu, K.; Tue, N.M.; Sudaryanto, A.; Devanathan, G.; Viet, P.H.; Tana, R.S.; Takahashi, S.; Subramanian, A.; et al. Soil contamination by brominated flame retardants in open waste dumping sites in Asian developing countries. Chemosphere 2013, 90, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zheng, J.; Bi, X.; Fu, J.; Wong, M. Distribution of PBDEs in air particles from an electronic waste recycling site compared with Guangzhou and Hong Kog, South China. Environ. Int. 2007, 33, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.H.; Liao, J.B.; Liu, X.; Wu, C.F.; Wu, H.Z.; Guan, Q.Q. Source, characteristics, environmental distribution and pollution control of PBDEs. Acta Sci. Circum. 2015, 35, 3025–3041. [Google Scholar]
- Motamedi, M.; Yerushalmi, L.; Haghighat, F.; Chen, Z. A critical review of water contamination by polybrominated diphenyl ethers (PBDE) and main degradation techniques. J. Environ. Chem. Eng. 2022, 10, 108196. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, S.; Wang, S.; Lv, J. In vitro biotransformation of PBDEs by root crude enzyme extracts: Potential role of nitrate reductase (NaR) and glutathione S-transferase (GST) in their debromination. Chemosphere 2013, 90, 1885–1892. [Google Scholar] [CrossRef]
- Jiang, X.; Bai, X.; Liu, X.; Wang, X.; Shiu, K.-K. Detecting low-brominated diphenyl ethers by highly sensitive biosensors based on the blocking effect on glucose oxidase. Sens. Diagn. 2023, 2, 176–187. [Google Scholar] [CrossRef]
- Verslycke, T.A.; Vethaak, A.D.; Arijs, K.; Janssen, C.R. Flame retardants, surfactants and organotins in sediment and mysid shrimp of the Scheldt estuary (The Netherlands). Environ. Pollut. 2005, 136, 19–31. [Google Scholar] [CrossRef]
- You, X.; Xi, J.; Cao, Y.; Zhang, J.; Luan, Y. 4-Bromodiphenyl Ether Induces Germ Cell Apoptosis by Induction of ROS and DNA Damage in Caenorhabditis elegans. Toxicol. Sci. 2017, 157, 510–518. [Google Scholar] [CrossRef]
- Zhuang, Y.A.; Ahn, S.; Luthy, R.G. Debromination of Polybrominated Diphenyl Ethers by Nanoscale Zerovalent Iron: Pathways, Kinetics, and Reactivity. Environ. Sci. Technol. 2010, 44, 8236–8242. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Wu, Y.; Ge, J.; Li, W.; Huo, X. Polybrominated diphenyl ethers in residential and agricultural soils from an electronic waste polluted region in South China: Distribution, compositional profile, and sources. Chemosphere 2014, 102, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, D.; Yi, R. Temporal and spatial distribution dynamics of PBDEs in agricultural soil of e-waste recycling area before and after emission control. J. Agro-Environ. Sci. 2021, 40, 1718–1728. [Google Scholar]
- Chen, X.; Mo, J.; Zhang, S.; Li, X.; Huang, T.; Zhu, Q.; Wang, S.; Chen, X.; Ge, R.-S. 4-Bromodiphenyl Ether Causes Adrenal Gland Dysfunction in Rats during Puberty. Chem. Res. Toxicol. 2019, 32, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, F.; Li, Z.; Yu, Y.; Yan, H.; Tahir, A.; Zheng, W.; Li, X.; Huang, T.; Ge, R.-S. Exposure to 4-bromodiphenyl ether during pregnancy blocks testis development in male rat fetuses. Toxicol. Lett. 2021, 342, 38–49. [Google Scholar] [CrossRef]
- Akortia, E.; Olukunle, O.I.; Daso, A.P.; Okonkwo, J.O. Soil concentrations of polybrominated diphenyl ethers and trace metals from an electronic waste dump site in the Greater Accra Region, Ghana: Implications for human exposure. Ecotoxicol. Environ. Saf. 2017, 137, 247–255. [Google Scholar] [CrossRef]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Hydrogen peroxide modulate photosynthesis and antioxidant systems in tomato (Solanum lycopersicum L.) plants under copper stress. Chemosphere 2019, 230, 544–558. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Heavy-metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. New Phytol. 1999, 143, 253–259. [Google Scholar] [CrossRef]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha; Murtaza, G.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, X.; Yang, Y.; Sun, Z.; Jian, X.; Zhao, Y.; Zhang, X. Evaluation of the toxic response induced by BDE-47 in a marine alga, Phaeodactylum tricornutum, based on photosynthesis-related parameters. Aquat. Toxicol. 2020, 227, 105588. [Google Scholar] [CrossRef]
- Liu, N.; Zhong, G.; Zhou, J.; Liu, Y.; Pang, Y.; Cai, H.; Wu, Z. Separate and combined effects of glyphosate and copper on growth and antioxidative enzymes in Salvinia natans (L.) All. Sci. Total Environ. 2019, 655, 1448–1456. [Google Scholar] [CrossRef]
- Gifford, E.M.; Foster, A.S. Morphology and Evolution of Vascular Plants; W.H. Freeman and Company: New York, NY, USA, 1989. [Google Scholar]
- Mandal, C.; Ghosh, N.; Dey, N.; Adak, M. Effects of putrescine on oxidative stress induced by hydrogen peroxide in Salvinia natans L. J. Plant Interact. 2014, 9, 550–558. [Google Scholar] [CrossRef]
- Jampeetong, A.; Muenrew, J. Interactive effects of NH4+ concentration and O2 availability on growth, morphology, and mineral allocation of hybrid Napier grass (Pennisetum purpureum×P. americanum cv. Pakchong1). Ecol. Eng. 2016, 91, 409–418. [Google Scholar] [CrossRef]
- Hasegawa, H.; Ueda, K.; Maki, T.; Rahman, M.M. Influence of phosphate and iron ions in selective uptake of arsenic species by water fern (Salvinia natans L.). Chem. Eng. J. 2008, 145, 179–184. [Google Scholar]
- Mukherjee, S.; Kumar, S. Adsorptive uptake of arsenic (V) from water by aquatic fern Salvinia natans. J. Water Supply Res. Technol. Aqua 2005, 54, 47–53. [Google Scholar] [CrossRef]
- Tian, L.Y.; Zhang, H.L.; Zhao, X.P.; Zhao, X.Y.; White, J.C.; Zhao, L.J.; Ji, R. CdS nanoparticles in soil induce metabolic reprogramming in broad bean (Vicia faba L.) roots and leaves. Environ. Sci. Nano 2020, 7, 93–104. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Shi, B.; Chai, Y.; Han, N.; Zhu, M.; Bian, H. Overexpression of HvHGGT enhances tocotrienol levels and antioxidant activity in Barley. J. Agric. Food Chem. 2017, 65, 5181–5187. [Google Scholar] [CrossRef]
- Wang, B.; Su, Y.; Tian, L.; Peng, S.; Ji, R. Heavy metals in face paints: Assessment of the health risks to Chinese opera actors. Sci. Total Environ. 2020, 724, 138163. [Google Scholar] [CrossRef]
- Yuan, W.; Xu, E.G.; Li, L.; Zhou, A.; Peijnenburg, W.J.; Grossart, H.-P.; Liu, W.; Yang, Y. Tracing and trapping micro- and nanoplastics: Untapped mitigation potential of aquatic plants? Water Res. 2023, 242, 120249. [Google Scholar] [CrossRef]
- Yaacoub, R.; Saliba, R.; Nsouli, B.; Khalaf, G.; Birlouez-Aragon, I. Formation of Lipid Oxidation and Isomerization Products during Processing of Nuts and Sesame Seeds. J. Agric. Food Chem. 2008, 56, 7082–7090. [Google Scholar] [CrossRef]
- Sarvajeet Singh, G.; Narendra, T. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar]
- Di Mascio, P.; Murphy, M.; Sies, H. Antioxidant defense systems: The role of carotenoids, tocopherols, and thiols. Am. J. Clin. Nutr. 1991, 53 (Suppl. S1), 194–200. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Stepanenko, A.; Kishchenko, O.; Xu, J.; Borisjuk, N. Duckweeds for phytoremediation of polluted water. Plants 2023, 12, 589. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.C.; Xie, H.C.; Li, K.; Zhang, S.Y. The effects of the phenol concentrations on photosynthetic parameters of Salix babylonica L. Photosynthetica 2015, 53, 430–435. [Google Scholar] [CrossRef]
- Qiu, N.; Zhang, W.; Yan, X.; Wang, R.; Tian, L.; Han, G.; Zhou, F. The toxicity of BDE-47 to the photosystem of Lemna minor fronds. Biol. Plant. 2020, 64, 591–597. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Wang, W.; Zhou, D.; Wang, L.; Corvini, P.F.-X.; Ji, R. Fate of lower-brominated diphenyl ethers (LBDEs) in a red soil—Application of 14C-labelling. Sci. Total Environ. 2020, 721, 137735. [Google Scholar] [CrossRef]
- Xu, X.; Huang, H.; Wen, B.; Wang, S.; Zhang, S. Phytotoxicity of brominated diphenyl ether-47 (BDE-47) and its hydroxylated and methoxylated analogues (6-OH-BDE-47 and 6-MeO-BDE-47) to maize (Zea mays L.). Chem. Res. Toxicol. 2015, 28, 510–517. [Google Scholar] [CrossRef]
- Zhao, Y.R.; Tang, X.X.; Quigg, A.; Lv, M.C.; Zhao, Y. The toxic mechanisms of BDE-47 to the marine diatom Thalassiosira pseudonana—A study based on multiple physiological processes. Aquat. Toxicol. 2019, 212, 20–27. [Google Scholar] [CrossRef]
- Deng, D.; Chen, H.X.; Wong, Y.S.; Tam, N.F.Y. Physiological response and oxidative transformation of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) by a Chlorella isolate. Sci. Total Environ. 2020, 744, 140869. [Google Scholar] [CrossRef]
- Källqvist, T.; Grung, M.; Tollefsen, K.E. Chronic toxicity of 2,4,2′,4′-tetrabromodiphenyl ether on the marine alga Skeletonema costatum and the crustacean Daphnia magna. Environ. Toxicol. Chem. 2006, 25, 1657–1662. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Li, Y.; Santschi, P.H.; Quigg, A. Response of photosynthesis and the antioxidant defense system of two microalgal species (Alexandrium minutum and Dunaliella salina) to the toxicity of BDE-47. Mar. Pollut. Bull. 2017, 124, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Mhadhbi, L.; Fumega, J.; Beiras, R. Toxicological Effects of Three Polybromodiphenyl Ethers (BDE-47, BDE-99 and BDE-154) on Growth of Marine Algae Isochrysis galbana. Water Air Soil Pollut. 2012, 223, 4007–4016. [Google Scholar] [CrossRef]
- Mobin, F.Z. Metal Uptake (Pb, Ni and Cu) by Duckweed and Cattails Under Different Citric Acid (CA) Concentrations. Master’s Thesis, East Texas A&M University, Commerce, TX, USA, 2024. [Google Scholar]
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci. Rep. 2019, 9, 5658. [Google Scholar] [CrossRef]
- Van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Duarte, B.; Caetano, M.; Almeida, P.R.; Vale, C.; Caçador, I. Accumulation and biological cycling of heavy metal in four salt marsh species, from Tagus estuary (Portugal). Environ. Pollut. 2010, 158, 1661–1668. [Google Scholar] [CrossRef] [PubMed]





| Concentration of BDE3 (mg L−1) | Day 1 | Day 4 | Day 14 |
|---|---|---|---|
| 0.5 | 9.79% | 72.5% | 92.4% |
| 5 | 2.49% | 50.8% | 91.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Long, B.; Zhu, M.; Zhang, S.; Liu, H.; Tian, L. Effects of BDE3 and the Co-Existence Copper on Photosynthesis and Antioxidative Enzymes in Salvinia natans (L.). Water 2025, 17, 1712. https://doi.org/10.3390/w17111712
Yao Y, Long B, Zhu M, Zhang S, Liu H, Tian L. Effects of BDE3 and the Co-Existence Copper on Photosynthesis and Antioxidative Enzymes in Salvinia natans (L.). Water. 2025; 17(11):1712. https://doi.org/10.3390/w17111712
Chicago/Turabian StyleYao, Yao, Bin Long, Mengjie Zhu, Simin Zhang, He Liu, and Liyan Tian. 2025. "Effects of BDE3 and the Co-Existence Copper on Photosynthesis and Antioxidative Enzymes in Salvinia natans (L.)" Water 17, no. 11: 1712. https://doi.org/10.3390/w17111712
APA StyleYao, Y., Long, B., Zhu, M., Zhang, S., Liu, H., & Tian, L. (2025). Effects of BDE3 and the Co-Existence Copper on Photosynthesis and Antioxidative Enzymes in Salvinia natans (L.). Water, 17(11), 1712. https://doi.org/10.3390/w17111712







