Freshwater Mussels as Multifaceted Ecosystem Engineers: Insights into Their Ecological Importance, Bioindication, and Economic Contributions
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
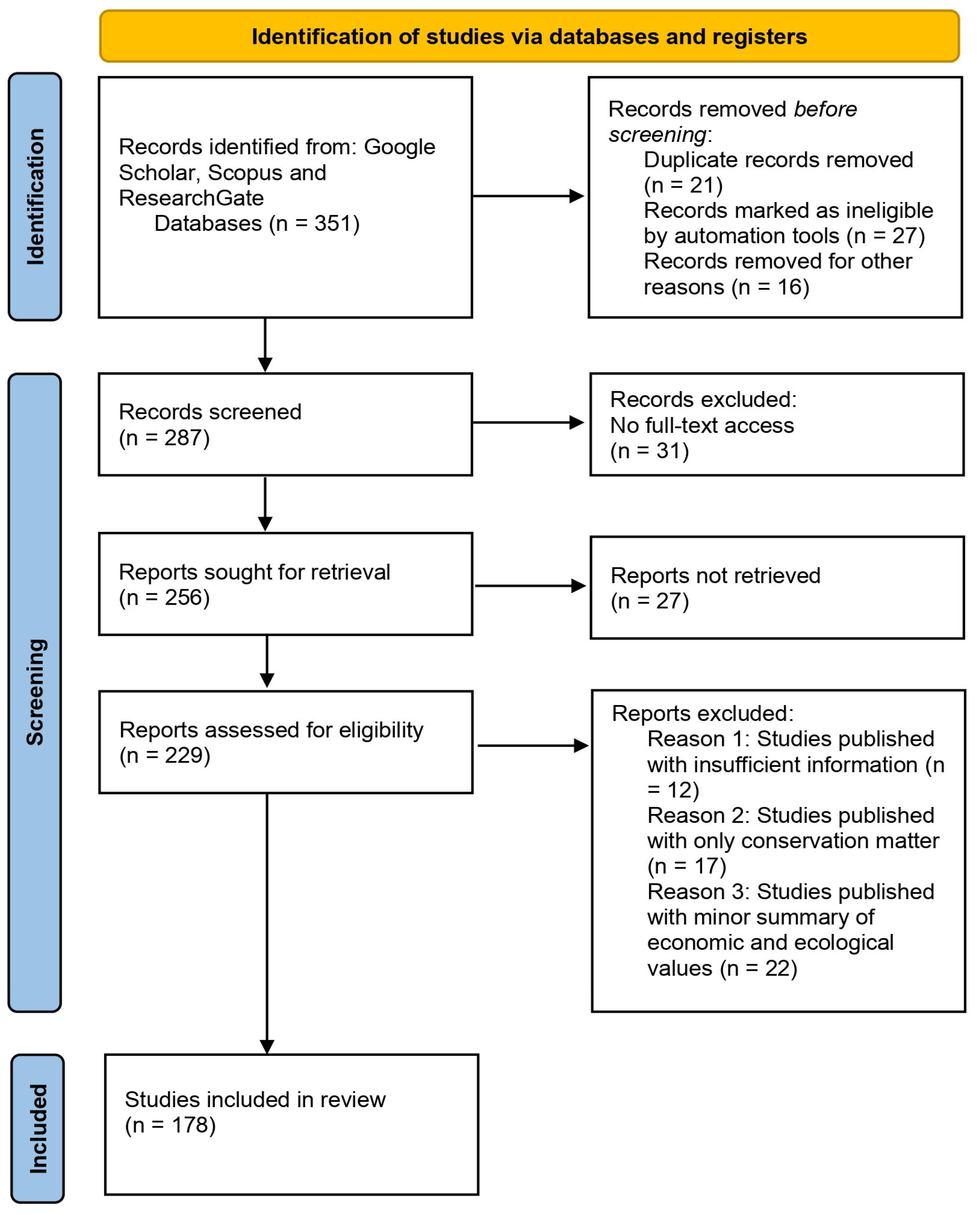
2.3. PRISMA Flow Diagram and Quality Assessment
3. Ecological Values of Freshwater Mussels
3.1. Water Filtration and Quality Improvement
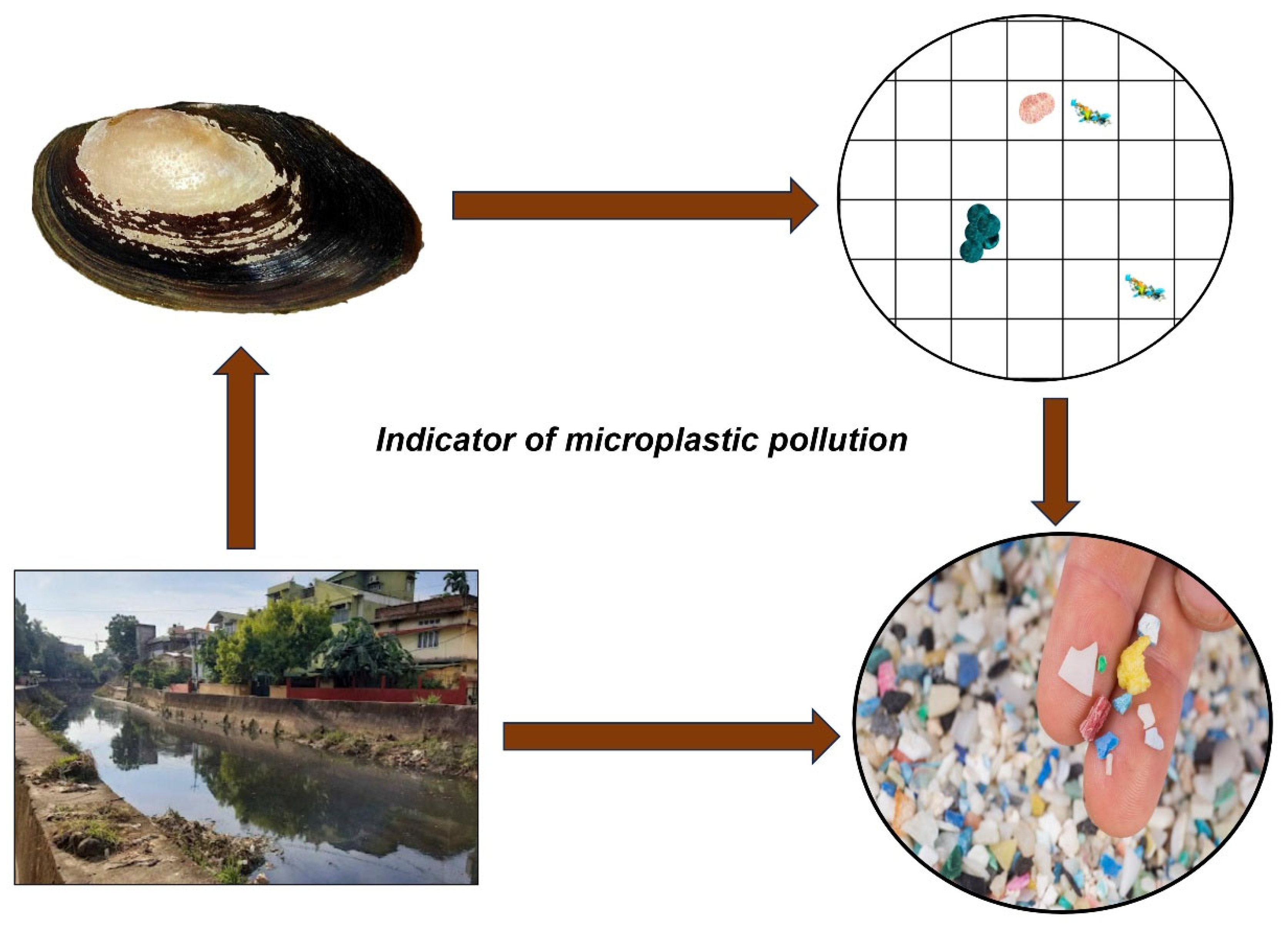
3.2. Bioindicator of Environment
3.3. Bioaccumulation of Pharmaceuticals and Personal Care Products (PPCPs)
3.4. Bio-Absorption of Metals
3.5. Nutrient Cycling and Energy Transfer
3.6. Habitat Structuring and Biodiversity Enhancement
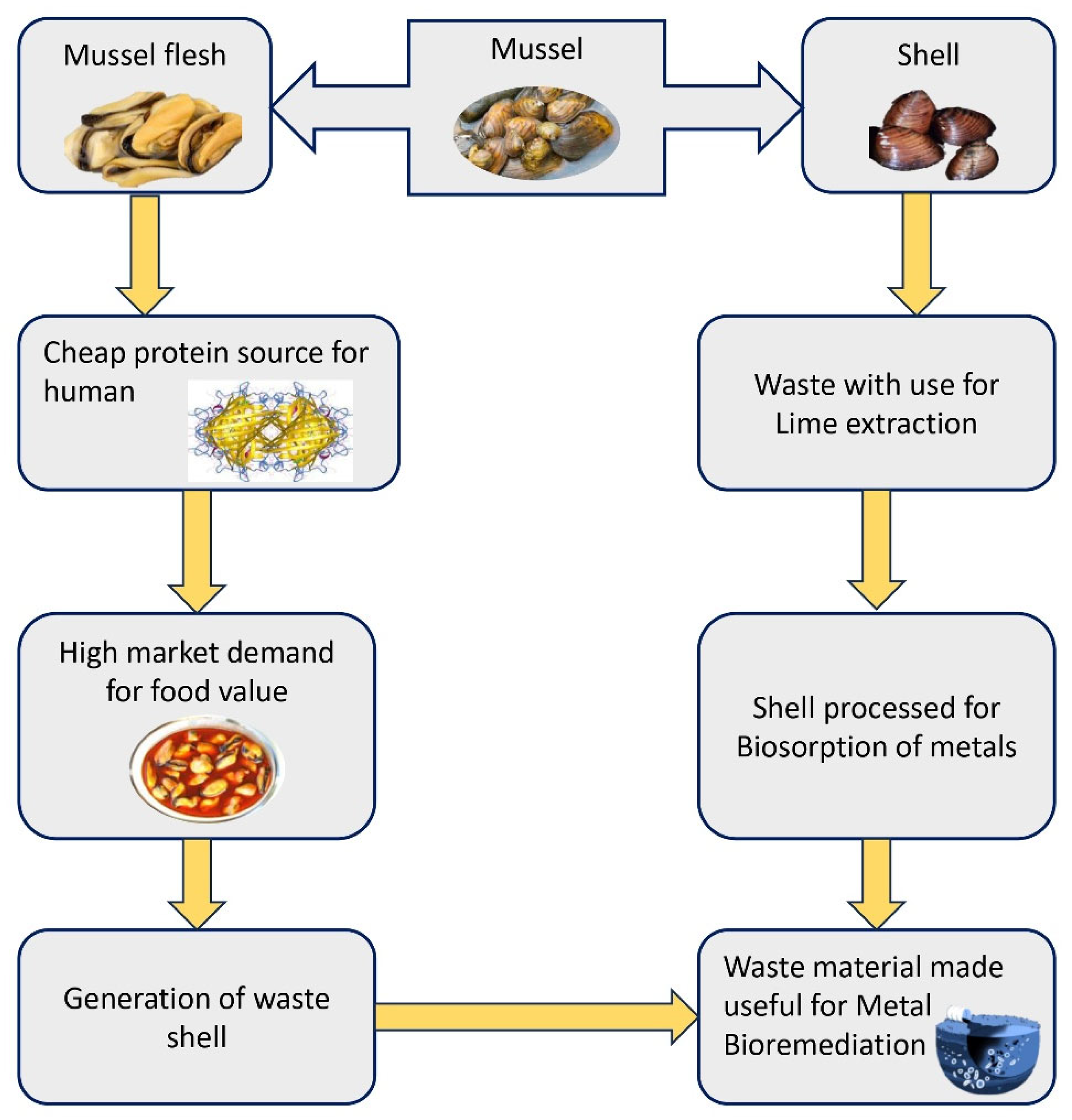
4. Economic Values of Freshwater Mussels
4.1. Mussel Flesh as Food and Its Bioactivities
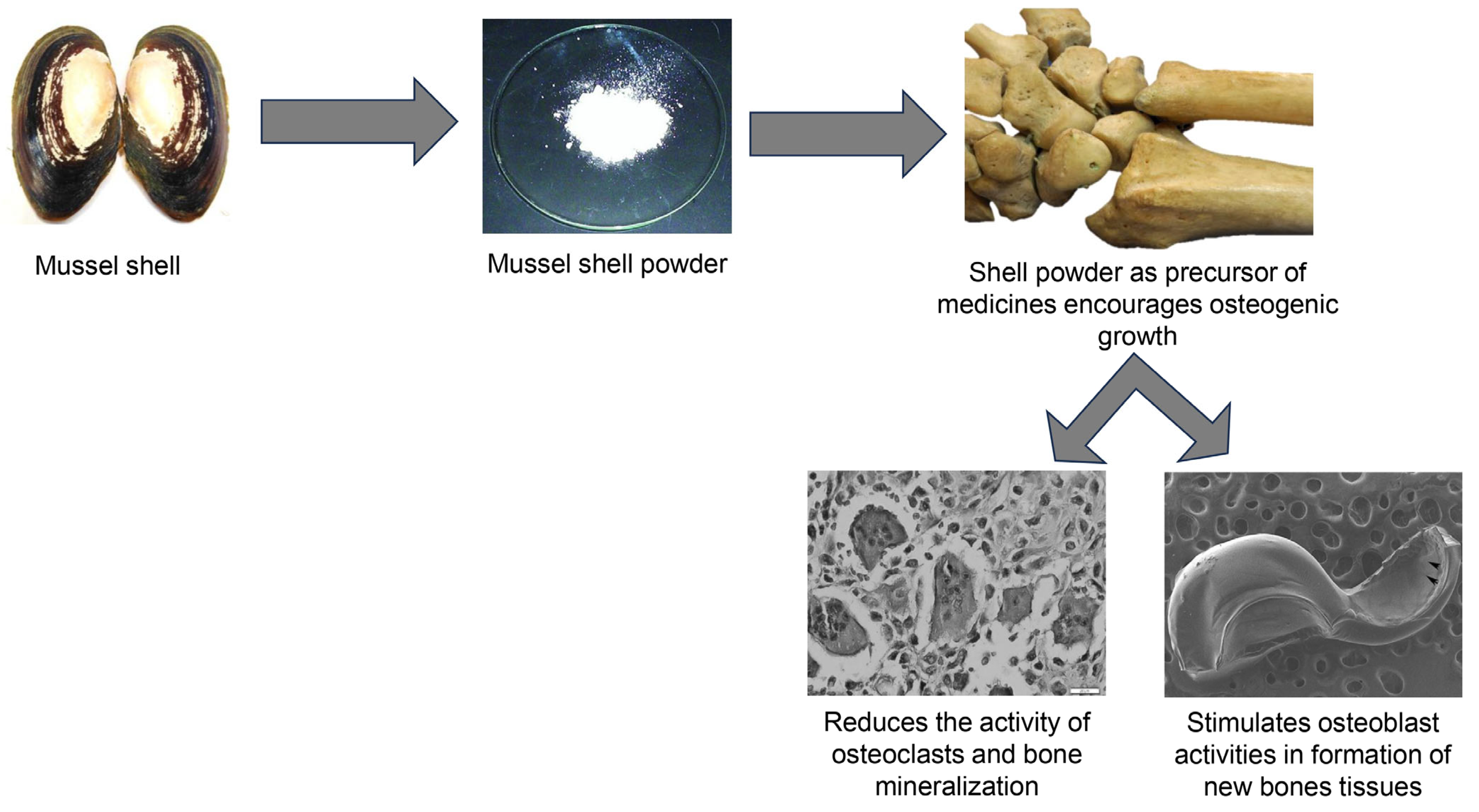
4.2. Shell Powder and Its Derivatives
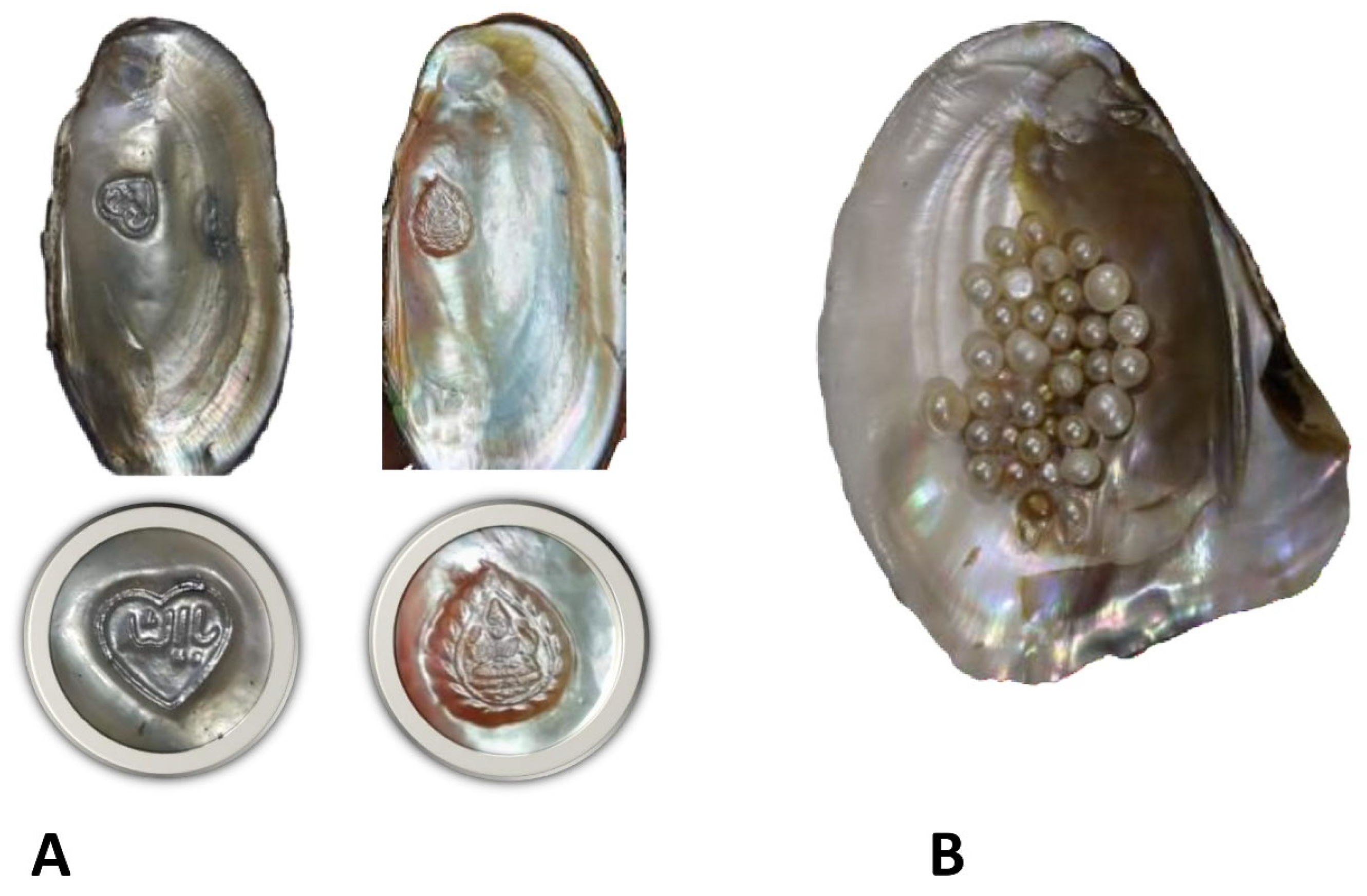
4.3. Pearl and Jewelry Industry
5. Threats to Freshwater Mussel Populations and Conservation Strategies
5.1. Habitat Destruction and Pollution
5.2. Climate Change, Overexploitation, and Invasive Species
| Threat | Global Examples and Data | Source(s) |
|---|---|---|
| Pollution |
| [115,150,151,152,153] |
| Habitat modification and degradation |
| [115,132,150,154,155,156] |
| Climate change |
| [132,151,157,158] |
| Invasive species |
| [150,151,159,160] |
| Overexploitation |
| [115,132,161] |
| Declining host fish populations |
| [132] |
| Emerging and novel pollutants |
| [151] |
| Declining calcium availability |
| [151] |
| Cumulative stressors |
| [151] |
5.3. Current Protection Measures
5.4. Habitat Protection and Restoration
5.5. Research, Monitoring, and Stakeholder Engagement
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thorp, J.H.; Rogers, D.C. (Eds.) Thorp and Covich’s Freshwater Invertebrates: Ecology and General Biology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1. [Google Scholar]
- Howard, D. A river mussel parasitic on a salamander. Nat. Hist. Misc. Chic. Acad. Sci. 1951, 77, 1–6. [Google Scholar]
- Zimmerman, L.L.; Neves, R.J. Effects of temperature on duration of viability for glochidia of freshwater mussels (Bivalvia: Unionidae). Am. Malacol. Bull. 2001, 17, 31–35. [Google Scholar]
- Watters, G.T.; O’Dee, S.H. Metamorphosis of freshwater mussel glochidia (Bivalvia: Unionidae) on amphibians and exotic fishes. Am. Midl. Nat. 1998, 139, 49–57. [Google Scholar] [CrossRef]
- Haag, W.R. Extreme longevity in freshwater mussels revisited: Sources of bias in age estimates derived from mark–recapture experiments. Freshw. Biol. 2009, 54, 1474–1486. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, J.; Bespalaya, Y.; Bódis, E.; Burlakova, L.; Van Damme, D.; et al. Conservation status of freshwater mussels in Europe state of the art and future challenges. Biol. Rev. 2017, 92, 572–607. [Google Scholar] [CrossRef]
- Allen, D.C.; Vaughn, C.C.; Kelly, J.F.; Cooper, J.T.; Engel, M.H. Bottom-up biodiversity effects increase resource subsidy flux between ecosystems. Ecology 2012, 93, 2165–2174. [Google Scholar] [CrossRef]
- Henderson, N.D.; Christian, A.D. Freshwater invertebrate assemblage composition and water quality assessment of an urban coastal watershed in the context of land-use land-cover and reach-scale physical habitat. Ecologies 2022, 3, 376–394. [Google Scholar] [CrossRef]
- Atkinson, C.L.; Vaughn, C.C.; Forshay, K.J.; Cooper, J.T. Aggregated filter-feeding consumers alter nutrient limitation: Consequences for ecosystem and community dynamics. Ecology 2013, 94, 1359–1369. [Google Scholar] [CrossRef]
- Strayer, D.L. Understanding how nutrient cycles and freshwater mussels (Unionida) affect each other. Hydrobiologia 2014, 735, 277–292. [Google Scholar] [CrossRef]
- Catherine, G.; Holly, R. Freshwater Mussels and Fish: A Timeless Love Affair|U.S. Fish & Wildlife Service. Available online: https://www.fws.gov/story/2023-05/freshwater-mussels-and-fish-timeless-love-affair (accessed on 27 October 2024).
- Strayer, D.L.; Smith, D.R. A Guide to Sampling Freshwater Mussel Populations; American Fisheries Society: Bethesda, MD, USA, 2003. [Google Scholar]
- Bril, J.S.; Durst, J.J.; Hurley, B.M.; Just, C.L.; Newton, T.J. Sensor data as a measure of native freshwater mussel impact on nitrate formation and food digestion in continuous-flow mesocosms. Freshw. Sci. 2014, 33, 417–424. [Google Scholar] [CrossRef]
- Modesto, V.; Ilarri, M.; Souza, A.T.; Lopes-Lima, M.; Douda, K.; Clavero, M.; Sousa, R. Fish and mussels: Importance of fish for freshwater mussel conservation. Fish Fish. 2018, 19, 244–259. [Google Scholar] [CrossRef]
- Haag, W.R. North American Freshwater Mussels Natural History, Ecology, and Conservation; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Prisma-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, C.C.; Hakenkamp, C.C. The functional role of burrowing bivalves in freshwater ecosystems. Freshw. Biol. 2001, 46, 1431–1446. [Google Scholar] [CrossRef]
- Elliott, P.; Aldridge, D.C.; Moggridge, G.D. Zebra mussel filtration and its potential uses in industrial water treatment. Water Res. 2008, 42, 1664–1674. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Nichols, S.J.; Spooner, D.E. Community and foodweb ecology of freshwater mussels. J. N. Am. Benthol. Soc. 2008, 27, 409–423. [Google Scholar] [CrossRef]
- Aldridge, D.C.; Fayle, T.M.; Jackson, N. Freshwater mussel abundance predicts the behaviour of mobile interstitial invertebrates in a soft sediment environment. Oecologia 2007, 151, 115–124. [Google Scholar]
- Howard, J.K.; Cuffey, K.M. Freshwater mussels in a California North Coast Range river: Occurrence, distribution, and controls. J. N. Am. Benthol. Soc. 2003, 22, 63–77. [Google Scholar] [CrossRef]
- Strayer, D.L.; Caraco, N.F.; Cole, J.J.; Findlay, S.; Pace, M.L. Transformation of freshwater ecosystems by bivalves: A case study of zebra mussels in the Hudson River. BioScience 1999, 49, 19–27. [Google Scholar] [CrossRef]
- Geist, J. Strategies for the conservation of endangered freshwater pearl mussels (Margaritifera margaritifera L.) a synthesis of Conservation Genetics and Ecology. Hydrobiologia 2010, 644, 69–88. [Google Scholar] [CrossRef]
- Buddensiek, V.; Engel, H.; Wachtler, K.; Wacker, K. Studies on the chemistry of artificial river water suitable for rearing young freshwater pearl mussels (Margaritifera margaritifera L.). Arch. Hydrobiol. 1993, 127, 87–107. [Google Scholar]
- Farris, J.L.; Van Hassel, J.H.; Newton, T.J. Biomonitoring with freshwater mussels. Environ. Toxicol. Chem. 1988, 7, 295–307. [Google Scholar]
- Blaise, C.; Gagné, F.; Pellerin, J.; Hansen, P.D.; Trottier, S. Molluscan shellfish biomarker study of the Quebec harbor dredging project. Environ. Toxicol. 2002, 17, 170–186. [Google Scholar] [CrossRef]
- Baldigo, B.P.; Sporn, L.A.; George, S.D.; Ball, J.A. Assessment of freshwater mussel health using physical, chemical, and biological indicators in the Upper Delaware River Basin, New York. Environ. Monit. Assess. 2017, 189, 459. [Google Scholar]
- Augspurger, T.; Keller, A.E.; Black, M.C.; Cope, W.G.; Dwyer, F.J. Water quality guidance for protection of freshwater mussels (Unionidae) from ammonia exposure. Environ. Toxicol. Chem. 2003, 22, 2569–2575. [Google Scholar] [CrossRef]
- Miller, A.K.; Reed, S.M.; Huffnagle, K. Microplastic ingestion by freshwater mussels in the Laurentian Great Lakes: Implications for ecological health and water quality monitoring. Environ. Pollut. 2017, 225, 52–59. [Google Scholar]
- Almeshal, W.; Takács, A.; Aradi, L.; Sandil, S.; Dobosy, P.; Záray, G. Comparison of freshwater mussels Unio tumidus and Unio crassus as biomonitors of microplastic contamination of Tisza River (Hungary). Environments 2022, 9, 122. [Google Scholar] [CrossRef]
- Bousselmi, A.; Khalloufi, N.; Bacha, O.; Alzwawy, A.G.; Nhili, A.; Mahmoudi, E.; Bejaoui, M. Behaviour, biochemical and histological responses of the freshwater mussels Unio ravoisieri exposed to wastewater from Wadi Guenniche (Northeastern Tunisia). Chem. Ecol. 2025, 41, 129–151. [Google Scholar] [CrossRef]
- Choudri, B.S.; Baawain, M. Effects of Pollution on Freshwater Organisms. Water Environ. Res. 2016, 88, 1672–1692. [Google Scholar] [CrossRef]
- Binelli, A.; Della Torre, C.; Magni, S.; Parolini, M. Does zebra mussel (Dreissena polymorpha) represent the freshwater counterpart of Mytilus in ecotoxicological studies? A critical review. Environ. Pollut. 2015, 196, 386–403. [Google Scholar] [CrossRef]
- Busch, D.; Schuchardt, B. The use of the freshwater mussel Dreissena polymorpha (Pallas) for biomonitoring heavy metals in limnic ecosystems: The Weser (FRG). Int. Ver. Theor. Angew. Limnol. Verhandlungen 1991, 24, 2261–2264. [Google Scholar] [CrossRef]
- Yevtushenko, N.Y.; Bren, N.V.; Sytnik, Y.M. Heavy metal contents in invertebrates of the Danube River. Water Sci. Technol. 1990, 22, 119–125. [Google Scholar] [CrossRef]
- Mersch, J.; Pihan, J.C. Simultaneous assessment of environmental impact on condition and trace metal availability in zebra mussels Dreissena polymorpha transplanted into the Wiltz River, Luxembourg. Comparison with the aquatic moss. Arch. Environ. Contam. Toxicol. 1993, 25, 353–364. [Google Scholar] [CrossRef]
- Kraak, M.H.; Martin, C.; Peeters, W.H.; De Kock, W.C. Biomonitoring of heavy metals in the Western European rivers Rhine and Meuse using the freshwater mussel Dreissena polymorpha. Environ. Pollut. 1991, 74, 101–114. [Google Scholar] [CrossRef]
- Giusti, F.; Oppi, E. Dreissena polymorpha (Pallas) nuovamente in Italia. (Bivalvia, Dreissenidae). Mem. Mus. Civ. Stor. Nat. Verona 1973, 20, 45–49. [Google Scholar]
- Camusso, M.; Balestrini, R.; Binelli, A. Use of zebra mussel (Dreissena polymorpha) to assess trace metal contamination in the largest Italian subalpine lakes. Chemosphere 2001, 44, 263–270. [Google Scholar] [CrossRef]
- Carrasco, L.; Díez, S.; Soto, D.X.; Catalan, J.; Bayona, J.M. Assessment of mercury and methylmercury pollution with zebra mussel (Dreissena polymorpha) in the Ebro River (NE Spain) impacted by industrial hazardous dumps. Sci. Total Environ. 2008, 407, 178–184. [Google Scholar] [CrossRef]
- Bacchetta, R.; Mantecca, P. DDT polluted meltwater affects reproduction in the mussel Dreissena polymorpha. Chemosphere 2009, 76, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Khazri, A.; Abidi, O.; Touaylia, S.; Belgacem, R.; Mezni, A.; Mahmoudi, E.; Beyrem, H.; Mohamed, D. Bisphenol a (BPA) aggravate the adverse effect on physiological and biochemical response in freshwater mussel potomida littoralis. Int. J. Environ. Health Res. 2025, 35, 140–151. [Google Scholar] [CrossRef]
- Berglund, E.; Fogelberg, V.; Nilsson, P.A.; Hollander, J. Microplastics in a freshwater mussel (Anodonta anatina) in Northern Europe. Sci. Total Environ. 2019, 697, 134192. [Google Scholar] [CrossRef]
- Della Torre, C.; Riccardi, N.; Magni, S.; Modesto, V.; Fossati, M.; Binelli, A. First comparative assessment of contamination by plastics and non-synthetic particles in three bivalve species from an Italian sub-alpine lake. Environ. Pollut. 2023, 330, 121752. [Google Scholar] [CrossRef]
- Zorita, I.; Ortiz-Zarragoitia, M.; Soto, M.; Cajaraville, M.P. Biomarkers in mussels from a copper site gradient (Visnes, Norway): An integrated biochemical, histochemical and histological study. Aquat. Toxicol. 2006, 78, S109–S116. [Google Scholar] [CrossRef] [PubMed]
- Binelli, A.; Ricciardi, F.; Riva, C.; Provini, A. New evidences for old biomarkers: Effects of several xenobiotics on EROD and AChE activities in Zebra mussel (Dreissena polymorpha). Chemosphere 2006, 62, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.; Rotteveel, S.; den Besten, P.J.; van Noort, P.C.M. In vivo exposure of Dreissena polymorpha mussels to the quinones menadione and lawsone: Menadione is more toxic to mussels than lawsone. J. Appl. Toxicol. 2004, 24, 135e141. [Google Scholar] [CrossRef]
- Farris, J.L.; Van Hassel, J.H. Freshwater Bivalve Ecotoxicology; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Naimo, T.J. A review of the effects of heavy metals on freshwater mussels. Ecotoxicology 1995, 4, 341–362. [Google Scholar] [CrossRef]
- Lemos, P.; Silva, P.; Sousa, C.A.; Duarte, A.J. Polluted Rivers—A Case Study in Porto, Portugal. Ecologies 2024, 5, 188–197. [Google Scholar] [CrossRef]
- Newton, T.J.; Bartsch, M.R. Lethal and sublethal effects of ammonia to juvenile Lampsilis mussels (Unionidae) in sediment and water-only exposures. Environ. Toxicol. Chem. 2007, 26, 2057–2065. [Google Scholar] [CrossRef]
- Spooner, D.E.; Vaughn, C.C. Context-dependent effects of freshwater mussels on stream benthic communities. Freshw. Biol. 2006, 51, 1016–1024. [Google Scholar] [CrossRef]
- Strayer, D.L.; Malcom, H.M. Shell decay rates of native and alien freshwater bivalves and implications for habitat engineering. Freshw. Biol. 2007, 52, 1611–1617. [Google Scholar] [CrossRef]
- Daughton, C.G. Pharmaceuticals and Personal Care Products in the Environment: Overarching Issues and Overview; ACS Publications: Washington, DC, USA, 2001. [Google Scholar]
- Dhodapkar, R.S.; Gandhi, K.N. Pharmaceuticals and personal care products in aquatic environment: Chemicals of emerging concern? In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Butterworth-Heinemann: Oxford, UK, 2019; pp. 63–85. [Google Scholar]
- Chandra, S.; Chaloupka, F.J. Seasonality in cigarette sales: Patterns and implications for tobacco control. Tob. Control 2003, 12, 105–110. [Google Scholar] [CrossRef]
- Buerge, I.J.; Poiger, T.; Müller, M.D.; Buser, H.R. Caffeine, an anthropogenic marker for wastewater contamination of surface waters. Environ. Sci. Technol. 2003, 37, 691–700. [Google Scholar] [CrossRef]
- de Solla, S.R.; Gilroy, È.; Klinck, J.; King, L.E.; McInnis, R.; Struger, J.; Backus, S. Bioaccumulation of pharmaceuticals and personal care products in wild and caged freshwater mussels. Chemosphere 2016, 146, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Chu, S.; Judt, C.; Li, H.; Oakes, K.D.; Servos, M.R.; Andrews, D.M. Antidepressants and their metabolites in municipal wastewater, and downstream exposure in an urban watershed. Environ. Toxicol. Chem. 2010, 29, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Persistence and Bioaccumulation Regulations. In Canada Gazette Part II; Government of Canada: Ottawa, ON, Canada, 2000; Volume 134, pp. 607–612. [Google Scholar]
- Hossain, A.; Bhattacharyya, S.R.; Aditya, G. Biosorption of cadmium by waste shell dust of fresh water mussel Lamellidens marginalis: Implications for metal bioremediation. ACS Sustain. Chem. Eng. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- de Paula, S.M.; Silveira, M. Studies on molluscan shells: Contributions from microscopic and analytical methods. Micron 2009, 40, 669–690. [Google Scholar] [CrossRef]
- Vaughn, C.C. Ecosystem services provided by freshwater mussels. Hydrobiologia 2018, 810, 15–27. [Google Scholar] [CrossRef]
- Atkinson, C.L.; Vaughn, C.C. Biogeochemical hotspots: Temporal and spatial scaling of the impact of freshwater mussels on ecosystem function. Freshw. Biol. 2015, 60, 563–574. [Google Scholar] [CrossRef]
- Spooner, D.E.; Vaughn, C.C. Species’ traits and environmental gradients interact to govern primary production in freshwater mussel communities. Oikos 2012, 121, 403–416. [Google Scholar] [CrossRef]
- Allen, D.C.; Vaughn, C.C. Complex hydraulic and substrate variables limit freshwater mussel species richness and abundance. J. N. Am. Benthol. Soc. 2010, 29, 383–394. [Google Scholar] [CrossRef]
- Howard, J.K.; Cuffey, K.M. The functional role of native freshwater mussels in the fluvial benthic environment. Freshw. Biol. 2006, 51, 460–474. [Google Scholar] [CrossRef]
- Hopper, G.W.; DuBose, T.P.; Gido, K.B.; Vaughn, C.C. Freshwater mussels alter fish distributions through habitat modifications at fine spatial scales. Freshw. Sci. 2019, 38, 702–712. [Google Scholar] [CrossRef]
- McCall, P.L.; Tevesz, M.J.S. The effects of benthos on physical properties of freshwater sediments. In Animal-Sediment Relations the Biogenic Alteration of Sediments; McCall, P.L., Tevesz, M.J.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 105–176. [Google Scholar]
- Ilarri, M.I.; Amorim, L.; Souza, A.T.; Sousa, R. Physical legacy of freshwater bivalves: Effects of habitat complexity on the taxonomical and functional diversity of invertebrates. Sci. Total Environ. 2018, 634, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Ilarri, M.; Souza, A.; Sousa, R. Contrasting decay rates of freshwater bivalves’ shells: Aquatic versus terrestrial habitats. Limnologica 2015, 51, 8–14. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Atkinson, C.L.; Julian, J.P. Multiple droughts lead to long-term losses in mussel-provided ecosystem services. Ecol. Evol. 2015, 5, 1291–1305. [Google Scholar] [CrossRef]
- Meyer, B.J.; Mann, N.J.; Lewis, J.L.; Milligan, G.C.; Sinclair, A.J.; Howe, P.R. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 2003, 38, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Linehan, L.G.; O’Connor, T.P.; Burnell, G. Seasonal variation in the chemical composition and fatty acid profile of Pacific oysters (Crassostrea gigas). Food Chem. 1999, 64, 211–214. [Google Scholar] [CrossRef]
- Sonowal, J.; Kardong, D. Nutritional evaluation of freshwater bivalve, Lamellidens spp. from the upper Brahmaputra basin, Assam with special reference to dietary essential amino acids, omega fatty acids and minerals. J. Environ. Biol. 2020, 41, 931–941. [Google Scholar] [CrossRef]
- Ulagesan, S.; Krishnan, S.; Nam, T.J.; Choi, Y.H. A review of bioactive compounds in oyster shell and tissues. Front. Bioeng. Biotechnol. 2022, 10, 913839. [Google Scholar] [CrossRef]
- Tan, K.; Lu, S.Y.; Tan, K.; Ransangan, J.; Cai, X.; Cheong, K.L. Bioactivity of polysaccharides derived from bivalves. Int. J. Biol. Macromol. 2023, 250, 126096. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.W.; Zhou, D.Y.; Li, T.; Yan, S.; Yang, J.F.; Li, D.M.; Dong, X.-P.; Murata, Y. Chemical composition and free radical scavenging activities of a sulphated polysaccharide extracted from abalone gonad (Haliotis discus Hannai Ino). Food Chem. 2010, 121, 712–718. [Google Scholar] [CrossRef]
- Yin, H.L.; Yuan, M.A.; Wang, L.; Sun, Y.M.; Yu, Y.H.; Zhu, B.W. The extraction of and scavenging hydroxyl free radicals by scallop viscus polysaccharide. Fish. Sci. 2007, 26, 255–258. [Google Scholar]
- Song, S.Y.; Sun, L.M.; Zhu, B.W.; Niu, H.L.; Yang, J.F. Antioxidant and immunomodulating activities of polysaccharide from scallop gonad. Food Sci. 2012, 33, 248–251. [Google Scholar]
- He, N.; Yang, X.; Jiao, Y.; Tian, L.; Zhao, Y. Characterisation of antioxidant and antiproliferative acidic polysaccharides from Chinese wolfberry fruits. Food Chem. 2012, 133, 978–989. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, H.; Zhang, Y.; Wang, H. Chemical properties and immunostimulatory activity of a water-soluble polysaccharide from the clam of Hyriopsis cumingii Lea. Carbohydr. Polym. 2009, 77, 365–369. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L.; Cha, Q.; Sun, Y.; Ye, H.; Zeng, X. Structural characterization and immunostimulatory activity of a homogeneous polysaccharide from Sinonovacula constricta. J. Agric. Food Chem. 2015, 63, 7986–7994. [Google Scholar] [CrossRef] [PubMed]
- Amornrut, C.; Toida, T.; Imanari, T.; Woo, E.R.; Park, H.; Linhardt, R.; Wu, S.J.; Kim, Y.S. A new sulfated β-galactan from clams with anti-HIV activity. Carbohydr. Res. 1999, 321, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Di, L.Q.; Li, J.S.; Hu, L.H.; Cheng, J.M.; Wu, H. Elaboration in type, primary structure, and bioactivity of polysaccharides derived from mollusks. Crit. Rev. Food Sci. Nutr. 2019, 59, 1091–1114. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.R.; Kim, W.S.; Kim, Y.S. Virus-cell fusion inhibitory activity for the polysaccharides from various Korean edible clams. Arch. Pharmacal Res. 2001, 24, 514–517. [Google Scholar] [CrossRef]
- Yu, N.; Liu, S.; Han, J.J. The deppressive effect of glycosaminoglycan from scallop on type-I herpes simplex virus. Acta Acad. Med. Qingdao Univ. 2008, 44, 111–113. [Google Scholar]
- Fan, Q.Y.; Li, C.P.; Xu, L.F.; Wang, K.X. Antiviral effect of scallop polysaccharide on duck Hepatitis B virus. Chin. J. Zoonoses 2012, 28, 248–251. [Google Scholar]
- Arumugam, M.; Garg, H.; Ajithkumar, T.; Shanmugam, A. Antiproliferative heparin (glycosaminoglycans) isolated from giant clam (Tridacna maxima) and green mussel (Perna viridis). Afr. J. Biotechnol. 2009, 8, 2394–2396. [Google Scholar]
- Matsue, H.; Takaya, Y.; Uchisawa, H.; Naraoka, T.; Okuzaki, B.; Narumi, F.; Ishida, K.; Sasaki, J. Antitumor peptidoglycan with new carbohydrate structure from squid Ink. In Food Factors for Cancer Prevention; Springer: Tokyo, Japan, 1997; pp. 331–336. [Google Scholar]
- Zhang, L.; Liu, W.; Han, B.; Sun, J.; Wang, D. Isolation and characterization of antitumor polysaccharides from the marine mollusk Ruditapes philippinarum. Eur. Food Res. Technol. 2008, 227, 103–110. [Google Scholar] [CrossRef]
- Du, T.T.; Cheng, Y.; Zhong, C.C. Structure modification and research on antitumor activity of mussel polysaccharide. J. Zhejiang Ocean. Univ. 2014, 2014, 354–357. [Google Scholar]
- Zhang, D.; Wang, C.; Wu, H.; Xie, J.; Du, L.; Xia, Z.; Cai, J.; Huang, Z.; Wei, D. Three sulphated polysaccharides isolated from the mucilage of mud snail, Bullacta exarata philippi: Characterization and antitumour activity. Food Chem. 2013, 138, 306–314. [Google Scholar] [CrossRef]
- Wang, H.T.; Liu, S.; Ju, C.X.; Yu, N. Study in the protection effects of oyster glycosaminoglycan on vascular endothelial cell injury. Prog. Mod. Biomed. 2006, 6, 33–35. [Google Scholar]
- Sun, F.; Liu, S. Effects of glycosaminoglycan from scallop skirt on foam cell formation and its function. J. Med. Postgrad. 2003. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/wpr-584630 (accessed on 18 January 2025).
- Heydari, S.; Pourashouri, P.; Shabanpour, B.; Shamsabadi, F.T.; Arabi, M.S. Evaluation of Freshwater Mussel (Anodonta cygnea) Protein Hydrolysates in Terms of Antibacterial Activity and Functional Properties. Waste Biomass Valorization 2024, 15, 4279–4290. [Google Scholar] [CrossRef]
- Hou, L.; Wang, Q.K.; He, Y.H.; Ren, D.D.; Song, Y.F.; Jiang, X.D.; Li, L.D. The extraction of oyster polysaccharide and its hepatoprotective effect against alcohol-induced hepatic injury in mice. Sci. Technol. Food Ind. 2014, 35, 356. [Google Scholar]
- Li, J.B.; Yuan, X.F.; Hou, G. Effects of mussel polysaccharide on alcoholic hepatic injury in mice. Food Sci. Technol. 2009, 2009, 188–190. [Google Scholar]
- Zhao, J.; Zhou, D.Y.; Yang, J.F.; Song, S.; Zhang, T.; Zhu, C.; Song, Y.-Q.; Yu, C.-X.; Zhu, B.W. Effects of abalone (Haliotis discus hannai Ino) gonad polysaccharides on cholecystokinin release in STC-1 cells and its signaling mechanism. Carbohydr. Polym. 2016, 151, 268–273. [Google Scholar] [CrossRef]
- Chakrabortya, I.; Royb, S.; Ghoshc, S.; Ghoshb, M.; Mukherjeed, D.C.; Sarkar, D. Solvent selection in extraction of bioactive and therapeutic components from Indian fresh water mussel Lamellidens marginalis. J. Indian Chem. Soc 2017, 94, 993–1008. [Google Scholar]
- Xing, R.; Qin, Y.; Guan, X.; Liu, S.; Yu, H.; Li, P. Comparison of Antifungal Activities of Scallop Shell, Oyster Shell and Their Pyrolyzed Products. Egypt. J. Aquat. Res. 2013, 39, 83–90. [Google Scholar] [CrossRef]
- Sadeghi, K.; Park, K.; Park, K.; Seo, J. Oyster Shell Disposal: Potential as a Novel Ecofriendly Antimicrobial Agent for Packaging: A Mini Review. Korean. J. Packag. Sci. Technol. 2019, 25, 57–62. [Google Scholar] [CrossRef]
- Tat Wai, K.; O’Sullivan, A.D.; Bello-Mendoza, R. Nitrogen and Phosphorus Removal from Wastewater Using Calcareous Waste Shells—A Systematic Literature Review. Environments 2024, 11, 119. [Google Scholar] [CrossRef]
- Upadhyay, A.; Thiyagarajan, V.; Thiyagarajan, V. Proteomic Characterization of Oyster Shell Organic Matrix Proteins (OMP). Bioinformation 2016, 12, 266–278. [Google Scholar] [CrossRef][Green Version]
- Choi, J.-S.; Lee, H.-J.; Jin, S.-K.; Lee, H.-J.; Choi, Y.-I. Effect of Oyster Shell Calcium Powder on the Quality of Restructured Pork Ham. Korean J. Food Sci. Anim. Resour. 2014, 34, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Lin, C.-L.; Li, C.-T.; Hwang, D.-F. Structural Transformation of Oyster, Hard Clam, and Sea Urchin Shells after Calcination and Their Antibacterial Activity against Foodborne Microorganisms. Fish. Sci. 2015, 81, 787–794. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, S.; Zhang, F.; Wang, R.; Zhang, T.; Zhao, Y.; Zeng, M. Extraction and characterization of matrix protein from pacific oyster (Crassostrea gigs) shell and its anti-osteoporosis properties in vitro and in vivo. Food Funct. 2021, 12, 9066–9076. [Google Scholar] [CrossRef]
- Miyamoto, H.; Miyashita, T.; Okushima, M.; Nakano, S.; Morita, T.; Matsushiro, A. A carbonic anhydrase from the nacreous layer in oyster pearls. Proc. Natl. Acad. Sci. USA 1996, 93, 9657–9660. [Google Scholar] [CrossRef]
- Hyung, J.H.; Ahn, C.B.; Je, J.Y. Blue mussel (Mytilus edulis) protein hydrolysate promotes mouse mesenchymal stem cell differentiation into osteoblasts through up-regulation of bone morphogenetic protein. Food Chem. 2018, 242, 156–161. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, B.; Li, J.; Pei, Y.; Li, B. Coordination-driven multilayer of phosvitin-polyphenol functional nanofibrous membranes: Antioxidant and biomineralization applications for tissue engineering. RSC Adv. 2016, 6, 98935–98944. [Google Scholar] [CrossRef]
- Claassen, C. Shells; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Anthony, J.L.; Downing, J.A. Exploitation trajectory of a declining fauna a century of freshwater mussel fisheries in North America. Can. J. Fish. Aquat. Sci. 2001, 58, 2071–2090. [Google Scholar] [CrossRef]
- Haag, W.R.; Warren, M.L. Effects of severe drought on freshwater mussel assemblages. Trans. Am. Fish. Soc. 2008, 137, 1165–1178. [Google Scholar] [CrossRef]
- Natural and Cultured Pearls Market Size, Share, Scope, Trends And Forecast 2030. (2024, July). Verified Market Reports. Available online: https://www.verifiedmarketreports.com/product/natural-and-cultured-pearls-market-size-and-forecast/ (accessed on 18 January 2025).
- Böhm, M.; Dewhurst-Richman, N.I.; Seddon, M.; Ledger, S.E.; Albrecht, C.; Allen, D.; Bogan, A.E.; Cordeiro, J.; Cummings, K.S.; Cuttelod, A.; et al. The conservation status of the world’s freshwater molluscs. Hydrobiologia 2021, 848, 3231–3254. [Google Scholar] [CrossRef]
- Watters, G.T. Freshwater mussels and water quality a review of the effects of hydrologic and instream habitat alterations. In Proceedings of the First Freshwater Mollusk Conservation Society Symposium, 2000. pp. 261–274. Available online: https://downloads.regulations.gov/FWS-R4-ES-2015-0142-0024/content.pdf (accessed on 18 January 2025).
- Baldan, D.; Kiesel, J.; Hauer, C.; Jähnig, S.C.; Hein, T. Increased sediment deposition triggered by climate change impacts freshwater pearl mussel habitats and metapopulations. J. Appl. Ecol. 2021, 58, 1933–1944. [Google Scholar] [CrossRef]
- Richman, N.I.; Böhm, M.; Adams, S.B.; Alvarez, F.; Bergey, E.A.; Bunn, J.J.; Burnham, Q.; Cordeiro, J.; Coughran, J.; Crandall, K.A.; et al. Multiple drivers of decline in the global status of freshwater crayfish (Decapoda: Astacidea). Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140060. [Google Scholar] [CrossRef]
- Vinarski, M.V.; Bolotov, I.N.; Aksenova, O.V.; Babushkin, E.S.; Bespalaya, Y.V.; Makhrov, A.A.; Nekhaev, I.O.; Vikhrev, I.V. Freshwater Mollusca of the Circumpolar Arctic: A review on their taxonomy, diversity and biogeography. Hydrobiologia 2021, 848, 2891–2918. [Google Scholar] [CrossRef]
- Lydeard, C.; Mayden, R.L. A diverse and endangered aquatic ecosystem of the Southeast United States. Conserv. Biol. 1995, 9, 800–805. [Google Scholar] [CrossRef]
- Zieritz, A.; Bogan, A.E.; Froufe, E.; Klishko, O.; Kondo, T.; Kovitvadhi, U.; Kovitvadhi, S.; Lee, J.H.; Lopes-Lima, M.; Pfeiffer, J.M.; et al. Diversity, biogeography and conservation of freshwater mussels (Bivalvia: Unionida) in East and Southeast Asia. Hydrobiologia 2018, 810, 29–44. [Google Scholar] [CrossRef]
- Köhler, F.; Seddon, M.; Bogan, A.E.; Tu, D.V.; Sri-Aroon, P.; Allen, D.; Allen, D.J.; Smith, K.G.; Darwall, W.R. The status and distribution of freshwater molluscs of the Indo-Burma region. In The Status and Distribution of Freshwater Biodiversity in Indo-Burma; IUCN: Gland, Switzerland, 2012; pp. 66–88. [Google Scholar]
- Keller, A.E.; Zam, S.G. The acute toxicity of selected metals to the freshwater mussel, Anodonta imbecillis, Ceriodaphnia dubia, and Pimephales promelas. Environ. Toxicol. Chem. 1991, 10, 1139–1147. [Google Scholar] [CrossRef]
- Auclair, J.; Turcotte, P.; Gagnon, C.; Peyrot, C.; Wilkinson, K.J.; Gagné, F. Toxicological effects of inorganic nanoparticle mixtures in freshwater mussels. Environments 2020, 7, 109. [Google Scholar] [CrossRef]
- He, F.; Bremerich, V.; Zarfl, C.; Geldmann, J.; Langhans, S.D.; David, J.N.; Darwall, W.; Tockner, K.; Jähnig, S.C. Freshwater megafauna diversity: Patterns, status and threats. Divers. Distrib. 2018, 24, 1395–1404. [Google Scholar] [CrossRef]
- Danellakis, D.; Ntaikou, I.; Kornaros, M.; Dailianis, S. Olive oil mill wastewater toxicity in the marine environment: Alterations of stress indices in tissues of mussel Mytilus galloprovincialis. Aquat. Toxicol. 2011, 101, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Belamy, T.; Legeay, A.; Etcheverria, B.; Cordier, M.A.; Gourves, P.Y.; Baudrimont, M. Acute toxicity of sodium chloride, nitrates, ortho-phosphates, cadmium, arsenic and aluminum for juveniles of the freshwater pearl mussel: Margaritifera margaritifera (L. 1758). Environments 2020, 7, 48. [Google Scholar] [CrossRef]
- Pipe, R.K.; Coles, J.A. Environmental contaminants influencing immunefunction in marine bivalve molluscs. Fish Shellfish. Immunol. 1995, 5, 581–595. [Google Scholar] [CrossRef]
- Livingstone, D.R.; Chipman, J.K.; Lowe, D.M.; Minier, C.; Pipe, R.K. Development of biomarkers to detect the effects of organic pollution on aquatic invertebrates: Recent molecular, genotoxic, cellular and immunological studies on the common mussel (Mytilus edulis L.) and other mytilids. Int. J. Environ. Pollut. 2000, 13, 56–91. [Google Scholar] [CrossRef]
- Funes, V.; Alhama, J.; Navas, J.I.; López-Barea, J.; Peinado, J. Ecotoxicological effects of metal pollution in two mollusc species from the Spanish South Atlantic littoral. Environ. Pollut. 2006, 139, 214–223. [Google Scholar] [CrossRef]
- Beniston, M.; Tol, R.S. The potential impacts of climate change on Europe. Energy Environ. 1998, 9, 365–381. [Google Scholar] [CrossRef]
- Hastie, L.C.; Cosgrove, P.J.; Ellis, N.; Gaywood, M.J. The threat of climate change to freshwater pearl mussel populations. AMBIO J. Hum. Environ. 2003, 32, 40–46. [Google Scholar] [CrossRef]
- Hastie, L.C.; Cosgrove, P.J. The decline of migratory salmonid stocks: A new threat to pearl mussels in Scotland. Freshw. Forum 2001, 15, 85–96. [Google Scholar]
- Young, M.R.; Williams, J.C. The reproductive biology of the freshwater pearl mussel Margaritifera margaritifera (Linn.) in Scotland I. Field studies. Arch. Hydrobiol. 1984, 99, 405–422. [Google Scholar]
- Dawson, A.G.; Smith, D.E.; Dawson, S. The Potential Impacts of Climate Change on Sea Levels Around Scotland; SNH Research, Survey and Development Report No. 178; Scottish Natural Heritage: Edinburgh, UK, 2001. [Google Scholar]
- Williams, J.D.; Warren, M.L.; Cummings, K.S.; Harris, J.L.; Neves, R.J. Conservation status of freshwater mussels of the United States and Canada. Fisheries 1993, 18, 6–22. [Google Scholar] [CrossRef]
- Haag, W.R.; Williams, J.D. Monitoring integrative and practical approaches for evaluating conservation status and management of freshwater mussels (Bivalvia unionidae). Freshw. Mollusk Biol. Conserv. 2014, 17, 17–28. [Google Scholar]
- Uliano-Silva, M.; Fernandes, F.F.C.F.; de Holanda, I.B.; Rebelo, M.F. Invasive species as a threat to biodiversity: The golden mussel Limnoperna fortunei approaching the Amazon River basin. Explor. Themes Aquat. Toxicol. 2013, 2013, 1–14. [Google Scholar]
- Mingyang, L.; Yunwei, J.; Kumar, S.; Stohlgren, T.J. Modeling potential habitats for alien species Dreissena polymorpha in Continental USA. Acta Ecol. Sin. 2008, 28, 4253–4258. [Google Scholar] [CrossRef]
- Drake, J.M.; Bossenbroek, J.M. The potential distribution of zebra mussels in the United States. BioScience 2004, 54, 931–941. [Google Scholar] [CrossRef]
- Karatayev, A.Y.; Boltovskoy, D.; Padilla, D.K.; Burlakova, L.E. The invasive bivalves Dreissena polymorpha and Limnoperna fortunei: Parallels, contrasts, potential spread and invasion impacts. J. Shellfish. Res. 2007, 26, 205–213. [Google Scholar] [CrossRef]
- Mansur, M.C.D.; Santos, C.P.D.; Darrigran, G.; Heydrich, I.; Callil, C.T.; Cardoso, F.R. Primeiros dados quali-quantitativos do mexilhão-dourado, Limnoperna fortunei (Dunker), no Delta do Jacuí, no Lago Guaíba e na Laguna dos Patos, Rio Grande do Sul, Brasil e alguns aspectos de sua invasão no novo ambiente. Rev. Bras. Zool. 2003, 20, 75–84. [Google Scholar] [CrossRef]
- Miehls, A.L.J.; Mason, D.M.; Frank, K.A.; Krause, A.E.; Peacor, S.D.; Taylor, W.W. Invasive species impacts on ecosystem structure and function: A comparison of Oneida Lake, New York, USA, before and after zebra mussel invasion. Ecol. Model. 2009, 220, 3194–3209. [Google Scholar] [CrossRef]
- Krause, A.E.; Frank, K.A.; Mason, D.M.; Ulanowicz, R.E.; Taylor, W.W. Compartments revealed in food-web structure. Nature 2003, 426, 282–285. [Google Scholar] [CrossRef]
- Strayer, D.L. Effects of alien species on freshwater mollusks in North America. J. N. Am. Benthol. Soc. 1999, 18, 74–98. [Google Scholar] [CrossRef]
- Grizzle, J.M.; Brunner, C.J. Infectious diseases of freshwater mussels and other freshwater bivalve mollusks. Rev. Fish. Sci. 2009, 17, 425–467. [Google Scholar] [CrossRef]
- Pereira, C.; Duarte, J.; Costa, P.; Braz, M.; Almeida, A. Bacteriophages in the control of Aeromonas sp. in aquaculture systems: An integrative view. Antibiotics 2022, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.P. Acute and Chronic effects of Nitrate Pollution on Fish and Freshwater Mussels. Ph.D. Dissertation, University of Georgia, Athens, GA, USA, 2018. [Google Scholar]
- Beggel, S.; Geist, J. Acute effects of salinity exposure on glochidia viability and host infection of the freshwater mussel Anodonta anatina (Linnaeus, 1758). Sci. Total Environ. 2015, 502, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Lima, M.; Burlakova, L.E.; Karatayev, A.Y.; Mehler, K.; Seddon, M.; Sousa, R. Conservation of freshwater bivalves at the global scale: Diversity, threats and research needs. Hydrobiologia 2018, 810, 1–14. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Hundley, J.; Mosby, D.; Rosenberger, A.; Bouska, K.L.; Simmons, B.; Lindner, G.; Roberts, A. Quantitative Survey of Freshwater Mussels (Unionoidea) and Assessment of Sediment Contamination in the Big River, Missouri. Fish and Wildlife Service Report. 2016. Available online: https://www.fws.gov/media/quantitative-survey-freshwater-mussels-unionoidea-and-assessment-sediment-contamination-big (accessed on 28 December 2024).
- Bringolf, R.B.; Cope, W.G.; Eads, C.B.; Lazaro, P.R.; Barnhart, M.C.; Shea, D. Acute and chronic toxicity of technical-grade pesticides to glochidia and juveniles of freshwater mussels (Unionidae). Environ. Toxicol. Chem. 2007, 26, 2086–2093. [Google Scholar] [CrossRef]
- World Commission on Dams. Dams and Development: A New Framework for Decision-Making: The Report of the World Commission on Dams; Earthscan: Oxford, UK, 2000. [Google Scholar]
- Master, L.L.; Flack, S.R.; Stein, B.A. (Eds.) Rivers of Life: Critical Watersheds for Protecting Freshwater Biodiversity; Nature Conservancy: Arlington, VA, USA, 1998; p. 71. [Google Scholar]
- Strayer, D.L.; Downing, J.A.; Haag, W.R.; King, T.L.; Layzer, J.B.; Newton, T.J.; Nichols, J.S. Changing perspectives on pearly mussels, North America’s most imperiled animals. BioScience 2004, 54, 429–439. [Google Scholar] [CrossRef]
- Pandolfo, T.J.; Cope, W.G.; Arellano, C.; Bringolf, R.B.; Barnhart, M.C.; Hammer, E. Upper thermal tolerances of early life stages of freshwater mussels. J. N. Am. Benthol. Soc. 2010, 29, 959–969. [Google Scholar] [CrossRef]
- Golladay, S.W.; Gagnon, P.; Kearns, M.; Battle, J.M.; Hicks, D.W. Response of freshwater mussel assemblages (Bivalvia: Unionidae) to a record drought in the Gulf Coastal Plain of southwestern Georgia. J. N. Am. Benthol. Soc. 2004, 23, 494–506. [Google Scholar] [CrossRef]
- Gurevitch, J.; Padilla, D.K. Are invasive species a major cause of extinctions? Trends Ecol. Evol. 2004, 19, 470–474. [Google Scholar] [CrossRef]
- Schloesser, D.W.; Nalepa, T.F.; Mackie, G.L. Zebra mussel infestation of unionid bivalves (Unionidae) in North America. Am. Zool. 1996, 36, 300–310. [Google Scholar] [CrossRef]
- Neves, R.J. Biological feasibility of freshwater mussel and pearl culture in Gulf Coast states. Gulf Mex. Sci. 1999, 17, 6. [Google Scholar] [CrossRef]
- Cosgrove, P.J.; Hastie, L.C. Conservation of threatened freshwater pearl mussel populations: River management, mussel translocation and conflict resolution. Biol. Conserv. 2001, 99, 183–190. [Google Scholar] [CrossRef]
- Valovirta, I. Conservation of Margaritifera margaritifera in Finland. Environ. Encount. Counc. Eur. 1990, 10, 59–63. [Google Scholar]
- Poff, N.L.; Zimmerman, J.K. Ecological responses to altered flow regimes: A literature review to inform the science and management of environmental flows. Freshw. Biol. 2010, 55, 194–205. [Google Scholar] [CrossRef]
- Guerra, D.; Plazzi, F.; Stewart, D.T.; Bogan, A.E.; Hoeh, W.R.; Breton, S. Evolution of sex-dependent mtDNA transmission in freshwater mussels (Bivalvia: Unionida). Sci. Rep. 2017, 7, 1551. [Google Scholar] [CrossRef]
- Ferreira-Rodríguez, N.; Sousa, R.; Pardo, I. Negative effects of Corbicula fluminea over native freshwater mussels. Hydrobiologia 2018, 810, 85–95. [Google Scholar] [CrossRef]
- Koehnken, L.; Rintoul, M.S.; Goichot, M.; Tickner, D.; Loftus, A.-C.; Acreman, M.C. Impacts of riverine sand mining on freshwater ecosystems: A review of the scientific evidence and guidance for future research. River Res. Appl. 2020, 36, 362–370. [Google Scholar] [CrossRef]
- Sousa, R.; Halabowski, D.; Labecka, A.M.; Douda, K.; Aksenova, O.; Bespalaya, Y.; Bolotov, I.; Geist, J.; Jones, H.A.; Konopleva, E.; et al. The role of anthropogenic habitats in freshwater mussel conservation. Glob. Change Biol. 2021, 27, 2298–2314. [Google Scholar] [CrossRef]
- Bogan, A.E. Global diversity of freshwater mussels (Mollusca, Bivalvia) in freshwater. Hydrobiologia 2008, 595, 139–147. [Google Scholar] [CrossRef]
- Richardson, J.S.; Béraud, E. Effects of riparian forest harvest on streams a meta-analysis. J. Appl. Ecol. 2014, 51, 1712–1721. [Google Scholar] [CrossRef]
- National Research Council. Hydrology, Ecology, and Fishes of the Klamath River Basin; The National Academies Press: Washington, DC, USA, 2008. [Google Scholar] [CrossRef]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Lydeard, C.; Cummings, K.S. (Eds.) Freshwater Mollusks of the World: A Distribution Atlas; JHU Press: Baltimore, MD, USA, 2019. [Google Scholar]
- Graf, D.L.; Cummings, K.S. A ‘big data’approach to global freshwater mussel diversity (Bivalvia: Unionoida), with an updated checklist of genera and species. J. Molluscan Stud. 2021, 87, eyaa034. [Google Scholar] [CrossRef]
- Aldridge, D.C.; Ollard, I.S.; Bespalaya, Y.V.; Bolotov, I.N.; Douda, K.; Geist, J.; Haag, W.R.; Klunzinger, M.W.; Lopes-Lima, M.; Mlambo, M.C.; et al. Freshwater mussel conservation: A global horizon scan of emerging threats and opportunities. Glob. Change Biol. 2023, 29, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Prié, V.; Valentini, A.; Lopes-Lima, M.; Froufe, E.; Rocle, M.; Poulet, N.; Taberlet, P.; Dejean, T. Environmental DNA metabarcoding for freshwater bivalves biodiversity assessment: Methods and results for the Western palearctic (European sub-region). Hydrobiologia 2021, 848, 2931–2950. [Google Scholar] [CrossRef]
- Freshwater Mollusk Conservation Society. A national strategy for the conservation of native freshwater mollusks. Freshw. Mollusk Biol. Conserv. 2016, 19, 1–21. [Google Scholar] [CrossRef]
- Galbraith, H.S.; Spooner, D.E.; Vaughn, C.C. Status of rare and endangered freshwater mussels in Southeastern Oklahoma. Southwest. Nat. 2008, 53, 45–50. [Google Scholar] [CrossRef]


| Conservation Measure | Current Major Research Directions and Protection Measures | Source(s) | |
|---|---|---|---|
| Legal protection and policy | Description | Regional examples for protection measures | [115,132] |
| National and international legal frameworks that restrict harmful activities, regulate exploitation, and establish conservation priorities. |
| ||
| Pollution control | Implementation of stricter regulations and improved management practices to reduce pollutant loads in freshwater systems. |
| [132] |
| Habitat restoration and flow management | Restoration of natural hydrological regimes and physical habitats through dam removals, re-meandering rivers, and environmental flow programs that mimic natural conditions. |
| [132,151,164] |
| Host fish conservation | Management practices aimed at restoring and supporting populations of key host fish species required for mussel larval development. |
| [132] |
| Advanced monitoring and research | Utilization of modern tools such as environmental DNA (eDNA), remote sensing, and next-generation sequencing to monitor populations, understand genetic diversity, and inform conservation strategies. |
| [151,165] |
| Invasive species management | Strategies designed to monitor, control, and reduce the impacts of non-native species on native mussel populations. |
| [70,151,166] |
| Protected areas and conservation prioritization | Identification of key biodiversity hotspots and establishment of protected areas to safeguard critical habitats from ongoing threats. |
| [115,122,150] |
| Global cooperation and public awareness | Collaborative efforts among scientists, managers, and policymakers to share best practices and develop coordinated strategies, complemented by increased public awareness campaigns. |
| [152,162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, A.K.; Rahman, A.; Hussain, S.; Singh, N.S. Freshwater Mussels as Multifaceted Ecosystem Engineers: Insights into Their Ecological Importance, Bioindication, and Economic Contributions. Water 2025, 17, 1629. https://doi.org/10.3390/w17111629
Verma AK, Rahman A, Hussain S, Singh NS. Freshwater Mussels as Multifaceted Ecosystem Engineers: Insights into Their Ecological Importance, Bioindication, and Economic Contributions. Water. 2025; 17(11):1629. https://doi.org/10.3390/w17111629
Chicago/Turabian StyleVerma, Akalesh Kumar, Aminur Rahman, Saddam Hussain, and Namram Sushindrajit Singh. 2025. "Freshwater Mussels as Multifaceted Ecosystem Engineers: Insights into Their Ecological Importance, Bioindication, and Economic Contributions" Water 17, no. 11: 1629. https://doi.org/10.3390/w17111629
APA StyleVerma, A. K., Rahman, A., Hussain, S., & Singh, N. S. (2025). Freshwater Mussels as Multifaceted Ecosystem Engineers: Insights into Their Ecological Importance, Bioindication, and Economic Contributions. Water, 17(11), 1629. https://doi.org/10.3390/w17111629







