Visible Light-Driven Z-Scheme CNQDs/Ag3PO4 Octopod-Shaped Nanostructures with Exposed {110} Facets for Enhanced Photocatalytic Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of CNQDs, Ag3PO4 and CNQDs/Ag3PO4
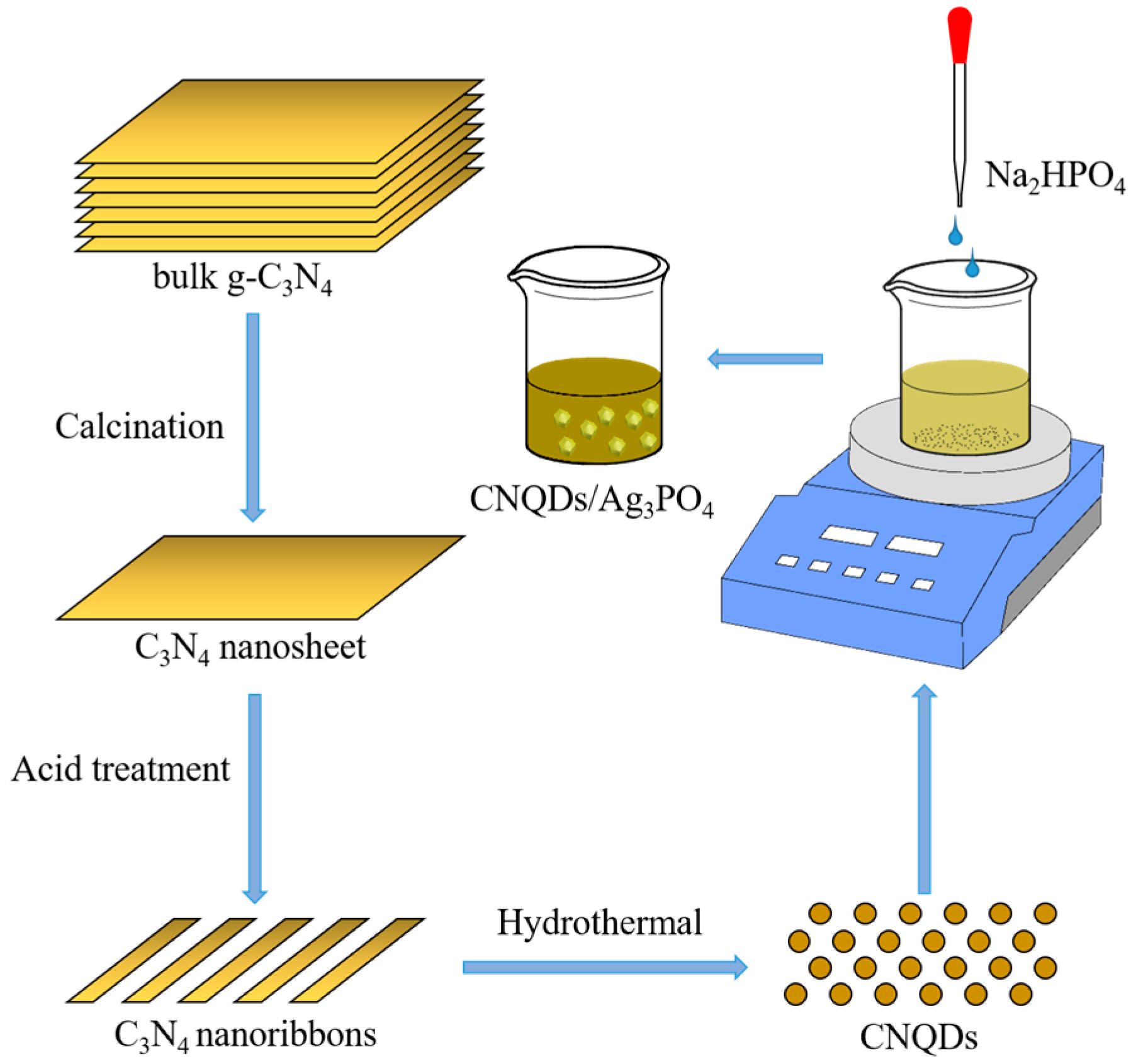
2.2.1. Synthesis of CNQDs
2.2.2. Synthesis of Ag3PO4
2.2.3. Synthesis of CNQDs/Ag3PO4
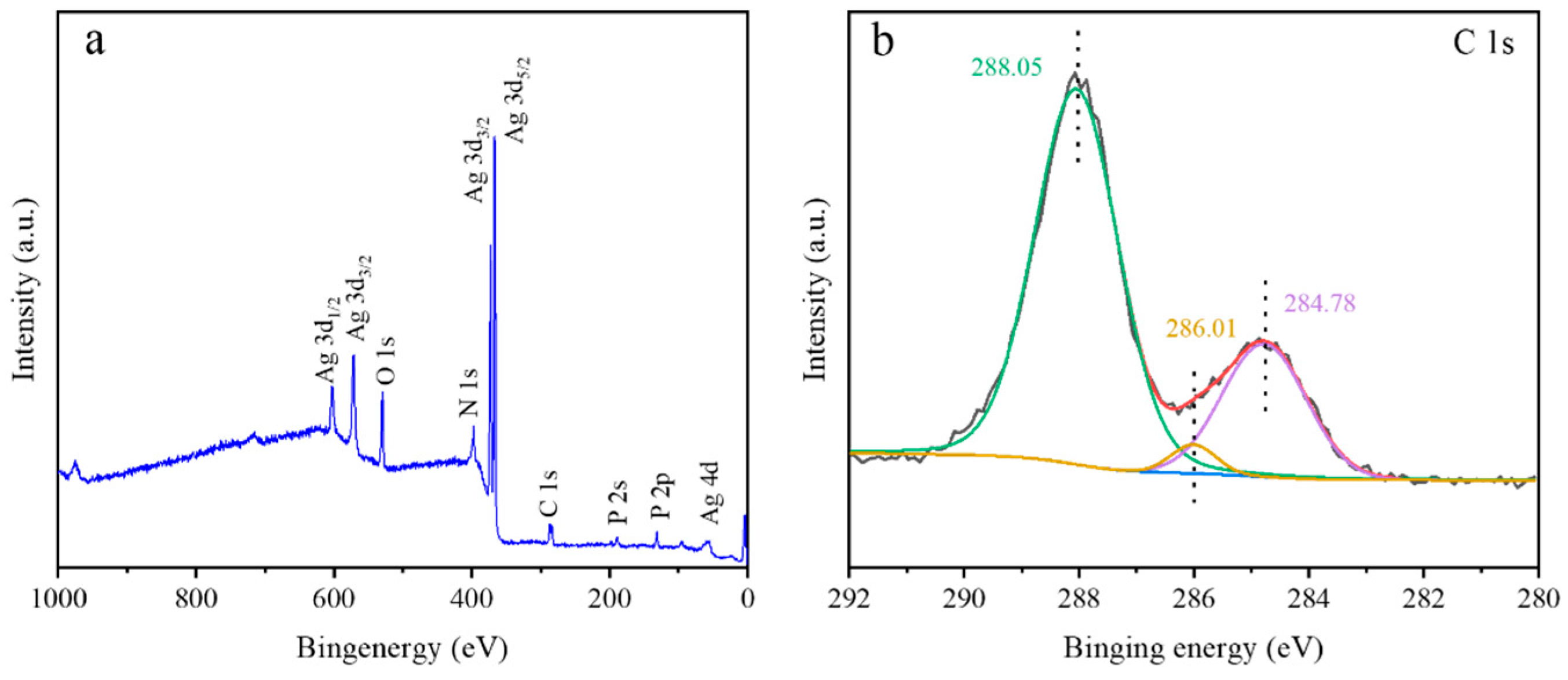
2.3. Characterization of the Photocatalysts
2.4. Photocatalytic Activity Tests
3. Results and Discussion
3.1. Morphology and Structure of Photocatalysts
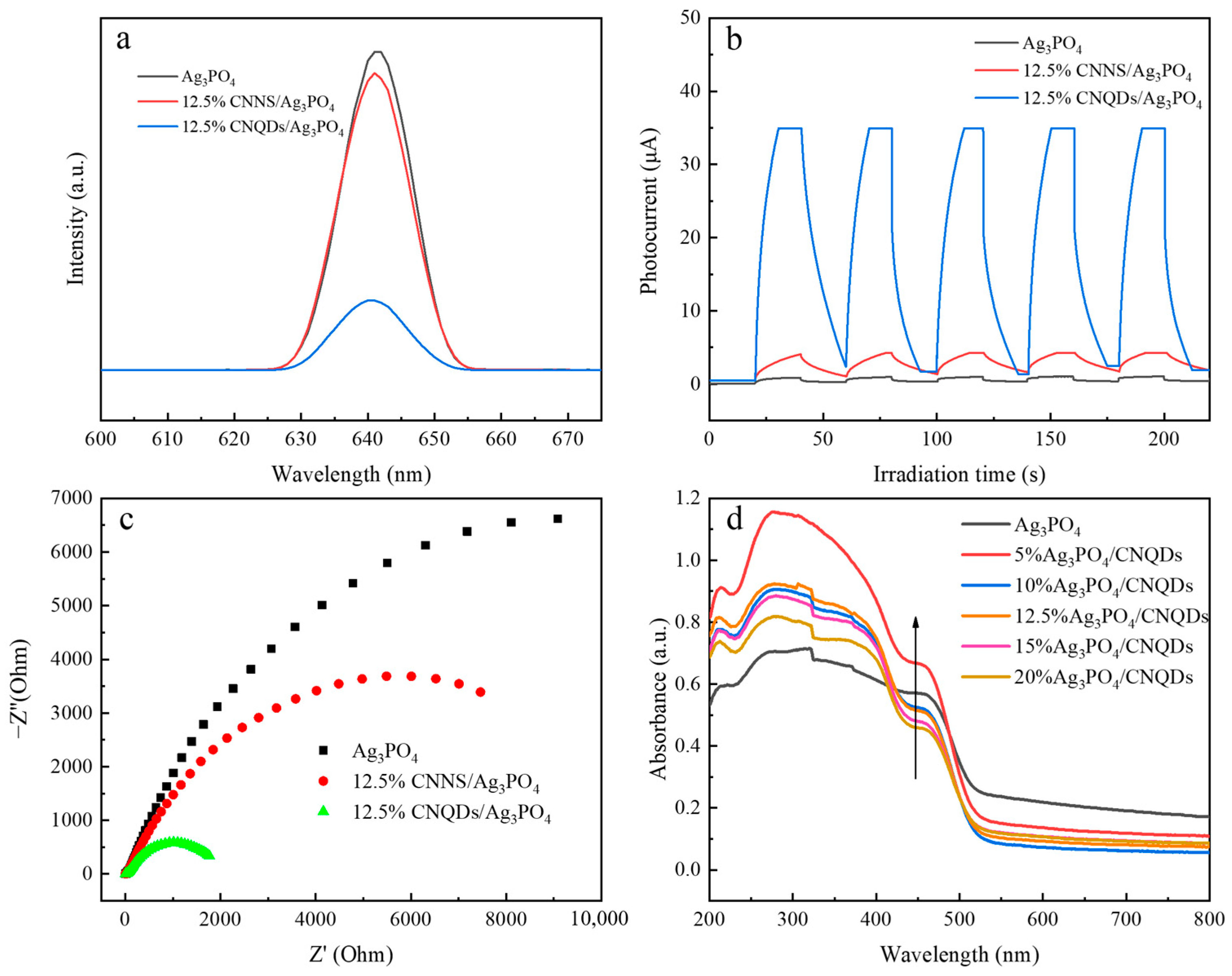
3.2. Photocatalytic Performance
3.3. Proposed Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, L.; Chu, R.; Hao, X.; Lei, Y.; Li, H.; Ma, D.; Wang, G.; Tung, C.-H.; Wang, Y. Ag3PO4 enables the generation of long-lived radical cations for visible light-driven [2 + 2] and [4 + 2] pericyclic reactions. Nat. Commun. 2024, 15, 979. [Google Scholar] [CrossRef] [PubMed]
- Sadhwani Alonso, J.J.; Vaswani Reboso, J.; Santiago, D.E. Treatment of Wastewater Using a Magnetically Recoverable Ag-Based Photocatalyst. Water 2025, 17, 232. [Google Scholar] [CrossRef]
- Wang, T.; Kumar, A.; Sharma, G.; Wang, S.; Jia, J.; Zheng, J.; Shi, H. Synergistic mechanisms of novel Z-Scheme N,S co-doped biochar-based Ag3PO4 composites for efficient removal of norfloxacin. Npj Clean Water 2024, 7, 97. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.; Ma, H.; Zhang, J.; Jiao, L.; Hao, H.; Liu, E.; Xu, L.; Wang, C.; Zhou, B.; et al. Dynamic construction of self-assembled supramolecular H12SubPcB-OPhCOOH/Ag3PO4 S-scheme arrays for visible photocatalytic oxidation of antibiotics. Appl. Catal. B-Environ. 2022, 318, 121882. [Google Scholar] [CrossRef]
- Chen, X.J.; Dai, Y.Z.; Wang, X.Y. Methods and mechanism for improvement of photocatalytic activity and stability of Ag3PO4: A review. J. Alloys Compd. 2015, 649, 910–932. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, E.; Qian, B.; Cai, M.; Bai, J.-Q.; Jiang, Y.; Chen, J.; Mao, C.-J.; Sun, S. Reinforcing Cd-S bonds through morphology engineering for enhanced intrinsic photocatalytic stability of CdS. J. Colloid. Interface Sci. 2025, 677, 963–973. [Google Scholar] [CrossRef]
- Zheng, C.; Guo, Y.; Zhang, C.; Cao, X.; Wan, J. Nitrogen-defective protonated porous C3N4 nanosheets for enhanced photocatalytic hydrogen production under visible light. Appl. Catal. B-Environ. 2025, 365, 124879. [Google Scholar] [CrossRef]
- Dong, C.; Wang, J.; Wu, K.-L.; Ling, M.; Xia, S.-H.; Hu, Y.; Li, X.; Ye, Y.; Wei, X.-W. Rhombic dodecahedral Ag3PO4architectures: Controllable synthesis, formation mechanism and photocatalytic activity. CrystEngComm 2016, 18, 1618–1624. [Google Scholar] [CrossRef]
- Bi, Y.; Ouyang, S.; Umezawa, N.; Cao, J.; Ye, J. Facet effect of single-crystalline Ag3PO4 sub-microcrystals on photocatalytic properties. J. Am. Chem. Soc. 2011, 133, 6490–6492. [Google Scholar] [CrossRef]
- Guo, R.R.; Fan, Y.T.; Tang, Y. Interesting Ag3PO4 concave rhombic dodecahedra: The same face with different morphologies and photocatalytic properties. RSC Adv. 2017, 7, 23977–23981. [Google Scholar] [CrossRef]
- Martin, D.J.; Liu, G.; Moniz, S.J.; Bi, Y.; Beale, A.M.; Ye, J.; Tang, J. Efficient visible driven photocatalyst, silver phosphate: Performance, understanding and perspective. Chem. Soc. Rev. 2015, 44, 7808–7828. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wang, Q.; Huang, L.; Quan, X.; Puma, G.L. Highly efficient and photostable conversion of bicarbonate to acetate by a pyrrolic nitrogen enriched Ag3PO4/g-C3N4 photocathode with an intermediate band level in photo-assisted microbial electrosynthesis systems. Chem. Eng. J. 2023, 471, 144673. [Google Scholar] [CrossRef]
- Chen, H.; Kang, C.; Zhao, S.; Wang, Z.; Liu, H.; Dong, D.; Qu, J.; Zheng, N.; Hua, X. Synergistic effect of oxygen vacancies and Z-scheme in Ag3PO4/FeSnO(OH)5 for ultrafast degradation of indomethacin via photogenerated h+. Sep. Purif. Technol. 2025, 366, 132802. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, Y.; Wu, Z. Photocatalytic Oxidative Coupling of Methane to Ethane Using Water and Oxygen on Ag3PO4-ZnO. Environ. Sci. Technol. 2023, 57, 11531–11540. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, H.; Yao, B.; Xiao, K.; Wang, Y. Hollow g-C3N4@Ag3PO4 Core–Shell Nanoreactor Loaded with Au Nanoparticles: Boosting Photothermal Catalysis in Confined Space. Small 2024, 20, 2308032. [Google Scholar] [CrossRef]
- Li, J.; Huang, W.; Yang, L.; Gou, G.; Zhou, C.; Li, L.; Li, N.; Liu, C.; Lai, B. Novel Ag3PO4 modified tubular carbon nitride with visible-light-driven peroxymonosulfate activation: A wide pH tolerance and reaction mechanism. Chem. Eng. J. 2022, 432, 133588. [Google Scholar] [CrossRef]
- Li, M.; Lai, C.; Yi, H.; Huang, D.; Qin, L.; Liu, X.; Li, B.; Liu, S.; Zhang, M.; Fu, Y.; et al. Multiple charge-carrier transfer channels of Z-scheme bismuth tungstate-based photocatalyst for tetracycline degradation: Transformation pathways and mechanism. J. Colloid. Interface Sci. 2019, 555, 770–782. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Wu, Y.; Duan, A.; Wang, D.; Yang, Q.; Fan, Y. Achieving simultaneous hydrogen evolution and organic pollutants degradation through the modification of Ag3PO4 using Cs2AgBiBr6 quantum dots and graphene hydrogel. Sep. Purif. Technol. 2022, 302, 122079. [Google Scholar] [CrossRef]
- Chen, G.; Wang, H.; Dong, W.; Huang, Y.; Zhao, Z.; Zeng, Y. Graphene dispersed and surface plasmon resonance-enhanced Ag3PO4 (DSPR-Ag3PO4) for visible light driven high-rate photodegradation of carbamazepine. Chem. Eng. J. 2021, 405, 126850. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, X.; Cai, M.; Cui, N.; Chen, G.; Zou, G. New insights into the efficient charge transfer of the modified-TiO2/Ag3PO4 composite for enhanced photocatalytic destruction of algal cells under visible light. Appl. Catal. B-Environ. 2022, 302, 120868. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhuang, Y.; Wang, L.; Tang, H.; Meng, X.; She, X. Constructing 0D/1D Ag3PO4/TiO2 S-scheme heterojunction for efficient photodegradation and oxygen evolution. Chin. J. Catal. 2022, 43, 2558–2568. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Zeng, G.; Xue, W.; Lei, L.; Xu, P.; Deng, R.; Li, J.; Cheng, M. In-situ synthesis of facet-dependent BiVO4/Ag3PO4/PANI photocatalyst with enhanced visible-light-induced photocatalytic degradation performance: Synergism of interfacial coupling and hole-transfer. Chem. Eng. J. 2020, 382, 122840. [Google Scholar] [CrossRef]
- Li, X.; Garlisi, C.; Guan, Q.; Anwer, S.; Al-Ali, K.; Palmisano, G.; Zheng, L. A review of material aspects in developing direct Z-scheme photocatalysts. Mater. Today 2021, 47, 75–107. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; An, C.; Wang, M. Constructing Z-Scheme 3D WO3@Co2SnO4 Heterojunction as Dual-Photocathode for Production of H2O2 and In-Situ Degradation of Organic Pollutants. Water 2024, 16, 406. [Google Scholar] [CrossRef]
- Bamiduro, G.J.; Zahran, E.M. Pd@Bi2Ru2O7/BiVO4 Z-Scheme Heterojunction Nanocomposite Photocatalyst for the Degradation of Trichloroethylene. ACS Appl. Mater. Interfaces 2023, 15, 59337–59347. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Li, Y.; Ho, W.; Yu, J. Fabrication and photocatalytic activity enhanced mechanism of direct Z-scheme g-C3N4/Ag2WO4 photocatalyst. Appl. Surf. Sci. 2017, 391, 175–183. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Madhusudan, P.; Shi, R.; Xiang, S.; Jin, M.; Chandrashekar, B.N.; Wang, J.; Wang, W.; Peng, O.; Amini, A.; Cheng, C. Construction of highly efficient Z-scheme ZnxCd1-xS/Au@g-C3N4 ternary heterojunction composite for visible-light-driven photocatalytic reduction of CO2 to solar fuel. Appl. Catal. B-Environ. 2021, 282, 119600. [Google Scholar] [CrossRef]
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. A Review of Direct Z-Scheme Photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.; Jaroniec, M. All-Solid-State Z-Scheme Photocatalytic Systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar]
- He, Y.; Zhang, L.; Teng, B.; Fan, M. New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel. Environ. Sci. Technol. 2015, 49, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Ning, X.; Zhang, T.; Chen, S.-F.; Fu, X. What is the transfer mechanism of photogenerated carriers for the nanocomposite photocatalyst Ag3PO4/g-C3N4, band-band transfer or a direct Z-scheme? Phys. Chem. Chem. Phys. 2015, 17, 11577–11585. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, G.; Li, N.; Chen, P. Quantum dots derived from two-dimensional materials and their applications for catalysis and energy. Chem. Soc. Rev. 2016, 45, 2239–2262. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Ghosh, D.; Kaley, N.M.; Pati, S.K. Photocatalytic Activity of g-C3N4 Quantum Dots in Visible Light: Effect of Physicochemical Modifications. J. Phys. Chem. C 2017, 121, 1982–1989. [Google Scholar] [CrossRef]
- Wang, W.; Yu, J.C.; Shen, Z.; Chan, D.K.L.; Gu, T. g-C3N4 quantum dots: Direct synthesis, upconversion properties and photocatalytic application. Chem. Commun. 2014, 50, 10148–10150. [Google Scholar] [CrossRef]
- Liu, S.; Yu, X.F.; Peng, Y.; Ding, X.; Cai, H.; Jin, J.; Li, Z.; Tang, H.; Yang, X. Atomically Dispersed Cobalt Anchored on Hollow Tubular Carbon Nitride Mediates Direct Electron Transfer and Oxygen-Related Active Species Path for Activation of Permonosulfate. Inorg. Chem. 2024, 63, 21260–21274. [Google Scholar] [CrossRef]
- Li, G.; Lian, Z.; Wang, W.; Zhang, D.; Li, H. Nanotube-confinement induced size-controllable g-C3N4 quantum dots modified single-crystalline TiO2 nanotube arrays for stable synergetic photoelectrocatalysis. Nano Energy 2016, 19, 446–454. [Google Scholar] [CrossRef]
- Liu, L.; Qi, Y.; Lu, J.; Lin, S.; An, W.; Liang, Y.; Cui, W. A stable Ag3PO4@g-C3N4 hybrid core@shell composite with enhanced visible light photocatalytic degradation. Appl. Catal. B-Environ. 2016, 183, 133–141. [Google Scholar] [CrossRef]
- Ma, J.; Zou, J.; Li, L.; Yao, C.; Zhang, T.; Li, D. Synthesis and characterization of Ag3PO4 immobilized in bentonite for the sunlight-driven degradation of Orange II. Appl. Catal. B-Environ. 2013, 134, 1–6. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis Of Nitrogen-Doped Graphene Films For Lithium Battery Application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Wu, Q.; Du, P.; Zhu, J.; Dai, S.; Yang, S. Incorporating Graphitic Carbon Nitride (g-C3N4) Quantum Dots into Bulk-Heterojunction Polymer Solar Cells Leads to Efficiency Enhancement. Adv. Funct. Mater. 2016, 26, 1719–1728. [Google Scholar] [CrossRef]
- Min, S.; Lu, G. Enhanced Electron Transfer from the Excited Eosin Y to mpg-C3N4 for Highly Efficient Hydrogen Evolution under 550 nm Irradiation. J. Phys. Chem. C 2012, 116, 19644–19652. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Ma, D.-K.; Zhuang, Y.; Zhang, X.; Chen, W.; Hong, L.-L.; Yan, Q.-X.; Yu, K.; Huang, S.-M. One-pot synthesis of N-doped carbon dots with tunable luminescence properties. J. Mater. Chem. 2012, 22, 16714–16718. [Google Scholar] [CrossRef]
- Yang, X.; Qin, J.; Jiang, Y.; Chen, K.; Yan, X.; Zhang, D.; Li, R.; Tang, H. Fabrication of P25/Ag3PO4/graphene oxide heterostructures for enhanced solar photocatalytic degradation of organic pollutants and bacteria. Appl. Catal. B-Environ. 2015, 166, 231–240. [Google Scholar] [CrossRef]
- Yang, Z.-M.; Liu, Y.-Y.; Xu, L.; Huang, G.-F.; Huang, W.-Q. Facile shape-controllable synthesis of Ag3PO4 photocatalysts. Mater. Lett. 2014, 133, 139–142. [Google Scholar] [CrossRef]
- Shao, N.; Hou, Z.; Zhu, H.; Wang, J.; François-Xavier, C.P. Novel 3D core-shell structured CQDs/Ag3PO4@Benzoxazine tetrapods for enhancement of visible-light photocatalytic activity and anti-photocorrosion. Appl. Catal. B-Environ. 2018, 232, 574–586. [Google Scholar] [CrossRef]
- Park, S.H.; Kwon, T.H.; Kim, K.; Annamalai, A.; Lee, M.J. Hierarchical Cu2ZnSnS4 nanostructures synthesised by a simple solvothermal reaction. Int. J. Nanotechnol. 2013, 10, 671–680. [Google Scholar] [CrossRef]
- Chen, P.; Wang, F.; Chen, Z.-F.; Zhang, Q.; Su, Y.; Shen, L.; Yao, K.; Liu, Y.; Cai, Z.; Lv, W.; et al. Study on the photocatalytic mechanism and detoxicity of gemfibrozil by a sunlight-driven TiO2/carbon dots photocatalyst: The significant roles of reactive oxygen species. Appl. Catal. B-Environ. 2017, 204, 250–259. [Google Scholar] [CrossRef]
- Fan, H.-B.; Ren, Q.-F.; Wang, S.-L.; Jin, Z.; Ding, Y. Synthesis of the Ag/Ag3PO4/diatomite composites and their enhanced photocatalytic activity driven by visible light. J. Alloys Compd. 2019, 775, 845–852. [Google Scholar] [CrossRef]
- Dong, L.; He, Y.; Li, T.; Cai, J.; Hu, W.; Wang, S.; Lin, H.; Luo, M.; Yi, X.; Zhao, L.; et al. A comparative study on the photocatalytic activities of two visible-light plasmonic photocatalysts: AgCl-SmVO4 and AgI-SmVO4 composites. Appl. Catal. A-Gen. 2014, 472, 143–151. [Google Scholar] [CrossRef]
- Bi, Y.; Ouyang, S.; Cao, J.; Ye, J. Facile synthesis of rhombic dodecahedral AgX/Ag3PO4 (X = Cl, Br, I) heterocrystals with enhanced photocatalytic properties and stabilities. Phys. Chem. Chem. Phys. 2011, 13, 10071–10075. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Quan, X.; Chen, S.; Zhao, H.; Zhang, Y. TiO2-carbon nanotube heterojunction arrays with a controllable thickness of TiO2 layer and their first application in photocatalysis. J. Photochem. Photobiol. A 2008, 200, 301–306. [Google Scholar] [CrossRef]
- Lim, J.; Monllor-Satoca, D.; Jang, J.S.; Lee, S.; Choi, W. Visible light photocatalysis of fullerol-complexed TiO2 enhanced by Nb doping. Appl. Catal. B-Environ. 2014, 152, 233–240. [Google Scholar] [CrossRef]
- Lei, Y.; Yu, Y.; Lei, X.; Liang, X.; Cheng, S.; Ouyang, G.; Yang, X. Assessing the Use of Probes and Quenchers for Understanding the Reactive Species in Advanced Oxidation Processes. Environ. Sci. Technol. 2023, 57, 5433–5444. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, Y.; Yang, Z.; Liu, Y.; Sun, X.; Li, L.; Cao, W.; Yang, J. A Novel Z-Scheme Ag3PO4/SrTiO3 Heterojunction for Highly Efficient Photocatalytic Degradation. Appl. Organomet. Chem. 2025, 39, e70041. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, Y.; Liu, Q.; Xu, M.; Hu, Y.; Chen, S. Synthesis of Ag3PO4/Ag/g-C3N4 Composite for Enhanced Photocatalytic Degradation of Methyl Orange. Molecules 2023, 28, 6082. [Google Scholar] [CrossRef]
- Lebogang, L.; Bosigo, R.; Lefatshe, K.; Muiva, C. Ag3PO4/nanocellulose composite for effective sunlight driven photodegradation of organic dyes in wastewater. Mater. Chem. Phys. 2019, 236, 121756. [Google Scholar] [CrossRef]
- Xia, Y.; He, Z.; Su, J. One-step construction of novel Ag3PO4/PbBiO2Br composite with enhanced photocatalytic activity. Mater. Res. Express. 2019, 6, 085909. [Google Scholar] [CrossRef]
- Liu, H.; Li, D.; Yang, X.; Li, H. Fabrication and characterization of Ag3PO4/TiO2 heterostructure with improved visible-light photocatalytic activity for the degradation of methyl orange and sterilization of E.coli. Mater. Technol. 2018, 34, 192–203. [Google Scholar] [CrossRef]
- Hu, Z.; Lyu, J.; Ge, M. Role of reactive oxygen species in the photocatalytic degradation of methyl orange and tetracycline by Ag3PO4 polyhedron modified with g-C3N4. Mater. Sci. Semicond. Process. 2020, 105, 104731. [Google Scholar] [CrossRef]



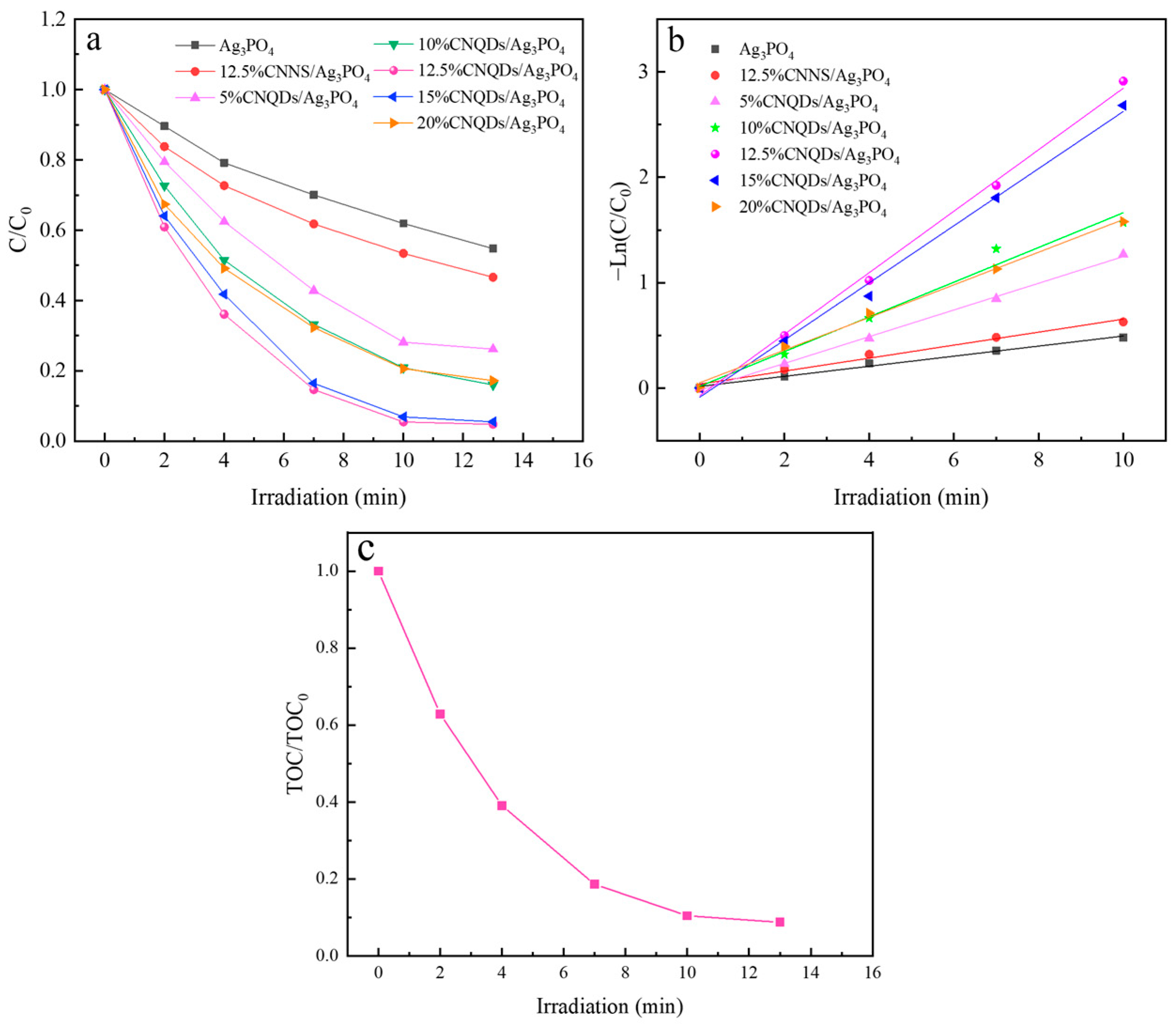

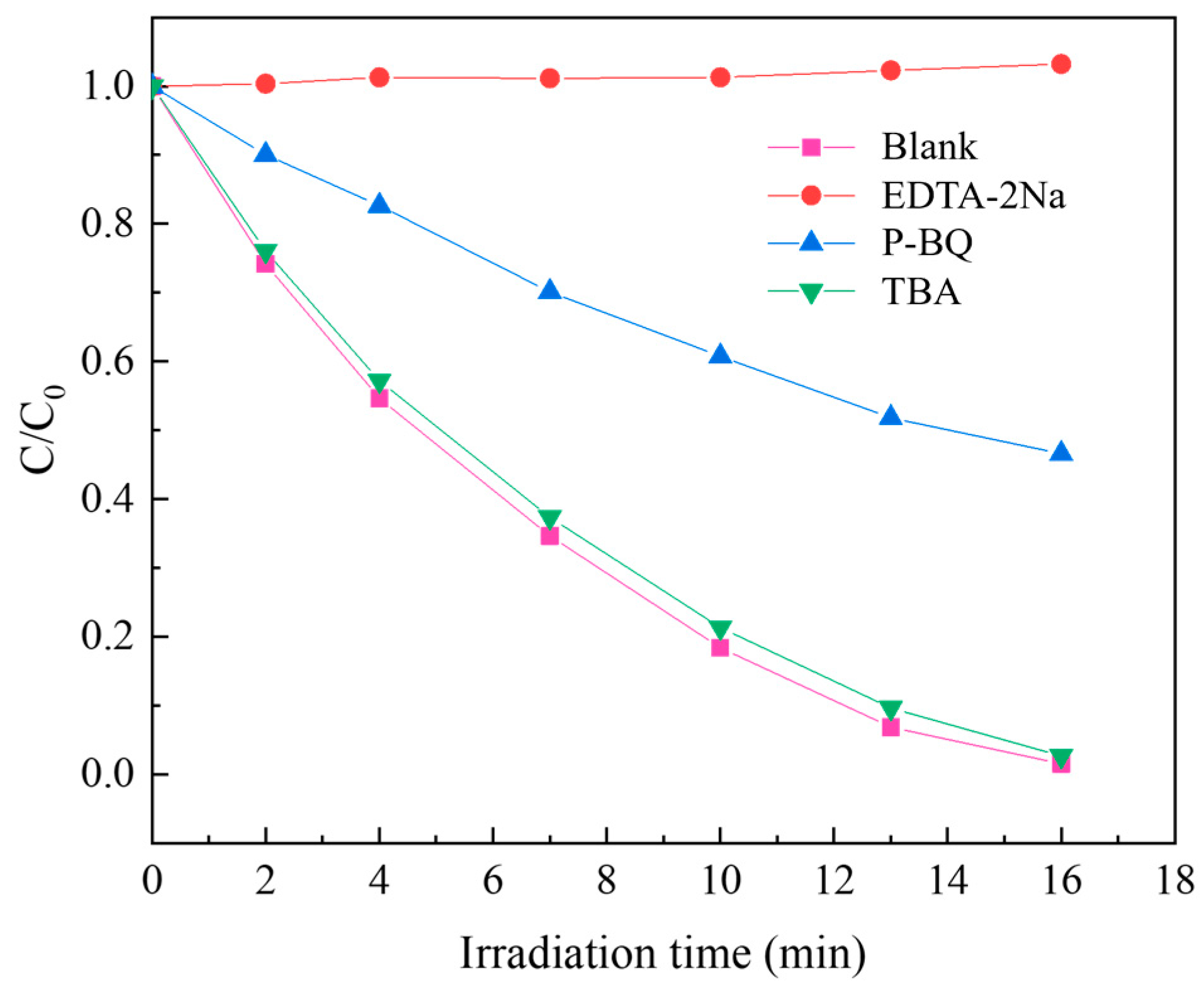

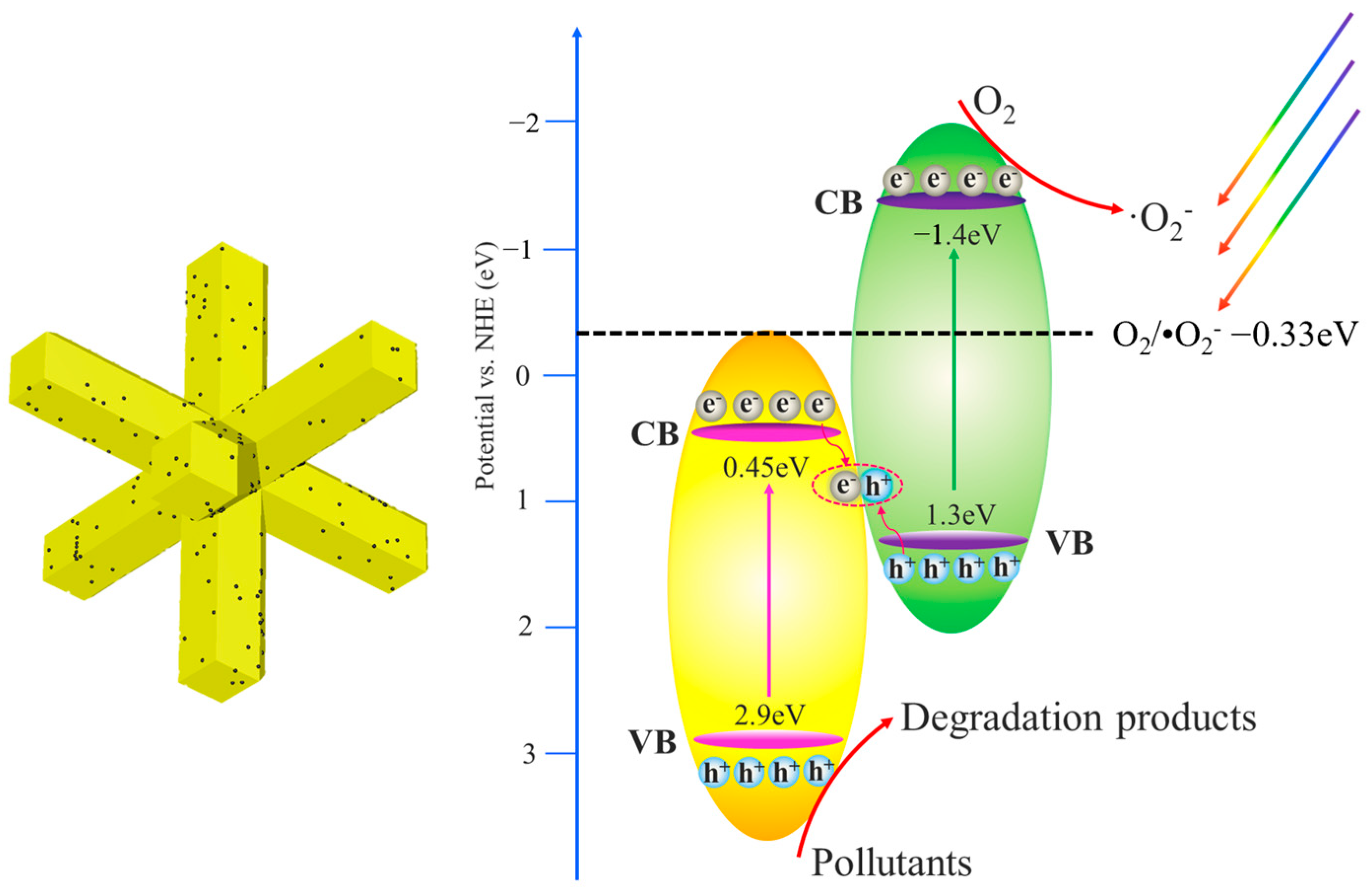
| Number | Photocatalyst | K (min−1) | R2 |
|---|---|---|---|
| 1 | Ag3PO4 | 0.0478 | 0.9918 |
| 2 | 12.5%CNNS/Ag3PO4 | 0.0616 | 0.9856 |
| 3 | 5%CNQDs/Ag3PO4 | 0.1267 | 0.9985 |
| 4 | 10%CNQDs/Ag3PO4 | 0.1646 | 0.9811 |
| 5 | 12.5%CNQDs/Ag3PO4 | 0.2916 | 0.9967 |
| 6 | 15%CNQDs/Ag3PO4 | 0.2710 | 0.9943 |
| 7 | 20%CNQDs/Ag3PO4 | 0.1548 | 0.9962 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Xiao, Y.; Wu, C.; Wang, J. Visible Light-Driven Z-Scheme CNQDs/Ag3PO4 Octopod-Shaped Nanostructures with Exposed {110} Facets for Enhanced Photocatalytic Degradation. Water 2025, 17, 1594. https://doi.org/10.3390/w17111594
Yin X, Xiao Y, Wu C, Wang J. Visible Light-Driven Z-Scheme CNQDs/Ag3PO4 Octopod-Shaped Nanostructures with Exposed {110} Facets for Enhanced Photocatalytic Degradation. Water. 2025; 17(11):1594. https://doi.org/10.3390/w17111594
Chicago/Turabian StyleYin, Xiaoze, Yuxin Xiao, Chaoyue Wu, and Jinnan Wang. 2025. "Visible Light-Driven Z-Scheme CNQDs/Ag3PO4 Octopod-Shaped Nanostructures with Exposed {110} Facets for Enhanced Photocatalytic Degradation" Water 17, no. 11: 1594. https://doi.org/10.3390/w17111594
APA StyleYin, X., Xiao, Y., Wu, C., & Wang, J. (2025). Visible Light-Driven Z-Scheme CNQDs/Ag3PO4 Octopod-Shaped Nanostructures with Exposed {110} Facets for Enhanced Photocatalytic Degradation. Water, 17(11), 1594. https://doi.org/10.3390/w17111594






