Abstract
Aerobic denitrifying bacteria can effectively cope with the challenge of dissolving nitrogen in wastewater. High-performance aerobic denitrifying bacteria were isolated using the plate streaking method and subsequently evaluated and identified based on nitrate removal efficiency, nitrite accumulation, growth characteristics, morphological analysis, and 16S rRNA sequencing. Results showed that strain N7 achieved a nitrate removal rate of 92.53% at 15 °C, with a maximum removal rate of 28.15 mg·L−1·h−1. Molecular identification confirmed this strain as Rhizobium pusense N7. Optimization experiments established the ideal conditions for Rhizobium pusense N7: sodium succinate as the carbon source, C/N ratio of 15:1, temperature at 30 °C, shaking speed at 100 rpm·min−1, and initial pH of 7.0. During the application process, Rhizobium pusense N7 demonstrated efficient nitrogen removal, eliminating 18.3% of nitrate, 71.5% of ammonia nitrogen, and 26.9% of total nitrogen (TN) from aquaculture wastewater within 24 h. This study offers a promising solution for the biological treatment of wastewater under low-temperature conditions.
1. Introduction
The aquaculture industry plays a significant role in the global food supply chain. Its rapid development has provided strong support to meet the growing demand for food in recent years [1]. However, excessive nitrogen levels have become one of the key challenges limiting the sustainable development of aquaculture. More complex and more diverse aquaculture environments have put forward higher requirements for water treatment technologies [2,3]. Therefore, the use of biological treatment for aquaculture wastewater, which is more adaptable and effective, is of great significance in promoting the development of the aquaculture industry in an environmentally friendly direction.
Aerobic denitrification technology is a common and effective method for treating aquaculture wastewater in a biological way. Aerobic denitrification bacteria reduce nitrification products such as ammonia nitrogen and nitrite to nitrogenous gases under aerobic conditions, removing nitrogen rapidly and improving water quality. The effect of their treatment is significant and long-lasting [4]. These properties make them widely used in the bioremediation of wastewater, aquaculture, and groundwater nitrate pollution. Aerobic denitrifying bacteria are widely found in soil, lakes, sediments, activated sludge and other environments [5,6,7]. They show strong environmental adaptability and high tolerance to pollutants [8]. Notably, Rhizobium sp. WS7 displayed high nitrogen removal efficiency at 15 °C, eliminating 98.73% of NH4⁺-N, 99.98% of NO3⁻-N, all NO2⁻-N (100%), and almost all mixed nitrogen (NH4⁺-N + NO3⁻-N) [9]. A biofilm system constructed by Lin et al. using heterotrophic nitrifying and aerobic denitrifying bacteria showed good nitrogen removal performance even under high-temperature conditions (50 °C) [10]. Pseudomonas mendocina 3–7 also demonstrated efficient nitrogen removal under oligotrophic conditions [11]. Heterotrophic nitrification aerobic denitrification (HNAD) bacteria are not only capable of rapid biofilm formation in moving bed bioreactors, but also tolerate low total organic carbon (TOC) and high salinity. Within 10 h, Zobellella B307 removed 90.9% of ammonia nitrogen and 97.1% of nitrate nitrogen [12]. Using Stenotrophomonas maltophilia DQ01 (an HNAD bacterium), researchers successfully started up a denitrification moving bed biofilm reactor attaining a remarkable simultaneous nitrification and denitrification efficiency of 94.21% and total nitrogen removal of 94.43% [13]. These studies collectively demonstrate the high nitrogen removal efficiency and strong environmental adaptability of aerobic denitrifying bacteria under various conditions, highlighting their potential for application in aquaculture wastewater treatment.
Although many aerobic denitrifying bacteria have been isolated, industrial wastewater treatment still primarily relies on anaerobic denitrification processes [14]. Through methods such as high-throughput sequencing of microorganisms, studies have identified the main groups of microorganisms in aerobic sludges to be Zoogloea, Paracoccus, Thaurea. Rhodobacter, etc., but most of them are difficult to isolate, purify and culture in the laboratory [15,16]. In addition, they are also sensitive to environmental factors such as temperature and dissolved oxygen [17].
Rhizobium is widespread in soil and easily available [18]. It is usually regarded as an aerobic nitrogen-fixing bacterium, but some studies have reported that it also has denitrifying ability [19]. Hidalgo-Garcia observed that Rhizobium etli was able to grow under aerobic and microaerobic conditions with nitrate as the sole nitrogen source [20]. Isolated strains like Rhizobium ZB612 have shown a nitrate removal efficiency of 60.34% [21]. At the genetic level, studies suggest that Rhizobium are capable of denitrification because they carry denitrification-related genes such as nirK and nirS, and exhibit activities of nitrate reductase and nitrite reductase [22,23]. When Rhizobium symbiotically interacts with plant roots, they can stimulate the plants′ absorption of nitrogen and phosphorus, indirectly reducing the nitrogen content in the water and enhancing purification effects [24]. Therefore, Rhizobium contributes significantly to bioremediation strategies and offers great potential for treating nitrogen-polluted water.
Despite the extensive research on Rhizobium′s denitrification capabilities, there is still a lack of comprehensive studies on its optimal culture conditions specifically for aquaculture wastewater treatment. This study aims to isolate and screen efficient aerobic denitrifying Rhizobium strains from soil fermentation broth and optimize their culture conditions to achieve high nitrogen removal efficiency. The findings are expected to provide a cost-effective and environmentally friendly solution for biological nitrogen removal in aquaculture wastewater, contributing to sustainable aquaculture practices.
2. Materials and Methods
2.1. Media
The Luria–Bertani (LB) medium, used for bacterial activation and strain preservation, consisted of tryptone (10 g/L), yeast extract (5 g/L), and NaCl (10 g/L). The Bromothymol Blue (BTB) medium, employed for isolation and cultivation, comprised KNO3 (1 g/L), KH2PO4 (1 g/L), CaCl2·2H2O (0.2 g/L), MgSO4·7H2O (1 g/L), FeSO4 (0.2 g/L), and sodium succinate (8.5 g/L), with the pH adjusted to 7.0 ± 0.5. All media were sterilized at 121 °C and 205.8 kPa before use. Tryptone and yeast extract were bought from OXOID, Basingstoke, UK. Other reagents were purchased from Sinopharm Chemical Reagent Co., Ltd, Shanghai, China.
2.2. Isolation and Screening
Samples were obtained from laboratory soil fermentation broth. Denitrifying bacteria were isolated by the spread plate method. The bacterial suspension was diluted with phosphate buffer (pH 7.2) and 1 mL aliquots of dilutions (10−5, 10−7, and 10−9) were spread onto BTB plates (BTB medium added to 2% agar) in triplicate. The plates were incubated at 30 °C for 3–5 days. After incubation, nine colonies surrounded by blue circles were identified as denitrifying bacteria. These nine colonies were selected and purified on the BTB plates using the streak plate method. Their physical properties (size, morphology, color, surface, bulge, transparency, texture) were recorded and preserved after numbering.
The strains were cultivated in BTB medium at 15 °C to preliminarily assess their denitrification capability under low-temperature conditions. The bacterial medium was sampled to detect nitrites and nitrates using a spectrophotometric method with a Flow Injection Analysis instrument (FIA, QC8500, HACH, Loveland, CO, USA). The denitrification rate was calculated using the formula: . C1 (mg·L−1) is the initial concentration of nitrate nitrogen before denitrification, c2 (mg·L−1) is the final concentration of nitrate nitrogen after denitrification, V (mL) is the volume of the medium, t (h) is the denitrification period. Bacterial growth was monitored by measuring turbidity increases at 600 nm using spectrophotometry. The strain with the highest denitrification rate, lowest nitrite accumulation and highest growth rate was chosen for further study.
2.3. Identification
An optical microscope was used to observe bacterial morphology. Biochemical tests were conducted according to Bergey’s Manual of Systematic Bacteriology (8th Edition), including Gram staining, sugar fermentation (glucose, lactose, mannitol), protein and amino acid metabolism tests (hydrogen sulfide, urease), and citrate, ornithine decarboxylase, arginine dehydrogenase, lysine decarboxylase and silymarin tests.
Bacterial DNA was extracted using a kit. PCR amplification was performed using forward primer 27F and reverse primer 1492R. Sequencing was completed by Shanghai Yuanshen Biomedical Technology Co., Ltd, Shanghai, China. The sequence was compared with the NCBI-BLAST database to identify the most similar genus. MEGA 11 software was used to construct a phylogenetic tree by the neighbor-joining method. Bootstrap analysis was chosen to calculate the support degree of each branch.
2.4. Denitrification Characteristics
Rhizobium pusense N7 was cultured in BTB medium without any additional BTB. The volume of the medium was 100 mL, and the culture period lasted for 48 h. In the laboratory cultivation process, shaking speed was used to reflect the dissolved oxygen concentration in the medium. This study explored the effects of various carbon sources (sodium succinate, glucose, sodium citrate, sodium acetate, mannitol), C/N ratios (5:1, 10:1, 15:1, 20:1, 25:1), temperatures (4 °C, 15 °C, 20 °C, 30 °C, 37 °C), shaker speeds (0 rpm, 50 rpm, 100 rpm, 200 rpm), and pH levels (5, 6, 7, 8, 9, 10) on the denitrification rate. The initial concentrations of NO3-N and NO2-N were 143.41 mg·L−1, 0 mg L−1, respectively. The concentration of nitrates in the medium was measured at 0 and 48 h. Nitrate removal rate was calculated using the formula: . c1 (mg L−1) is the initial concentration of nitrate nitrogen before denitrification (143.41 mg L−1), c2 (mg·L−1) is the final concentration of nitrate nitrogen after denitrification, t (h) is the denitrification period. All experiments were conducted in triplicate.
2.5. Application of Removing Nitrogen from Aquaculture Wastewater
Wastewater from black carp was taken into conical flasks (nitrate 7.87 mg·L−1, nitrite 0.23 mg·L−1, ammonia 2.03 mg·L−1, total nitrogen 10.14 mg·L−1, pH 7.84). Rhizobium pusense N7 was added and cultured at a concentration of 107 CFU·mL⁻¹ under optimal denitrification conditions (30 °C, shaker speed 100 rpm) for 24 h. Sodium succinate was added to achieve a C/N ratio of 15:1. Nitrate, nitrite, ammonia and TN concentrations were determined using a spectrophotometric method with a Flow Injection Analysis instrument (FIA, QC8500, HACH, Loveland, CO, USA).
2.6. Statistical Analysis
Data are presented as mean ± standard error of the mean (SE). One-way ANOVA was performed using SPSS software (Version 26.0) to analyze the differences between groups. Equation calculations are done in Microsoft Excel 2019. Statistical analysis and plotting were performed using SigmaPlot (Version 14.0, Systat Software Inc., San Jose, CA, USA).
3. Results
3.1. Isolation of Denitrifying Bacteria
A total of nine denitrifying bacteria strains were isolated and purified (Table 1). Based on the colony morphology of the microorganisms on the plate, six strains (N1, N3, N5, N7, N8, and N9) were identified as bacteria, while the remaining strains (N2, N4, and N6) were identified as fungi. The denitrification rates of these six bacterial strains were analyzed under aerobic conditions at 15 °C (Figure 1). The nitrate removal rates of strains N1, N5, and N8 were consistently below 20%, while the rates of strains N3, N7, and N9 were above 50%. The final nitrate removal rates for strains N3, N7, and N9 were 89.94%, 92.24%, and 70.13%, respectively. Therefore, strains N3, N7, and N9 were selected for further study.

Table 1.
Deposit number and colony morphology record form.
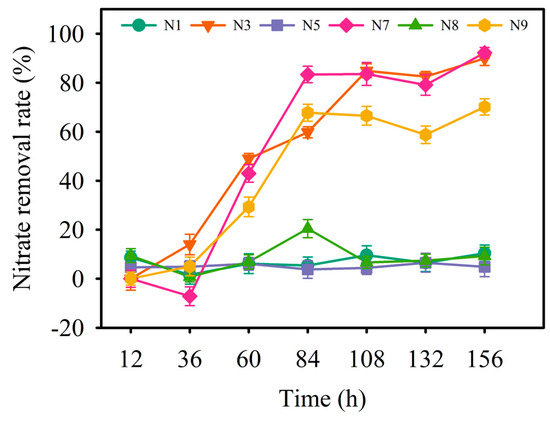
Figure 1.
Line plot of nitrate removal rates of six denitrifying bacteria strains at 15 °C.
As shown in Figure 2a, strain N3 was in a logarithmic phase from 0 to 60 h and then entered a stationary phase, with the absorbance at 600 nm reaching approximately 0.6. The denitrification rate of this strain increased rapidly during the logarithmic growth phase and then decreased upon entering the stationary phase. At 15 °C, the denitrification rate reached 22.34 mg·L−1·h−1 at 60 h and nitrite accumulation was below 20 mg·L−1 (Figure 2a). At 30 °C, the denitrification rate was up to 63.07 mg·L−1·h−1 at 36 h. Nitrite concentration peaked at 96 mg·L−1 at the same time (Figure 3a)
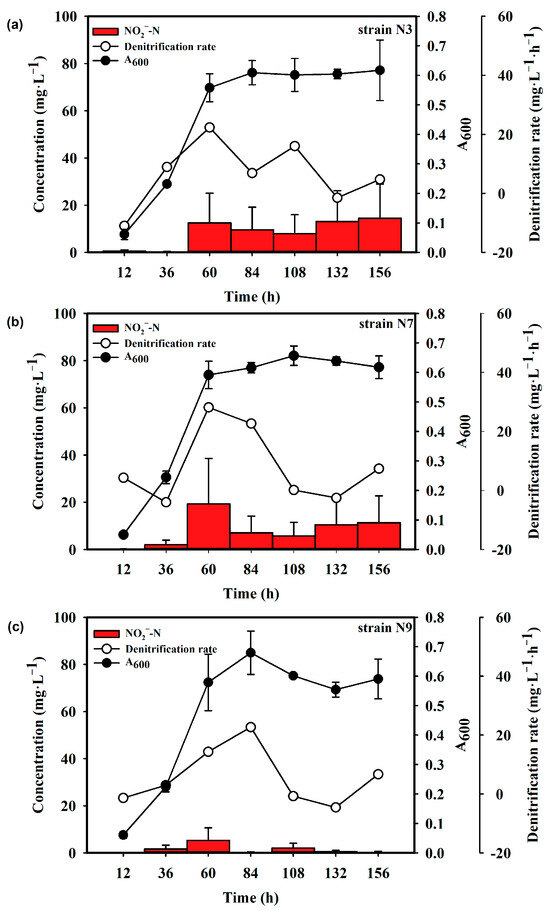
Figure 2.
Denitrification rate and growth curve of strains (a) N3, (b) N7 and (c) N9 at 15 °C.
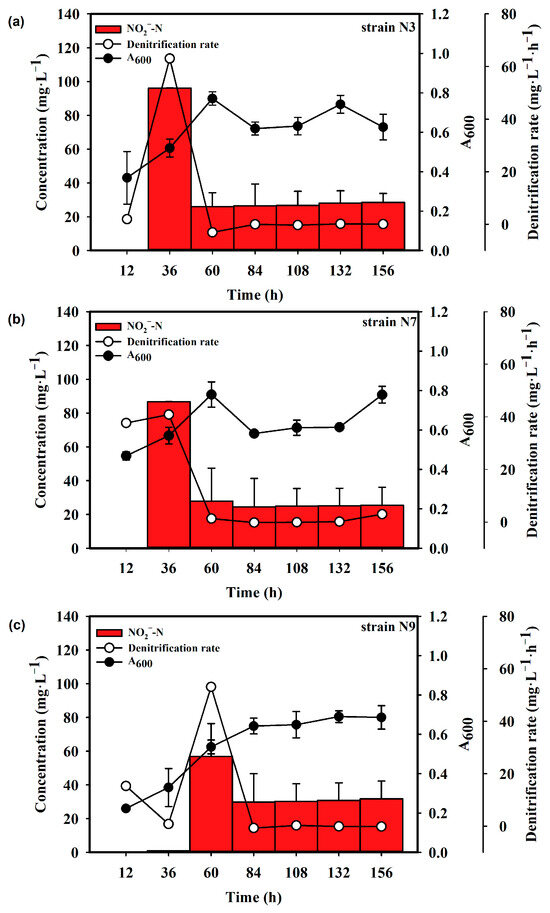
Figure 3.
Denitrification rate and growth curve of strains (a) N3, (b) N7 and (c) N9 at 30 °C.
Strain N7 was also in the logarithmic growth phase from 0 to 60 h, achieving a maximum absorbance of approximately 0.6. At 15 °C, the denitrification rate reached 28.15 mg·L−1·h−1 at 60 h and then decreased. Nitrite accumulation did not exceed 20 mg·L−1 (Figure 2b). At 30 °C, the denitrification rate was 37.67 mg·L−1·h−1 at 12 h and 40.89 mg·L−1·h−1 at 36 h. After 60 h, the denitrification rate was almost 0 mg·L−1·h−1. Nitrite apparently accumulated up to 86.7 mg·L−1 at 36 h, then dropped (Figure 3b).
Strain N9 exhibited a longer logarithmic growth phase, lasting from 0 to 84 h. At 15 °C, the denitrification rate continued to increase during this phase, reaching a maximum of 22.69 mg·L−1·h−1 at 84 h. Nitrite accumulation was minimal, with the highest content being 5.31 mg·L−1 at 60 h (Figure 2c). At 30 °C, strain N9 showed its peak denitrification rate, which achieved 53.09 mg·L−1·h−1, and highest nitrite accumulation at 60 h (Figure 3c).
These results indicate that strain N7 exhibited the highest denitrification rate and the fastest growth rate at 15 °C. It was the first to start the denitrification process at 30 °C.
3.2. Identification of Strain N7
Strain N7 was identified as a Gram-negative bacterium. Physiological and biochemical tests revealed the following characteristics: It tested negative for glucose and lactose fermentation but positive for mannitol fermentation in sugar (alcohol, glycoside) fermentation tests. In proteins and amino acid metabolism tests, it did not break down sulfur-containing amino acids to produce hydrogen sulfide. However, it tested positive for urease activity and could decompose urea to produce a large amount of ammonia. Ornithine decarboxylase, lysine decarboxylase, and salicylate tests were positive, while arginine dehydrogenase and citrate tests were negative (Table 2).

Table 2.
Physiological and biochemical identification results of strain N7.
The 16S rRNA sequencing of strain N7 yielded a sequence length of 1349 bp (GenBank: PV413231.1). The similarity between strain N7 and Rhizobium pusense 92 was more than 99%. The constructed phylogenetic tree is shown in Figure 4, confirming the identification of strain N7 as Rhizobium pusense. Therefore, we named this strain Rhizobium pusense N7.
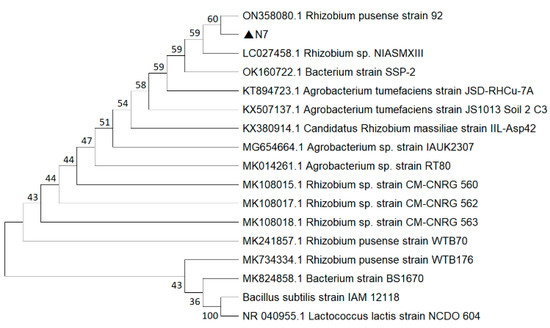
Figure 4.
Phylogenetic tree of strain N7 based on 16S rRNA sequence.
3.3. Characteristics of Denitrification
3.3.1. Carbon Source
As shown in Figure 5a, sodium succinate achieved complete nitrate removal (98.79%) with little intermediate nitrite accumulation, representing the most efficient denitrification among all carbon sources. Under other carbon source conditions, the nitrate removal rate was only between 4.01–5.05%. Therefore, sodium succinate is the optimal carbon source for Rhizobium pusense N7.
3.3.2. C/N Ratio
After 48 h of cultivation, the nitrate removal rate in all groups exceeded 97%. As depicted in Figure 5b, the nitrite accumulation column displayed a continuous downward trend across the C/N ratio gradient from 5 to 25. When the C/N ratio was above 15, Rhizobium pusense N7 could completely transform both nitrate and nitrite. However, when the C/N ratio was below 15, although nitrates were removed, nitrite accumulation occurred. Hence, the optimal C/N ratio for Rhizobium pusense N7 should be above 15.
3.3.3. Temperature
The graph (Figure 5c) depicts a temperature-dependent nitrate removal efficiency, showing minimal removal (0%) at 4 °C that sharply increased to 99.97% at 30 °C. The system began to accumulate nitrite at 37 °C, with a concentration of 34.2 mg/L. Thus, the optimal temperature for Rhizobium pusense N7 is 30 °C.
3.3.4. Shaking Speed
At shaking speeds of 0–50 rpm/min, nitrates were not reduced, with residual amounts ranging between 89.06 and 109.88 mg/L. When the shaking speed reached 100 rpm/min or more, the nitrate removal rate was between 96.34–99.21%. At shaking speeds of 100 and 200 rpm/min, the nitrite concentrations were 69.10 mg/L and 50.90 mg/L, respectively (Figure 5d). Therefore, the optimal shaking speed for cultivating Rhizobium pusense N7 is 100 rpm/min.
3.3.5. pH
When the pH was between 4 and 6, the nitrate removal rate was between 4.72 and 12.15%. When the pH rose above 7, the nitrate removal rate rapidly increased to 97.03–101.58% (Figure 5e). Considering that the denitrification process can raise the pH of the medium, the optimal pH for cultivating Rhizobium pusense N7 is 7.
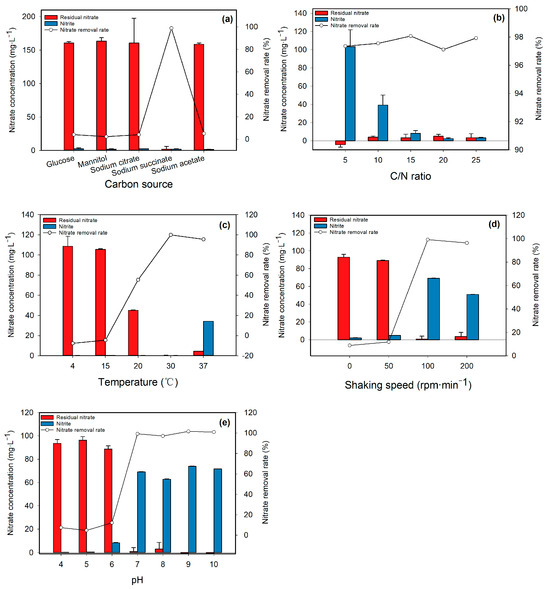
Figure 5.
Effects of carbon source (a), C/N ratio (b), temperature (c), shaking speed (d) and pH (e) on nitrate removal rate of Rhizobium pusense N7.
3.4. Effect of Removing Nitrogen from Aquaculture Wastewater
Changes in nitrate, nitrite, ammonia and TN concentration are presented in Figure 6. Within 24 h, nitrate levels decreased by 18.3%, while ammonia nitrogen exhibited a reduction of 71.5%. TN removal efficiency reached 26.9%. Nitrite concentrations increased slightly from 0.23 mg·L−1 to 0.40 mg·L−1.
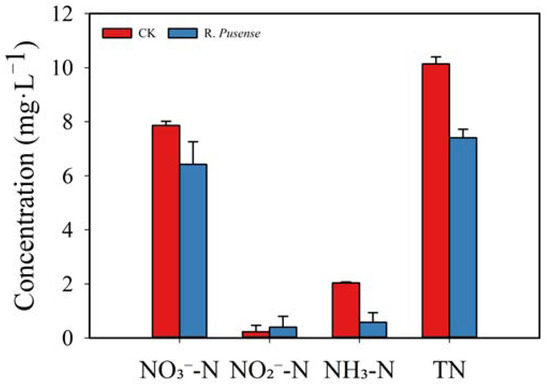
Figure 6.
Changes in nitrate (NO3⁻-N), nitrite (NO2⁻-N), ammonia (NH3-N) and total nitrogen (TN) concentrations in aquaculture wastewater.
4. Discussion
4.1. Isolation of Bacteria
Under optimal conditions, aerobic denitrifying bacteria can nearly completely remove nitrate. Rhizobium pusense N7 demonstrated exceptional efficiency, with a nitrate removal rate of 99.97% at 30 °C. Similarly, the total nitrogen removal efficiency of Zobellella endophytica W14 was 95.22% [25]. Considering nitrate removal speed, Rhizobium pusense N7 was able to achieve a maximum rate of 28.15 mg·L−1·h−1 while the highest NO3⁻-N and NO2⁻-N removal rate was 60.08 mg·L−1·h−1 in Chen’s autotrophic denitrification system [26]. The nitrate removal rate of strain SNDM1 was 4.88 mg·L−1·h−1 [27].
Low-temperature wastewater treatment poses significant challenges, particularly in cold-water aquaculture systems. The optimum culture temperature for cold-water fish is 10~18 °C, which is lower than the temperature at which most aerobic denitrifying bacteria can denitrify [28,29]. Despite these constraints, certain bacterial strains demonstrate good denitrification efficiency under cold conditions. Rhizobium pusense N7 achieved nitrate removal rates of 92.24% (140 mg·L−1) at 15 °C. The Antarctic isolate Priestia aryabhattai KX-3 exhibits exceptional performance, removing 96.0% of nitrate (40 mg·L⁻¹) at 10 °C [30]. Similarly, Pseudomonas extremaustralis Y39-6 and Pseudomonas veronii DH-3 show removal efficiencies of 63% (10 °C) and 96.89% (10 °C), respectively, within 48 h, highlighting their potential for low-temperature wastewater treatment applications [31,32].
Bacterial growth rate is another key factor in strain selection, as rapid denitrification often correlates with robust microbial proliferation. Studies suggest that inoculating denitrifying bacteria into recirculating water systems can accelerate biofilm formation, thereby shortening the water treatment cycle [33,34]. For example, Rhizobium pusense N7 enters its logarithmic growth phase within 12 h post-inoculation, aligning with the timeframe required for biofilm establishment [35]. This rapid growth enhances its practical applicability in environmental remediation.
4.2. Effect of Environmental Conditions on Nitrate Removal
4.2.1. Carbon Source
The results indicated that sodium succinate is the optimal carbon source for Rhizobium pusense N7. Succinates are the optimal carbon source for many denitrifying bacteria, including many Rhizobium species. Rhizobium leguminosarum grows significantly faster in succinate than in glucose or mannitol [36]. Sodium succinates serve as the optimal carbon source for denitrifying bacteria Psychrobacter sp. and Marinobacter sp. [37,38]. Nitrate removal of 79.63% was achieved when sodium succinate was used as carbon source in denitrification bioreactor [39]. Succinates, being intermediates of the tricarboxylic acid (TCA) cycle and having smaller and simpler structures, serve as optimal carbon sources for most heterotrophic denitrifying bacteria by facilitating easier utilization for energy provision [40,41].
4.2.2. C/N Ratio
The aerobic denitrification process involves the stepwise reduction of NO3⁻ to N2 (NO3⁻ → NO2⁻ → NO → N2O → N2). These reactions are coupled with microbial aerobic respiration, where glycolysis and the TCA cycle supply essential electron carriers such as NADH and FADH₂ and energy (ATP) [42,43]. Thus, sufficient carbon sources are critical for effective aerobic denitrification. Our results demonstrate that when the C/N ratio falls below 5, nitrate is completely depleted, but nitrite accumulates significantly (103.37 mg·L−1). This observation clearly demonstrates that the aerobic denitrification process was arrested after the initial step (NO3⁻ → NO2⁻), with subsequent reduction steps (NO2⁻ → NO → N2O → N2) failing to proceed due to insufficient reducing power. When the C/N ratio reached 15, no significant nitrite accumulation was observed, indicating that 15 represents the optimal C/N ratio for denitrification.
4.2.3. Temperature
Temperature significantly influences denitrification performance. Most aerobic denitrifying bacteria perform best between 28~37 °C [10,44]. Once the temperature falls below 15 °C, the rate of denitrification is greatly reduced. The nitrate removal rate by Rhizobium pusense N7 reached its peak of 99.97% at 30 °C, while it significantly decreased to only 4.52% at 15 °C. Our results are consistent with those reported for Pseudomonas mendocina 3–7, whose nitrate removal efficiency declined sharply from 86.0% at 30 °C to 9.2% at 10 °C [11]. Arthrobacter spp. isolated from burdock fields demonstrate adaptability across a wider range. They maintain activity at 15~40 °C despite an optimum of 30 °C [45]. Additionally, Volokita et al. found that the denitrification rate increased by 60% when the temperature increased from 19 °C to 24 °C. The denitrification rate was two times faster at 14 °C than at 30 °C [46].
4.2.4. Shaking Speed
Dissolved oxygen (DO) presents a major challenge in conventional nitrogen removal systems [47]. While aquaculture wastewater requires high DO levels to sustain fish, traditional denitrification depends on anoxic conditions, as oxygen inhibits nitrate uptake and denitrifying enzymes [48]. This requires additional equipment to create anoxic zones, increasing system complexity and operating costs. Rhizobium pusense N7 exhibited high denitrification rates at shaking speeds ranging from 100 to 200 r/min. Similarly, Pseudomonas BB-17 and Paracoccus sp. QD-19 show optimal performance at 180 r/min and 140 r/min, respectively, demonstrating adaptability to oxygen-rich environments [49,50].
4.2.5. pH
pH further influences denitrification by modulating the activity of key enzymes such as membrane-bound nitrate reductase (NAR) and periplasmic nitrate reductase (NAP) and altering nitrate’s ionic form in water [51,52,53]. This leads to a reduction in the biological conversion of nitrate. In practice, the alkalinity generated during denitrification can offset pH drops from nitrification, reducing the need for external pH adjustment and lowering treatment costs [54].
5. Conclusions
This study aimed to isolate and screen efficient aerobic denitrifying bacteria to provide an environmentally friendly solution for biological nitrogen removal in aquaculture wastewater. We obtained a Rhizobium strain in a soil fermentation broth. Compared to other reported aerobic denitrifying bacteria, Rhizobium pusense N7 demonstrated superior performance in nitrate removal at 15 °C. Its nitrate removal rate at 15 °C was 92.53%, and the maximum removal rate could reach 28.15 mg·L−1·h−1. Optimization experiments revealed that sodium succinate was the most effective carbon source for the denitrification of Rhizobium pusense N7, with a C/N ratio of more than 15 being essential for optimal performance. The strain exhibited the highest nitrate removal efficiency at a temperature of 30 °C, a pH of 7, and a shaking speed of 100 rpm/min. During the application process, Rhizobium pusense N7 demonstrated efficient nitrogen removal, eliminating 18.3% of nitrate, 71.5% of ammonia nitrogen, and 26.9% of total nitrogen (TN) from aquaculture wastewater within 24 h. Future research should focus on developing effective bioremediation strategies based on this strain and the interactions between Rhizobium pusense N7 and other microbial communities in wastewater treatment systems. The discovery of Rhizobium pusense N7 represents a significant contribution to the bioremediation field. It is a promising candidate for practical applications in cold environments.
Author Contributions
Conceptualization, Y.L. and Y.Z.; Methodology, M.S., Z.K. and R.Z.; Validation, M.S. and Y.F.; Resources, Y.L. and Y.Z.; Data curation, S.L.; Writing—original draft, S.L.; Writing—review & editing, S.L. and R.Z.; Visualization, Z.K. and Y.F.; Validation, M.S. and Y.F.; Supervision, Y.L., Y.Z. and R.Z.; Funding acquisition, Y.L. and R.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Public Welfare Research Key Project of Huzhou City (2019GZ28) and the Basic Public Welfare Research Project of Zhejiang Province (LQ21C190001).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xue, S.; Xu, W.; Wei, J.; Sun, J. Impact of environmental bacterial communities on fish health in marine recirculating aquaculture systems. Vet. Microbiol. 2017, 203, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jiang, R.; Qiu, J.; Liu, J.; Shao, L.; Zhang, J.; Liu, Q.; Jiang, Z.; Wang, H.; He, W.; et al. How to control pollution from tailwater in large scale aquaculture in China: A review. Aquaculture 2024, 590, 741085. [Google Scholar] [CrossRef]
- Quijano, G.; Arcila, J.S.; Buitrón, G. Microalgal-bacterial aggregates: Applications and perspectives for wastewater treatment. Biotechnol. Adv. 2017, 35, 772–781. [Google Scholar] [CrossRef] [PubMed]
- David, F.S.; Proença, D.C.; Valenti, W.C. Nitrogen budget in integrated aquaculture systems with Nile tilapia and Amazon River prawn. Aquacult. Int. 2017, 25, 1733–1746. [Google Scholar] [CrossRef]
- Vargas-Albores, F.; Martínez-Córdova, L.R.; Hernández-Mendoza, A.; Cicala, F.; Lago-Lestón, A.; Martínez-Porchas, M. Therapeutic modulation of fish gut microbiota, a feasible strategy for aquaculture? Aquaculture 2021, 544, 737050. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Q.; Sun, H.; Jia, L.; Zhao, L.; Wu, W. Metagenomic analyses of microbial structure and metabolic pathway in solid-phase denitrification systems for advanced nitrogen removal of wastewater treatment plant effluent: A pilot-scale study. Water Res. 2021, 196, 117067. [Google Scholar] [CrossRef] [PubMed]
- Giatsis, C.; Sipkema, D.; Smidt, H.; Heilig, H.; Benvenuti, G.; Verreth, J.; Verdegem, M. The impact of rearing environment on the development of gut microbiota in tilapia larvae. Sci. Rep. 2015, 5, 18206. [Google Scholar] [CrossRef]
- Castignetti, D.; Hollocher, T.C. Heterotrophic nitrification among denitrifiers. Appl. Environ. Microbiol. 1984, 47, 620–623. [Google Scholar] [CrossRef]
- Wei, B.; Luo, X.; Ma, W.; Lv, P. Biological nitrogen removal and metabolic characteristics of a novel cold-resistant heterotrophic nitrification and aerobic denitrification Rhizobium sp. WS7. Bioresour. Technol. 2022, 362, 127756. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, J.; He, L.; He, X.; Pan, Z.; Wang, Y.; He, Q. High-temperature biofilm system based on heterotrophic nitrification and aerobic denitrification treating high-strength ammonia wastewater: Nitrogen removal performances and temperature-regulated metabolic pathways. Bioresour. Technol. 2022, 344, 126184. [Google Scholar] [CrossRef]
- Zhu, L.; Ding, W.; Feng, L.-j.; Dai, X.; Xu, X.-Y. Characteristics of an aerobic denitrifier that utilizes ammonium and nitrate simultaneously under the oligotrophic niche. Environ. Sci. Pollut. Res. 2012, 19, 3185–3191. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Chen, X.; Bai, J.; Li, B.; Li, H.; Huang, X. Bioaugmentation performance for moving bed biofilm reactor (MBBR) treating mariculture wastewater by an isolated novel halophilic heterotrophic nitrification aerobic denitrification (HNAD) strain (Zobellella B307). J. Environ. Manag. 2022, 325, 116566. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhou, M.; Chen, Y.; Hu, Y.; Luo, J. Insight into short-cut of simultaneous nitrification and denitrification process in moving bed biofilm reactor: Effects of carbon to nitrogen ratio. Chem. Eng. J. 2020, 400, 125905. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, C.; Rong, H.; Zheng, G.; Zhao, L. The effect of dissolved oxygen concentration (DO) on oxygen diffusion and bacterial community structure in moving bed sequencing batch reactor (MBSBR). Water Res. 2017, 108, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.Y.A.; Gill, L.; Monleon, A.; Pronk, M.; van Loosdrecht, M.; Saikaly, P.E.; Ali, M. Genome-resolved metatranscriptomics unveils distinct microbial functionalities across aggregate sizes in aerobic granular sludge. Environ. Sci. Ecotechnol. 2025, 25, 100560. [Google Scholar] [CrossRef]
- Tian, L.; Feng, K.; Qin, G.; Tong, X.; Feng, X.; Xu, X.; Zhu, L. Enhancing nitrogen removal of aerobic granular sludge with optimization of dissolved oxygen spatial distribution. J. Water Process Eng. 2022, 49, 103005. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, F.; Huang, Z.; Liu, M.; Mu, X.; Zhang, X.; Giglio, G.L.; Peng, Z. The impact of organic loading rate on the cultivation and stability of aerobic granular sludge in continuous flow reactor. Int. Biodeterior. Biodegrad. 2025, 198, 106016. [Google Scholar] [CrossRef]
- Shameem, M.R.; Sonali, J.M.I.; Kumar, P.S.; Rangasamy, G.; Gayathri, K.V.; Parthasarathy, V. Rhizobium mayense sp. Nov., an efficient plant growth-promoting nitrogen-fixing bacteria isolated from rhizosphere soil. Environ. Res. 2023, 220, 115200. [Google Scholar] [CrossRef]
- Safonov, A.V.; Babich, T.L.; Sokolova, D.S.; Grouzdev, D.S.; Tourova, T.P.; Poltaraus, A.B.; Zakharova, E.V.; Merkel, A.Y.; Novikov, A.P.; Nazina, T.N. Microbial community and in situ bioremediation of groundwater by nitrate removal in the zone of a radioactive waste surface repository. Front Microbiol. 2018, 9, 1985. [Google Scholar] [CrossRef]
- Hidalgo-Garcia, A.; Tortosa, G.; Pacheco, P.J.; Gates, A.J.; Richardson, D.J.; Bedmar, E.J.; Girard, L.; Torres, M.J.; Delgado, M.J. Rhizobium etli is able to emit nitrous oxide by connecting assimilatory nitrate reduction with nitrite respiration in the bacteroids of common bean nodules. J. Plant Interact. 2023, 18, 2251511. [Google Scholar] [CrossRef]
- Zou, Y.Y.; Zhang, Y.; Li, M.Z.; Mei, R.W.; Wei, Y.F.; Ding, L.X. Isolation and identification of a heterotropgic nitrification-aerobic denitrification bacterium and its denitrification ability. China Environ. Sci. 2016, 36, 887–893. Available online: https://kns.cnki.net/kcms2/article/abstract?v=fSCzX0TVvUjLExBLO09sX70OU-mEbMnTeMXXOwHGabPSFcPyQYKG-BlQtfiHWytFWuu3z06m5g82Tpr1kBF9keweI7Nv4zALoELMEoeidZupzGppSxYoH0YS39kSLrdi5QAmoT-1YxlnPuSbqExNfs6ouZ9WkMyI2Pdnox77GgwkcOv3BEBxIA==&uniplatform=NZKPT&language=CHS (accessed on 7 August 2015). (In Chinese).
- Hidalgo-Garcia, A.; Torres, M.J.; Sales, A.; Bedmar, E.J.; Girard, L.; Delgado, M.J. Rhizobium etli produces nitrous oxide by coupling the assimilatory and denitrification pathways. Front. Microbiol. 2019, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, M.; Anahi Gomez, M. Denitrifying capacity of rhizobial strains of Argentine soils and herbicide sensitivity. Ann. Microbiol. 2013, 63, 1563–1570. [Google Scholar] [CrossRef]
- Lu, Y.; Kronzucker, H.J.; Shi, W. Stigmasterol root exudation arising from Pseudomonas inoculation of the duckweed rhizosphere enhances nitrogen removal from polluted waters. Environ. Pollut. 2021, 287, 117587. [Google Scholar] [CrossRef]
- Hu, N.; Li, Y.; Yin, J.; Ren, Z.; Li, J.; Zhao, J.; Wang, L.; Wu, L. A novel Zobellella endophytica W14 strain for nitrogen removal from hypersaline wastewater through simultaneous nitrification and denitrification. J. Environ. Manag. 2024, 371, 123171. [Google Scholar] [CrossRef]
- Chen, C.; Song, M.; Huang, G.; Li, R. Foam FeSO₄ modified limestone sulfur concrete for non-stink and high-rate nitrogen and phosphorus removal from wastewater. Water Res. 2025, 271, 122996. [Google Scholar] [CrossRef]
- Xu, M.J.; Cui, Y.W. Simultaneous aerobic nitrogen and phosphorus removal by novel halotolerant fungus Mucor circinelloides SNDM1: Function and metabolism pathway. Bioresour. Technol. 2024, 410, 131257. [Google Scholar] [CrossRef]
- Idenyi, J.N.; Abdallah, H.; Adeyemi, A.D.; Huber, D.H.; Gannam, A.; Sealey, W.; Igwe, D.O.; Eya, J.C. Optimizing growth and mitochondrial function in rainbow trout, Oncorhynchus mykiss through eco-friendly dietary and changes in water temperature regimen strategies. Aquaculture 2025, 595, 741591. [Google Scholar] [CrossRef]
- Korus, J.; Filgueira, R.; Grant, J. Influence of temperature on the behaviour and physiology of Atlantic salmon (Salmo salar) on a commercial farm. Aquaculture 2024, 589, 740978. [Google Scholar] [CrossRef]
- Kang, X.; Zhao, X.; Song, X.; Wang, D.; Shi, G.; Duan, X.; Chen, X.; Shen, G. Nitrogen removal by a novel strain Priestia aryabhattai KX-3 from East Antarctica under alkaline pH and low-temperature conditions. Process Biochem. 2023, 130, 674–684. [Google Scholar] [CrossRef]
- Lara-Moreno, A.; Vargas-Ordóñez, A.; Villaverde, J.; Madrid, F.; Carlier, J.D.; Santos, J.L.; Alonso, E.; Morillo, E. Bacterial bioaugmentation for paracetamol removal from water and sewage sludge. Genomic approaches to elucidate biodegradation pathway. J. Hazard. Mater. 2024, 480, 136128. [Google Scholar] [CrossRef]
- Zarei, M.; Yousefvand, A.; Maktabi, S.; Pourmahdi Borujeni, M.; Mohammadpour, H. Identification, phylogenetic characterisation and proteolytic activity quantification of high biofilm-forming Pseudomonas fluorescens group bacterial strains isolated from cold raw milk. Int. Dairy J. 2020, 109, 104787. [Google Scholar] [CrossRef]
- Tamminen, M.; Spaak, J.; Tlili, A.; Eggen, R.; Stamm, C.; Raesaenen, K. Wastewater constituents impact biofilm microbial community in receiving streams. Sci. Total Environ. 2022, 807, 151080. [Google Scholar] [CrossRef]
- Xu, W.; Xu, Y.; Su, H.; Hu, X.; Yang, K.; Wen, G.; Cao, Y. Characteristics of ammonia removal and nitrifying microbial communities in a hybrid biofloc-RAS for intensive Litopenaeus vannamei culture: A Pilot-Scale Study. Water 2020, 12, 3000. [Google Scholar] [CrossRef]
- Tang, B.; Yu, C.; Bin, L.; Zhao, Y.; Feng, X.; Huang, S.; Fu, F.; Ding, J.; Chen, C.; Li, P.; et al. Essential factors of an integrated moving bed biofilm reactor–membrane bioreactor: Adhesion characteristics and microbial community of the biofilm. Bioresour. Technol. 2016, 211, 574–583. [Google Scholar] [CrossRef]
- Finan, T.M.; Wood, J.M.; Jordan, D.C. Succinate transport in Rhizobium leguminosarum. J. Bacteriol. 1981, 148, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, Y.; Sun, G.; Gao, X.; Zhang, Q.; Liu, Z. Denitrification characteristics of a marine origin psychrophilic aerobic denitrifying bacterium. J. Environ. Sci. 2011, 23, 1888–1893. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Y.; Liu, Y.; Gao, X.Y.; Ai, G.M.; Miao, L.L.; Liu, Z.P. Characterization of a marine origin aerobic nitrifying–denitrifying bacterium. J Biosci. Bioeng. 2012, 114, 33–37. [Google Scholar] [CrossRef]
- Shao, S.; Zhong, J.; Wang, C.; Pan, D.; Wu, X. Performance of simultaneous nitrification–denitrification and denitrifying phosphorus and manganese removal by driving a single-stage moving bed biofilm reactor based on manganese redox cycling. Bioresour. Technol. 2022, 362, 127846. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Y.; Liu, H.; Xi, C.; Song, L. A novel heterotrophic nitrifying and aerobic denitrifying bacterium, Zobellella taiwanensis DN-7, can remove high-strength ammonium. Appl. Microbiol. Biotechnol. 2016, 100, 4219–4229. [Google Scholar] [CrossRef]
- Shu, H.; Sun, H.; Huang, W.; Zhao, Y.; Ma, Y.; Chen, W.; Sun, Y.; Chen, X.; Zhong, P.; Yang, H.; et al. Nitrogen removal characteristics and potential application of the heterotrophic nitrifying-aerobic denitrifying bacteria Pseudomonas mendocina S16 and Enterobacter cloacae DS’5 isolated from aquaculture wastewater ponds. Bioresour. Technol. 2022, 345, 126541. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Lu, J.; Qin, Y.; Zhou, M.; Tan, Y.; Wu, P.; Zhao, J. A critical review of heterotrophic nitrification and aerobic denitrification process: Influencing factors and mechanisms. J. Water Process Eng. 2023, 54, 103995. [Google Scholar] [CrossRef]
- Hu, J.; Yang, X.; Deng, X.; Liu, X.; Yu, J.; Chi, R.; Xiao, C. Isolation and nitrogen removal efficiency of the heterotrophic nitrifying-aerobic denitrifying strain K17 from a rare earth element leaching site. Front. Microbiol. 2022, 13, 905409. [Google Scholar] [CrossRef]
- Ren, Y.X.; Yang, L.; Liang, X. The characteristics of a novel heterotrophic nitrifying and aerobic denitrifying bacterium, Acinetobacter junii YB. Bioresour. Technol. 2014, 171, 1–9. [Google Scholar] [CrossRef]
- Sun, L.; Ge, Q.L.; Cao, W.P.; Zhang, H.F.; Zhang, J.K.; Zhang, H.; Dong, Y.W.; Li, Q.N.; Wang, Y.H. Screening and characterization study on an aerobic denitrifying bacteria. J. Henan Polytech. Univ. (Nat. Sci.) 2017, 36, 79–85. (In Chinese) [Google Scholar] [CrossRef]
- Volokita, M.; Belkin, S.; Abeliovich, A.; Soares, M.I.M. Biological denitrification of drinking water using newspaper. Water Res. 1996, 30, 965–971. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Liu, M.; Liu, C.; Yin, Z. Simultaneous nitrogen and phosphorus removal from black water: Effects of dissolved oxygen level and sludge concentration on full-scale performance and bacterial community dynamics. J. Water Process Eng. 2024, 57, 104735. [Google Scholar] [CrossRef]
- Davies, K.J.P.; Lloyd, D.; Boddy, L. The effect of oxygen on denitrification in Paracoccus denitrificans and Pseudomonas aeruginosa. Microbiology 1989, 135, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Du, L.B.; Liu, S.Y.; Weng, M.S.; Shu, H.; Chen, Q.H. Isolation and identification of an efficient aerobic denitrifying bacterium. Biotechnol. Bull. 2019, 35, 202–209. Available online: https://biotech.aiijournal.com/EN/10.13560/j.cnki.biotech.bull.1985.2019-0339 (accessed on 19 April 2019). (In Chinese).
- Zhang, Y.H.; Dong, X.B.; Liu, X.Y.; Xu, J.Q.; Xu, Z.L. Isolation of a novel heterotrophic nitrification-aerobic denitrification bacterium Paracoccus sp. QD-19 and its characterization of removing nitrogen. Biotechnol. Bull. 2023, 39, 301–310. Available online: https://biotech.aiijournal.com/EN/abstract/abstract13790.shtml (accessed on 6 July 2022). (In Chinese).
- AbdelGawwad, M.R.; Mahmutović, E.; Al Farraj, D.A.; Elshikh, M.S. In silico prediction of silver nitrate nanoparticles and nitrate reductase A (NAR A) interaction in the treatment of infectious disease causing clinical strains of E. coli. J. Infect. Public Health 2020, 13, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Qiao, S.; Zhou, J.; Bhatti, Z. Effects of redox mediators on nitrogen removal performance by denitrifying biomass and the activity of Nar and Nir. Chem. Eng. J. 2014, 257, 90–97. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Huang, H.K.; Tseng, S.K. Nitrate reduction by Citrobacter diversus under aerobic environment. Appl. Microbiol. Biotechnol. 2001, 55, 90–94. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).