A Bench-Scale Woodchip-Enhanced Bioelectrochemical Denitrification Remediation Wall for Simulating Nitrate-Contaminated Groundwater In Situ Treatment
Abstract
1. Introduction
2. Materials and Methods
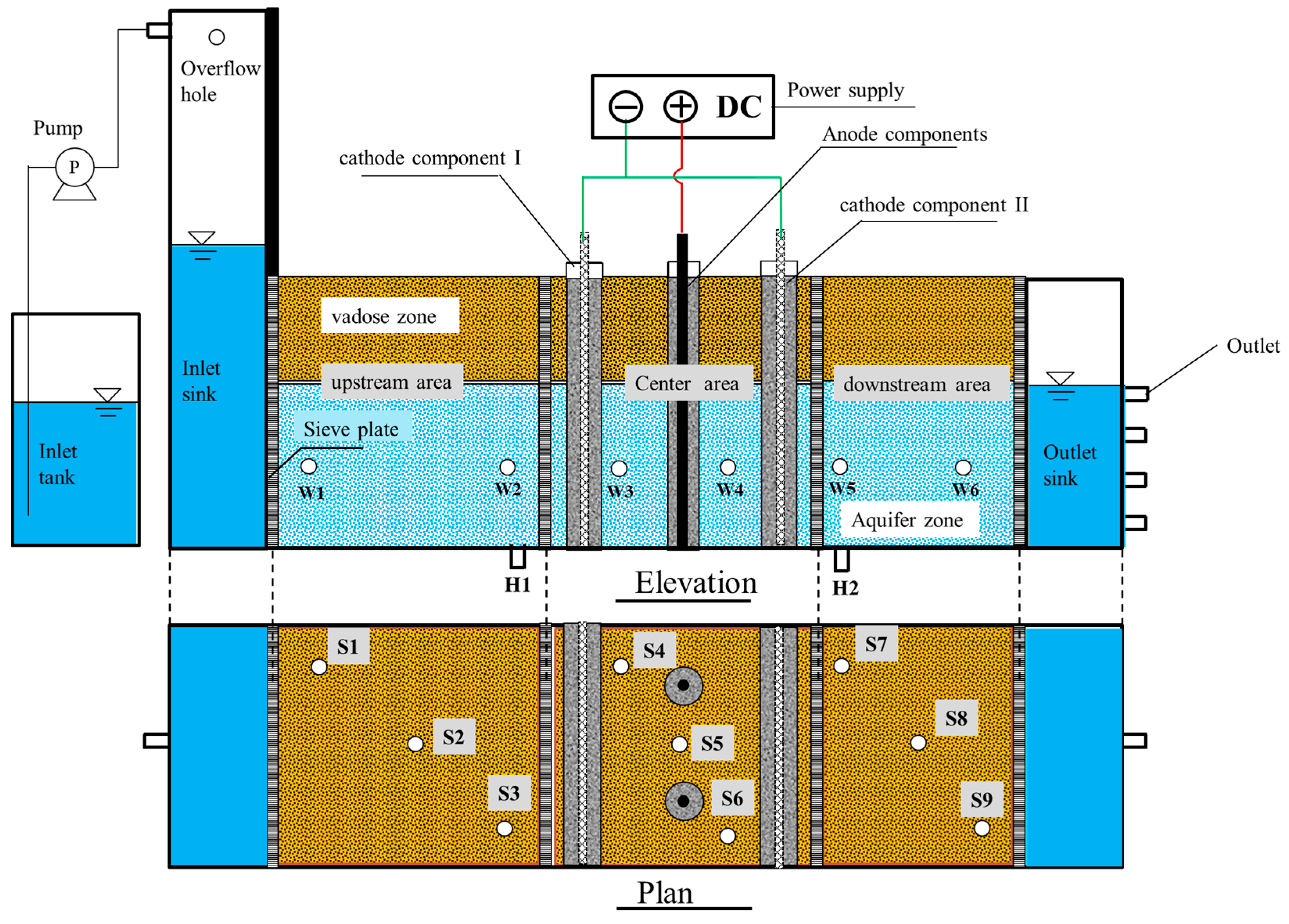
2.1. isBER Construction and Inoculation
2.2. Groundwater and Soil
2.3. Experimental Start-Up and Operation
2.4. Samples and Analyses
2.4.1. Water Quality and Soil Indicators
2.4.2. Microbial Analysis
2.4.3. Permeability Analysis
3. Results and Discussion
3.1. Denitrification Performance
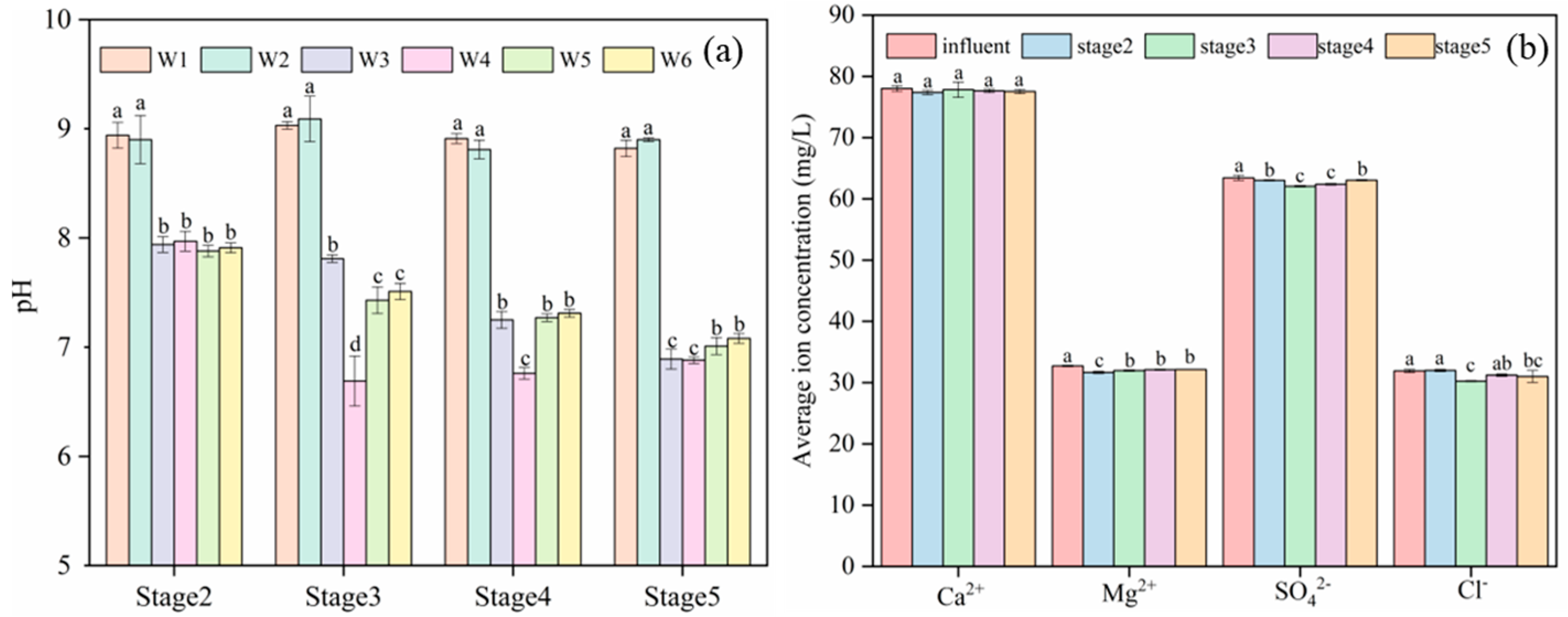
3.2. Changes in pH and Ions
3.3. Permeability of the Grid
3.4. Microbiological Analysis
3.4.1. Changes in Microbial Community of Electrode Components
3.4.2. The Bacterial Count in Effluent
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, H.; Kang, W.D.; Li, Y.R.; Li, Z. Fluoride and nitrate contamination of groundwater in the Loess Plateau, China: Sources and related human health risks. Environ. Pollut. 2021, 286, 117287. [Google Scholar] [CrossRef]
- Egbi, C.D.; Anornu, G.K.; Ganyaglo, S.Y.; Appiah-Adjei, E.K.; Li, S.L.; Dampare, S.B. Nitrate contamination of groundwater in the Lower Volta River Basin of Ghana: Sources and related human health risks. Ecotoxicol. Environ. Saf. 2020, 191, 110227. [Google Scholar] [CrossRef] [PubMed]
- Borah, P.; Borah, G.; Malakar, A. Mitigating nitrate contamination in groundwater: A comprehensive review of in-situ approaches. Groundw. Sustain. Dev. 2025, 28, 101406. [Google Scholar] [CrossRef]
- Lawrinenko, M.; Rhea, L.; Forshay, K.J.; Lee, T.R.; White, M.; Wilkin, R.T. Long-term field study of nitrate and ammonium remediation using a permeable reactive barrier at a livestock feeding operation. J. Environ. Manag. 2025, 380, 124962. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.H.; Zheng, T.Y.; Guo, B.; Tao, Y.H.; Liu, L.C.; Yan, N.; Zheng, X.L. Coupling of polyhydroxybutyrate and zero-valent iron for enhanced treatment of nitrate pollution within the Permeable Reactive Barrier and its downgradient aquifer. Water Res. 2024, 250, 121060. [Google Scholar] [CrossRef]
- Zou, L.; Xu, J.; Liu, H.; Zhou, Z.; Chen, Y.; Wang, X.; Wang, H.; Zou, Y. The efficiency of enhanced nitrogen and phosphorus removal in a vertical flow constructed wetland using alkaline modified corn cobs as a carbon source. Environ. Technol. Innov. 2024, 35, 103690. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnol. Adv. 2016, 34, 1103–1112. [Google Scholar] [CrossRef]
- Law, J.Y. Opportunities and Challenges to Use Electrical Stimulation for Enhanced Denitrification in Woodchip Bioreactors; Iowa State University: Ames, IA, USA, 2017. [Google Scholar]
- Li, W.W.; Yu, H.Q. Electro-assisted groundwater bioremediation: Fundamentals, challenges and future perspectives. Bioresour. Technol. 2015, 196, 677–684. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Kuroda, M. Electric prompting and control of denitrification. Biotechnol. Bioeng. 1993, 42, 535–537. [Google Scholar] [CrossRef]
- Tong, S.; Liu, H.Y.; Feng, C.P.; Chen, N.; Zhao, Y.; Xu, B.C.; Zhao, J.M.; Zhu, M. Stimulation impact of electric currents on heterotrophic denitrifying microbial viability and denitrification performance in high concentration nitrate-contaminated wastewater. J. Environ. Sci. 2019, 77, 363–371. [Google Scholar] [CrossRef]
- Law, J.Y.; Soupir, M.L.; Raman, D.R.; Moorman, T.B.; Ong, S.K. Electrical stimulation for enhanced denitrification in woodchip bioreactors: Opportunities and challenges. Ecol. Eng. 2018, 110, 38–47. [Google Scholar] [CrossRef]
- Liu, H.; Chen, N.; Feng, C.; Tong, S.; Li, R. Impact of electro-stimulation on denitrifying bacterial growth and analysis of bacterial growth kinetics using a modified Gompertz model in a bio-electrochemical denitrification reactor. Bioresour. Technol. 2017, 232, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.J.; Chen, N.; Zhao, J.M.; Yang, C.; Feng, C.P. Rice husk-intensified cathode driving bioelectrochemical reactor for remediating nitrate-contaminated groundwater. Sci. Total Environ. 2022, 837, 155917. [Google Scholar] [CrossRef]
- Yang, C.; Liu, T.; Chen, N.; Tong, S.; Feng, C. Performance and mechanism of a novel woodchip embedded biofilm electrochemical reactor (WBER) for nitrate-contaminated wastewater treatment. Chemosphere 2021, 276, 130250. [Google Scholar] [CrossRef]
- Xu, X.; Zou, Y.; Pan, H.; Gu, B. Safeguarding Groundwater Nitrate within Regional Boundaries in China. Environ. Sci. Technol. 2025, 59, 467–477. [Google Scholar] [CrossRef]
- Freeze, R.A. Three-Dimensional, Transient, Saturated-Unsaturated Flow in a Groundwater Basin. Water Resour. Res. 1971, 7, 347–366. [Google Scholar] [CrossRef]
- Pous, N.; Koch, C.; Vilà-Rovira, A.; Balaguer, M.D.; Colprim, J.; Mühlenberg, J.; Müller, S.; Harnisch, F.; Puig, S. Monitoring and engineering reactor microbiomes of denitrifying bioelectrochemical systems. RSC Adv. 2015, 5, 68326–68333. [Google Scholar] [CrossRef]
- Mena, E.; Ruiz, C.; Villaseñor, J.; Rodrigo, M.A.; Cañizares, P. Biological permeable reactive barriers coupled with electrokinetic soil flushing for the treatment of diesel-polluted clay soil. J. Hazard. Mater. 2015, 283, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Feng, C.P.; Chen, N.; Zhang, B.G.; Hao, C.B.; Peng, T.; Zhu, X. A bench-scale denitrification wall for simulating the in-situ treatment of nitrate-contaminated groundwater. Ecol. Eng. 2014, 73, 536–544. [Google Scholar] [CrossRef]
- Yang, C.; Feng, C.P.; Chen, N.; Deng, Y.; Hu, W.W.; Xue, L.J. Development of a novel palm fiber biofilm electrode reactor (PBER) for nitrate-contaminated wastewater treatment: Performance and mechanism. Environ. Sci.-Water Res. 2020, 6, 839–850. [Google Scholar] [CrossRef]
- SEPA. Water and Wastewater Monitoring Analysis Method; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Zhao, J.M.; Feng, C.P.; Tong, S.; Chen, N.; Dong, S.S.; Peng, T.; Jin, S.L. Denitrification behavior and microbial community spatial distribution inside woodchip-based solid-phase denitrification (W-SPD) bioreactor for nitrate-contaminated water treatment. Bioresour. Technol. 2018, 249, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Irvin, S. The Comparison of Alkalinity and ORP as Indicators for Nitrification and Denitrification in a Sequencing Batch Reactor (SBR). Biochem. Eng. J. 2007, 34, 248–255. [Google Scholar] [CrossRef]
- Zhang, J.; Quast, T.; Eid, B.; Chen, Y.-T.; Zerdoumi, R.; Dieckhöfer, S.; Junqueira, J.R.C.; Seisel, S.; Schuhmann, W. In-situ electrochemical reconstruction and modulation of adsorbed hydrogen coverage in cobalt/ruthenium-based catalyst boost electroreduction of nitrate to ammonia. Nat. Commun. 2024, 15, 8583. [Google Scholar] [CrossRef]
- Chen, H.; Tang, M.; He, L.; Xiao, X.; Yang, F.; He, Q.; Sun, S.; Gao, Y.; Zhou, L.; Li, Y.; et al. Exploring the impact of fulvic acid on electrochemical hydrogen-driven autotrophic denitrification system: Performance, microbial characteristics and mechanism. Bioresour. Technol. 2024, 412, 131432. [Google Scholar] [CrossRef]
- Du, X.; Zhang, H.; Sullivan, K.P.; Gogoi, P.; Deng, Y. Electrochemical Lignin Conversion. ChemSusChem 2020, 13, 4318–4343. [Google Scholar] [CrossRef]
- Glass, C.; Silverstein, J.A. Denitrification kinetics of high nitrate concentration water: pH effect on inhibition and nitrite accumulation. Water Res. 1998, 32, 831–839. [Google Scholar] [CrossRef]
- Nakhla, G.; Zhu, J. Impact of calcium on biofilm morphology, structure, detachment and performance in denitrifying fluidized bed bioreactors (DFBBRs). Chem. Eng. J. 2013, 232, 183–195. [Google Scholar] [CrossRef]
- Celmer, D.; Oleszkiewicz, J.A.; Cicek, N. Impact of shear force on the biofilm structure and performance of a membrane biofilm reactor for tertiary hydrogen-driven denitrification of municipal wastewater. Water Res. 2008, 42, 3057–3065. [Google Scholar] [CrossRef]
- Mahara, Y.M.A.; Habermehl, T.; Hasegawa, K.; Nakata, T.; Ransley, R. Groundwater dating by estimation of groundwater flow velocity and dissolved 4He accumulation rate calibrated by 36Cl in the Great Artesian Basin, Australia. Earth Planet. Sc. Lett. 2009, 287, 43–56. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Wang, Z.; Liang, H.; Du, Z.; Zhang, Y.; Lu, H.; Peng, Y. Metagenomic insights into nitrite accumulation in sulfur-based denitrification systems utilizing different electron donors: Functional microbial communities and metabolic mechanisms. Water Res. 2025, 270, 122805. [Google Scholar] [CrossRef]
- Kinnunen, O.; Kruglova, A.; Jensen, M.M.; Kuokkanen, A.; Smets, B.F.; Mikola, A. Shift in activated sludge microbiomes associated with nitrite accumulation and high nitrous oxide emissions. Environ. Res. 2025, 277, 121591. [Google Scholar] [CrossRef] [PubMed]
- Thrash, J.C.; Coates, J.D. Direct and indirect electrical stimulation of microbial metabolism. Environ. Sci. Technol. 2008, 42, 3921–3931. [Google Scholar] [CrossRef]
- Kaviraj, M.; Kumar, U.; Chatterjee, S.; Parija, S.; Padbhushan, R.; Nayak, A.; Gupta, V.V. Dissimilatory nitrate reduction to ammonium (DNRA): A unique biogeochemical cycle to improve nitrogen (N) use efficiency and reduce N-loss in rice paddy. Rhizosphere 2024, 30, 100875. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, C.; Hong, S.; Hao, H.; Yang, Y. Behavior of solid carbon sources for biological denitrification in groundwater remediation. Water Sci. Technol. 2012, 65, 1696–1704. [Google Scholar] [CrossRef]
- White, C.; Antell, E.; Schwartz, S.L.; Lawrence, J.E.; Keren, R.; Zhou, L. Synergistic interactions between anammox and dissimilatory nitrate reducing bacteria sustains reactor performance across variable nitrogen loading ratios. Front. Microbiol. 2023, 14, 1243410. [Google Scholar] [CrossRef]
- Wan, Y.; Li, R.; Yao, K.; Peng, C.; Wang, W.; Li, N.; Wang, X. Bioelectro-barriers prevent nitrate leaching into groundwater via nitrogen retention. Water Res. 2024, 249, 120988. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dong, H.; Wang, Q.; Yang, J.; Zhai, X.; Lin, M.; Liu, K.; Yang, Q. Efficient nitrate removal via microorganism-iron oxide co-evolution on biocathode surface. Bioelectrochemistry 2024, 163, 108889. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Bai, J.; Liu, Z.; Yan, D. High efficiency of inorganic nitrogen removal by integrating biofilm-electrode with constructed wetland: Autotrophic denitrifying bacteria analysis. Bioresour. Technol. 2015, 227, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Robertson, W.D. Nitrate removal rates in woodchip media of varying age. Ecol. Eng. 2010, 36, 1581–1587. [Google Scholar] [CrossRef]
- Christianson, L.E.; Feyereisen, G.W.; Hay, C.; Tschirner, U.W.; Kult, K.; Wickramarathne, N.M.; Hoover, N.; Soupir, M.L. Denitrifying bioreactor woodchip recharge: Media properties after nine years. Trans. ASABE 2020, 63, 407–416. [Google Scholar] [CrossRef]
- Parpot, P.; Bettencourt, A.P.; Carvalhoa, A.M.; Carvalhoe, M. Biomass conversion: Attempted electrooxidation of lignin for vanillin production. J. Appl. Electrochem. 2000, 30, 727–731. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Flora, J.R.V.; Suidan, M.T.; Kuroda, M. Modeling of electrochemically-activated denitrifying biofilms. Water Res. 1994, 28, 1077–1086. [Google Scholar] [CrossRef]
- Fu, F.S.; Zhang, R.D.; Luo, H.P.; Liu, G.L.; Bai, Y.P. Autotrophic biocathode for high efficient sulfate reduction in microbial electrolysis cells. Bioresour. Technol. 2014, 167, 462–468. [Google Scholar] [CrossRef]
- Otwell, A.E.; Carr, A.V.; Majumder, E.L.W.; Ruiz, M.K.; Wilpiszeski, R.L.; Hoang, L.T.; Webb, B.; Turkarslan, S.; Gibbons, S.M.; Elias, D.A.; et al. Sulfur metabolites play key system-level roles in modulating denitrification. mSystems 2021, 6, e01025-20. [Google Scholar] [CrossRef]
- Kuang, P.; Natsui, K.; Einaga, Y.; Feng, C.; Deng, Y. Annealing enhancement in stability and performance of copper modified boron-doped diamond (Cu-BDD) electrode for electrochemical nitrate reduction. Diam. Relat. Mater. 2021, 114, 108310. [Google Scholar] [CrossRef]
- Cunningham, A.B.; Characklis, W.G.; Abedeen, F.; Crawford, D. Influence of biofilm accumulation on porous media hydrodynamics. Environ. Sci. Technol. 1991, 25, 1305–1311. [Google Scholar] [CrossRef]
- Iqbal, A.; Ligeng, J.; Mo, Z.; Adnan, M.; Lal, R.; Zaman, M.; Usman, S.; Hua, T.; Imran, M.; Pan, S.-G.; et al. Substation of vermicompost mitigates Cd toxicity, improves rice yields and restores bacterial community in a Cd-contaminated soil in Southern China. J. Hazard. Mater. 2024, 5, 465. [Google Scholar] [CrossRef]
- Almeida, G.S.S.; Saito, V.S.; Sartori, M. Experimental effects of multiple agricultural stressors on diversity and size structure of subtropical stream macroinvertebrates. Environ. Adv. 2025, 20, 100630. [Google Scholar] [CrossRef]
- Hassan, M.; Zhu, G.; Yang, Z. The removal performance of nitrates in the novel 3D-BERS with GAC and diversity of immobilized microbial communities treating nitrate-polluted water: Effects of pH and COD/NO3−-N ratio. Environ. Eng. Res. 2022, 27, 200526. [Google Scholar] [CrossRef]
- Li, C.; Pan, G.; Wang, X.; Qiang, X.; Qiang, T. The effects of non-metallic organic tanning agents on the microbial community structure in wastewater. J. Clean. Prod. 2020, 279, 123553. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, D.; Morrison, L.; Ge, Z.; Zhan, X.; Li, R. Nutrient removal through pyrrhotite autotrophic denitrification: Implications for eutrophication control. Sci. Total Environ. 2019, 662, 287–296. [Google Scholar] [CrossRef]
- Liang, B.; Wang, L.Y.; Mbadinga, S.M.; Liu, J.F.; Mu, B.Z. Anaerolineaceae and Methanosaeta turned to be the dominant microorganisms in alkanes-dependent methanogenic culture after long-term of incubation. Amb. Express. 2015, 5, 37. [Google Scholar] [CrossRef]
- Mao, Y.; Yu, X.; Tong, Z. Characterization of Thauera-dominated hydrogen-oxidizing autotrophic denitrifying microbial communities by using high-throughput sequencing. Bioresour. Technol. 2013, 128, 703–710. [Google Scholar] [CrossRef]
- Freddi, L.; de la Garza-García, J.A.; Al Dahouk, S.; Occhialini, A.; Köhler, S. Brucella spp. are facultative anaerobic bacteria under denitrifying conditions. Microbiol. Spectr. 2023, 11, 02767. [Google Scholar] [CrossRef]
- Vanderstraeten, J.; Lamote, B.; da Fonseca, M.J.M.; De Groote, P.; Briers, Y. Conversion of the free Cellvibrio japonicus xyloglucan degradation system to the cellulosomal mode. Appl. Microbiol. Biotechnol. 2022, 106, 5495–5509. [Google Scholar] [CrossRef]
- Underwood, J.C.; Harvey, R.W.; Metge, D.W.; Repert, D.A.; Baumgartner, L.K.; Smith, R.L.; Roane, T.M.; Barber, L.B. Effects of the Antimicrobial Sulfamethoxazole on Groundwater Bacterial Enrichment. Environ. Sci. Technol. 2011, 45, 3096–3101. [Google Scholar] [CrossRef] [PubMed]
- Cloete, T.; Thantsha, M.; Maluleke, M.; Kirkpatrick, R. The antimicrobial mechanism of electrochemically activated water against Pseudomonas aeruginosa and Escherichia coli as determined by SDS-PAGE analysis. J. Appl. Microbiol. 2010, 107, 379–384. [Google Scholar] [CrossRef] [PubMed]



| Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | |
|---|---|---|---|---|---|
| Current density (mA/m2) | 0 | 0 | 350 | 350 | 350 |
| flow rate (L/d) | 0.99 | 0.99 | 0.99 | 1.98 | 3.96 |
| Running time (d) | 0~15 | 16~30 | 31~45 | 46~60 | 61~75 |
| Sample | Sobs | Shannon | Ace | Chao | Coverage |
|---|---|---|---|---|---|
| Original sludge | 344 | 4.09 | 378.27 | 390.16 | 0.997875 |
| Cathode component I | 301 | 2.42 | 369.48 | 352.41 | 0.998078 |
| Anode component | 422 | 4.16 | 481.42 | 468.72 | 0.997738 |
| Cathode component II | 312 | 2.99 | 387.83 | 412.63 | 0.997556 |
| Water Sample | The Bacterial Count (CFU/mL) |
|---|---|
| The actual groundwater | 1.13 × 102~3.45 × 102 |
| Effluent at 5 cm/d | 3.10 × 102~7.51 × 102 |
| Effluent at 10 cm/d | 2.18 × 103~2.74 × 103 |
| Effluent at 20 cm/d | 3.72 × 105~5.21 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Cao, Y.; Feng, C. A Bench-Scale Woodchip-Enhanced Bioelectrochemical Denitrification Remediation Wall for Simulating Nitrate-Contaminated Groundwater In Situ Treatment. Water 2025, 17, 1593. https://doi.org/10.3390/w17111593
Yang C, Cao Y, Feng C. A Bench-Scale Woodchip-Enhanced Bioelectrochemical Denitrification Remediation Wall for Simulating Nitrate-Contaminated Groundwater In Situ Treatment. Water. 2025; 17(11):1593. https://doi.org/10.3390/w17111593
Chicago/Turabian StyleYang, Chen, Yiheng Cao, and Chuanping Feng. 2025. "A Bench-Scale Woodchip-Enhanced Bioelectrochemical Denitrification Remediation Wall for Simulating Nitrate-Contaminated Groundwater In Situ Treatment" Water 17, no. 11: 1593. https://doi.org/10.3390/w17111593
APA StyleYang, C., Cao, Y., & Feng, C. (2025). A Bench-Scale Woodchip-Enhanced Bioelectrochemical Denitrification Remediation Wall for Simulating Nitrate-Contaminated Groundwater In Situ Treatment. Water, 17(11), 1593. https://doi.org/10.3390/w17111593






