Anthropogenic Release of Per- and Polyfluoroalkyl Substances into Surface Water Systems: Distribution Characteristics and Environmental Persistence Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Pretreatment
2.3. Instrumental Analysis
2.4. Nontarget Screening Strategy
2.5. Persistence Assessment
2.6. Quality Control and Quality Assurance
3. Results
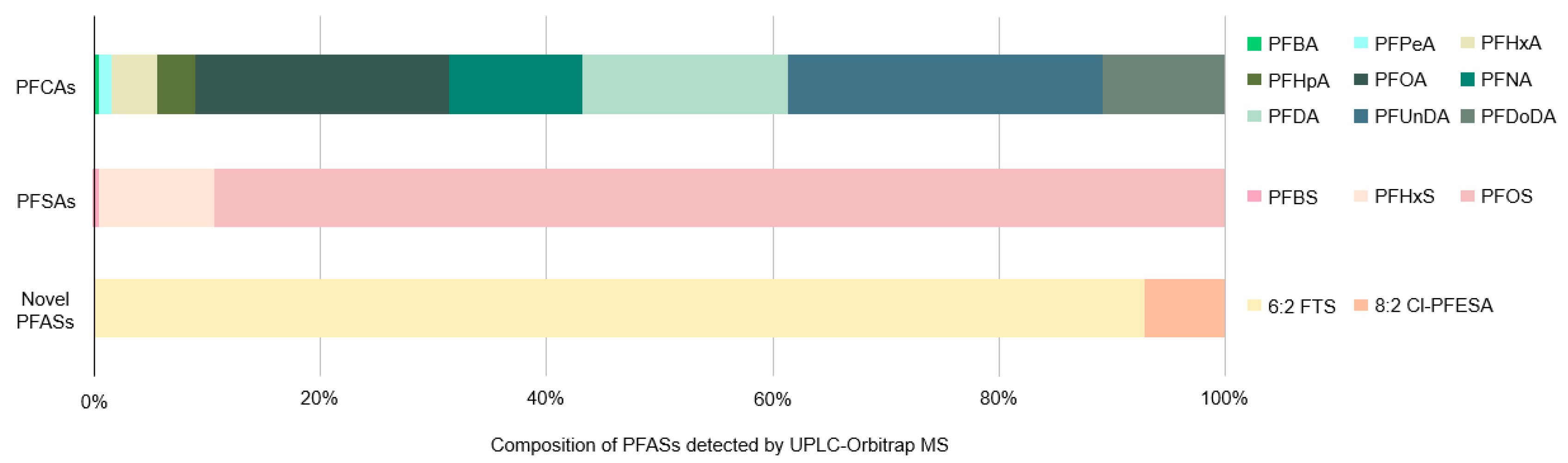
3.1. Detection and Identification of PFASs
3.2. Distribution Characteristics of Different Functional Areas
3.3. Persistence Assessment of PFASs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PFASs | Per- and polyfluoroalkyl substances |
| PFCAs | Perfluoroalkyl carboxylic acids |
| PFSAs | Perfluoroalkyl sulfonic acids |
| PFBA | Perfluorobutanoic acid |
| PFPeA | Perfluoropentanoic acid |
| PFHxA | Perfluorohexanoic acid |
| PFOA | Perfluorooctanoic acid |
| PFNA | Perfluorononanoic acid |
| PFDA | Perfluorodecanoic acid |
| PFUnDA | Perfluoroundecanoic acid |
| PFDoDA | Perfluorododecanoic acid |
| PFBS | Perfluorobutane sulfonate |
| PFHxS | Perfluorohexane sulfonate |
| PFOS | Perfluorooctane sulfonate |
| 6:2 FTS | 6:2 Fluorotelomer sulfonate |
| 8:2 Cl-PFESA | 8:2 Chlorinated polyfluoroalkyl ether sulfonic acid |
References
- Kotthoff, M.; Müller, J.; Jürling, H.; Schlummer, M.; Fiedler, D. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ. Sci. Pollut. Res. 2015, 22, 14546–14559. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.A.; Field, J.A. Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ. Sci. Technol. 2000, 34, 3864–3870. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, N.Y.; Zhu, X.B.; Guo, H.W.; Jiang, J.G.; Wang, X.B.; Shi, W.; Wu, J.C.; Yu, H.X.; Wei, S. Suspect and Nontarget Screening of Per- and Polyfluoroalkyl Substances in Wastewater from a Fluorochemical Manufacturing Park. Environ. Sci. Technol. 2018, 52, 11007–11016. [Google Scholar] [CrossRef]
- Briels, N.; Ciesielski, T.M.; Herzke, D.; Jaspers, V.L.B. Developmental Toxicity of Perfluorooctanesulfonate (PFOS) and Its Chlorinated Polyfluoroalkyl Ether Sulfonate Alternative F-53B in the Domestic Chicken. Environ. Sci. Technol. 2018, 52, 12859–12867. [Google Scholar] [CrossRef]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef]
- Martínez, R.; Herrero-Nogareda, L.; Van Antro, M.; Campos, M.P.; Casado, M.; Barata, C.; Piña, B.; Navarro-Martín, L. Morphometric signatures of exposure to endocrine disrupting chemicals in zebrafish eleutheroembryos. Aquat. Toxicol. 2019, 214, 105232. [Google Scholar] [CrossRef]
- Pan, Y.T.; Zhang, H.X.; Cui, Q.Q.; Sheng, N.; Yeung, L.W.Y.; Guo, Y.; Sun, Y.; Dai, J.Y. First Report on the Occurrence and Bioaccumulation of Hexafluoropropylene Oxide Trimer Acid: An Emerging Concern. Environ. Sci. Technol. 2017, 51, 9553–9560. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Wang, X.B.; Yang, M.M.; Wei, S.; Li, Y.Q. Integrated Analysis of Per- and Polyfluoroalkyl Substance Exposure and Metabolic Profiling of Elderly Residents Living near Industrial Plants. Environ. Sci. Technol. 2024, 58, 4104–4114. [Google Scholar] [CrossRef] [PubMed]
- Rotander, A.; Kärrman, A.; Toms, L.M.L.; Kay, M.; Mueller, J.F.; Ramos, M.J.G. Novel Fluorinated Surfactants Tentatively Identified in Firefighters Using Liquid Chromatography Quadrupole Time-of-Flight Tandem Mass Spectrometry and a Case-Control Approach. Environ. Sci. Technol. 2015, 49, 2434–2442. [Google Scholar] [CrossRef]
- Liu, Y.N.; Richardson, E.S.; Derocher, A.E.; Lunn, N.J.; Lehmler, H.J.; Li, X.S.; Zhang, Y.F.; Cui, J.Y.; Cheng, L.H.; Martin, J.W. Hundreds of Unrecognized Halogenated Contaminants Discovered in Polar Bear Serum. Angew. Chem. Int. Ed. 2018, 57, 16401–16406. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.L.; Bu, D.; Fu, J.; Wang, M.L.; Zhou, W.; Gu, L.Y.; Fu, Y.L.; Cong, Z.Y.; Hu, B.Y.; et al. Trophic Magnification of Short-Chain Per- and Polyfluoroalkyl Substances in a Terrestrial Food Chain from the Tibetan Plateau. Environ. Sci. Technol. Lett. 2022, 9, 147–152. [Google Scholar] [CrossRef]
- Yu, N.Y.; Wen, H.Z.; Wang, X.B.; Yamazaki, E.; Taniyasu, S.; Yamashita, N.; Yu, H.X.; Wei, S. Nontarget Discovery of Per- and Polyfluoroalkyl Substances in Atmospheric Particulate Matter and Gaseous Phase Using Cryogenic Air Sampler. Environ. Sci. Technol. 2020, 54, 3103–3113. [Google Scholar] [CrossRef]
- Li, Y.Q.; Yang, Y.J.; Wang, X.B. Identification, annotation and toxicity estimation of organic pollutants in human serum via non-target analysis. Environ. Pollut. 2025, 367, 125577. [Google Scholar] [CrossRef]
- Gomis, M.I.; Wang, Z.Y.; Scheringer, M.; Cousins, I.T. A modeling assessment of the physicochemical properties and environmental fate of emerging and novel per- and polyfluoroalkyl substances. Sci. Total Environ. 2015, 505, 981–991. [Google Scholar] [CrossRef]
- Jiao, Z.Y.; Yu, N.Y.; Mao, J.D.; Yang, Q.; Jiao, L.P.; Wang, X.B.; Shi, W.; Yu, H.X.; Wei, S. The occurrence, tissue distribution, and PBT potential of per- and polyfluoroalkyl substances in the freshwater organisms from the Yangtze river via nontarget analysis. J. Hazard. Mater. 2023, 458, 131868. [Google Scholar] [CrossRef]
- Hoke, R.A.; Ferrell, B.D.; Ryan, T.; Sloman, T.L.; Green, J.W.; Nabb, D.L.; Mingoia, R.; Buck, R.C.; Korzeniowski, S.H. Aquatic hazard, bioaccumulation and screening risk assessment for 6:2 fluorotelomer sulfonate. Chemosphere 2015, 128, 258–265. [Google Scholar] [CrossRef]
- Chen, F.F.; Yin, S.S.; Kelly, B.C.; Liu, W.P. Chlorinated Polyfluoroalkyl Ether Sulfonic Acids in Matched Maternal, Cord, and Placenta Samples: A Study of Transplacental Transfer. Environ. Sci. Technol. 2017, 51, 6387–6394. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D.; Boethling, R.; Howard, P.; Stiteler, W. Estimating biodegradation half-lives for use in chemical screening. Chemosphere 2006, 63, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.S. Fluorotechnology Is Critical to Modern Life: The FluoroCouncil Counterpoint to the Madrid Statement. Environ. Health Pers. 2015, 123, A112–A113. [Google Scholar] [CrossRef]
- Li, Y.; Fletcher, T.; Mucs, D.; Scott, K.; Lindh, C.H.; Tallving, P.; Jakobsson, K. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup. Environ. Med. 2018, 75, 46–51. [Google Scholar] [CrossRef]
- He, Y.X.; Lv, D.; Li, C.H.; Liu, X.Q.; Liu, W.D.; Han, W.C. Human exposure to F-53B in China and the evaluation of its potential toxicity: An overview. Environ. Int. 2022, 161, 107108. [Google Scholar] [CrossRef] [PubMed]
- Munoz, G.; Budzinski, H.; Babut, M.; Lobry, J.; Selleslagh, J.; Tapie, N.; Labadie, P. Temporal variations of perfluoroalkyl substances partitioning between surface water, suspended sediment, and biota in a macrotidal estuary. Chemosphere 2019, 233, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.S.C.; Szostek, B.; DeRito, C.M.; Madsen, E.L. Investigating the biodegradability of perfluorooctanoic acid. Chemosphere 2010, 80, 176–183. [Google Scholar] [CrossRef]
- Zhang, L.C.; Wang, M.Y.; Zhang, M.Q.; Yang, D.J. Per- and polyfluoroalkyl substances in Chinese surface waters: A review. Ecoto. Environ. Safe 2023, 262, 115178. [Google Scholar] [CrossRef]
- Delle Site, A. Factors affecting sorption of organic compounds in natural sorbent/water systems and sorption coefficients for selected pollutants. A review. J. Phys. Chem. Ref. Data 2001, 30, 187–439. [Google Scholar] [CrossRef]
- Meng, L.; Song, B.; Zhong, H.; Ma, X.; Wang, Y.; Ma, D.; Lu, Y.; Gao, W.; Wang, Y.; Jiang, G. Legacy and emerging per- and polyfluoroalkyl substances (PFAS) in the Bohai Sea and its inflow rivers. Environ. Int. 2021, 156, 106735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Boucher, J.M.; Scheringer, M.; Cousins, I.T.; Hungerbühler, K. Toward a Comprehensive Global Emission Inventory of C4-C10 Perfluoroalkanesulfonic Acids (PFSAs) and Related Precursors: Focus on the Life Cycle of C6- and C10-Based Products and Ongoing Industrial Transition. Environ. Sci. Technol. 2017, 51, 4482–4493. [Google Scholar] [CrossRef]
- Pétré, M.A.; Genereux, D.P.; Koropeckyj-Cox, L.; Knappe, D.R.U.; Duboscq, S.; Gilmore, T.E.; Hopkins, Z.R. Per- and Polyfluoroalkyl Substance (PFAS) Transport from Groundwater to Streams near a PFAS Manufacturing Facility in North Carolina, USA. Environ. Sci. Technol. 2021, 55, 5848–5856. [Google Scholar] [CrossRef]
- Blaine, A.C.; Rich, C.D.; Sedlacko, E.M.; Hundal, L.S.; Kumar, K.; Lau, C.; Mills, M.A.; Harris, K.M.; Higgins, C.P. Perfluoroalkyl Acid Distribution in Various Plant Compartments of Edible Crops Grown in Biosolids-Amended soils. Environ. Sci. Technol. 2014, 48, 7858–7865. [Google Scholar] [CrossRef]
- Skutlarek, D.; Exner, M.; Färber, H. Perfluorinated surfactants in surface and drinking water. Environ. Sci. Pollut. Res. 2006, 13, 299–307. [Google Scholar]
- Peter, L.G.; Lee, L.S. Sources and Pathways of PFAS Occurrence in Water Sources: Relative Contribution of Land-Applied Biosolids in an Agricultural Dominated Watershed. Environ. Sci. Technol. 2025, 59, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Tan, D.F.; Geng, Y.; Wang, L.; Peng, Y.; He, Z.Y.; Xu, Y.P.; Liu, X.W. Perfluorinated Compounds in Greenhouse and Open Agricultural Producing Areas of Three Provinces of China: Levels, Sources and Risk Assessment. Int. J. Environ. Res. Public Heal. 2016, 13, 1224. [Google Scholar] [CrossRef] [PubMed]
- D’Hollander, W.; Herzke, D.; Huber, S.; Hajslova, J.; Pulkrabova, J.; Brambilla, G.; De Filippis, S.P.; Bervoets, L.; de Voogt, P. Occurrence of perfluorinated alkylated substances in cereals, salt, sweets and fruit items collected in four European countries. Chemosphere 2015, 129, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Beyer-Robson, J.; Johnson, D.D.; Knott, N.A.; Bowles, K.C. Bioaccumulation of perfluoroalkyl substances in exploited fish and crustaceans: Spatial trends across two estuarine systems. Mar. Pollut. Bull. 2018, 131, 303–313. [Google Scholar] [CrossRef]
- Taylor, M.D.; Johnson, D.D. Preliminary investigation of perfluoroalkyl substances in exploited fishes of two contaminated estuaries. Mar. Pollut. Bull. 2016, 111, 509–513. [Google Scholar] [CrossRef]
- Davis, S.N.; Klumker, S.M.; Mitchell, A.A.; Coppage, M.A.; Labonté, J.M.; Quigg, A. Life in the PFAS lane: The impact of perfluoroalkyl substances on photosynthesis, cellular exudates, nutrient cycling, and composition of a marine microbial community. Sci. Total Environ. 2024, 927, 171977. [Google Scholar] [CrossRef]
- Gebbink, W.A.; van Asseldonk, L.; van Leeuwen, S.P.J. Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands. Environ. Sci. Technol. 2017, 51, 11057–11065. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Li, Y. Anthropogenic Release of Per- and Polyfluoroalkyl Substances into Surface Water Systems: Distribution Characteristics and Environmental Persistence Analysis. Water 2025, 17, 1589. https://doi.org/10.3390/w17111589
Sun M, Li Y. Anthropogenic Release of Per- and Polyfluoroalkyl Substances into Surface Water Systems: Distribution Characteristics and Environmental Persistence Analysis. Water. 2025; 17(11):1589. https://doi.org/10.3390/w17111589
Chicago/Turabian StyleSun, Miaomiao, and Yuqian Li. 2025. "Anthropogenic Release of Per- and Polyfluoroalkyl Substances into Surface Water Systems: Distribution Characteristics and Environmental Persistence Analysis" Water 17, no. 11: 1589. https://doi.org/10.3390/w17111589
APA StyleSun, M., & Li, Y. (2025). Anthropogenic Release of Per- and Polyfluoroalkyl Substances into Surface Water Systems: Distribution Characteristics and Environmental Persistence Analysis. Water, 17(11), 1589. https://doi.org/10.3390/w17111589




