Processes of Groundwater Contamination in Coastal Aquifers in Sri Lanka: A Geochemical and Isotope-Based Approach
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
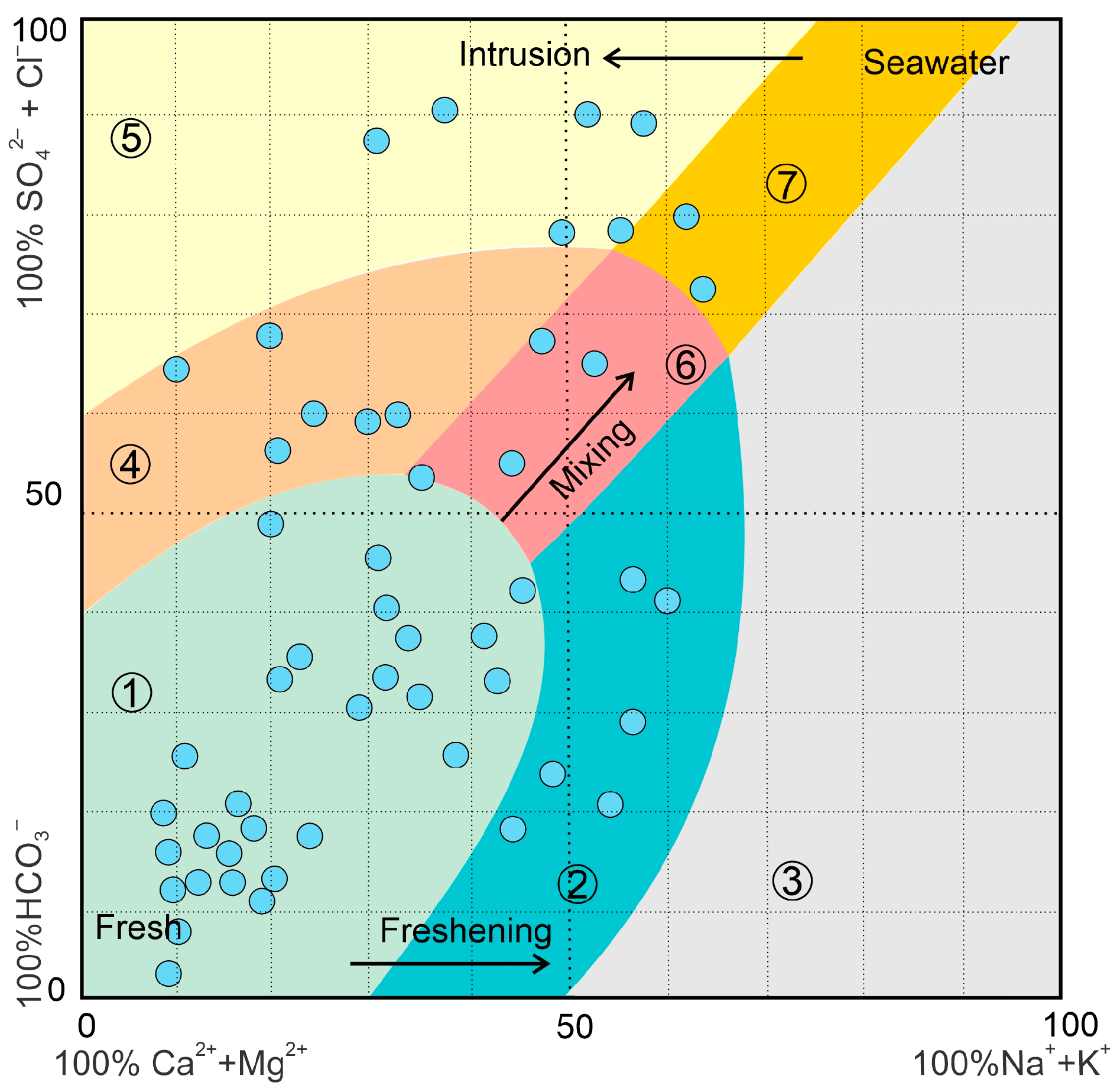
3.1. Groundwater Suitability
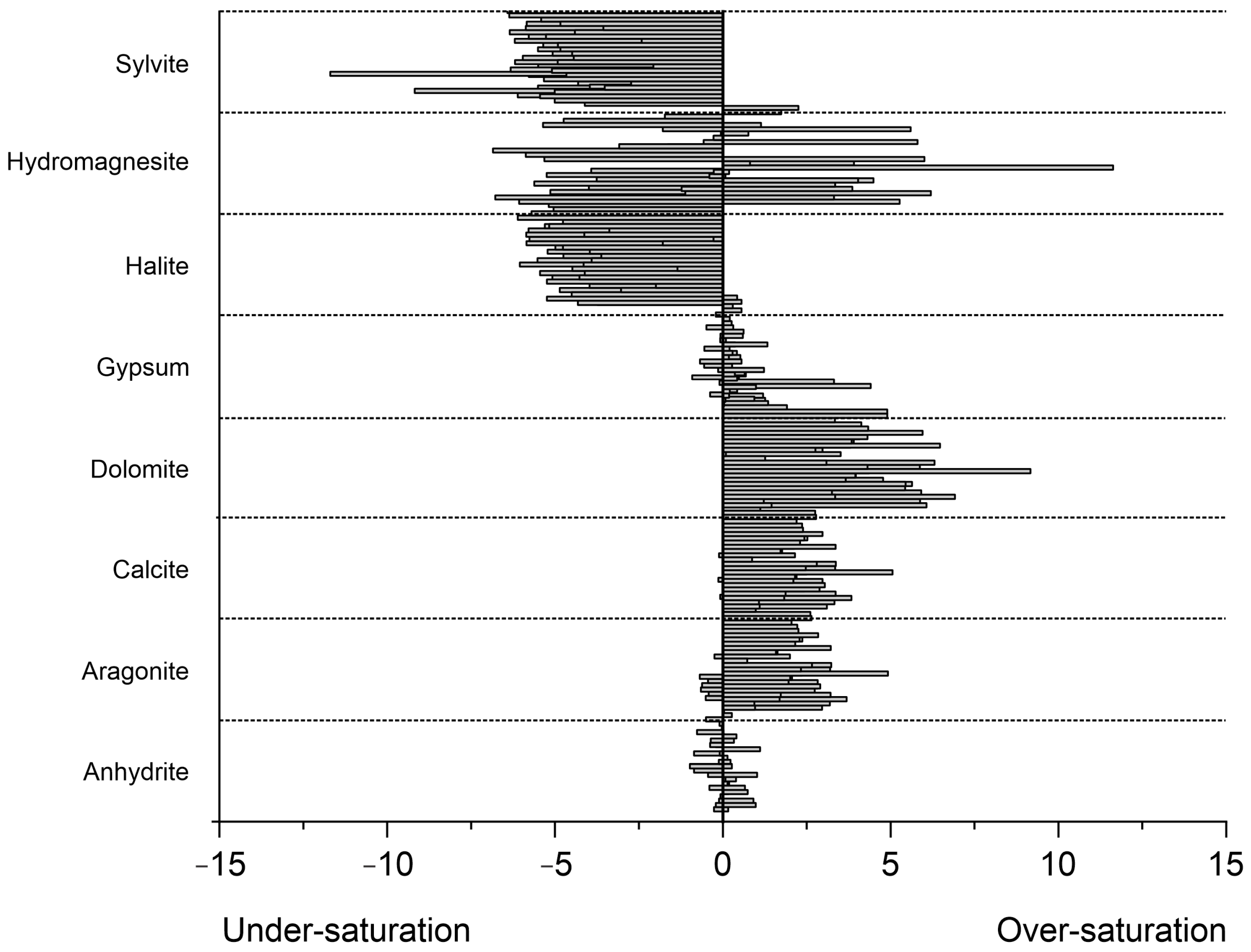
3.2. Saturation Index of Groundwater
3.3. Isotope Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grönwall, J.; Danert, K. Regarding groundwater and drinking water access through a human rights lens: Self-supply as a norm. Water 2020, 12, 419. [Google Scholar] [CrossRef]
- Mukherjee, A.; Scanlon, B.R.; Aureli, A.; Langan, S.; Guo, H.; McKenzie, A. (Eds.) Global groundwater: From scarcity to security through sustainability and solutions. In Global Groundwater; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 1; pp. 3–20. [Google Scholar]
- Pradhan, B.; Chand, S.; Chand, S.; Rout, P.R.; Naik, S.K. Emerging groundwater contaminants: A comprehensive review on their health hazards and remediation technologies. Groundw. Sustain. Dev. 2023, 20, 100868. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Kanel, S.R.; Nakarmi, A. Groundwater pollution: Occurrence, detection, and remediation of organic and inorganic pollutants. Water Environ. Res. 2020, 92, 1659–1668. [Google Scholar] [CrossRef]
- Alfarrah, N.; Walraevens, K. Groundwater overexploitation and seawater intrusion in coastal areas of arid and semi-arid regions. Water 2018, 10, 143. [Google Scholar] [CrossRef]
- Han, D.; Currell, M.J. Review of drivers and threats to coastal groundwater quality in China. Sci. Total Environ. 2022, 806, 150913. [Google Scholar] [CrossRef]
- Boumaiza, L.; Chesnaux, R.; Drias, T.; Walter, J.; Huneau, F.; Garel, E.; Knoeller, K.; Stumpp, C. Identifying groundwater degradation sources in a Mediterranean coastal area experiencing significant multi-origin stresses. Sci. Total Environ. 2020, 746, 141203. [Google Scholar] [CrossRef]
- Winid, B.; Maruta, M. Assessment of Groundwater (Main Usable Aquifer) Vulnerability to Seawater Intrusion in the Polish Baltic Coastal Region. Water 2025, 17, 336. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Liu, C.; Zhang, S.; Yao, F.; Yang, G.; Hou, W. Geochemical Exploration Techniques with Deep Penetration: Implications for the Exploration of Concealed Potash Deposits in the Covered Area on the Southern Margin of the Kuqa Basin. Water 2025, 17, 298. [Google Scholar] [CrossRef]
- Eggenkamp, H. The Geochemistry of Stable Chlorine and Bromine Isotopes; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Sebastian, S.; Górecka, A.; Wojtal-Frankiewicz, A. The effects of road salt on freshwater ecosystems and solutions for mitigating chloride pollution—A review. Sci. Total Environ. 2022, 805, 150289. [Google Scholar] [CrossRef]
- Xaza, A.; Mapoma, H.W.T.; Abiye, T.A.; Clarke, S.; Kanyerere, T. Investigating Seawater Intrusion in Republic of South Africa’s Heuningnes, Cape Agulhas Using Hydrogeochemistry and Seawater Fraction Techniques. Water 2023, 15, 2141. [Google Scholar] [CrossRef]
- Ratnayake, A.S.; Perera, U.L.H.P. Coastal zone management in Sri Lanka: A lesson after recent naval accidents. Mar. Pollut. Bull. 2022, 182, 113994. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, C.B.; Chandrajith, R. The hydrogeological and geochemical characteristics of groundwater of Sri Lanka. In Groundwater of South Asia; Mukherjee, A., Ed.; Springer: Singapore, 2018; pp. 405–428. [Google Scholar] [CrossRef]
- Bandara, U.G.C.; Diyabalanage, S.; Barth, J.A.C.; Chandrajith, R. Recharging mechanisms and seawater intrusion in karst aquifers in northwest Sri Lanka, based on hydrogeochemistry and water isotopes. ACS EST Water 2023, 3, 1678–1686. [Google Scholar] [CrossRef]
- Jayathunga, K.; Diyabalanage, S.; Frank, A.H.; Chandrajith, R.; Barth, J.A.C. Influences of seawater intrusion and anthropogenic activities on shallow coastal aquifers in Sri Lanka: Evidence from hydrogeochemical and stable isotope data. Environ. Sci. Pollut. Res. 2020, 27, 23002–23014. [Google Scholar] [CrossRef]
- Bandara, U.G.C.; Diyabalanage, S.; Hanke, C.; van Geldern, R.; Barth, J.A.C.; Chandrajith, R. Arsenic-rich shallow groundwater in sandy aquifer systems buffered by rising carbonate waters: A geochemical case study from Mannar Island, Sri Lanka. Sci. Total Environ. 2018, 633, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Amarathunga, U.; Diyabalanage, S.; Bandara, U.G.C.; Chandrajith, R. Environmental factors controlling arsenic mobilization from sandy shallow coastal aquifer sediments in the Mannar Island, Sri Lanka. Appl. Geochem. 2019, 100, 152–159. [Google Scholar] [CrossRef]
- Jayasekera, D.L.; Kaluarachchi, J.J.; Villholth, K.G. Groundwater stress and vulnerability in rural coastal aquifers under competing demands: A case study from Sri Lanka. Environ. Monit. Assess. 2011, 176, 13–30. [Google Scholar] [CrossRef]
- Sivashanthini, K.; Dhanushka Dilini, J.; Shivatharshini, Y.; Gunaalan, K. Quantitative analysis of trace metal In water and soft tissues of Balanus Amphitrite In Nandikadal lagoon, Sri Lanka, 2019. In Proceedings of the 2nd International Conference on Research in Science, Engineering and Technology, Paris, France, 22–24 November 2019. [Google Scholar]
- Abbasi, T.; Abbasi, S.A. (Eds.) Water-Quality Indices: Looking Back, Looking Ahead. In Water Quality Indices; Elsevier: Amsterdam, The Netherlands, 2012; Chapter 16; pp. 353–356. [Google Scholar]
- Sutadian, A.D.; Muttil, N.; Yilmaz, A.G.; Perera, B.J.C. Development of river water quality indices—A review. Environ. Monit. Assess. 2015, 188, 58. [Google Scholar] [CrossRef]
- Uddin, M.G.; Nash, S.; Olbert, A.I. A review of water quality index models and their use for assessing surface water quality. Ecol. Indic. 2021, 122, 107218. [Google Scholar] [CrossRef]
- Brown, R.M.; McClelland, N.I.; Deininger, R.A.; O’Connor, M.F. A Water Quality Index-Crashing the Psychological Barrier. In Indicators of Environmental Quality; Springer: Boston, MA, USA, 1972; pp. 173–182. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 3rd ed.; World Health Organization: Geneva, Switzerland, 2011; p. 216. [Google Scholar]
- Bijay, S.; Craswell, E. Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Han, D.; Post, V.E.A.; Song, X. Groundwater salinization processes and reversibility of seawater intrusion in coastal carbonate aquifers. J. Hydrol. 2015, 531, 1067–1080. [Google Scholar] [CrossRef]
- Reay, W.G. Septic tank impacts on ground water quality and nearshore sediment nutrient flux. Groundwater 2004, 42, 1079–1089. [Google Scholar] [CrossRef]
- Huang, G.; Han, D.; Song, J.; Li, L.; Pei, L. A sharp contrasting occurrence of iron-rich groundwater in the Pearl River Delta during the past dozen years (2006–2018): The genesis and mitigation effect. Sci. Total Environ. 2022, 829, 154676. [Google Scholar] [CrossRef]
- Antony, S.; Dev, V.V.; Kaliraj, S.; Ambili, M.S.; Krishnan, K.A. Seasonal variability of groundwater quality in coastal aquifers of Kavaratti Island, Lakshadweep Archipelago, India. Groundw. Sustain. Dev. 2020, 11, 100377. [Google Scholar] [CrossRef]
- Mora, A.; Mahlknecht, J.; Ledesma-Ruiz, R.; Sanford, W.E.; Lesser, L.E. Dynamics of major and trace elements during seawater intrusion in a coastal sedimentary aquifer impacted by anthropogenic activities. J. Contam. Hydrol. 2020, 232, 103653. [Google Scholar] [CrossRef]
- Gopinath, S.; Srinivasamoorthy, K.; Saravanan, K.; Prakash, R. Tracing groundwater salinization using geochemical and isotopic signature in Southeastern coastal Tamilnadu, India. Chemosphere 2019, 236, 124305. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.R.; Van Camp, M.; Hossain, D.; Islam, M.; Ahmed, N.; Karim, M.; Bhuiyan, A.Q.; Walraevens, K. Groundwater salinization and freshening processes in coastal aquifers from southwest Bangladesh. Sci. Total Environ. 2021, 779, 146339. [Google Scholar] [CrossRef]
- Skrzypek, G.; Dogramaci, S.; Grierson, P.F. Geochemical and hydrological processes controlling groundwater salinity of a large inland wetland of northwest Australia. Chem. Geol. 2013, 357, 164–177. [Google Scholar] [CrossRef]
- Howcroft, W.; Cartwright, I.; Fifield, L.K.; Cendón, D.I. Differences in groundwater and chloride residence times in saline groundwater: The Barwon River Catchment of Southeast Australia. Chem. Geol. 2017, 451, 154–168. [Google Scholar] [CrossRef]
- Magazinovic, R.S.; Nicholson, B.C.; Mulcahy, D.E.; Davey, D.E. Bromide levels in natural waters: Its relationship to levels of both chloride and total dissolved solids and the implications for water treatment. Chemosphere 2004, 57, 329–335. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Davis, S.N.; Whittemore, D.O.; Fabryka-Martin, J. Uses of chloride/bromide ratios in studies of potable water. Groundwater 1998, 36, 338–350. [Google Scholar] [CrossRef]
- Katz, B.G.; Griffin, D.W.; McMahon, P.B.; Harden, H.S.; Wade, E.; Hicks, R.W.; Chanton, J.P. Fate of efluent-borne contaminants beneath septic tank drainfields overlying a karst aquifer. J. Environ. Qual. 2010, 39, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Vengosh, A.; Pankratov, I. Chloride/bromide and chloride/fluoride ratios of domestic sewage effluents and associated contaminated ground water. Groundwater 1998, 36, 815–824. [Google Scholar] [CrossRef]
- Katz, B.G.; Eberts, S.M.; Kauffman, L.J. Using Cl/Br ratios and other indicators to assess potential impacts on groundwater quality from septic systems: A review and examples from principal aquifers in the United States. J. Hydrol. 2011, 397, 151–166. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water; US Geological Survey Water Supply Paper; Geological Survey: Alexandria, VA, USA, 1992. [Google Scholar]
- Chandrajith, R.; Bandara, U.G.C.; Diyabalanage, S.; Senaratne, S.; Barth, J.A.C. Application of water quality index as a vulnerability indicator to determine seawater intrusion in unconsolidated sedimentary aquifers in a tropical coastal region of Sri Lanka. Groundw. Sustain. Dev. 2022, 19, 100831. [Google Scholar] [CrossRef]
- Udeshani, W.A.C.; Dissanayake, H.M.K.P.; Gunatilake, S.K.; Chandrajith, R. Assessment of groundwater quality using water quality index (WQI): A case study of a hard rock terrain in Sri Lanka. Groundw. Sustain. Dev. 2020, 11, 100421. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geol. Surv. Tech. Methods 2013, 6, 497. [Google Scholar]
- Edirisinghe, E.A.N.V.; Karunarathne, G.R.R.; Tilakarathna, I.A.N.D.P.; Gunasekara, J.D.C.; Priyadarshanee, K.S.G.S. Isotope and chemical assessment of natural water in the Jaffna Peninsula in northern Sri Lanka for groundwater development aspects. Isot. Environ. Health Stud. 2020, 56, 205–219. [Google Scholar] [CrossRef]



| Percentile | |||||||
|---|---|---|---|---|---|---|---|
| Unit | Minimum | Maximum | Mean | 25% (Q1) | 50% (Q2) | 75% (Q3) | |
| Temp | °C | 27.4 | 35.9 | 29.2 | 28.5 | 29.1 | 29.7 |
| pH | 5.53 | 9.16 | 7.10 | 6.57 | 7.24 | 7.50 | |
| EC | µS/cm | 167 | 11140 | 1323 | 337 | 449 | 988 |
| HCO3 | mg/L | 24.9 | 902 | 156 | 68.1 | 132 | 195 |
| Cl | mg/L | 8.56 | 2755 | 215.8 | 17.5 | 43.2 | 154.6 |
| F− | mg/L | 0.002 | 1.150 | 0.164 | 0.026 | 0.059 | 0.138 |
| SO42− | mg/L | 3.18 | 231 | 53.4 | 14.0 | 33.3 | 75.38 |
| Br− | mg/L | 0.07 | 2.94 | 0.55 | 0.19 | 0.35 | 0.68 |
| NO3− | mg/L | 2.21 | 21.69 | 7.82 | 4.87 | 6.20 | 10.62 |
| Na | mg/L | 4.78 | 1308 | 88.6 | 13.2 | 31.9 | 59.4 |
| K | mg/L | 0.40 | 72.4 | 7.02 | 1.50 | 2.45 | 5.26 |
| Ca | mg/L | 10.5 | 548 | 85.8 | 35.9 | 49.6 | 89.0 |
| Mg | mg/L | 0.18 | 96.1 | 13.7 | 3.88 | 6.51 | 18.2 |
| Al | µg/L | 4.24 | 416 | 35.8 | 15.2 | 22.9 | 37.5 |
| Mn | µg/L | 0.78 | 709 | 76.8 | 3.14 | 27.9 | 64.6 |
| Fe | µg/L | 110 | 4279 | 1383 | 748 | 1183 | 1622 |
| Sr | µg/L | 41.9 | 2370 | 400 | 131 | 281 | 452 |
| Ba | µg/L | 5.68 | 710 | 107 | 19.7 | 40.5 | 134 |
| δ2H | ‰ | −61.1 | −0.69 | −37.4 | −45.1 | −36.7 | −33.8 |
| δ18O | ‰ | −8.94 | 1.46 | −5.68 | −6.82 | −5.81 | −5.22 |
| Type of Water Quality | WQI Range | Number of Samples | % of Samples |
|---|---|---|---|
| Excellent | <25 | 2 | 4 |
| Good | 26–50 | 8 | 16 |
| Poor | 51–75 | 20 | 39 |
| Very poor (Inferior) | 76–100 | 5 | 10 |
| Unsuitable | >100 | 16 | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratnayake, M.S.; Senarathne, S.L.; Diyabalanage, S.; Bandara, C.; Wickramarathne, S.; Chandrajith, R. Processes of Groundwater Contamination in Coastal Aquifers in Sri Lanka: A Geochemical and Isotope-Based Approach. Water 2025, 17, 1571. https://doi.org/10.3390/w17111571
Ratnayake MS, Senarathne SL, Diyabalanage S, Bandara C, Wickramarathne S, Chandrajith R. Processes of Groundwater Contamination in Coastal Aquifers in Sri Lanka: A Geochemical and Isotope-Based Approach. Water. 2025; 17(11):1571. https://doi.org/10.3390/w17111571
Chicago/Turabian StyleRatnayake, Movini Sathma, Sachintha Lakshan Senarathne, Saranga Diyabalanage, Chaminda Bandara, Sudeera Wickramarathne, and Rohana Chandrajith. 2025. "Processes of Groundwater Contamination in Coastal Aquifers in Sri Lanka: A Geochemical and Isotope-Based Approach" Water 17, no. 11: 1571. https://doi.org/10.3390/w17111571
APA StyleRatnayake, M. S., Senarathne, S. L., Diyabalanage, S., Bandara, C., Wickramarathne, S., & Chandrajith, R. (2025). Processes of Groundwater Contamination in Coastal Aquifers in Sri Lanka: A Geochemical and Isotope-Based Approach. Water, 17(11), 1571. https://doi.org/10.3390/w17111571









