Biofilm Characteristics and Microbial Community Structure in Pipeline Systems Using Tea Polyphenols as Disinfectant
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental System Design
2.2. Water Quality Parameters and Operating Conditions
2.3. Biofilm Characterization Methods
2.3.1. SEM Analysis
2.3.2. AFM Analysis
2.3.3. CLSM Analysis
2.4. CODMn Determination
2.5. BDOC Determination
2.6. DNA Extraction and Sequencing
3. Results and Discussion
3.1. Effects of Tea Polyphenols on Water Quality
3.2. Biofilm Morphological Characteristics
3.2.1. Surface Morphology
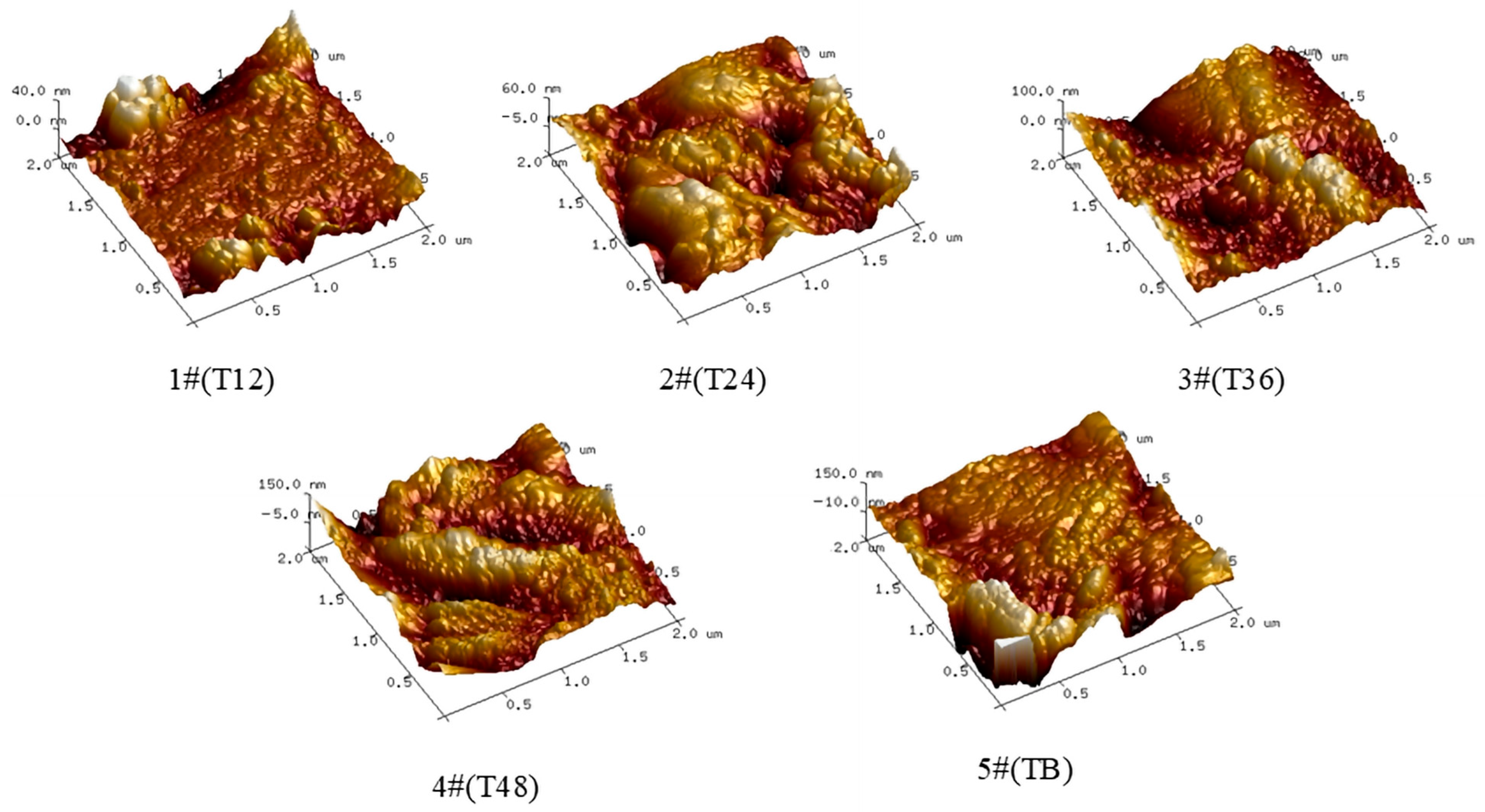
3.2.2. Roughness and Thickness Analysis
3.2.3. Spatial Structure Characteristics
3.3. Microbial Community Analysis
3.3.1. Community Diversity Analysis
3.3.2. Community Composition Analysis
3.3.3. Functional Characteristics and Ecological Significance of Dominant Genera
4. Conclusions
- Tea polyphenol disinfection significantly influenced the structural evolution of biofilms in pipeline systems: with increasing water age (12 h to 48 h), the surface roughness increased from 5.57 nm to 32.8 nm, and the biofilm thickness increased from 40 nm to 150 nm, establishing a quantitative relationship between tea polyphenol concentration and biofilm morphological characteristics.
- Tea polyphenol disinfection maintained excellent biological stability in water distribution systems through a dual regulatory mechanism: (i) direct antimicrobial action effectively controlled microbial proliferation, maintaining total bacterial counts below 45 CFU/mL across all water age conditions; (ii) the modulation of organic matter metabolism pathways maintained CODMn values consistently below 0.75 mg/L and BDOC at approximately 0.21 mg/L throughout the distribution network.
- Tea polyphenols promoted the formation of a more diverse and resilient microbial ecosystem by selectively inhibiting specific bacterial genera. This ecological regulation mechanism not only suppressed the excessive proliferation of potential pathogens (maintaining Legionella at levels below 0.21% except for a temporary increase at 36 h water age) but also enhanced microbial community diversity (Shannon index of 2.847 at 36 h water age compared to 1.336 in the control group). The community structure evolved from a single-dominant-genus pattern (Methylophilus at 54.41% at 12 h) to a balanced multi-genus coexistence structure at 48 h (Methylophilus at 24.33%, unclassified_Saprospiraceae at 21.70%, and Hydrogenophaga at 16.52%), indicating the capacity of tea polyphenols to foster a balanced microbial ecosystem.
- Tea polyphenols as a disinfectant for ultrafiltration effluent are most suitable for water distribution systems with water ages not exceeding 36 h. Under these conditions, the maintained effective residual concentration (≥2.2 mg/L) achieves optimal balance between microbial control efficacy and ecological diversity, providing both microbiological safety and system stability for drinking water distribution. And from the perspective of microbial ecological distribution, the microbial safety of water in the pipeline network is better at the distal point than at the starting point after disinfection with tea polyphenols.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Jiang, L.; Li, H.; Zhang, J.; Sun, T.; Dong, Y.; Ding, N. Disinfection kinetics of peracetic acid inactivation of pathogenic bacteria in water. Water Cycle 2022, 3, 79–85. [Google Scholar] [CrossRef]
- Xiao, R.; Deng, Y.; Xu, Z.; Chu, W. Disinfection Byproducts and Their Precursors in Drinking Water Sources: Origins, Influencing Factors, and Environmental Insights. Engineering 2024, 36, 36–50. [Google Scholar] [CrossRef]
- Li, W.; Han, J.; Zhang, X.; Chen, G.; Yang, Y. Contributions of Pharmaceuticals to DBP Formation and Developmental Toxicity in Chlorination of NOM-containing Source Water. Environ. Sci. Technol. 2023, 57, 18775–18787. [Google Scholar] [CrossRef] [PubMed]
- Duque-Sarango, P.; Delgado-Armijos, N.; Romero-Martínez, L.; Cruz, D.; Pinos-Vélez, V. Advancing Waterborne Fungal Spore Control: UV-LED Disinfection Efficiency and Post-Treatment Reactivation Analysis. Water 2025, 17, 922. [Google Scholar] [CrossRef]
- Guo, K.; Wu, Z.; Chen, C.; Fang, J. UV/Chlorine Process: An Efficient Advanced Oxidation Process with Multiple Radicals and Functions in Water Treatment. Acc. Chem. Res. 2022, 55, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ateia, M.; Awfa, D.; Yoshimura, C. Regrowth of bacteria after light-based disinfection—What we know and where we go from here. Chemosphere 2021, 268, 128850. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, C.; Jin, J.; Yang, W.; Wang, Z. Current Understanding on Antibacterial Mechanisms and Research Progress of Tea Polyphenols as a Supplementary Disinfectant for Drinking Water. J. Water Health 2022, 20, 1611–1628. [Google Scholar] [CrossRef]
- Feng, C.; Wei, T.; Qing, S.; Han, F.; Tao, X. Application of Tea Polyphenols and Their Effects on Ultrafiltration Effluent Disinfection and Microbial Risk. Water 2021, 13, 2559. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, H.; Jiang, W.-B.; Li, X.-Q.; Eng, X.-J. Effects of Catechins on Growth Performance, Serum Antioxidant Indices, and Muscle Quality of Grass Carp. Chin. J. Anim. Nutr. 2020, 32, 836–846. [Google Scholar]
- Luo, D.-L.; Li, X.-Y.; Cao, S.; Ba, L.-J. Comparison on Several Antioxidant Components and Activity for Different Kinds of Guizhou Tea. Food Res. Dev. 2019, 49, 35–41. [Google Scholar]
- Ma, J.; Du, Z.; Gao, S.; Zang, J. Tea Polyphenols-Mediated Supramolecular Architectures: Design and Applications. Trends Food Sci. Technol. 2024, 152, 104665. [Google Scholar] [CrossRef]
- Cai, J.; Ye, R.; Jia, K.; Sun, W.; Zhao, L.-P. Review on Extraction and Antibacterial Activity of Tea Polyphenols. Chem. Reag. 2020, 42, 105–114. [Google Scholar]
- Wei, T.; Qing, S.; Feng, C.; Yao, R.; Zhu, N.; Xu, Z.; Wang, Y. Microbial Characterizations of Water Using Tea Polyphenols as a Disinfectant for Effluent Treatment after the Ultrafiltration Process. AQUA Water Infrastruct. Ecosyst. Soc. 2021, 70, 1170–1180. [Google Scholar] [CrossRef]
- Feng, C.; Wang, T.; Wang, C.; Chen, X.; Guo, Z.; Chen, Z. Disinfection Effects and Operating Conditions of Tea Polyphenols Combined with Ozone. Ozone Sci. Eng. 2020, 42, 551–557. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Feng, C.-M.; Wang, C.-Z.; Wang, J.-L.; Sun, J.-Y. Disinfection Effect of Ultraviolet-Tea Polyphenols Combination on Water Supply Networks. China Environ. Sci. 2020, 40, 1563–1569. [Google Scholar]
- Afonso, A.C.; Saavedra, M.J.; Gomes, I.B.; Simões, M.; Simões, L.C. Current Microbiological Challenges in Drinking Water. J. Water Process Eng. 2025, 72, 107614. [Google Scholar] [CrossRef]
- Hemdan, B.A.; El-Taweel, G.E.; Goswami, P.; Pant, D.; Sevda, S. The role of biofilm in the development and dissemination of ubiquitous pathogens in drinking water distribution systems: An overview of surveillance, outbreaks, and prevention. World J. Microbiol. Biotechnol. 2021, 37, 36. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.M.; Gomes, I.B.; Simões, L.C.; Simões, M. A review of research advances on disinfection strategies for biofilm control in drinking water distribution systems. Water Res. 2024, 253, 121273. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, L.; Zhou, L.; Zhao, Z. Impacts of antibiotics on biofilm bacterial community and disinfection performance on simulated drinking water supply pipe wall. Environ. Pollut. 2021, 288, 117736. [Google Scholar] [CrossRef]
- Huang, C.; Ginn, T.R.; Clark, G.G.; Zaki, F.R.; Won, J.; Boppart, S.A.; Nguyen, T.H. Phosphate-Based Corrosion Inhibition in Drinking Water Systems and Effects on Disinfectant Decay and Biofilm Growth. Environ. Eng. Sci. 2023, 40, 634–644. [Google Scholar] [CrossRef]
- Javed, A.; Amjad, H.; Hashmi, I.; Miran, W. Investigating the Influence of Tank Material and Residual Chlorine on the Proliferation of Bacterial Biofilm Growth in the Drinking Water Storage Systems. J. Water Sanit. Hyg. Dev. 2025, 15, 305–321. [Google Scholar] [CrossRef]
- Blyth, W.E.; Shahsavari, E.; Aburto-Medina, A.; Ball, A.S.; Osborn, A.M. Variation in the Structure and Composition of Bacterial Communities within Drinking Water Fountains in Melbourne, Australia. Water 2022, 14, 908. [Google Scholar] [CrossRef]
- Kitajima, M.; Cruz, M.C.; Williams, R.B.H.; Wuertz, S.; Whittle, A.J. Microbial Abundance and Community Composition in Biofilms on In-Pipe Sensors in a Drinking Water Distribution System. Sci. Total Environ. 2021, 766, 142314. [Google Scholar] [CrossRef]
- Lin, W.-F.; Yu, Z.-S.; Chen, X.; Zhang, H.-X. Progress in Biofilm Reactors and Molecular Biology Research Methods for Water Supply Networks. Environ. Sci. Technol. 2012, 35, 71–78. [Google Scholar]
- Wang, Q.; Lu, Y.; Yang, W.; Liu, Z. Study on Conditions for Determining Permanganate Index in Water by Spectrophotometry. Nonferrous Met. Equip. 2020, 34, 36–39. [Google Scholar]
- Xin, C.; Khu, S.-T.; Wang, T.; Zuo, X.; Zhang, Y. Effect of Flow Fluctuation on Water Pollution in Drinking Water Distribution Systems. Environ. Res. 2024, 246, 118142. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X. Green Synthesis of Modified Polyethylene Packing Supported Tea Polyphenols-NZVI for Nitrate Removal from Wastewater: Characterization and Mechanisms. Sci. Total Environ. 2022, 806, 150596. [Google Scholar] [CrossRef]







| Parameter | Values | Instruments | Methods |
|---|---|---|---|
| pH | 7.6 ± 0.2 | FiveGo Single-Channel Portable pH Meter (METTLER TOLEDO, Greifensee, Switzerland) | |
| Temperature (℃) | 17.9 ± 0.6 | FiveGo Single-Channel Portable pH Meter(METTLER TOLEDO, Greifensee, Switzerland) | |
| Chroma (degree) | 1 ± 1 | PFXi-995 High-Precision Automatic((The Tintometer Ltd., Amesbury, Wiltshire, UK)) | |
| Turbidity (NTU) | 0.084 ± 0.015 | HACH-2100ANTurbidity Meter(HACH Instrument Corporation, Loveland, CO, USA) | |
| CODMn (mg/L) | 0.73 ± 0.11 | DR6000 (HACH Instrument Corporation, Loveland, CO, USA) | Wang [24] |
| Total number of bacteria (CFU/mL) | 2 ± 2 | Plate Counting |
| BAR | Residual Concentrations of Tea Polyphenols (mg/L) | Water Age (h) |
|---|---|---|
| 1#(T12) | 3.6 | 12 |
| 2#(T24) | 2.8 | 24 |
| 3#(T36) | 2.2 | 36 |
| 4#(T48) | 1.5 | 48 |
| 5#(TB) | 0 | 48 |
| BAR | Ra (nm) | Max Thickness (nm) | Water Age (h) |
|---|---|---|---|
| 1#(T12) | 5.57 | 40 | 12 |
| 2#(T24) | 13.9 | 60 | 24 |
| 3#(T36) | 21.3 | 100 | 36 |
| 4#(T48) | 32.8 | 150 | 48 |
| 5#(TB) | 32.1 | 150 | 48 |
| BAR | Water Age | Shannon | Simpson | Chao | ACE |
|---|---|---|---|---|---|
| 1#(T12) | 12 h | 2.08 | 0.31 | 147.00 | 140.47 |
| 2#(T24) | 24 h | 2.12 | 0.19 | 134.58 | 133.65 |
| 3#(T36) | 36 h | 2.85 | 0.10 | 146.11 | 142.24 |
| 4#(T48) | 48 h | 2.54 | 0.14 | 144.07 | 144.67 |
| 5#(Blank) | 48 h | 1.34 | 0.43 | 147.00 | 142.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Luo, J.; Yang, T.; Li, Y.; Li, Y.; Feng, C. Biofilm Characteristics and Microbial Community Structure in Pipeline Systems Using Tea Polyphenols as Disinfectant. Water 2025, 17, 1545. https://doi.org/10.3390/w17101545
Wang Z, Luo J, Yang T, Li Y, Li Y, Feng C. Biofilm Characteristics and Microbial Community Structure in Pipeline Systems Using Tea Polyphenols as Disinfectant. Water. 2025; 17(10):1545. https://doi.org/10.3390/w17101545
Chicago/Turabian StyleWang, Ziwei, Jiacheng Luo, Tongtong Yang, Ying Li, Yihao Li, and Cuimin Feng. 2025. "Biofilm Characteristics and Microbial Community Structure in Pipeline Systems Using Tea Polyphenols as Disinfectant" Water 17, no. 10: 1545. https://doi.org/10.3390/w17101545
APA StyleWang, Z., Luo, J., Yang, T., Li, Y., Li, Y., & Feng, C. (2025). Biofilm Characteristics and Microbial Community Structure in Pipeline Systems Using Tea Polyphenols as Disinfectant. Water, 17(10), 1545. https://doi.org/10.3390/w17101545





