Application of Zero-Valent Iron and Its Derivatives in the Removal of Toxic Metal Ions from Groundwater
Abstract
1. Introduction
2. Zero-Valent Iron and Its Derivatives
2.1. Zero-Valent Iron
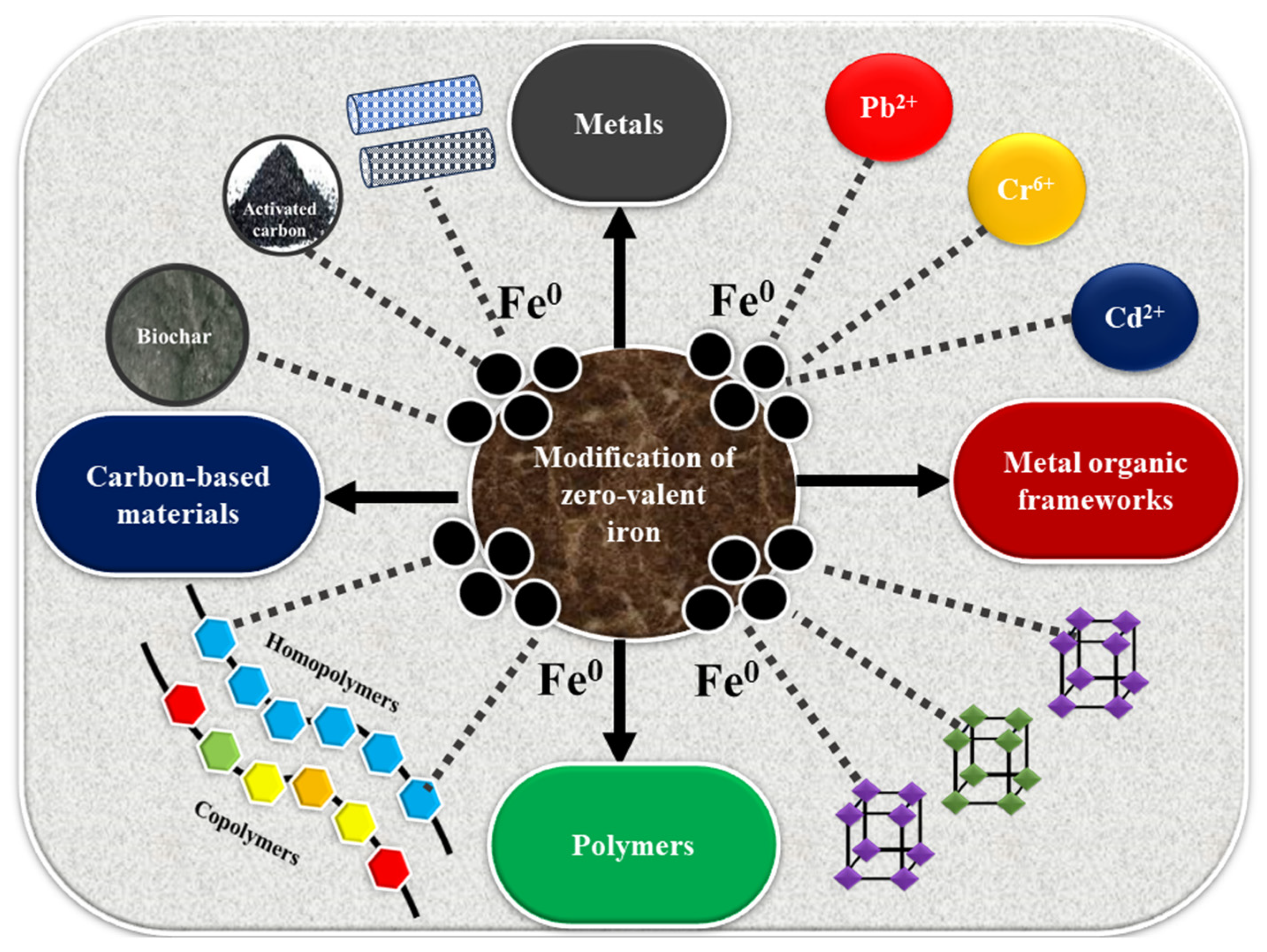
2.2. Modifications of ZVI
2.2.1. ZVI Doping with Metals
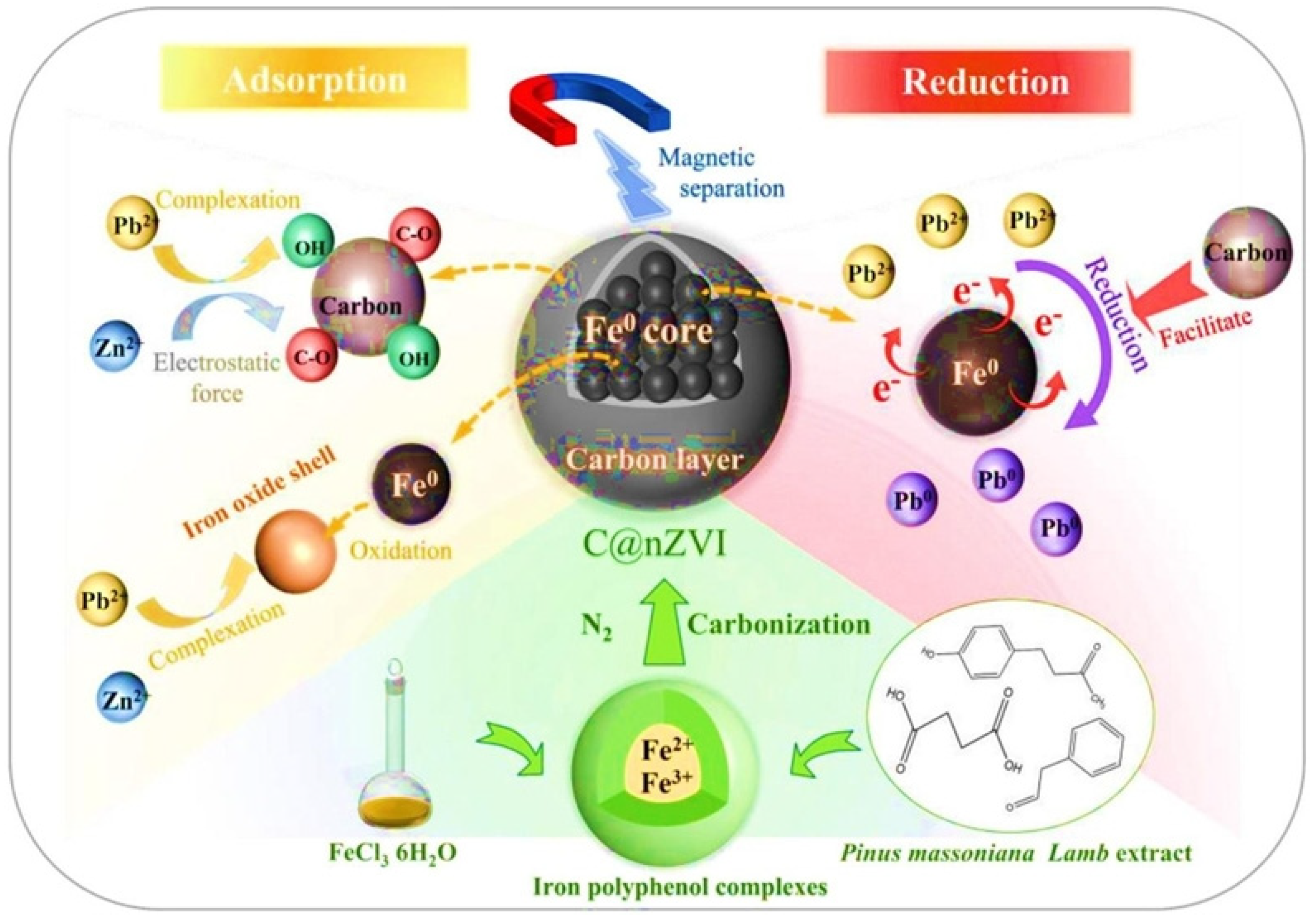
2.2.2. ZVI Modification with Different Carbon-Based Materials
3. Application of Zero-Valent Iron and Its Derivatives in the Removal of Toxic Metal Ions from Groundwater and Its Mechanism
3.1. Zero-Valent Iron Used as a Permeable Reactive Barrier for Metal Ion Removal
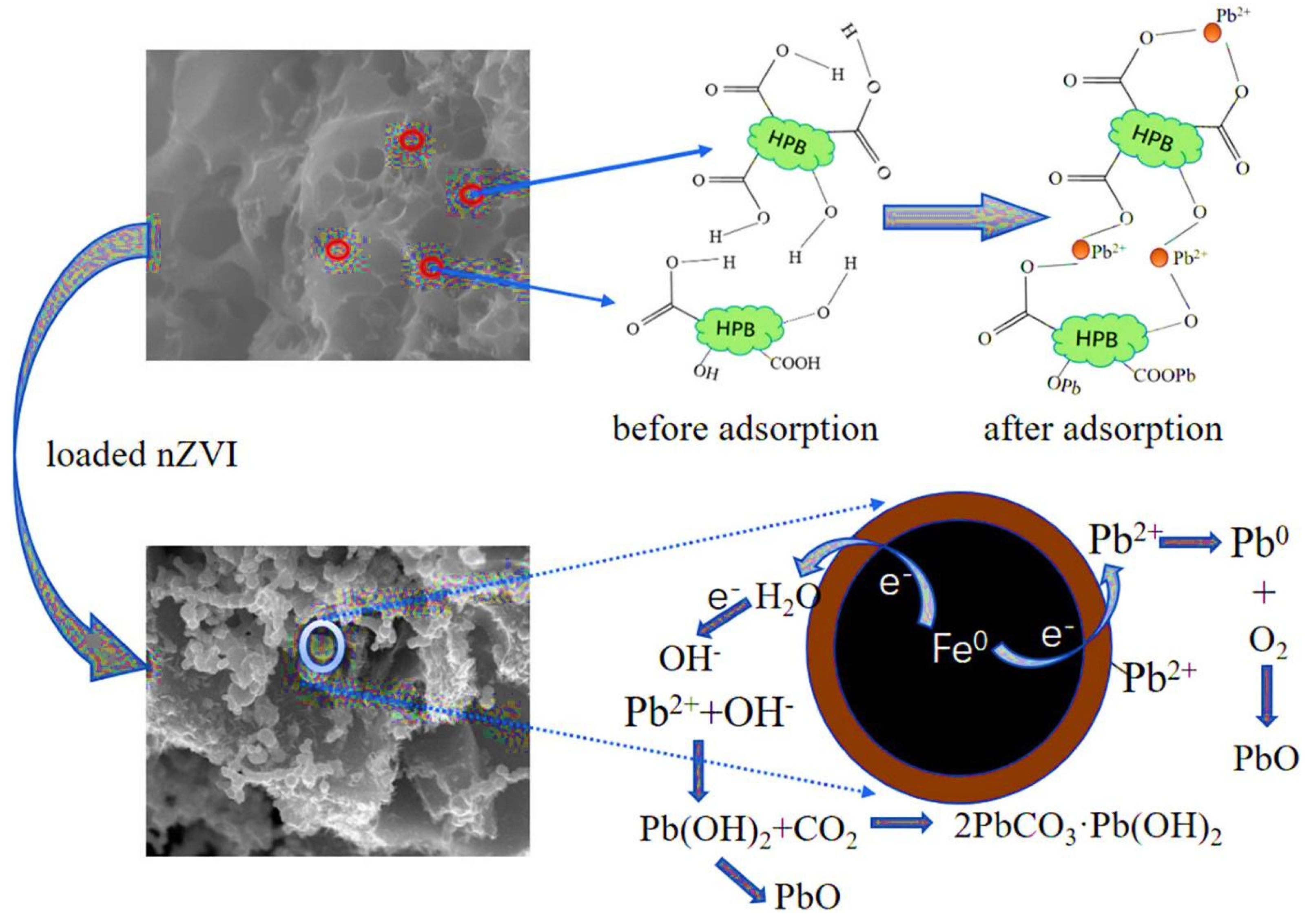
3.2. Zero-Valent Iron (ZVI) and Its Modifications for Groundwater Remediation
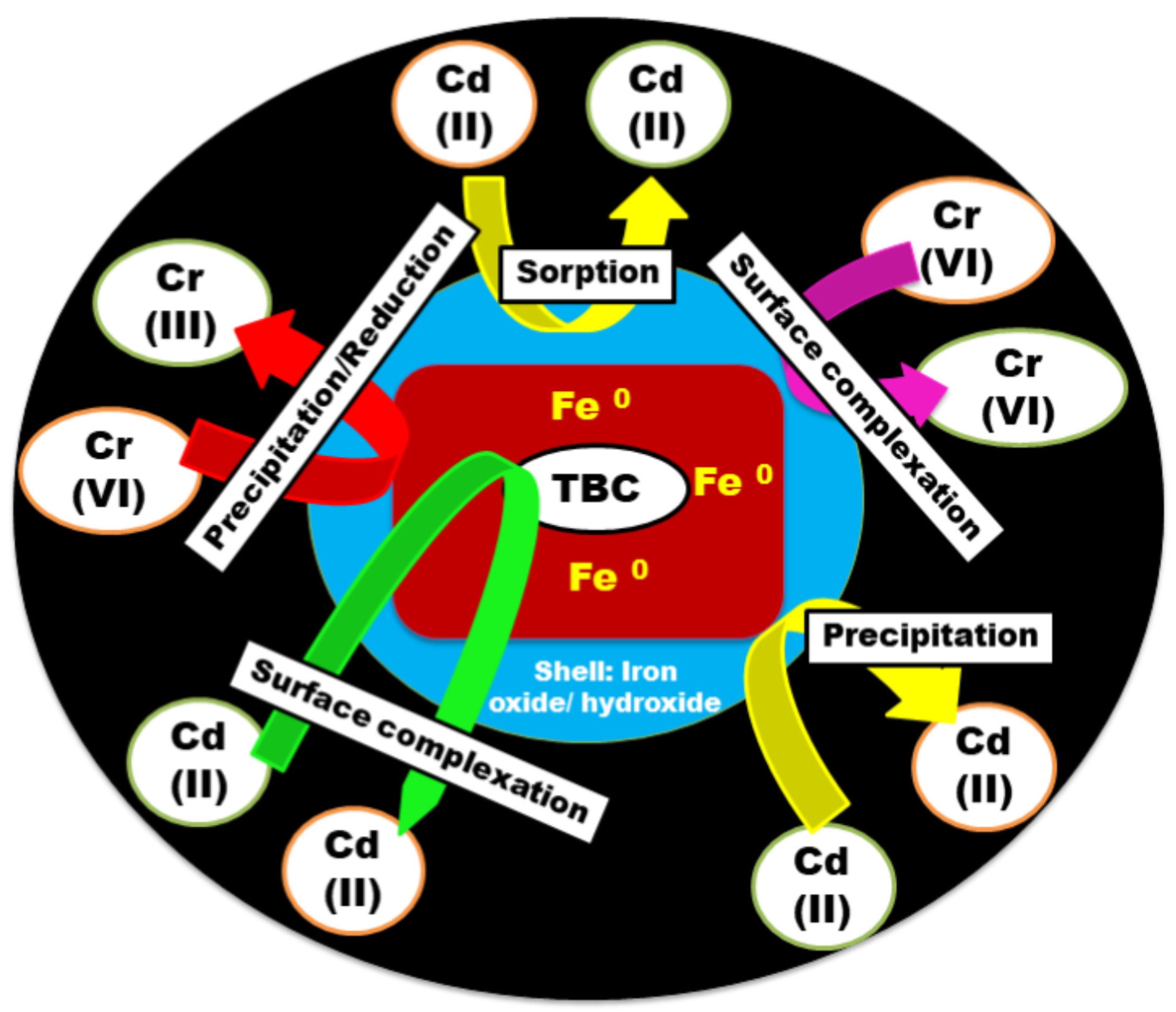
3.3. Mechanism and Factors Affecting Adsorption of Metal Ion Using Zero-Valent Iron
4. Future Perspectives
4.1. Enhancing Long-Term Stability and Reactivity
4.2. Optimizing Composite Materials for Selective Adsorption
4.3. Improving Regeneration and Reusability
4.4. Field-Scale Applications and Cost Considerations
4.5. Integration with Advanced Remediation Technologies
4.6. Real Aquifer Conditions and Field-Deployable Systems
4.7. Green Synthesis of ZVI Using Plant Extracts
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Liu, M.; Wu, X.; Chen, C.; Wang, Q.; Wen, T.; Wang, X. Synthesizing the composites of graphene oxide-wrapped polyaniline hollow microspheres for high-performance supercapacitors. Sci. Adv. Mater. 2013, 5, 1686–1693. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Zhang, Z.; Li, Y.; Dong, Y.; Sun, Y. Synthesis of multi-walled carbon nanotube–hydroxyapatite composites and its application in the sorption of Co(II) from aqueous solutions. J. Mol. Liq. 2013, 179, 46–53. [Google Scholar] [CrossRef]
- Ren, X.; Yang, S.; Shao, D.; Tan, X. Retention of Pb(II) by a low-cost magnetic composite prepared by environmentally-friendly plasma technique. Sep. Sci. Technol. 2013, 48, 1211–1219. [Google Scholar] [CrossRef]
- Qiu, X.; Fang, Z.; Yan, X.; Gu, F.; Jiang, F. Emergency remediation of simulated chromium (VI)-polluted river by nanoscale zero-valent iron: Laboratory study and numerical simulation. Chem. Eng. J. 2012, 193, 358–365. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, S.; Chen, Z.; Megharaj, M.; Naidu, R. Kaolinite-supported nanoscale zero-valent iron for removal of Pb2+ from aqueous solution: Reactivity, characterization and mechanism. Water Res. 2011, 45, 3481–3488. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Kim, D.-G.; Shin, H.-S. Mechanism study of nitrate reduction by nano zero valent iron. J. Hazard. Mater. 2011, 185, 1513–1521. [Google Scholar] [CrossRef]
- Ryu, A.; Jeong, S.-W.; Jang, A.; Choi, H. Reduction of highly concentrated nitrate using nanoscale zero-valent iron: Effects of aggregation and catalyst on reactivity. Appl. Catal. B Environ. 2011, 105, 128–135. [Google Scholar] [CrossRef]
- Luo, S.; Qin, P.; Shao, J.; Peng, L.; Zeng, Q.; Gu, J.-D. Synthesis of reactive nanoscale zero valent iron using rectorite supports and its application for Orange II removal. Chem. Eng. J. 2013, 223, 1–7. [Google Scholar] [CrossRef]
- Shirin, S.; Balakrishnan, V.K. Using chemical reactivity to provide insights into environmental transformations of priority organic substances: The Fe0-mediated reduction of acid blue 129. Environ. Sci. Technol. 2011, 45, 10369–10377. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Tokumura, M.; Nakajima, K.; Kawase, Y. Phenol removal using zero-valent iron powder in the presence of dissolved oxygen: Roles of decomposition by the Fenton reaction and adsorption/precipitation. J. Hazard. Mater. 2012, 201, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Segura, Y.; Martínez, F.; Melero, J.A.; Molina, R.; Chand, R.; Bremner, D.H. Enhancement of the advanced Fenton process (Fe0/H2O2) by ultrasound for the mineralization of phenol. Appl. Catal. B Environ. 2012, 113, 100–106. [Google Scholar] [CrossRef]
- Neumann, A.; Kaegi, R.; Voegelin, A.; Hussam, A.; Munir, A.K.M.; Hug, S.J. Arsenic removal with composite iron matrix filters in Bangladesh: A field and laboratory study. Environ. Sci. Technol. 2013, 47, 4544–4554. [Google Scholar] [CrossRef]
- Klas, S.; Kirk, D.W. Advantages of low pH and limited oxygenation in arsenite removal from water by zero-valent iron. J. Hazard. Mater. 2013, 252, 77–82. [Google Scholar] [CrossRef]
- Yin, W.; Wu, J.; Li, P.; Wang, X.; Zhu, N.; Wu, P.; Yang, B. Experimental study of zero-valent iron induced nitrobenzene reduction in groundwater: The effects of pH, iron dosage, oxygen and common dissolved anions. Chem. Eng. J. 2012, 184, 198–204. [Google Scholar] [CrossRef]
- Gu, C.; Jia, H.; Li, H.; Teppen, B.J.; Boyd, S.A. Synthesis of highly reactive subnano-sized zero-valent iron using smectite clay templates. Environ. Sci. Technol. 2010, 44, 4258–4263. [Google Scholar] [CrossRef]
- Su, C.; Puls, R.W.; Krug, T.A.; Watling, M.T.; O’Hara, S.K.; Quinn, J.W.; Ruiz, N.E. A two and half-year-performance evaluation of a field test on treatment of source zone tetrachloroethene and its chlorinated daughter products using emulsified zero valent iron nanoparticles. Water Res. 2012, 46, 5071–5084. [Google Scholar] [CrossRef]
- Dorathi, P.J.; Kandasamy, P. Dechlorination of chlorophenols by zero valent iron impregnated silica. J. Environ. Sci. 2012, 24, 765–773. [Google Scholar] [CrossRef]
- Choi, K.; Lee, W. Enhanced degradation of trichloroethylene in nano-scale zero-valent iron Fenton system with Cu(II). J. Hazard. Mater. 2012, 211, 146–153. [Google Scholar] [CrossRef]
- Kustov, L.M.; Finashina, E.D.; Shuvalova, E.V.; Tkachenko, O.P.; Kirichenko, O.A. Pd–Fe nanoparticles stabilized by chitosan derivatives for perchloroethene dechlorination. Environ. Int. 2011, 37, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-B.; Zhang, W.-X. Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environ. Sci. Technol. 1997, 31, 2154–2156. [Google Scholar] [CrossRef]
- Sweeny, K.; Fischer, J. Reductive Degradation of Halogenated Pesticides. U.S. Patent No. 3,640,821, 8 February 1972. [Google Scholar]
- Galdames, A.; Ruiz-Rubio, L.; Orueta, M.; Sánchez-Arzalluz, M.; Vilas-Vilela, J.L. Zero-valent iron nanoparticles for soil and groundwater remediation. Int. J. Environ. Res. Public Health 2020, 17, 5817. [Google Scholar] [CrossRef]
- Yang, L.; Jin, X.; Owens, G.; Chen, Z. Nitrogen-doped carbon enhances Fe0 activation for efficient tetrabromobisphenol a decontamination and its removal mechanism. Chem. Eng. J. 2024, 482, 148839. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, L.; Liu, Y.; Liu, Y.; Wen, J. Degradation of 1, 3, 6, 8-tetrabromocarbazole by sulfidated zero-valent iron activated peroxydisulfate: Mechanistic insight and transformation pathways. Chem. Eng. J. 2023, 458, 141439. [Google Scholar] [CrossRef]
- Khuntia, B.K.; Anwar, M.F.; Alam, T.; Samim, M.; Kumari, M.; Arora, I. Synthesis and characterization of zero-valent iron nanoparticles, and the study of their effect against the degradation of DDT in soil and assessment of their toxicity against collembola and ostracods. ACS Omega 2019, 4, 18502–18509. [Google Scholar] [CrossRef]
- Yan, Z.; Ouyang, J.; Wu, B.; Liu, C.; Wang, H.; Wang, A.; Li, Z. Nonmetallic modified zero-valent iron for remediating halogenated organic compounds and heavy metals: A comprehensive review. Environ. Sci. Ecotechnology 2024, 21, 100417. [Google Scholar] [CrossRef]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Sun, Y.; Guan, X.; Wang, J.; Meng, X.; Xu, C.; Zhou, G. Effect of weak magnetic field on arsenate and arsenite removal from water by zerovalent iron: An XAFS investigation. Environ. Sci. Technol. 2014, 48, 6850–6858. [Google Scholar] [CrossRef]
- Ullah, S.; Faiz, P.; Leng, S. Synthesis, Mechanism, and Performance Assessment of Zero-Valent Iron for Metal-Contaminated Water Remediation: A Review. CLEAN–Soil Air Water 2020, 48, 2000080. [Google Scholar] [CrossRef]
- Patra, S.; Pranudta, A.; Chanlek, N.; Nguyen, T.T.; Nhat, N.H.; El-Moselhy, M.M.; Padungthon, S. Denitrification of nitrate in regeneration waste brine using hybrid cation exchanger supported nanoscale zero-valent iron with/without palladium nanoparticles. Chemosphere 2023, 310, 136851. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Chin, Y.-P.; Ahn, J.-Y.; Wei-Haas, M.; McAdams, B.; Hwang, I. Reciprocal influences of dissolved organic matter and nanosized zero-valent iron in aqueous media. Chemosphere 2018, 193, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Namakka, M.; Rahman, R.; Said, K.A.B.M.; Muhammad, A. Insights into micro-and nano-zero valent iron materials: Synthesis methods and multifaceted applications. RSC Adv. 2024, 14, 30411–30439. [Google Scholar] [CrossRef]
- Di, L.; Chen, X.; Lu, J.; Zhou, Y.; Zhou, Y. Removal of heavy metals in water using nano zero-valent iron composites: A review. J. Water Process Eng. 2023, 53, 103913. [Google Scholar] [CrossRef]
- Namakka, M.; Rahman, M.R.; Said, K.A.M.B.; Mannan, M.A.; Patwary, A.M. A review of nanoparticle synthesis methods, classifications, applications, and characterization. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100900. [Google Scholar] [CrossRef]
- Yan, W.; Lien, H.-L.; Koel, B.E.; Zhang, W.-X. Iron nanoparticles for environmental clean-up: Recent developments and future outlook. Environ. Sci. Process. Impacts 2013, 15, 63–77. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, S.; Meng, Y.; Qiu, Y.; Chen, M.; Ma, L.; Li, L.; Liu, Y.; Yang, B. Advances in modified zero-valent iron materials: Synthesis methods, field studies, practical applications and challenges. Chem. Eng. J. 2024, 502, 157832. [Google Scholar] [CrossRef]
- Qin, Y.; Li, G.; Zhang, L.; An, T. Protocatechuic acid promoted catalytic degradation of rhodamine B with Fe@ Fe2O3 core-shell nanowires by molecular oxygen activation mechanism. Catal. Today 2019, 335, 144–150. [Google Scholar] [CrossRef]
- Hu, Y.-B.; Zhang, M.; Li, X.-Y. Improved longevity of nanoscale zero-valent iron with a magnesium hydroxide coating shell for the removal of Cr (VI) in sand columns. Environ. Int. 2019, 133, 105249. [Google Scholar] [CrossRef]
- Zhou, N.; Gong, K.; Hu, Q.; Cheng, X.; Zhou, J.; Dong, M.; Wang, N.; Ding, T.; Qiu, B.; Guo, Z. Optimizing nanocarbon shell in zero-valent iron nanoparticles for improved electron utilization in Cr (VI) reduction. Chemosphere 2020, 242, 125235. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, P.; Hu, Y.; Zan, R. Application of weak magnetic field coupling with zero-valent iron for remediation of groundwater and wastewater: A review. J. Clean. Prod. 2020, 262, 121341. [Google Scholar] [CrossRef]
- Fang, Y.; Wen, J.; Zhang, H.; Wang, Q.; Hu, X. Enhancing Cr (VI) reduction and immobilization by magnetic core-shell structured NZVI@ MOF derivative hybrids. Environ. Pollut. 2020, 260, 114021. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Zhang, C.-H.; Xu, J.-H.; Li, L.; Li, D.; Wu, Q.; Ma, L.-M. Strategies to enhance the reactivity of zero-valent iron for environmental remediation: A review. J. Environ. Manag. 2022, 317, 115381. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Z.; Wang, H.; Yang, H.; Wen, T.; Wang, S.; Hu, B.; Wang, X. Removal of organic compounds by nanoscale zero-valent iron and its composites. Sci. Total Environ. 2021, 792, 148546. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Ma, Z.; Carroll, K.C.; Zhao, X.; Huo, Z. Cadmium removal mechanistic comparison of three Fe-based nanomaterials: Water-chemistry and roles of Fe dissolution. Front. Environ. Sci. Eng. 2022, 16, 151. [Google Scholar] [CrossRef]
- Xue, C.; Wu, J.; Wang, K.; Yi, Y.; Fang, Z.; Cheng, W.; Fang, J. Effects of different types of biochar on the properties and reactivity of nano zero-valent iron in soil remediation. Front. Environ. Sci. Eng. 2021, 15, 1–13. [Google Scholar] [CrossRef]
- Zhou, L.; Li, A.; Ma, F.; Zhao, H.; Deng, F.; Pi, S.; Tang, A.; Yang, J. Combining high electron transfer efficiency and oxidation resistance in nZVI with coatings of microbial extracellular polymeric substances to enhance Sb (V) reduction and adsorption. Chem. Eng. J. 2020, 395, 125168. [Google Scholar] [CrossRef]
- Song, M.; Hu, X.; Gu, T.; Zhang, W.-X.; Deng, Z. Nanocelluloses affixed nanoscale Zero-Valent Iron (nZVI) for nickel removal: Synthesis, characterization and mechanisms. J. Environ. Chem. Eng. 2022, 10, 107466. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, S.; Li, Z.; Li, H.; Shaban, M.; Chen, C. Nanoscale zero-valent iron composites for uranium-contaminated water treatment and environmental remediation: A review. Environ. Sci. Nano 2025, 12, 20–40. [Google Scholar] [CrossRef]
- He, F.; Li, Z.; Shi, S.; Xu, W.; Sheng, H.; Gu, Y.; Jiang, Y.; Xi, B. Dechlorination of excess trichloroethene by bimetallic and sulfidated nanoscale zero-valent iron. Environ. Sci. Technol. 2018, 52, 8627–8637. [Google Scholar] [CrossRef]
- Pandey, K.; Sharma, S.; Saha, S. Advances in design and synthesis of stabilized zero-valent iron nanoparticles for groundwater remediation. J. Environ. Chem. Eng. 2022, 10, 107993. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, J.; Gao, F.; Wang, D.; Lyu, H.; Tang, J. Zero-valent iron-based materials for enhanced reductive removal of contaminants: From the trial-and-error synthesis to rational design. Appl. Catal. B Environ. Energy 2024, 365, 124901. [Google Scholar] [CrossRef]
- Cheng, S.-F.; Wu, S.-C. Feasibility of using metals to remediate water containing TCE. Chemosphere 2001, 43, 1023–1028. [Google Scholar] [CrossRef]
- Lien, H.-L.; Zhang, W.-X. Nanoscale iron particles for complete reduction of chlorinated ethenes. Colloids Surf. A Physicochem. Eng. Asp. 2001, 191, 97–105. [Google Scholar] [CrossRef]
- Pan, M.; Liu, J.; Yang, H.; Zhang, W.; Huang, K. One-pot synthesis of N-doped porous carbon supported zero-valent iron nanomaterials for efficient removal of U(VI). Chem. Eng. J. 2025, 505, 159870. [Google Scholar] [CrossRef]
- Yang, L.; Jin, X.; Lin, Q.; Owens, G.; Chen, Z. Enhanced adsorption and reduction of Pb(II) and Zn(II) from mining wastewater by carbon@nano-zero-valent iron (C@nZVI) derived from biosynthesis. Sep. Purif. Technol. 2023, 311, 123249. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Yang, Y.; Han, Y.; Wang, T.; Chen, J.; Tsang, D.C. Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J. Hazard. Mater. 2020, 383, 121240. [Google Scholar] [CrossRef]
- Tian, H.; Wu, Q.; Wu, Q.; Sun, C.; Zhang, R.; Wei, J.; Xu, H.; Liu, Z.; Huang, C.; Wang, P. Carbon nanotubes mediate electron transfer between Shewanella oneidenis MR-1 and nano zero-valent iron to enhance the elimination of Cr (VI) from aqueous media. Sep. Purif. Technol. 2024, 341, 126909. [Google Scholar] [CrossRef]
- Xiao, J.; Pang, Z.; Zhou, S.; Chu, L.; Rong, L.; Liu, Y.; Li, J.; Tian, L. The mechanism of acid-washed zero-valent iron/activated carbon as permeable reactive barrier enhanced electrokinetic remediation of uranium-contaminated soil. Sep. Purif. Technol. 2020, 244, 116667. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Sun, Y.; Cheng, K.; Li, J.; Tsang, D.C. Fabrication and characterization of hydrophilic corn stalk biochar-supported nanoscale zero-valent iron composites for efficient metal removal. Bioresour. Technol. 2018, 265, 490–497. [Google Scholar] [CrossRef]
- Tan, X.-F.; Liu, Y.-G.; Gu, Y.-L.; Xu, Y.; Zeng, G.-M.; Hu, X.-J.; Liu, S.-B.; Wang, X.; Liu, S.-M.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-Y.; Seo, Y.-D.; Ryu, K.-S.; Park, D.-J.; Lee, S.-H. Redox and catalytic properties of biochar-coated zero-valent iron for the removal of nitro explosives and halogenated phenols. Environ. Sci. Process. Impacts 2017, 19, 711–719. [Google Scholar] [CrossRef]
- Su, Y.-F.; Cheng, Y.-L.; Shih, Y.-H. Removal of trichloroethylene by zerovalent iron/activated carbon derived from agricultural wastes. J. Environ. Manag. 2013, 129, 361–366. [Google Scholar] [CrossRef]
- Li, S.; Yang, F.; Li, J.; Cheng, K. Porous biochar-nanoscale zero-valent iron composites: Synthesis, characterization and application for lead ion removal. Sci. Total Environ. 2020, 746, 141037. [Google Scholar] [CrossRef]
- Wei, A.; Ma, J.; Chen, J.; Zhang, Y.; Song, J.; Yu, X. Enhanced nitrate removal and high selectivity towards dinitrogen for groundwater remediation using biochar-supported nano zero-valent iron. Chem. Eng. J. 2018, 353, 595–605. [Google Scholar] [CrossRef]
- Rajput, M.K.; Hazarika, R.; Sarma, D. Zerovalent iron decorated tea waste derived porous biochar [ZVI@TBC] as an efficient adsorbent for Cd(II) and Cr(VI) removal. J. Environ. Chem. Eng. 2023, 11, 110279. [Google Scholar] [CrossRef]
- Weisener, C.G.; Sale, K.S.; Smyth, D.J.A.; Blowes, D.W. Field Column Study Using Zerovalent Iron for Mercury Removal from Contaminated Groundwater. Environ. Sci. Technol. 2005, 39, 6306–6312. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Su, C.; Ford, R.G.; Paul, C.J. Chromium-Removal Processes during Groundwater Remediation by a Zerovalent Iron Permeable Reactive Barrier. Environ. Sci. Technol. 2005, 39, 4599–4605. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Abbas, T.R.; Jassam, S.H. Removal of zinc from contaminated groundwater by zero-valent iron permeable reactive barrier. Desalin. Water Treat. 2015, 55, 1586–1597. [Google Scholar] [CrossRef]
- Njaramba, L.K.; Park, J.-B.; Lee, C.-S.; Nzioka, A.M.; Kim, Y.-J. Permeable Reactive Barriers with Zero-Valent Iron and Pumice for Remediation of Groundwater Contaminated with Multiple Heavy Metals. Environ. Eng. Sci. 2020, 38, 245–255. [Google Scholar] [CrossRef]
- Kornilovych, B.; Wireman, M.; Ubaldini, S.; Guglietta, D.; Koshik, Y.; Caruso, B.; Kovalchuk, I. Uranium Removal from Groundwater by Permeable Reactive Barrier with Zero-Valent Iron and Organic Carbon Mixtures: Laboratory and Field Studies. Metals 2018, 8, 408. [Google Scholar] [CrossRef]
- Song, I.-G.; Kang, Y.-G.; Kim, J.-H.; Yoon, H.; Um, W.Y.; Chang, Y.-S. Assessment of sulfidated nanoscale zerovalent iron for in-situ remediation of cadmium-contaminated acidic groundwater at a zinc smelter. J. Hazard. Mater. 2023, 441, 129915. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, M.; Li, M.; Liu, X. Cr(VI) removal from groundwater using double surfactant-modified nanoscale zero-valent iron (nZVI): Effects of materials in different status. Sci. Total Environ. 2020, 717, 137112. [Google Scholar] [CrossRef]
- Li, Z.; Xu, S.; Xiao, G.; Qian, L.; Song, Y. Removal of hexavalent chromium from groundwater using sodium alginate dispersed nano zero-valent iron. J. Environ. Manag. 2019, 244, 33–39. [Google Scholar] [CrossRef]
- Amiri, M.J.; Abedi-koupai, J.; Eslamian, S. Adsorption of Hg(II) and Pb(II) ions by nanoscale zero valent iron supported on ostrich bone ash in a fixed-bed column system. Water Sci. Technol. 2017, 76, 671–682. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, F.; Liu, X.; Li, M. Removal of Cr(VI) by glutaraldehyde-crosslinked chitosan encapsulating microscale zero-valent iron: Synthesis, mechanism, and longevity. J. Environ. Sci. 2024, 142, 115–128. [Google Scholar] [CrossRef]
- Qin, H.; Xu, L.; Qin, L.; Kang, B.; Zha, F.; Wang, Q.; Huang, K. Removal of Cu(II) by sodium hexametaphosphate and nano zero-valent iron modified calcium bentonite: Characteristic, adsorption performance and mechanism. J. Environ. Manag. 2024, 358, 120866. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, Z.; Yan, Y.; Feng, M.; Wang, Y.; Wang, J. Preparation of chitosan-loaded zero-valent iron-coated quartz sand and study of its ability to remove Cr(VI) in groundwater. Desalin. Water Treat. 2023, 284, 101–115. [Google Scholar] [CrossRef]
- Kanel, S.R.; Choi, H. Removal of Arsenic from Groundwater by Industrial Byproducts and Its Comparison with Zero-Valent Iron. J. Hazard. Toxic Radioact. Waste 2017, 21, 04016028. [Google Scholar] [CrossRef]
- Anang, E.; Liu, H.; Fan, X. Compositional transformation of Ni2+ and Fe0 during the removal of Ni2+ by nanoscale zero-valent iron and the implications to groundwater remediation. Water Sci. Technol. 2023, 88, 2409–2422. [Google Scholar] [CrossRef]
- Fu, R.; Yang, Y.; Xu, Z.; Zhang, X.; Guo, X.; Bi, D. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 2015, 138, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Azzam, A.M.; El-Wakeel, S.T.; Mostafa, B.B.; El-Shahat, M. Removal of Pb, Cd, Cu and Ni from aqueous solution using nano scale zero valent iron particles. J. Environ. Chem. Eng. 2016, 4, 2196–2206. [Google Scholar] [CrossRef]
- Pullin, H.; Crane, R.; Morgan, D.; Scott, T. The effect of common groundwater anions on the aqueous corrosion of zero-valent iron nanoparticles and associated removal of aqueous copper and zinc. J. Environ. Chem. Eng. 2017, 5, 1166–1173. [Google Scholar] [CrossRef]
- Yin, L.; Liu, L.; Lin, S.; Owens, G.; Chen, Z. Synthesis and characterization of Nanoscale Zero-Valent Iron (nZVI) as an adsorbent for the simultaneous removal of As(III) and As(V) from groundwater. J. Water Process Eng. 2022, 47, 102677. [Google Scholar] [CrossRef]
- Tesnim, D.; Hédi, B.A.; Ridha, D.; Cid-Samamed, A. Green low-cost synthesis of zero-valent iron nanoparticles from Palm Petiole Extract for Cr(VI) removal from water. Environ. Sci. Pollut. Res. 2024, 31, 44272–44288. [Google Scholar] [CrossRef]
- Qasim, G.H.; Lee, S.; Lee, W.; Han, S. Reduction and removal of aqueous Hg(II) using indium-modified zero-valent iron particles. Appl. Catal. B Environ. 2020, 277, 119198. [Google Scholar] [CrossRef]
- He, L.; Huang, D.; He, Z.; Yang, X.; Yue, G.; Zhu, J.; Astruc, D.; Zhao, P. Nanoscale zero-valent iron intercalated 2D titanium carbides for removal of Cr(VI) in aqueous solution and the mechanistic aspect. J. Hazard. Mater. 2020, 388, 121761. [Google Scholar] [CrossRef]
- Jin, G.; Zhang, Z.; Li, R.; Chen, C.; Tang, H.; Li, L.; Barry, D. Transport of zinc ions in the hyporheic zone: Experiments and simulations. Adv. Water Resour. 2020, 146, 103775. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, G.; Zhang, P.; Galindo-Torres, S.; Scheuermann, A.; Li, L. An efficient framework for particle-fluid interaction using Discrete Element Lattice Boltzmann Method: Coupling scheme and periodic boundary condition. Comput. Fluids 2020, 208, 104613. [Google Scholar] [CrossRef]

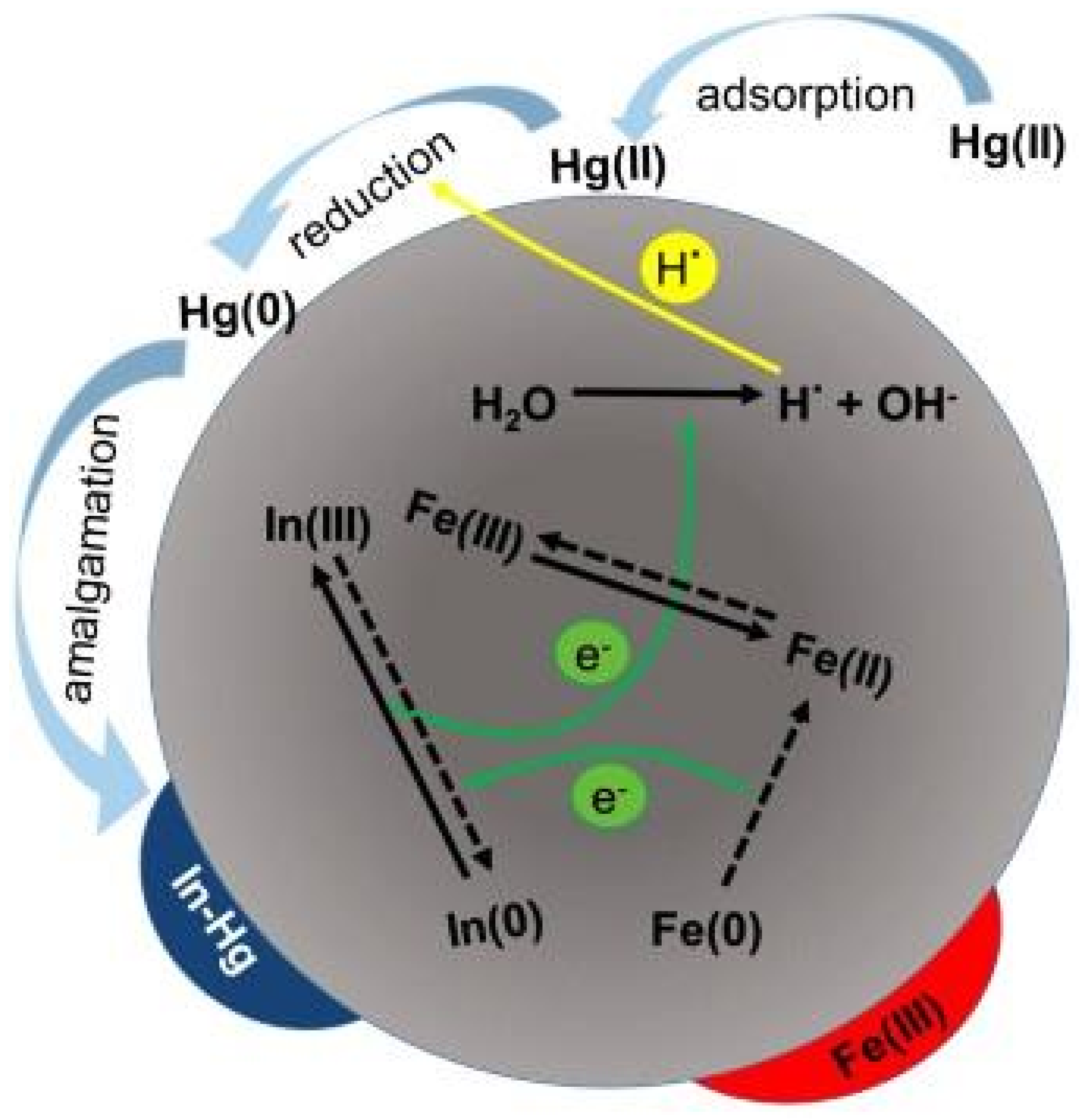
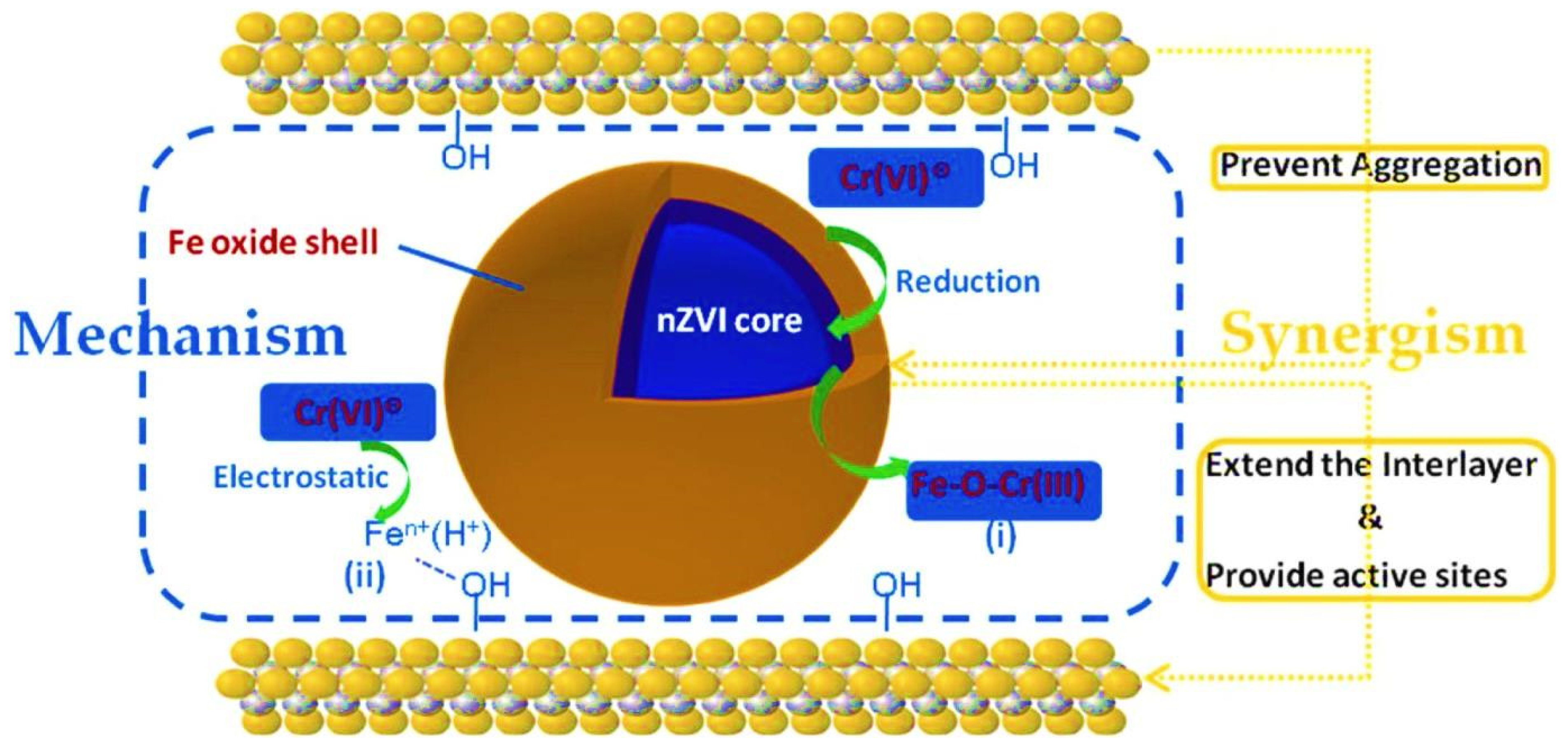
| Study | Metal Ion Removed | Study Type | ZVI Type | ZVI Use | Removal Efficiency | Mechanism | Challenges | References |
|---|---|---|---|---|---|---|---|---|
| Field column experiment (Hg removal) | Mercury (Hg) | Column test | mZVI | Alone | Reduced from ~40 μg/L to <0.1 μg/L (2-year system) | Formation of HgS on ZVI surface (cinnabar/metacinnabar) | Removal concentrated in first 10 cm, potential clogging | [68] |
| Full-scale PRB (Cr removal, Elizabeth City) | Chromium (Cr(VI)) | Full scale | mZVI | Alone | >99% (from >1500 μg/L to <1 μg/L) | Cr(VI) reduction to Cr(III) and sequestration in iron-bearing minerals | PRB performance linked to geochemical gradients | [69] |
| Zinc removal study | Zinc (Zn(II)) | Batch + modeling | mZVI | Alone | 91% under optimized conditions | Sorption onto ZVI, Langmuir adsorption behavior | Iron hydroxide formation, pore clogging over time | [70] |
| ZVI–Pumice PRB study | Arsenic (As), Mn, Fe, Zn | Column test | mZVI | Mixture with pumice | Efficient removal for 90 days | Enhanced adsorption, improved fluid dynamics | Clogging in ZVI system, breakthrough in pumice-only system | [71] |
| PRB for uranium remediation (Zhovty Vody) | Uranium (U) | Pilot scale | mZVI | Mixture with sand, organics (bone meal, sawdust) | 50–80% removal over two years | ZVI–sand and organic-carbon-based mixtures facilitating U immobilization | Limited microbial activity in organic-carbon-only systems | [72] |
| Sulfidated nZVI PRB (South Korea) | Cd, Ni, Al, Zn | Column test | S-nZVI | Alone | 99.8% Cd removal, Zn/Fe reduction via NaHCO3 treatment | FeS interactions forming CdS, ZnS, and NaHCO3-induced precipitation | Potential microbial toxicity, need for sequential treatment | [73] |
| Zero-Valent Iron + Key Modification [Test Type] | Metal Ion | Removal Efficiency and Other Parameters | Mechanism, Advantages, and Key Challenges | Reference |
|---|---|---|---|---|
| Double surfactant + nZVI (Polymer stabilization) [Batch] | Cr (VI) | 99.5% (231.75 mg/g) Pseudo 2nd-order kinetics Langmuir Isotherm | Adsorption + Reduction + Immobilization, high adsorption capacity (231.75 mg/g), rapid removal, and agglomeration in absence of stabilizers | [74] |
| Sodium alginate + nZVI (Sodium alginate stabilization) [Batch and Column] | Cr (VI) | 96.4% | Reduction, improved stability, high dispersibility, and limited pH range for peak efficiency | [75] |
| Ostrich bone ash + nZVI (OBA support) [Column] | Hg (II) and Pb (II) | 5 to 9 pH | Adsorption + Reduction, prevents oxidation and aggregation, performance depends on pH, bed height | [76] |
| Glutaraldehyde + chitosan + mZVI (Chitosan encapsulation) [Batch and column] | Cr (VI) | 243.63 mg/g Langmuir 2 pH | Reduction + Electrostatic attraction, high reusability, mesoporous structure, cost of chitosan-based support | [77] |
| Calcium bentonite + SHMP + nZVI | Cu (II) | 97.41% | Adsorption + Ion exchange + Reduction, stable against acidic/oxidative conditions, SHMP addition may affect large-scale application | [78] |
| Quartz sand + chitosan + nZVI [Batch] | Cr (IV) | 91.6% Pseudo 2nd-order kinetics Langmuir Isotherm | Adsorption + Reduction, PRB application potential, retained efficiency over cycles, SO42− inhibition | [79] |
| nZVI [Batch] | Ni(II) | - | Fe-Ni alloy formation reduces toxicity, requires oxygen, redox optimization | [81] |
| nZVI (Anion Interaction Study) [Batch] | Cu, Zn | - | NO3− enhances stability, Cl− SO42− induce corrosion, and long-term retention affected by anions | [84] |
| Sepiolite + nZVI [Batch] | Cr (IV) and Pb (II) | 6 pH Pseudo 2nd-order kinetics Langmuir Isotherm and Freundlich Isotherm | Adsorption + Reduction, prevents agglomeration, improved stability, cost and availability of sepiolite | [82] |
| nZVI [Batch] | Pb(II), Cd(II), Cu(II), and Ni(II) | Pseudo 1st-order Langmuir Isotherm | Adsorption + Co-precipitation, multi-metal adsorption potential, efficiency drops at high pH | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, Y.; Verma, A.; Bhaskaralingam, A.; Dhiman, P.; Wang, T.; Kumar, A.; Sharma, G. Application of Zero-Valent Iron and Its Derivatives in the Removal of Toxic Metal Ions from Groundwater. Water 2025, 17, 1524. https://doi.org/10.3390/w17101524
Verma Y, Verma A, Bhaskaralingam A, Dhiman P, Wang T, Kumar A, Sharma G. Application of Zero-Valent Iron and Its Derivatives in the Removal of Toxic Metal Ions from Groundwater. Water. 2025; 17(10):1524. https://doi.org/10.3390/w17101524
Chicago/Turabian StyleVerma, Yaksha, Akshay Verma, Aishwarya Bhaskaralingam, Pooja Dhiman, Tongtong Wang, Amit Kumar, and Gaurav Sharma. 2025. "Application of Zero-Valent Iron and Its Derivatives in the Removal of Toxic Metal Ions from Groundwater" Water 17, no. 10: 1524. https://doi.org/10.3390/w17101524
APA StyleVerma, Y., Verma, A., Bhaskaralingam, A., Dhiman, P., Wang, T., Kumar, A., & Sharma, G. (2025). Application of Zero-Valent Iron and Its Derivatives in the Removal of Toxic Metal Ions from Groundwater. Water, 17(10), 1524. https://doi.org/10.3390/w17101524













