Abstract
Zero-valent iron (ZVI), particularly in its nanoscale form (nZVI), is now considered a highly promising material for the remediation of toxic metal ions from polluted groundwater owing to its strong reductive potential, significant surface area, and reactive behavior. This review systematically explores the application of pristine and modified ZVI systems—including doped ZVI, bio-stabilized composites, and ZVI supported on advanced materials like MXene and nanocellulose—for effective treatment of water containing metal species like As(III/V), Hg(II), Cr(VI), and Ni(II). Emphasis is placed on understanding the underlying mechanisms, including redox reactions, surface complexation, and synergistic adsorption–reduction pathways. Key factors affecting adsorption efficiency—such as pH, temperature, ZVI dosage, and competing ions—are thoroughly analyzed, alongside adsorption kinetics and isotherm models. Modified ZVI composites exhibit enhanced stability, selectivity, and reusability, demonstrating promising performance even in complex aqueous environments. Despite significant progress, challenges such as nanoparticle passivation, limited field-scale data, and potential toxicity of byproducts remain. The review concludes by highlighting future research directions focused on improving material longevity, regeneration efficiency, selective adsorption, and integration with other advanced remediation technologies for sustainable and scalable groundwater treatment.
1. Introduction
In some parts of the world, ecological damage is becoming worse. One of the essential elements needed to keep life going is water. Driven by rapid urbanization and industrial growth, global water consumption doubles approximately every 15 years. As a result, more than 2 billion people, or over 40% of the global population, still lack access to safe and clean water, according to the World Health Organization (WHO). Millions of people’s health around the world is being impacted by the rising concentrations of several impurities in groundwater and wastewater brought on by industrial and urban activity. Therefore, the cleanup of groundwater is crucial [1]. Rapid industrialization has led to an elevated need worldwide for metal utilization in a variety of products, including steel, automotive fuels, storage batteries, colorants, photographic films, various explosives, coating substances, and the aerospace and automotive industries [2,3,4,5].
Among emerging remediation technologies, zero-valent iron (ZVI) has gained significant attention due to its abundance, non-toxicity, affordability, and ease of application. Zero-valent iron (ZVI) has gained significant attention over the past ten years owing to its non-toxicity, abundance, affordability, ease of production, and low maintenance requirements for its reduction process. With a typical redox potential of E0 = −0.44 V, ZVI is a reactive metal. Therefore, it works well as a reducing agent when reacting with oxidized pollutants like Cr (VI). ZVI removes contaminants by transferring electrons directly to the pollutants, converting them into less harmful or non-toxic forms through a reduction process. However, in the presence of dissolved oxygen (DO), ZVI can donate electrons to generate H2O2, which is a key intermediate in Fenton-like advanced oxidation processes (AOPs). These reactions are primarily relevant in aerobic systems where oxygen is either naturally present or deliberately introduced—for instance, through oxygen insufflation or aeration setups. Such oxidative pathways are distinct from traditional anaerobic PRB (permeable reactive barrier) systems, where the absence of oxygen inhibits H2O2 formation and reductive mechanisms dominate.
ZVI has been extensively applied to the remediation of pollutants such as toxic metals [6,7], nitrate [8,9], dyes [10,11], phenol [12,13], arsenic [14,15], nitro aromatic compounds (NACs) [16,17], and chlorinated organic compounds (COCs) [18,19,20,21]. The versatility of ZVI stems from its ability to act through a combination of reduction, adsorption, and precipitation mechanisms, which are highly influenced by environmental conditions.
ZVI can be integrated into various remediation technologies such as in situ permeable reactive barriers (PRBs), slurry injections, and batch reactors, where performance varies with geochemical and hydraulic conditions. However, practical issues like the agglomeration of nZVI and clogging in PRB systems using mZVI need careful material design and delivery optimization. Depending on the application method, the performance of ZVI varies. For example, micro-scale ZVI (mZVI) is frequently used in PRBs due to its durability and longevity but may cause pore clogging over time. On the other hand, nanoscale ZVI (nZVI) offers high reactivity and better dispersion for in situ injection but suffers from challenges like agglomeration and rapid oxidation. These drawbacks necessitate tailored modification strategies and application-specific optimization to ensure effective groundwater remediation. Particularly, nZVI shows promise for in situ treatment of difficult-to-access aquifers, such as fractured bedrock, deep confined zones, and urban subsurfaces, where traditional pump-and-treat methods or excavation is not feasible due to cost or physical limitations [22].
The growing number of journal publications that have been published recently indicates that there is a growing interest in using ZVI to remove toxins from groundwater. Over the past decade, zero-valent iron (ZVI) has been widely applied to remediate various major contaminants in groundwater, including trichloroethylene (TCE), nitrate, arsenic, hexavalent chromium (Cr(VI)), phenol, and nitrobenzene (NB).
Although several reviews have addressed the use of ZVI for environmental applications, most have focused on either its general reductive capabilities or specific pollutant types. This review provides a comprehensive and up-to-date synthesis (2019–2025) of advancements in both pristine and modified ZVI systems, with a focus on toxic metal removal from groundwater. We begin by summarizing the fundamental properties of ZVI and its derivatives and then discuss various modification strategies—including surface functionalization, sulfidation, metal doping, and carbon-based supports (e.g., biochar)—to enhance reactivity, stability, and selectivity.
We critically evaluate adsorption–reduction mechanisms, operational parameters (e.g., pH, temperature, DO, ionic strength), and material performance under both laboratory and field conditions. Special emphasis is placed on regeneration strategies, long-term stability, and scalability to inform practical deployment. By comparing lab-scale findings with field-scale outcomes, this review aims to bridge the gap between bench research and real-world implementation, offering a novel and integrated perspective on ZVI-based groundwater remediation.
Among various materials investigated for in situ remediation of contaminated groundwater, zero-valent iron (ZVI) has received significant attention due to its strong redox potential and low cost. The following section explores the core properties and mechanisms underpinning ZVI-based remediation.
2. Zero-Valent Iron and Its Derivatives
2.1. Zero-Valent Iron
Zero-valent metals were first recognized for their environmental applications in 1972. Over time, research demonstrated their effectiveness in degrading pollutants, particularly trichloroethylene (TCE), with zero-valent iron (ZVI) emerging as the most widely and commonly used metal [23,24]. It has attracted considerable interest in the field of environmental removal owing to its strong reducing ability, low cost, easy availability, and broad applicability [25,26]. ZVI, known for its high reactivity and standard reduction potential of −0.44 V, is extensively employed in the reductive elimination of stubborn contaminant pollutants such as halogenated organic compounds (HOCs), nitroaromatic hydrocarbons, dyes, phenolic compounds, and heavy metals [24,27,28]. These hydroxyl radicals are particularly crucial as they can rapidly degrade a wide range of organic contaminants, enhancing ZVI’s remediation efficiency [24]. However, several factors influence the reactivity of zero-valent iron (ZVI) with toxic metal ions, including surface area, aging, and pH. The general literature suggests that ZVI’s reactivity is most effective in slightly acidic to neutral pH (5–7) and under low to moderate oxygen conditions (1–10 mg/L). These factors are critical in optimizing ZVI’s pollutant degradation capacity. A larger surface area provides more reactive sites, enhancing the metal removal efficiency. However, as ZVI ages, its ability to reduce toxic metal ions decreases because of the loss of reactive sites. The solution pH also plays a crucial role—a low pH accelerates iron corrosion, while a high pH leads to the formation of iron oxy (hydroxides), which reduces ZVI’s reactivity. Zero-valent iron functions as a sorbent, a reducing agent, and a coagulant, making it effective for treating surface and groundwater contaminants [29,30,31]. The effectiveness of ZVI in pollutant degradation is largely influenced by its interaction with dissolved oxygen in water. Initially, ZVI reacts with oxygen to form hydrogen peroxide (H2O2) (Equation (1)). The resulting hydrogen peroxide can then be further broken down into water by ZVI (Equation (2)) or take part in the Fenton reaction with Fe2+, leading to the formation of highly reactive hydroxyl radicals (·OH) (Equation (3)).
As a sorbent, the solid corrosion products of ZVI, such as iron oxides and hydroxides, provide active surfaces for the adsorption of contaminants, especially heavy metals. As a reducing agent, both Fe0 (metallic iron) and Fe2+ (ferrous iron) participate in redox reactions to chemically transform and detoxify pollutants, particularly those that are redox-sensitive, such as Cr(VI), nitro-aromatics, and halogenated organics. ZVI also acts as a coagulant through the formation of iron hydroxides in situ, which destabilize and aggregate colloidal particles and dissolved pollutants, aiding in their removal via sedimentation. Additionally, ZVI enables removal through precipitation and co-precipitation mechanisms. In precipitation, contaminants such as metals form insoluble compounds and settle out of solution. In co-precipitation, contaminants are physically or chemically incorporated into the iron oxides formed during ZVI corrosion, leading to their immobilization and removal from water. These combined mechanisms make ZVI a powerful and multifunctional material for environmental remediation.
ZVI is generally available in two main forms: micron-sized and nano-scale zero-valent iron (nZVI). Micro zero-valent iron (mZVI) is a fine black powder made from iron in its zero-oxidation state. It has a large surface area and strong chemical reactivity, which make it useful in various environmental and engineering applications like soil and groundwater remediation, battery technology, sensors, and especially biomedical applications, especially for removing contaminants [32,33,34]. On the other hand, nZVI comprises black powder produced from micro-ZVI using chemical and physical methods. With particle sizes ranging from 1 to 100 nm, nZVI offers a higher surface area, increased reactivity, and numerous active sites [34,35,36]. These properties exhibit superior performance due to their larger surface area, increased number of active sites, and enhanced reactivity, making them more effective for environmental cleanup efforts. However, their effectiveness may vary depending on site-specific conditions, such as aquifer characteristics, and the remediation approach employed, including permeable reactive barriers (PRBs) or injection-based technologies [37,38]. A key advancement in ZVI technology (refers to various remediation approaches that utilize zero-valent iron, including permeable reactive barriers (PRBs), slurry or nanoparticle injections, and in situ treatment systems designed for the removal of heavy metals from groundwater and soil) is the development of core-shell structured ZVI, which has gained significant attention due to its superior reactivity. This structure consists of a ZVI (Fe0) core surrounded by a protective shell. The shell can be composed of oxide layers (such as Fe–Fe2O3 nanowires) or coatings of carbon-based materials and other metal oxides/hydroxides (e.g., Mg (OH)2). The protective shell in the core–shell-structured ZVI@C nanoparticles is formed during the thermal carbonization process, where carbon precursors (e.g., glucose, sucrose, starch) are pyrolyzed under controlled heating conditions. Key factors such as temperature, dwell time, and carbon-to-iron ratio influence the shell formation, porosity, and stability in acidic environments [39,40,41]. The Fe core functions as an electron donor during pollutant degradation, while the oxide shell provides adsorption sites. Additionally, if the shell exhibits good electrical conductivity, it facilitates electron transfer between ZVI and contaminants, enhancing reaction efficiency. The creation of a core–shell structure effectively prevents the aggregation of nZVI particles by providing steric hindrance, which inhibits particle agglomeration. This structural design helps maintain the high reactivity of the particles and improves overall degradation performance by ensuring better dispersion and accessibility of the active sites [42,43,44].
nZVI’s core–shell structure plays a crucial role in contaminant removal, as it provides highly reactive surfaces for adsorption and transformation via reduction or oxidation processes, depending on the type of contaminant [45]. In the case of heavy metal removal, nZVI’s effectiveness is largely attributed to two mechanisms. First, its core–shell structure enhances interactions by offering more reactive sites, enabling the shell to adsorb metal ions and be chemically modified to improve affinity. Second, the iron oxide shell establishes a distinct interface with heavy metals in the environment, accelerating reaction rates and enhancing removal efficiency. These properties make nZVI a highly promising material for advanced water treatment and pollution remediation [46,47]. However, despite its advantages, nZVI faces challenges in practical applications such as groundwater remediation, industrial wastewater treatment, and in situ chemical reduction of toxic metal ions. However, under certain conditions, particularly in oxygen-rich aqueous environments, excessive oxidation of the Fe0 core can lead to the formation of a dense passivation layer composed of iron oxides (e.g., magnetite, goethite). While a thin oxide shell can aid in adsorption, a thick passivation layer may hinder electron transfer, thereby limiting the particle’s reducing capacity for redox-sensitive pollutants. Additionally, nZVI particles tend to aggregate due to their magnetic properties, leading to decreased reactivity, stability, and adsorption efficiency [48,49,50]. These limitations hinder its long-term effectiveness, necessitating modifications to improve its performance in environmental applications. Addressing these challenges through structural improvements and surface modifications can optimize nZVI’s potential for sustainable and efficient pollutant removal.
While conventional ZVI offers substantial promise, its performance in natural environments is often limited by passivation and aggregation. Therefore, a wide range of modifications have been developed to enhance its applicability, as discussed in the subsequent section.
2.2. Modifications of ZVI
To address the shortcomings of zero-valent iron (ZVI), numerous refinement strategies have been introduced to improve its stability, increase its reactivity, and boost overall performance. These include surface coatings, doping with metals, and composite formation with carbon-based materials, which help reduce oxidation, prevent aggregation, and improve pollutant removal performance. Building upon polymer-stabilized systems, another promising approach involves mineral-supported ZVI composites, which provide structural stability and improve dispersion in subsurface environments. Figure 1 shows various modifications of ZVI.
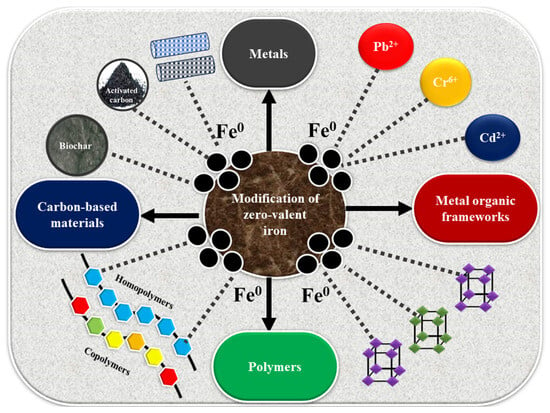
Figure 1.
Various modifications of zero-valent iron using different materials.
2.2.1. ZVI Doping with Metals
Doping zero-valent iron (ZVI)—particularly in its nanoscale (nZVI) and microscale (mZVI) forms—with transition or noble metals enhances its reactivity, stability, and resistance to oxidation. Metals such as Pd, Cu, silver Ag, and Ni act as catalysts, improving electron transfer and accelerating contaminant removal. These doped ZVI nanoparticles exhibit better performance in reducing pollutants, minimizing agglomeration, and extending their longevity in environmental applications [38]. Additionally, metal doping can modify surface properties, increasing selectivity and efficiency in wastewater treatment and remediation processes [51,52]. The physicochemical properties of metal-modified zero-valent iron, including its shape, size, composition, and surface chemistry, vary based on several factors. These include the choice of dopant metal, the doping technique used, the ratio of metal doping, and the sequence in which doping occurs [53]. Studies indicate that the addition of micron-sized Pd(0), Cu(0), or Ni(0) powders can reestablish Fe(0) particles whose reactivity has declined due to surface deactivation. This secondary metal layer prevents oxidation and enhances ZVI’s overall reactivity, ensuring sustained pollutant removal efficiency [38,54,55]. For example, Pan et al. explored the removal of U(VI) using a nitrogen-doped porous carbon nanoscale zero-valent iron (nZVI) composite. Their findings indicated that the synthesized Fe0@N-PCM-H2O2 demonstrated a remarkable removal capacity of 847.5 mg/g for uranyl ions in contaminated water. Moreover, the composite exhibited minimal iron leaching, demonstrating its stability and efficiency in uranium removal. Additionally, recyclability tests showed that Fe0@N-PCM-H2O2 retained 50.5% of its initial adsorption capacity after four cycles, indicating a reasonable level of reusability. The observed decline in adsorption efficiency was primarily attributed to the oxidation of Fe0 and the partial retention of U(VI) on the surface of the material [56].
Yang et al. [57] conducted a study to evaluate the adsorption and reduction of Pb(II) and Zn(II) using a carbon-supported nano zero-valent iron (carbon@nZVI) composite. Their results revealed that the material achieved an exceptional sorption ability of 98.37 mg/g for Pb(II) and 26.38 mg/g for Zn(II). The adsorption of both metal ions closely followed the Langmuir isotherm model, as indicated by high correlation coefficients (R2 = 0.991 for Pb(II) and R2 = 0.994 for Zn(II)), implying monolayer coverage on the adsorbent surface. Furthermore, the adsorption kinetics were best described by the non-linear pseudo-second-order model, with R2 values of 0.995 for Pb(II) and 0.959 for Zn(II), highlighting the predominance of chemical adsorption in the removal process. The proposed adsorption mechanisms, illustrated in Figure 2, include complexation, electrostatic attraction, and redox reactions, all of which contribute to the efficient removal of Pb(II) and Zn(II) from mining wastewater [57].
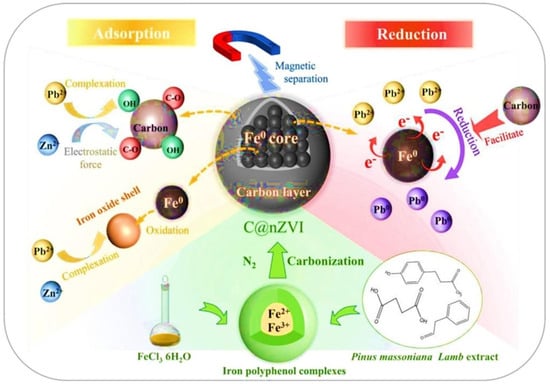
Figure 2.
Proposed mechanism of Pb(II) and Zn(II) by C@nZVI [57], reproduced with permission from Elsevier.
In addition to biopolymer and mineral modifications, hybrid systems integrating biochar and inorganic supports have shown synergistic effects, offering multifunctional pathways for metal removal.
2.2.2. ZVI Modification with Different Carbon-Based Materials
Carbon-based materials, made primarily of carbon in solid or powdered form, have gained significant attention because of their large surface area, excellent heat resistance, strong mechanical properties, and porous structure. These materials include both traditional types, like activated carbon and biochar, as well as newer materials such as graphene and its related forms, graphene oxide, reduced graphene oxide, and functionalized graphene nanosheets. They also include advanced nanostructures like single-walled and multi-walled carbon nanotubes (CNTs), carbon nano-fibers (CNFs), fullerenes, and carbon dots. When used to support nano zero-valent iron (nZVI), these materials enhance its stability and provide additional active sites. However, incorporating zero-valent iron (ZVI) with carbon-based materials has proven to be an effective approach to enhancing its efficiency in environmental remediation. Carbon materials such as biochar [58], carbon nanotubes [59], and activated carbon [60] enhance ZVI’s stability, conductivity, and surface activity, reducing agglomeration and increasing pollutant removal efficiency [61]. These modifications create more reactive sites, improve dispersion, and facilitate electron transfer, making ZVI more effective in degrading stubborn contaminants like polychlorinated biphenyls (PCBs), pentachlorophenol, and hexavalent chromium [62,63,64]. Additionally, the enhanced hydrophobic nature of carbon-modified ZVI improves its interaction with contaminants, further boosting its performance in water treatment [28].
For instance, Li et al. explored the removal of Pb(II) ions using a porous biochar/nZVI composite. Their findings demonstrated a high adsorption capacity, reaching a maximum uptake of 480.9 mg/g. The study also examined the mechanisms involved in Pb(II) removal (depicted in Figure 3), revealing multiple processes, including reduction, complexation, and co-precipitation. These interactions were confirmed through analyses using FTIR, XRD, and XPS [65].
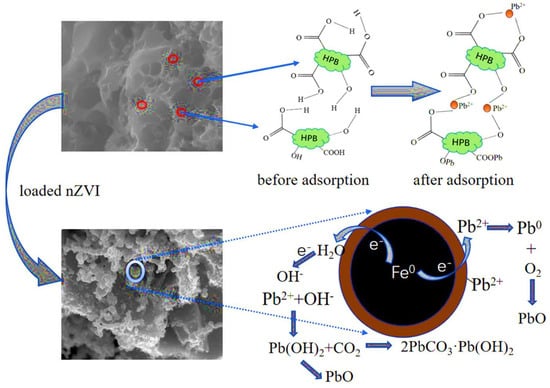
Figure 3.
Schematic representation of possible Pb(II) adsorption mechanisms on nZVI-HPB [65], reproduced with permission from Elsevier.
Wei et al. evaluated the effectiveness of nano zero-valent iron supported on biochar (nZVI/BC) for nitrate removal. Their findings demonstrated that the composite exhibited an excellent removal efficiency, ranging from 75.0% to 97.0%, across a wide pH spectrum (2–12). When applied to actual groundwater samples, nZVI/BC outperformed both standalone Fe0 nanoparticles and biochar. The product analysis revealed that 60.1% of the eliminated nitrate was transformed into nitrogen gas. XPS results showed a notable increase in surface magnetite due to the oxidation of Fe0 by nitrate. As the reaction progressed, the redox potential stabilized near −210 mV, and the pH maintained a range of 8–9, creating favorable conditions for the selective conversion of nitrate to nitrogen gas [66].
Rajput et al. investigated the effectiveness of a zero-valent iron-decorated porous biochar derived from tea waste (ZVI@TBC) for the removal of cadmium and hexavalent chromium from aqueous solutions. The composite demonstrated notable adsorption capacities, reaching 186.2 mg/g for Cr(VI) and 19.1 mg/g for Cd(II). An analysis of adsorption kinetics and isotherms suggested that chemisorption governed the uptake of both metal ions. The widespread use of the pseudo-second-order model in such systems reflects its suitability for chemisorption-dominated processes; however, it is important to note that in cases where physical adsorption or pore diffusion mechanisms prevail, models such as the pseudo-first-order or intraparticle diffusion model may offer a better fit (for studies following a different kinetic model, refer to Table given below). While pseudo-second-order (PSO) kinetics are commonly observed in ZVI-based systems due to chemisorption, deviations arise under specific experimental conditions. For example, at low pH values, surface protonation enhances electrostatic attraction with anionic species, sometimes resulting in pseudo-first-order (PFO) behavior. In environments with a high ionic strength, the compression of the electrical double layer may hinder chemisorption and promote pore diffusion-controlled processes. Similarly, at elevated initial contaminant concentrations or in systems utilizing porous ZVI composites, intra-particle diffusion or multilayer adsorption can become rate-limiting. These variations underscore the need to consider experimental conditions when interpreting kinetic models in ZVI-based remediation studies. Thus, selecting an appropriate kinetic model should be guided by both statistical fitting and mechanistic insights derived from material characterization and speciation studies. Also, thermodynamic parameters revealed that the sorption process occurred spontaneously and was endothermic in nature. The investigation of sorption mechanisms (illustrated in Figure 4) revealed that reduction, co-precipitation, and electrostatic interactions played key roles in the elimination of toxic metal ions. This study highlights the potential of tea biomass and ZVI-based composites in developing efficient and environmentally friendly adsorbents for heavy metal remediation [67].
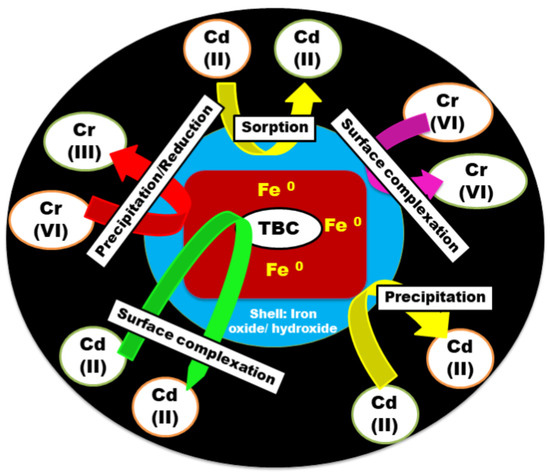
Figure 4.
Schematic illustration of adsorption mechanisms between ZVI@TBC and Cd(II)/Cr(VI) species.
Comparative evaluation: The modification strategies for zero-valent iron (ZVI)—namely, surface coatings, metal doping, and carbon-based composite formation—each offer unique benefits and face specific limitations. Surface coatings such as polymers or oxides provide a protective layer that enhances ZVI’s stability and reduces oxidation; however, they may sometimes hinder reactivity by limiting contact between iron and pollutants. Metal doping, particularly with noble or transition metals like Pd, Cu, or Ni, significantly improves electron transfer and reactivity. While highly effective, this approach can be costly and may involve complex synthesis steps, limiting its large-scale application. Composites with carbon-based materials (e.g., biochar, CNTs, activated carbon) strike a balance between performance and cost. These materials improve dispersion, increase active surface area, and facilitate electron mobility, often using low-cost and renewable resources. However, achieving uniform distribution and stable attachment of ZVI onto carbon supports can be technically challenging. Overall, while each strategy enhances the efficiency of ZVI for environmental remediation, carbon-based composites are generally considered more scalable and economically viable, whereas metal doping offers superior performance for targeted, high-value applications.
3. Application of Zero-Valent Iron and Its Derivatives in the Removal of Toxic Metal Ions from Groundwater and Its Mechanism
Groundwater contamination due to toxic metal ions is a pressing environmental issue, primarily driven by industrial discharges, mining activities, and improper waste disposal. While zero-valent iron (ZVI) and its derivatives have shown great promise for in situ groundwater remediation, they are not without challenges. Notably, nanoscale ZVI (nZVI) often suffers from agglomeration due to magnetic and van der Waals interactions, which reduces its reactive surface area and limits contaminant removal efficiency. Additionally, the clogging of permeable reactive barriers (PRBs) can occur over time due to the accumulation of corrosion products and mineral precipitates, obstructing groundwater flow and diminishing long-term performance.
Among the conventional remediation technologies such as ion exchange, membrane filtration, and chemical precipitation, zero-valent iron (ZVI) and its derivatives have gained particular attention due to their dual functionality in both reduction and adsorption processes. Unlike other methods, ZVI offers a cost-effective, environmentally benign, and efficient approach to the removal of toxic metal ions from groundwater. These materials effectively remove heavy metals through adsorption, reduction, and oxidation mechanisms, making them promising candidates for in situ groundwater treatment. This section explores the application of ZVI-based materials (Figure 5), including their use in permeable reactive barriers (PRBs) and various modifications aimed at enhancing their reactivity, stability, and long-term efficiency.
Figure 5.
Conceptual framework of ZVI-driven strategies for toxic metal elimination.
3.1. Zero-Valent Iron Used as a Permeable Reactive Barrier for Metal Ion Removal
Permeable reactive barriers (PRBs) employing micro-scale zero-valent iron (mZVI) have been widely used in the classical configuration for in situ groundwater remediation, where a trench is filled with reactive material and installed perpendicular to groundwater flow. This passive setup allows contaminated water to pass through and undergo treatment via reduction and adsorption processes. In contrast, nanoscale ZVI (nZVI) is typically applied via direct injection or as part of composite slurries, providing enhanced reactivity and mobility for site-specific applications. More than 100 nZVI-based in situ remediation projects have been implemented across North America, Taiwan, Europe, and China [35]. However, field-scale applications often deviate from lab-scale expectations due to variable groundwater flow, pH, temperature, oxygen content, and competing ions, which impact performance and longevity [35].
While field applications of PRBs have demonstrated promising results, the long-term performance of these systems remains a critical consideration. Factors such as passivation of ZVI, clogging due to the accumulation of reaction byproducts, and the effects of fluctuating geochemical conditions (e.g., pH, temperature, and competing ions) can significantly impact the lifespan and effectiveness of PRBs. For example, studies have indicated that the lifespan of PRBs can vary from a few years to over a decade, depending on site-specific conditions, with failure rates often linked to the buildup of passive iron oxides and decreased reactivity over time. Quantitative data on PRB lifespan, particularly in diverse groundwater environments, are limited, but ongoing research suggests that optimized operational conditions and regular monitoring may extend the effectiveness of these systems [68,69,70,71,72,73].
Passive in situ remediation, such as permeable reactive barriers (PRBs), offers a cost-effective alternative to conventional groundwater treatment. Field column experiments have demonstrated the effectiveness of zero-valent iron (ZVI) for in situ groundwater remediation. While chlorinated solvents are the most commonly studied contaminants in PRB configurations, specific studies have also reported successful mercury removal using ZVI, highlighting its versatility under certain conditions [68]. Over a 6-week study, simulating 2- and 10-year treatment periods, mercury concentrations were reduced from ~40 μg/L to <0.1 μg/L in the 2-year system and 0.5–4 μg/L in the 10-year system. Removal was concentrated in the first 10 cm of the reactive column, with SEM/EDS and XAS confirming mercury accumulation as HgS (cinnabar/metacinnabar) on the ZVI surface. This highlights ZVI’s potential for long-term metal immobilization in groundwater treatment.
A full-scale zerovalent iron (ZVI) permeable reactive barrier (PRB) installed in 1996 successfully reduced Cr(VI) concentrations in groundwater from >1500 μg/L to <1 μg/L after eight years of operation (Elizabeth City, NC) [69]. Chromium removal primarily occurred at the PRB’s leading edge and upgradient aquifer due to geochemical gradients from ZVI corrosion. XANES spectroscopy confirmed Cr reduction to Cr(III), with sequestration facilitated by secondary iron sulfide minerals. These findings highlight the role of iron-bearing mineral products in enhancing Cr remediation through redox reactions and Fe(II) release.
A study investigated the application of zero-valent iron (ZVI) in permeable reactive barriers (PRBs) for zinc removal from contaminated groundwater. Batch equilibrium tests evaluated the impact of contact time, pH, ZVI dosage, initial conc. of metal, and agitation speed, achieving 91% Zn(II) removal under optimal conditions (3 h, pH 5, 10 g/100 mL ZVI, 50 mg/L Zn(II), 200 rpm) [70]. Sorption data closely aligned with the Langmuir isotherm model (R2 = 0.9887). A one-dimensional numerical model (COMSOL Multiphysics, finite difference method) simulated Zn(II) transport and PRB efficiency, confirming PRB’s effectiveness in restricting contaminant plume migration. However, iron hydroxide formation and pore clogging may reduce long-term PRB efficiency. The study highlights ZVI as a cost-effective material for PRBs, with further research needed to enhance long-term functionality. These selected case studies collectively emphasize the dual nature of ZVI-based PRBs—while they demonstrate promising contaminant removal efficiencies across a range of metals, they also highlight the real-world operational limitations that arise over time. Challenges such as reactivity loss due to passivation, formation of secondary mineral phases (e.g., iron sulfides or hydroxides), pore clogging, and geochemical changes in the subsurface can significantly affect long-term barrier performance. Understanding these factors is crucial for optimizing PRB design, predicting longevity, and improving the sustainability of ZVI applications in groundwater remediation. Future research should focus on developing ZVI composites with enhanced stability, integrating real-time monitoring systems, and refining modeling tools to better simulate field conditions and predict system behavior over extended timeframes.
A permeable reactive barrier (PRB) system incorporating zero-valent iron (ZVI) and pumice in an asymmetrical packing configuration was evaluated for its effectiveness in removing metals and metalloids from groundwater. The study assessed contaminant retention, water flow dynamics, and reactivity in a packed bed system. Synthetic groundwater containing arsenic, manganese, iron, and zinc was treated using ZVI–pumice and pumice-only columns [71]. The ZVI–pumice system maintained efficient removal for 90 days, while the pumice-only setup exhibited a breakthrough of Zn and Mn within 8 days, indicating weaker adsorption. Notably, clogging was observed in the ZVI system, attributed to the formation of iron corrosion products, precipitation of metal hydroxides, and gas evolution, all of which can reduce porosity and hinder flow. The irregular packing structure improved fluid movement and contaminant capture, suggesting that bed geometry plays a critical role in maintaining hydraulic performance and extending PRB longevity.
Zhovty Vody, Ukraine, faces severe groundwater contamination from uranium and iron mining. A permeable reactive barrier (PRB) was designed using zero-valent iron (ZVI), phosphate materials, and organic carbon for contaminant removal [72]. The PRB media consisted of various formulations, including powdered ZVI mixed with sand (inorganic mixture), and combinations of powdered ZVI, gravel, bone meal, sawdust, and microbial activators (organic–inorganic mixture), aimed at enhancing both chemical and biological remediation. Laboratory tests showed that ZVI and ZVI-based mixtures achieved the highest uranium removal efficiency, with finely dispersed ZVI the performing best. A pilot-scale PRB using cylindrical columns filled with zero-valent iron (ZVI) reactive materials was installed to reduce costs [72]. The cylindrical reactive materials consisted mainly of scrap iron and direct-reduced iron particles of varying sizes, ranging from 0.1 mm to 2 mm. These materials provided a large reactive surface area and were packed into cylindrical columns to optimize groundwater flow and uranium removal efficiency. Over two years, uranium concentrations dropped from 0.38 mg/L to 0.07–0.15 mg/L. The best performance was achieved utilizing ZVI–sand and organic-carbon-based mixtures. Organic carbon alone was less effective due to limited microbial activity. The PRB remained effective despite complex hydrogeological conditions. Uranium levels were reduced by 50%, demonstrating the PRB’s long-term viability for mining site remediation.
Sulfidated nanoscale zero-valent iron (S-nZVI) has shown strong potential as a reactive material in permeable reactive barriers (PRBs) in remediating heavy metal-contaminated groundwater. A study on acidic groundwater from a zinc smelter site in Seokpo, South Korea, revealed that S-nZVI effectively removed contaminants such as Cd, Ni, Al, and Zn, with a notable 99.8% removal of Cd due to FeS surface interactions forming stable CdS and ZnS. MINEQL+ modeling and spectroscopic analyses confirmed the metal removal mechanisms [73]. Additionally, a sequential NaHCO3 treatment further reduced Zn and Fe concentrations while mitigating microbial toxicity. This study provides empirical evidence supporting the application of S-nZVI in PRBs for in-situ treatment of groundwater with high concentrations of coexisting toxic metal ions (Table 1).

Table 1.
Comparative analysis of ZVI-based PRB studies.
ZVI-based permeable reactive barriers (PRBs) have proven highly effective for in situ groundwater remediation, demonstrating significant removal efficiencies for heavy metals like Hg, Cr, Zn, Cd, and U. Mechanisms vary from precipitation (HgS formation) to redox reactions (Cr(VI) to Cr(III)) and adsorption Zn(II), Cd(II). However, long-term performance is often hindered by iron passivation, pore clogging, and geochemical influences. Sulfidated nanoscale ZVI (S-nZVI) enhances removal efficiency by stabilizing metal sulfides, while composite PRBs (e.g., ZVI–pumice, ZVI–carbon) improve permeability and reactivity. Sequential treatments, such as NaHCO3 flushing, further optimize contaminant removal. Future investigations should prioritize environmentally friendly enhancements, biologically supported PRB systems, and comprehensive field-scale trials to validate their durability, operational efficiency, and economic viability in long-term groundwater remediation.
3.2. Zero-Valent Iron (ZVI) and Its Modifications for Groundwater Remediation
Zero-valent iron (ZVI) has been extensively investigated for its potential in groundwater remediation due to its ability to reduce and immobilize various contaminants, including heavy metals and organic pollutants. However, unmodified nZVI often faces challenges such as particle agglomeration, passivation, and reduced long-term stability. To overcome these limitations, researchers have explored various ZVI modifications, including polymer-stabilized ZVI, biochar-supported ZVI, and sulfidated ZVI, each offering enhanced reactivity and selectivity.
Nanoscale zero-valent iron (nZVI) modified with polyvinylpyrrolidone and sodium oleate exhibited enhanced Cr(VI) removal efficiency, particularly in suspension form. The process followed pseudo-second-order kinetics and the Langmuir model, achieving 99.5% removal within 2 min with enhanced capacity of 231.75 mg/g. Acidic and anaerobic conditions favored Cr (VI) reduction to Cr(III), making it a promising in situ groundwater remediation approach [74]. The modification improved nZVI’s adsorption and reduction capabilities while utilizing cost-effective materials.
Sodium alginate (SA)-modified nano zero-valent iron (SA-nZVI) was developed to enhance Cr(VI) removal from contaminated groundwater by preventing nanoparticle agglomeration and sedimentation [75]. In comparison to conventional nZVI and CMC-nZVI, SA-nZVI demonstrated enhanced stability, improved dispersion, and greater mobility, as confirmed by zeta potential measurements and column experiments. Under experimental conditions of pH 6.0, room temperature (25 °C), and ambient dissolved oxygen levels, SA-nZVI achieved a Cr(VI) removal efficiency of 96.4% within 20 min. Additionally, NO3− ions showed minimal interference with Cr(VI) removal. XPS analysis revealed that Cr(VI) was reduced to Cr(III), followed by the formation of Cr(OH)3 precipitates. These findings underscore the potential of SA-nZVI as a highly effective and stable nanomaterial for in-situ remediation of Cr(VI)-contaminated groundwater across a range of environmental conditions.
A newly developed composite adsorbent, consisting of ostrich bone ash integrated with nanoscale zero-valent iron (OBA/nZVI), was utilized for the simultaneous removal and reduction of Hg(II) and Pb(II) in a fixed-bed column setup [76]. The inclusion of nZVI improved the material’s performance by minimizing oxidation and preventing particle aggregation. Key operational factors—such as pH, bed height, flow rate, and initial metal ion concentration—were found to significantly affect adsorption efficiency. Elevated pH levels and greater bed height led to delayed breakthrough times. The metal removal efficiency followed the sequence: OBA/nZVI-Hg(II) > OBA/nZVI-Pb (II) > OBA-Pb (II) > OBA-Hg (II). Overall, the findings indicate that OBA/nZVI is a promising candidate for effectively treating water contaminated with Hg(II) and Pb(II) within a pH range of 5 to 9.
A porous glutaraldehyde-crosslinked chitosan matrix (mZVI/GCS) was developed to encapsulate microscale zero-valent iron (mZVI) for enhanced Cr (VI) elimination from groundwater [77]. The mesoporous structure (average pore diameter of 8.775 nm) minimized passivation, improving reactivity. Batch studies demonstrated high Cr (VI) removal across a broad pH (2–10) and temperature (5–35 °C) range, with minimal interference from coexisting ions. The material retained 90.41% efficiency over eight reuse cycles, and its Langmuir adsorption capacity (243.63 mg/g) surpassed many previously reported adsorbents. Column studies revealed a 6.4-fold increase in Cr (VI) removal capacity compared to mZVI alone, highlighting mZVI/GCS as a promising remediation material for groundwater.
A novel material, NZVI/SHMP-CaB, was synthesized by modifying calcium bentonite (CaB) with sodium hexametaphosphate (SHMP) and nanoscale zero-valent iron (nZVI) for Cu(II) removal [78]. SHMP improved dispersion and reduced flocculation, while NZVI was stabilized, preventing agglomeration. The optimal composition (2% SHMP, 4% nZVI) achieved 97.41% Cu(II) removal efficiency. The adsorption process conformed to a pseudo-second-order kinetic model and aligned well with the Freundlich isotherm, with mechanisms involving surface adsorption, ion exchange, and reduction to Cu0 and Cu2O. The formation of FePO4 enhanced resistance to acidic and oxidative environments, making nZVI/SHMP-CaB a promising adsorbent for the remediation of heavy metal pollutants in both soil and aquatic environments.
A novel quartz sand chitosan zero-valent iron (QS-CTS@ZVI) composite was developed as a permeable reactive barrier (PRB) medium for groundwater Cr (VI) removal [79]. The material was synthesized using chitosan-modified ZVI coated on quartz sand and characterized using SEM, XRD, FTIR, and TGA-DSC. Adsorption studies revealed that at optimal conditions (ZVI:CTS = 1:1, QS:ZVI = 1:2, pH = 3, 5 h reaction time, 200 mg/L Cr(VI), 3.5 g/L dosage, 25 °C), QS-CTS@ZVI achieved 91.6% Cr(VI) and 90.9% total Cr removal from groundwater. The material retained an ~80% efficiency after three cycles, but SO42− significantly inhibited Cr(VI) removal. The adsorption process adhered to pseudo-second-order kinetic behavior and aligned with the Langmuir isotherm model, indicating that the adsorption occurred primarily as a uniform monolayer on the surface.
A study evaluating ZVI for As(III) removal from groundwater demonstrated its effectiveness as an adsorbent [80]. Adsorption followed pseudo-first-order kinetics, with rate constants varying from 0.080 to 0.52 h−1, and a removal capacity of 1.2 mg As(III)/g (Langmuir isotherm) at 1 mg/L As(III) and 15 °C. While bicarbonate had no impact on adsorption, silicate and phosphate (100 mM) reduced adsorption by up to 23%. Desorption was negligible in deionized water but reached ~18% in phosphate solutions, indicating potential competitive effects in real groundwater systems. These findings reinforce ZVI’s strong potential for arsenic remediation in groundwater treatment applications.
A recent study on nanoscale zero-valent iron (nZVI) for Ni(II) elimination from groundwater provided valuable insights into the compositional and structural transformations occurring during the process [81]. The study found that Fe0 undergoes oxidation to form lepidocrocite (γ-FeOOH), goethite (α-FeOOH), and magnetite (Fe3O4), while Ni(II) is reduced to Fe0·7Ni0·3 alloy and Fe–Ni composite (trevorite—NiFe2O4). The transformation mechanism involves Fe0 redox reactions with Ni(II), water, and dissolved oxygen, leading to the formation of Fe oxides and Fe–Ni complexes. Notably, the toxicity and bioavailability of these transformed products were significantly reduced, further reinforcing the potential of nZVI for safe and effective groundwater remediation.
A study evaluated the performance of sepiolite-supported nanoscale zero-valent iron (S-nZVI) for removing Cr(VI) and Pb(II) from groundwater, showing notable efficiency in both adsorption and reduction [82]. The removal process involved two main steps: initial adsorption of Cr(VI) and Pb(II) onto the S-nZVI surface, followed by their reduction to Cr(III) and elemental Pb(0), respectively. The incorporation of nZVI onto sepiolite minimized particle agglomeration, thereby enhancing both its stability and reactivity. Kinetic analysis indicated a pseudo-first-order reaction, while the adsorption behavior aligned with both Langmuir and the Freundlich isotherm models. These findings underscore the potential of S-nZVI for in situ treatment of groundwater contaminated with heavy metals.
A study on nZVI revealed its strong capability to remove Pb(II), Cd(II), Cu(II), and Ni(II) from aqueous solutions [83]. The adsorption process followed pseudo-second-order kinetics, with Pb(II) exhibiting the fastest uptake, followed in order by Cu(II), Cd(II), and Ni(II). Structural and surface analyses using SEM, TEM, XRD, and FTIR indicated the presence of FeOOH on the nZVI surface, which contributed to improved adsorption performance. pH played a key role in metal ion removal, with optimal adsorption occurring at pH levels below 6.5. These results suggest that nZVI is an effective material for the in situ remediation of contaminated groundwater, capable of simultaneously targeting multiple metal ions and maintaining performance over repeated use.
A study on the effects of typical groundwater anions (Cl−, NO3−, SO42−, and HCO3−) on nZVI corrosion and toxic metal removal revealed significant effects on both corrosion behavior and metal retention [84]. While Cu and Zn were initially removed efficiently, long-term retention was strongly influenced by anion type. Cl− and SO42− induced pitting corrosion, leading to metal desorption, whereas NO3− passivated the nZVI surface, preventing desorption. XRD analysis confirmed different iron hydroxide formations depending on anion presence. The study suggests that NO3− could enhance nZVI stability, improving its long-term application for in situ groundwater removal.
Zero-valent iron and its derivatives play a crucial role in addressing groundwater contamination through adsorption, reduction, and oxidation mechanisms. PRBs utilizing ZVI-based materials have shown significant success in real-world applications, despite challenges such as clogging, passivation, and secondary pollution. Modifications such as polymer coatings, biochar composites, and sulfide functionalization have significantly improved stability, reactivity, and efficiency. Future research should focus on optimizing material performance, understanding long-term transformation mechanisms, and ensuring environmental safety for large-scale applications. Advancements in ZVI-based technologies will contribute to the development of sustainable and cost-effective groundwater remediation solutions.
Here is a comparative Table 2 summarizing the performance of different ZVI-based materials for groundwater remediation. This table provides a structured overview of different ZVI-based materials, highlighting their modifications, removal efficiencies, kinetic models, advantages, and limitations.

Table 2.
Comparison of modified ZVI materials for heavy metal remediation.
While numerous studies have demonstrated the effectiveness of modified zero-valent iron (ZVI) materials in batch experiments, translating these findings into real-world scenarios necessitates validation through column studies. Batch tests are instrumental in providing preliminary insights into adsorption kinetics, equilibrium isotherms, and the influence of operational parameters. However, they do not adequately replicate the dynamic conditions of subsurface environments. In contrast, column experiments are essential for evaluating hydraulic behavior, breakthrough curves, and the long-term performance of ZVI-based systems—parameters that are critical for the design and deployment of permeable reactive barriers (PRBs). Consequently, there is a pressing need to shift focus toward column-based evaluations to bridge the gap between laboratory-scale studies and field applications. This is especially important for advanced materials, which have shown significant potential in contaminant remediation. By incorporating column testing into future research, a more realistic assessment of material performance under groundwater flow conditions can be achieved, ultimately enhancing the practical feasibility and scalability of ZVI-based remediation technologies [68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83].
The studies on ZVI and its modifications for groundwater remediation demonstrate significant advancements in material design to overcome inherent limitations such as agglomeration, passivation, and limited reactivity. The incorporation of stabilizers (e.g., polyvinylpyrrolidone, sodium alginate, and sodium hexametaphosphate) has enhanced the stability and dispersion of ZVI nanoparticles, improving their adsorption and reduction capacities. Additionally, bio-based supports like chitosan and biochar have shown promise in maintaining long-term efficiency and preventing secondary contamination. However, the interaction of ZVI with competing groundwater anions (e.g., Cl−, SO42−, NO3−) poses a challenge, influencing its corrosion behavior and heavy metal retention. Moreover, nZVI exhibits superior performance due to its high surface area; its high reactivity also raises concerns regarding rapid oxidation and a short lifespan, necessitating further research into long-term stability and regeneration strategies. A scientific approach integrating material modification, kinetic modeling, and real-world application testing is essential for optimizing ZVI-based systems for large-scale groundwater remediation.
Comparing the performance of different ZVI modifications reveals that polymer-stabilized and bio-supported ZVI materials generally exhibit higher removal efficiencies and better reusability than unmodified ZVI. For example, SA-nZVI and QS-CTS@ZVI demonstrate over 90% Cr(VI) removal with enhanced dispersibility and structural integrity. Similarly, nZVI/SHMP-CaB achieves a high Cu(II) removal efficiency while maintaining stability in acidic and oxidative environments. Studies on heavy metal adsorption kinetics indicate that most ZVI-based materials follow pseudo-second-order models, suggesting chemisorption-driven mechanisms. Additionally, equilibrium data often fit both Langmuir and Freundlich isotherms, indicating the occurrence of both monolayer and multilayer adsorption. However, performance varies with environmental conditions, including pH levels, competing ions, and reusability. Materials like mZVI/GCS and OBA/nZVI exhibit excellent retention of efficiency over multiple cycles, making them viable for long-term applications. Overall, while significant progress has been made in improving ZVI-based materials for groundwater remediation, future research should focus on field-scale applications, cost-effectiveness, and sustainability to ensure practical implementation.
3.3. Mechanism and Factors Affecting Adsorption of Metal Ion Using Zero-Valent Iron
Zero-valent iron (ZVI), especially in its nanoscale form (nZVI), is a promising candidate for heavy metal remediation due to its high surface area, strong reductive capacity, and surface reactivity. The metal ion removal mechanism by ZVI generally involves three principal pathways: adsorption onto the surface, reduction to a less toxic/insoluble form, and co-precipitation with iron corrosion products. The efficiency and dominant mechanism can vary significantly with material modifications, operating parameters, and target contaminants. Upon introduction into aqueous solutions, ZVI undergoes oxidation, forming Fe2+ and Fe3+ species while generating hydroxides and iron oxides (FeOOH, Fe3O4). These corrosion products play a vital role in surface adsorption and complexation with metal ions. Simultaneously, Fe0 acts as an electron donor, enabling the redox transformation of metal ions to less mobile or insoluble forms. For instance, Cr(VI) is reduced to Cr(III) and Hg(II) to elemental Hg0. The formed oxides and hydroxides provide reactive sites for ion exchange, electrostatic interaction, and complex formation, significantly contributing to the total sorption capacity.
In one study, the simultaneous elimination of As(III) and As(V) by nZVI was systematically investigated under varying conditions to elucidate the adsorption mechanism [85]. It was observed that pH played a critical role, with As(V) removal being highly efficient (>99.9%) at pH 4 and 7 due to favorable electrostatic attraction and surface complexation, while As(III) removal was more efficient at pH 10, attributed to its anionic form and enhanced surface interactions. Temperature also positively influenced the adsorption, with higher removal efficiencies at elevated temperatures (313 K), indicating an endothermic process. Increasing the nZVI dose enhanced both the oxidation capacity and availability of adsorption sites, significantly accelerating the removal kinetics. Conversely, higher initial concentrations reduced removal efficiency due to limited active sites on the nZVI surface. Overall, the findings demonstrated that the adsorption process adhered to the Langmuir isotherm and aligned with pseudo-second-order kinetic behavior, indicating monolayer adsorption governed by chemisorption. Thermodynamic evaluation revealed that the reaction occurred spontaneously and absorbed heat, implying an endothermic nature, primarily facilitated by electrostatic attraction and surface complexation mechanisms.
Zero-valent iron nanoparticles (P-ZVI), synthesized via green methods using palm petiole extract, exhibit excellent Cr(VI) removal efficiency owing to their large surface area and redox reactivity [86]. The adsorption behavior conforms to a pseudo-second-order kinetic model and aligns with the Langmuir isotherm, indicating chemisorption and monolayer adsorption. Optimal removal occurs at pH 5, where electrostatic attraction and Cr(VI) reduction are favored. Temperature positively influences adsorption, making the process spontaneous and endothermic. Additionally, P-ZVI demonstrates good reusability and potential for scale-up in wastewater treatment.
In another study, In-doped zero-valent iron (In-ZVI) was synthesized to improve the reactivity and durability of ZVI for Hg(II) removal. The mechanism involved a combination of surface adsorption, redox reactions, and the formation of an In-Hg amalgam. Initially, Hg(II) was adsorbed onto the ZVI surface, followed by partial reduction by Fe0 and surface-bound Fe2+ (Figure 6) [87]. The presence of In significantly enhanced the electron transfer via coupled redox reactions between In0/In3+ and Fe3+/Fe2+, which facilitated the generation of reactive atomic hydrogen (·H) from water. This ·H played a critical role in accelerating the reduction of Hg(II) to Hg0. The reduced mercury then interacted with In0 to form a stable amalgam, contributing to the sustained removal performance. The study demonstrated that In-ZVI maintained a high Hg(II) removal efficiency over repeated cycles, highlighting the synergistic effect of In doping on both the reactivity and longevity of ZVI.
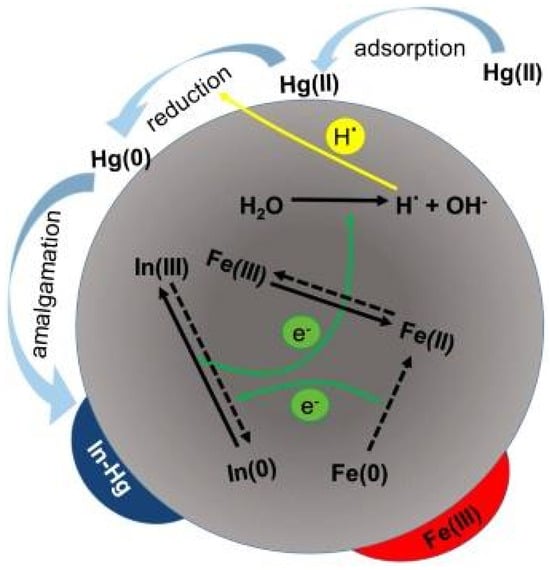
Figure 6.
Illustration depicting the proposed pathway for Hg (II) reduction and removal by In-ZVI under oxygen-rich conditions. Reprinted with permission from [87].
The removal of metal ions like Cr(VI) by zero-valent iron (ZVI), particularly when integrated with MXene-based structures like Alk-Ti3C2, occurs through a synergistic mechanism involving both reduction and surface adsorption [88] (Figure 7). In acidic media, Fe0 in ZVI reduces Cr(VI) to Cr(III), which subsequently forms Fe–O–Cr(III) complexes on the surface of composites. The intercalation of nanoscale ZVI within Alk-Ti3C2 enhances interlayer spacing, increases active site availability, and prevents ZVI aggregation, thereby improving adsorption efficiency. Factors such as pH, temperature, and the presence of coexisting ions significantly influence the process, with optimal removal observed at a low pH due to the enhanced corrosion of ZVI and exposure of reactive Fe0 sites. This dual functionality of reduction and adsorption underscores the potential of ZVI-MXene composites for efficient and stable heavy metal ion remediation in complex aqueous environments.
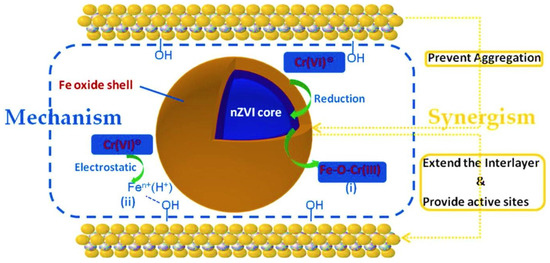
Figure 7.
The underlying mechanism and synergistic interfacial effects within the nZVI embedded Alk-Ti3C2 matrix. Reprinted with permission from Elsevier [88].
An exemplary study highlighting the adsorption mechanism and influencing factors for metal ion elimination using ZVI involved the development of nanocellulose (NC)-modified nZVI composites. Here, NC served as a stabilizing matrix to immobilize and disperse nZVI particles, effectively preventing their aggregation due to magnetic interactions and high surface energy [49,89,90]. Among various NC types, cellulose nanocrystal (CNC)-nZVI exhibited the most uniform dispersion, leading to superior Ni(II) removal efficiency (98.5%) compared to bare nZVI (89.9%). The removal mechanism was attributed to the synergistic effect of NC adsorption through surface functional groups and nZVI-mediated reduction of Ni(II), with the process following pseudo-second-order kinetics. Structural characterization (FTIR, XPS, XRD) confirmed strong interfacial interactions—electrostatic forces, hydrogen bonding, and coordination-covalent bonds—between NC and nZVI. The “hollow-out” morphology observed post-reaction supported the Kirkendall effect, indicating effective electron transfer and Ni(II) reduction at the NC-nZVI interface. Factors like pH, dosage, initial Ni(II) concentration, and interference ions significantly affected the removal performance, with optimal removal occurring at pH 5.0. CNC’s high crystallinity and negatively charged sulfate ester groups enhanced ion exchange and binding affinity toward Ni(II), making CNC-nZVI particularly robust in real wastewater matrices. This study underscores the critical role of support material geometry and functionalization in tuning nZVI behavior for efficient heavy metal remediation. The reviewed studies demonstrate that ZVI and its modified forms (e.g., In-ZVI, NC-nZVI, MXene-ZVI) exhibit a high efficiency for metal ion removal due to combined mechanisms of reduction and adsorption. Adsorption efficiency is strongly influenced by pH (optimal acidic range), temperature (endothermic nature), ZVI dose, and initial metal ion concentration. Functionalized supports like nanocellulose and MXene enhance ZVI dispersion, prevent aggregation, and improve reactivity and recyclability. Critically, while kinetics and thermodynamics are well-studied, limited emphasis is placed on long-term stability, regeneration under real wastewater conditions, and toxicity of by products (e.g., Cr(III), Hg0), which warrant further investigation for practical application.
The dominant sorption mechanisms involved in the removal of metal ions using zero-valent iron (ZVI) and its modified forms encompass a variety of interrelated pathways. Primarily, surface adsorption plays a crucial role, occurring through electrostatic interactions or surface complexation between the metal ions and reactive iron species or corrosion products. In parallel, reductive transformation is a key mechanism, where Fe0 or the atomic hydrogen generated from water reduction acts as an electron donor, converting toxic metal ions into less harmful or elemental forms. Another important pathway is the precipitation and co-precipitation of metal ions with iron hydroxides (Fe(OH)3) or iron oxides formed during ZVI corrosion, which immobilizes the metals in insoluble forms [65,73,83,85,86]. In doped ZVI systems, such as indium-doped ZVI (In-ZVI), a unique mechanism involves the formation of metal alloys or amalgams—for example, between In0 and reduced Hg0—which enhances the stability and durability of metal sequestration. Furthermore, in composite systems incorporating materials like MXene or nanocellulose, synergistic interfacial effects come into play. These composites prevent ZVI aggregation, increase surface area, and facilitate electron transfer, thereby improving both adsorption and reduction efficiencies. Overall, these mechanisms are strongly modulated by the physicochemical properties of the ZVI system, including surface modifications, particle morphology, and the nature of the support materials, all of which influence reactivity, electron flow, and dispersion in aqueous environments.
4. Future Perspectives
The advancements in zero-valent iron (ZVI) and its modified forms for groundwater remediation have led to notable improvements in adsorption capacity, stability, and reusability. Despite advancements, key research gaps remain in ZVI-based remediation, including limited field-scale validation, insufficient regeneration efficiency, and inadequate selectivity in complex contaminant matrices. Developing robust sulfidation protocols, multifunctional composites, and hybrid PRB systems is essential. Addressing these gaps will enhance the real-world applicability and sustainability of ZVI technologies.
4.1. Enhancing Long-Term Stability and Reactivity
A major limitation of ZVI-based systems is the rapid passivation and agglomeration of particles, which diminish their reactivity. To overcome this, future research should prioritize specific stabilization techniques such as sulfidation protocols that reduce surface oxidation, incorporation of carbonaceous supports (e.g., biochar, graphene oxide) to prevent aggregation, and polymer functionalization to improve colloidal stability. Emphasis should also be placed on evaluating these strategies under dynamic groundwater conditions to better reflect field behavior.
4.2. Optimizing Composite Materials for Selective Adsorption
Although ZVI composites show broad-spectrum contaminant removal, their selectivity in complex water matrices is limited. Research should focus on synthesizing engineered nanocomposites with functionalized surfaces (e.g., thiol, amine, or phosphate groups) to preferentially bind targeted metal ions or organic pollutants. Particular attention should be given to systems with high ionic interference, such as those containing Cl−, NO3−, or SO42−, to ensure performance in realistic scenarios.
4.3. Improving Regeneration and Reusability
Sustained performance over multiple treatment cycles remains a challenge due to structural deterioration. To address this, field-scalable regeneration techniques should be developed, including electrochemical reduction, mild acid washing, and bio-assisted regeneration using reducing microbes. These approaches should be evaluated not only for recovery efficiency but also for their compatibility with in situ conditions and cost-effectiveness.
4.4. Field-Scale Applications and Cost Considerations
Most existing studies are limited to batch or small-scale tests. To bridge the lab-to-field gap, it is essential to implement pilot-scale permeable reactive barriers (PRBs) using modified ZVI materials under actual groundwater flow and chemistry conditions. Performance should be assessed based on breakthrough curves, hydraulic conductivity, and longevity under natural organic matter and geochemical variability. Additionally, life-cycle assessments and cost–benefit analyses should guide the development of economically viable synthesis and deployment strategies.
4.5. Integration with Advanced Remediation Technologies
ZVI should be integrated with other remediation strategies to form hybrid treatment systems that exploit synergistic effects. To maximize the efficiency of groundwater remediation, future studies should explore the integration of ZVI and its derivatives—not merely as adsorbents but also as redox-active materials—with complementary treatment approaches such as bioremediation, photocatalysis, and electrochemical methods. These hybrid systems could deliver synergistic effects, enhancing contaminant transformation and removal while minimizing secondary waste generation. For instance, ZVI-biological PRBs combining ZVI with sulfate-reducing bacteria could simultaneously remove metals and degrade organics. Similarly, coupling ZVI with photocatalysts or electrochemical systems could improve transformation kinetics and reduce secondary pollution. Designing modular, multi-functional systems is a priority area for future investigation.
4.6. Real Aquifer Conditions and Field-Deployable Systems
Most existing studies have been conducted under controlled laboratory conditions, which do not accurately mimic the complex hydrogeochemistry of natural aquifers. Therefore, future research should prioritize field-deployable ZVI systems that account for heterogeneous flow, variable pH, co-contaminants, and real-time fluctuations. Integrating ZVI composites into permeable reactive barriers (PRBs) and in situ injection systems can significantly enhance practical applicability for groundwater remediation.
4.7. Green Synthesis of ZVI Using Plant Extracts
In recent years, the green synthesis of ZVI using plant-derived extracts has emerged as a sustainable alternative to conventional chemical reduction methods. These eco-friendly approaches not only eliminate the need for hazardous reducing agents but also offer surface-functionalized ZVI particles with enhanced reactivity. Future studies should focus on optimizing reaction parameters, identifying suitable plant species, and scaling up these green synthesis routes for large-scale environmental applications.
While considerable progress has been made in developing modified ZVI materials, advancing their stability, selectivity, reusability, and scalability is essential for real-world applications. Future work should emphasize targeted material design, field-scale validation, and integration with complementary technologies. Collaborative, interdisciplinary efforts are crucial to translate these innovations into sustainable groundwater remediation solutions.
The diverse range of ZVI modifications discussed highlights the rapid evolution of this field and its relevance to sustainable groundwater remediation. The concluding section synthesizes these findings and outlines key directions for future research.
5. Conclusions
Zero-valent iron (ZVI) and its modified forms have emerged as powerful materials for the remediation of heavy metal-contaminated groundwater due to their strong reducing ability, cost-effectiveness, and environmental compatibility. However, challenges such as rapid passivation, agglomeration, and limited mobility have hindered the widespread application of bare ZVI. To overcome these limitations, various modification strategies—including polymer stabilization, support on biochar or natural minerals, sulfidation, and hybrid composite formation—have been employed, significantly enhancing ZVI’s stability, reactivity, and selectivity. Studies across batch and column setups have demonstrated the improved performance of these materials in removing toxic metals such as Cr(VI), Pb(II), Hg(II), As(III), Cu(II), Ni(II), and Cd(II) under diverse environmental conditions. Despite the promising outcomes, further research is essential to understand long-term transformation pathways, environmental risks of secondary products, and scalability for real-field applications. A multidisciplinary approach integrating material innovation, process optimization, and field validation will be pivotal for the sustainable deployment of ZVI-based technologies in groundwater remediation.
Author Contributions
Conceptualization, G.S.; methodology, Y.V., A.V. and A.B.; validation, Y.V., A.V. and A.B.; formal analysis, Y.V., A.V. and A.B.; investigation, Y.V., A.V. and A.B; data curation, Y.V., A.V. and A.B.; writing—original draft preparation, Y.V., A.V. and A.B.; writing—review and editing, P.D., A.K., T.W. and G.S.; Supervision, G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Key Research and Development Program of Shaanxi Province (2023-LL-QY-42).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Liu, M.; Wu, X.; Chen, C.; Wang, Q.; Wen, T.; Wang, X. Synthesizing the composites of graphene oxide-wrapped polyaniline hollow microspheres for high-performance supercapacitors. Sci. Adv. Mater. 2013, 5, 1686–1693. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Zhang, Z.; Li, Y.; Dong, Y.; Sun, Y. Synthesis of multi-walled carbon nanotube–hydroxyapatite composites and its application in the sorption of Co(II) from aqueous solutions. J. Mol. Liq. 2013, 179, 46–53. [Google Scholar] [CrossRef]
- Ren, X.; Yang, S.; Shao, D.; Tan, X. Retention of Pb(II) by a low-cost magnetic composite prepared by environmentally-friendly plasma technique. Sep. Sci. Technol. 2013, 48, 1211–1219. [Google Scholar] [CrossRef]
- Qiu, X.; Fang, Z.; Yan, X.; Gu, F.; Jiang, F. Emergency remediation of simulated chromium (VI)-polluted river by nanoscale zero-valent iron: Laboratory study and numerical simulation. Chem. Eng. J. 2012, 193, 358–365. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, S.; Chen, Z.; Megharaj, M.; Naidu, R. Kaolinite-supported nanoscale zero-valent iron for removal of Pb2+ from aqueous solution: Reactivity, characterization and mechanism. Water Res. 2011, 45, 3481–3488. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Kim, D.-G.; Shin, H.-S. Mechanism study of nitrate reduction by nano zero valent iron. J. Hazard. Mater. 2011, 185, 1513–1521. [Google Scholar] [CrossRef]
- Ryu, A.; Jeong, S.-W.; Jang, A.; Choi, H. Reduction of highly concentrated nitrate using nanoscale zero-valent iron: Effects of aggregation and catalyst on reactivity. Appl. Catal. B Environ. 2011, 105, 128–135. [Google Scholar] [CrossRef]
- Luo, S.; Qin, P.; Shao, J.; Peng, L.; Zeng, Q.; Gu, J.-D. Synthesis of reactive nanoscale zero valent iron using rectorite supports and its application for Orange II removal. Chem. Eng. J. 2013, 223, 1–7. [Google Scholar] [CrossRef]
- Shirin, S.; Balakrishnan, V.K. Using chemical reactivity to provide insights into environmental transformations of priority organic substances: The Fe0-mediated reduction of acid blue 129. Environ. Sci. Technol. 2011, 45, 10369–10377. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Tokumura, M.; Nakajima, K.; Kawase, Y. Phenol removal using zero-valent iron powder in the presence of dissolved oxygen: Roles of decomposition by the Fenton reaction and adsorption/precipitation. J. Hazard. Mater. 2012, 201, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Segura, Y.; Martínez, F.; Melero, J.A.; Molina, R.; Chand, R.; Bremner, D.H. Enhancement of the advanced Fenton process (Fe0/H2O2) by ultrasound for the mineralization of phenol. Appl. Catal. B Environ. 2012, 113, 100–106. [Google Scholar] [CrossRef]
- Neumann, A.; Kaegi, R.; Voegelin, A.; Hussam, A.; Munir, A.K.M.; Hug, S.J. Arsenic removal with composite iron matrix filters in Bangladesh: A field and laboratory study. Environ. Sci. Technol. 2013, 47, 4544–4554. [Google Scholar] [CrossRef]
- Klas, S.; Kirk, D.W. Advantages of low pH and limited oxygenation in arsenite removal from water by zero-valent iron. J. Hazard. Mater. 2013, 252, 77–82. [Google Scholar] [CrossRef]
- Yin, W.; Wu, J.; Li, P.; Wang, X.; Zhu, N.; Wu, P.; Yang, B. Experimental study of zero-valent iron induced nitrobenzene reduction in groundwater: The effects of pH, iron dosage, oxygen and common dissolved anions. Chem. Eng. J. 2012, 184, 198–204. [Google Scholar] [CrossRef]
- Gu, C.; Jia, H.; Li, H.; Teppen, B.J.; Boyd, S.A. Synthesis of highly reactive subnano-sized zero-valent iron using smectite clay templates. Environ. Sci. Technol. 2010, 44, 4258–4263. [Google Scholar] [CrossRef]
- Su, C.; Puls, R.W.; Krug, T.A.; Watling, M.T.; O’Hara, S.K.; Quinn, J.W.; Ruiz, N.E. A two and half-year-performance evaluation of a field test on treatment of source zone tetrachloroethene and its chlorinated daughter products using emulsified zero valent iron nanoparticles. Water Res. 2012, 46, 5071–5084. [Google Scholar] [CrossRef]
- Dorathi, P.J.; Kandasamy, P. Dechlorination of chlorophenols by zero valent iron impregnated silica. J. Environ. Sci. 2012, 24, 765–773. [Google Scholar] [CrossRef]
- Choi, K.; Lee, W. Enhanced degradation of trichloroethylene in nano-scale zero-valent iron Fenton system with Cu(II). J. Hazard. Mater. 2012, 211, 146–153. [Google Scholar] [CrossRef]
- Kustov, L.M.; Finashina, E.D.; Shuvalova, E.V.; Tkachenko, O.P.; Kirichenko, O.A. Pd–Fe nanoparticles stabilized by chitosan derivatives for perchloroethene dechlorination. Environ. Int. 2011, 37, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-B.; Zhang, W.-X. Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environ. Sci. Technol. 1997, 31, 2154–2156. [Google Scholar] [CrossRef]
- Sweeny, K.; Fischer, J. Reductive Degradation of Halogenated Pesticides. U.S. Patent No. 3,640,821, 8 February 1972. [Google Scholar]
- Galdames, A.; Ruiz-Rubio, L.; Orueta, M.; Sánchez-Arzalluz, M.; Vilas-Vilela, J.L. Zero-valent iron nanoparticles for soil and groundwater remediation. Int. J. Environ. Res. Public Health 2020, 17, 5817. [Google Scholar] [CrossRef]
- Yang, L.; Jin, X.; Owens, G.; Chen, Z. Nitrogen-doped carbon enhances Fe0 activation for efficient tetrabromobisphenol a decontamination and its removal mechanism. Chem. Eng. J. 2024, 482, 148839. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, L.; Liu, Y.; Liu, Y.; Wen, J. Degradation of 1, 3, 6, 8-tetrabromocarbazole by sulfidated zero-valent iron activated peroxydisulfate: Mechanistic insight and transformation pathways. Chem. Eng. J. 2023, 458, 141439. [Google Scholar] [CrossRef]
- Khuntia, B.K.; Anwar, M.F.; Alam, T.; Samim, M.; Kumari, M.; Arora, I. Synthesis and characterization of zero-valent iron nanoparticles, and the study of their effect against the degradation of DDT in soil and assessment of their toxicity against collembola and ostracods. ACS Omega 2019, 4, 18502–18509. [Google Scholar] [CrossRef]
- Yan, Z.; Ouyang, J.; Wu, B.; Liu, C.; Wang, H.; Wang, A.; Li, Z. Nonmetallic modified zero-valent iron for remediating halogenated organic compounds and heavy metals: A comprehensive review. Environ. Sci. Ecotechnology 2024, 21, 100417. [Google Scholar] [CrossRef]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Sun, Y.; Guan, X.; Wang, J.; Meng, X.; Xu, C.; Zhou, G. Effect of weak magnetic field on arsenate and arsenite removal from water by zerovalent iron: An XAFS investigation. Environ. Sci. Technol. 2014, 48, 6850–6858. [Google Scholar] [CrossRef]
- Ullah, S.; Faiz, P.; Leng, S. Synthesis, Mechanism, and Performance Assessment of Zero-Valent Iron for Metal-Contaminated Water Remediation: A Review. CLEAN–Soil Air Water 2020, 48, 2000080. [Google Scholar] [CrossRef]
- Patra, S.; Pranudta, A.; Chanlek, N.; Nguyen, T.T.; Nhat, N.H.; El-Moselhy, M.M.; Padungthon, S. Denitrification of nitrate in regeneration waste brine using hybrid cation exchanger supported nanoscale zero-valent iron with/without palladium nanoparticles. Chemosphere 2023, 310, 136851. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Chin, Y.-P.; Ahn, J.-Y.; Wei-Haas, M.; McAdams, B.; Hwang, I. Reciprocal influences of dissolved organic matter and nanosized zero-valent iron in aqueous media. Chemosphere 2018, 193, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Namakka, M.; Rahman, R.; Said, K.A.B.M.; Muhammad, A. Insights into micro-and nano-zero valent iron materials: Synthesis methods and multifaceted applications. RSC Adv. 2024, 14, 30411–30439. [Google Scholar] [CrossRef]
- Di, L.; Chen, X.; Lu, J.; Zhou, Y.; Zhou, Y. Removal of heavy metals in water using nano zero-valent iron composites: A review. J. Water Process Eng. 2023, 53, 103913. [Google Scholar] [CrossRef]
- Namakka, M.; Rahman, M.R.; Said, K.A.M.B.; Mannan, M.A.; Patwary, A.M. A review of nanoparticle synthesis methods, classifications, applications, and characterization. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100900. [Google Scholar] [CrossRef]
- Yan, W.; Lien, H.-L.; Koel, B.E.; Zhang, W.-X. Iron nanoparticles for environmental clean-up: Recent developments and future outlook. Environ. Sci. Process. Impacts 2013, 15, 63–77. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, S.; Meng, Y.; Qiu, Y.; Chen, M.; Ma, L.; Li, L.; Liu, Y.; Yang, B. Advances in modified zero-valent iron materials: Synthesis methods, field studies, practical applications and challenges. Chem. Eng. J. 2024, 502, 157832. [Google Scholar] [CrossRef]
- Qin, Y.; Li, G.; Zhang, L.; An, T. Protocatechuic acid promoted catalytic degradation of rhodamine B with Fe@ Fe2O3 core-shell nanowires by molecular oxygen activation mechanism. Catal. Today 2019, 335, 144–150. [Google Scholar] [CrossRef]
- Hu, Y.-B.; Zhang, M.; Li, X.-Y. Improved longevity of nanoscale zero-valent iron with a magnesium hydroxide coating shell for the removal of Cr (VI) in sand columns. Environ. Int. 2019, 133, 105249. [Google Scholar] [CrossRef]
- Zhou, N.; Gong, K.; Hu, Q.; Cheng, X.; Zhou, J.; Dong, M.; Wang, N.; Ding, T.; Qiu, B.; Guo, Z. Optimizing nanocarbon shell in zero-valent iron nanoparticles for improved electron utilization in Cr (VI) reduction. Chemosphere 2020, 242, 125235. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, P.; Hu, Y.; Zan, R. Application of weak magnetic field coupling with zero-valent iron for remediation of groundwater and wastewater: A review. J. Clean. Prod. 2020, 262, 121341. [Google Scholar] [CrossRef]
- Fang, Y.; Wen, J.; Zhang, H.; Wang, Q.; Hu, X. Enhancing Cr (VI) reduction and immobilization by magnetic core-shell structured NZVI@ MOF derivative hybrids. Environ. Pollut. 2020, 260, 114021. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Zhang, C.-H.; Xu, J.-H.; Li, L.; Li, D.; Wu, Q.; Ma, L.-M. Strategies to enhance the reactivity of zero-valent iron for environmental remediation: A review. J. Environ. Manag. 2022, 317, 115381. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Z.; Wang, H.; Yang, H.; Wen, T.; Wang, S.; Hu, B.; Wang, X. Removal of organic compounds by nanoscale zero-valent iron and its composites. Sci. Total Environ. 2021, 792, 148546. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Ma, Z.; Carroll, K.C.; Zhao, X.; Huo, Z. Cadmium removal mechanistic comparison of three Fe-based nanomaterials: Water-chemistry and roles of Fe dissolution. Front. Environ. Sci. Eng. 2022, 16, 151. [Google Scholar] [CrossRef]
- Xue, C.; Wu, J.; Wang, K.; Yi, Y.; Fang, Z.; Cheng, W.; Fang, J. Effects of different types of biochar on the properties and reactivity of nano zero-valent iron in soil remediation. Front. Environ. Sci. Eng. 2021, 15, 1–13. [Google Scholar] [CrossRef]
- Zhou, L.; Li, A.; Ma, F.; Zhao, H.; Deng, F.; Pi, S.; Tang, A.; Yang, J. Combining high electron transfer efficiency and oxidation resistance in nZVI with coatings of microbial extracellular polymeric substances to enhance Sb (V) reduction and adsorption. Chem. Eng. J. 2020, 395, 125168. [Google Scholar] [CrossRef]
- Song, M.; Hu, X.; Gu, T.; Zhang, W.-X.; Deng, Z. Nanocelluloses affixed nanoscale Zero-Valent Iron (nZVI) for nickel removal: Synthesis, characterization and mechanisms. J. Environ. Chem. Eng. 2022, 10, 107466. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, S.; Li, Z.; Li, H.; Shaban, M.; Chen, C. Nanoscale zero-valent iron composites for uranium-contaminated water treatment and environmental remediation: A review. Environ. Sci. Nano 2025, 12, 20–40. [Google Scholar] [CrossRef]
- He, F.; Li, Z.; Shi, S.; Xu, W.; Sheng, H.; Gu, Y.; Jiang, Y.; Xi, B. Dechlorination of excess trichloroethene by bimetallic and sulfidated nanoscale zero-valent iron. Environ. Sci. Technol. 2018, 52, 8627–8637. [Google Scholar] [CrossRef]
- Pandey, K.; Sharma, S.; Saha, S. Advances in design and synthesis of stabilized zero-valent iron nanoparticles for groundwater remediation. J. Environ. Chem. Eng. 2022, 10, 107993. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, J.; Gao, F.; Wang, D.; Lyu, H.; Tang, J. Zero-valent iron-based materials for enhanced reductive removal of contaminants: From the trial-and-error synthesis to rational design. Appl. Catal. B Environ. Energy 2024, 365, 124901. [Google Scholar] [CrossRef]
- Cheng, S.-F.; Wu, S.-C. Feasibility of using metals to remediate water containing TCE. Chemosphere 2001, 43, 1023–1028. [Google Scholar] [CrossRef]
- Lien, H.-L.; Zhang, W.-X. Nanoscale iron particles for complete reduction of chlorinated ethenes. Colloids Surf. A Physicochem. Eng. Asp. 2001, 191, 97–105. [Google Scholar] [CrossRef]
- Pan, M.; Liu, J.; Yang, H.; Zhang, W.; Huang, K. One-pot synthesis of N-doped porous carbon supported zero-valent iron nanomaterials for efficient removal of U(VI). Chem. Eng. J. 2025, 505, 159870. [Google Scholar] [CrossRef]
- Yang, L.; Jin, X.; Lin, Q.; Owens, G.; Chen, Z. Enhanced adsorption and reduction of Pb(II) and Zn(II) from mining wastewater by carbon@nano-zero-valent iron (C@nZVI) derived from biosynthesis. Sep. Purif. Technol. 2023, 311, 123249. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Yang, Y.; Han, Y.; Wang, T.; Chen, J.; Tsang, D.C. Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J. Hazard. Mater. 2020, 383, 121240. [Google Scholar] [CrossRef]
- Tian, H.; Wu, Q.; Wu, Q.; Sun, C.; Zhang, R.; Wei, J.; Xu, H.; Liu, Z.; Huang, C.; Wang, P. Carbon nanotubes mediate electron transfer between Shewanella oneidenis MR-1 and nano zero-valent iron to enhance the elimination of Cr (VI) from aqueous media. Sep. Purif. Technol. 2024, 341, 126909. [Google Scholar] [CrossRef]
- Xiao, J.; Pang, Z.; Zhou, S.; Chu, L.; Rong, L.; Liu, Y.; Li, J.; Tian, L. The mechanism of acid-washed zero-valent iron/activated carbon as permeable reactive barrier enhanced electrokinetic remediation of uranium-contaminated soil. Sep. Purif. Technol. 2020, 244, 116667. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Sun, Y.; Cheng, K.; Li, J.; Tsang, D.C. Fabrication and characterization of hydrophilic corn stalk biochar-supported nanoscale zero-valent iron composites for efficient metal removal. Bioresour. Technol. 2018, 265, 490–497. [Google Scholar] [CrossRef]
- Tan, X.-F.; Liu, Y.-G.; Gu, Y.-L.; Xu, Y.; Zeng, G.-M.; Hu, X.-J.; Liu, S.-B.; Wang, X.; Liu, S.-M.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-Y.; Seo, Y.-D.; Ryu, K.-S.; Park, D.-J.; Lee, S.-H. Redox and catalytic properties of biochar-coated zero-valent iron for the removal of nitro explosives and halogenated phenols. Environ. Sci. Process. Impacts 2017, 19, 711–719. [Google Scholar] [CrossRef]
- Su, Y.-F.; Cheng, Y.-L.; Shih, Y.-H. Removal of trichloroethylene by zerovalent iron/activated carbon derived from agricultural wastes. J. Environ. Manag. 2013, 129, 361–366. [Google Scholar] [CrossRef]
- Li, S.; Yang, F.; Li, J.; Cheng, K. Porous biochar-nanoscale zero-valent iron composites: Synthesis, characterization and application for lead ion removal. Sci. Total Environ. 2020, 746, 141037. [Google Scholar] [CrossRef]
- Wei, A.; Ma, J.; Chen, J.; Zhang, Y.; Song, J.; Yu, X. Enhanced nitrate removal and high selectivity towards dinitrogen for groundwater remediation using biochar-supported nano zero-valent iron. Chem. Eng. J. 2018, 353, 595–605. [Google Scholar] [CrossRef]
- Rajput, M.K.; Hazarika, R.; Sarma, D. Zerovalent iron decorated tea waste derived porous biochar [ZVI@TBC] as an efficient adsorbent for Cd(II) and Cr(VI) removal. J. Environ. Chem. Eng. 2023, 11, 110279. [Google Scholar] [CrossRef]
- Weisener, C.G.; Sale, K.S.; Smyth, D.J.A.; Blowes, D.W. Field Column Study Using Zerovalent Iron for Mercury Removal from Contaminated Groundwater. Environ. Sci. Technol. 2005, 39, 6306–6312. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Su, C.; Ford, R.G.; Paul, C.J. Chromium-Removal Processes during Groundwater Remediation by a Zerovalent Iron Permeable Reactive Barrier. Environ. Sci. Technol. 2005, 39, 4599–4605. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Abbas, T.R.; Jassam, S.H. Removal of zinc from contaminated groundwater by zero-valent iron permeable reactive barrier. Desalin. Water Treat. 2015, 55, 1586–1597. [Google Scholar] [CrossRef]
- Njaramba, L.K.; Park, J.-B.; Lee, C.-S.; Nzioka, A.M.; Kim, Y.-J. Permeable Reactive Barriers with Zero-Valent Iron and Pumice for Remediation of Groundwater Contaminated with Multiple Heavy Metals. Environ. Eng. Sci. 2020, 38, 245–255. [Google Scholar] [CrossRef]
- Kornilovych, B.; Wireman, M.; Ubaldini, S.; Guglietta, D.; Koshik, Y.; Caruso, B.; Kovalchuk, I. Uranium Removal from Groundwater by Permeable Reactive Barrier with Zero-Valent Iron and Organic Carbon Mixtures: Laboratory and Field Studies. Metals 2018, 8, 408. [Google Scholar] [CrossRef]
- Song, I.-G.; Kang, Y.-G.; Kim, J.-H.; Yoon, H.; Um, W.Y.; Chang, Y.-S. Assessment of sulfidated nanoscale zerovalent iron for in-situ remediation of cadmium-contaminated acidic groundwater at a zinc smelter. J. Hazard. Mater. 2023, 441, 129915. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, M.; Li, M.; Liu, X. Cr(VI) removal from groundwater using double surfactant-modified nanoscale zero-valent iron (nZVI): Effects of materials in different status. Sci. Total Environ. 2020, 717, 137112. [Google Scholar] [CrossRef]
- Li, Z.; Xu, S.; Xiao, G.; Qian, L.; Song, Y. Removal of hexavalent chromium from groundwater using sodium alginate dispersed nano zero-valent iron. J. Environ. Manag. 2019, 244, 33–39. [Google Scholar] [CrossRef]
- Amiri, M.J.; Abedi-koupai, J.; Eslamian, S. Adsorption of Hg(II) and Pb(II) ions by nanoscale zero valent iron supported on ostrich bone ash in a fixed-bed column system. Water Sci. Technol. 2017, 76, 671–682. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, F.; Liu, X.; Li, M. Removal of Cr(VI) by glutaraldehyde-crosslinked chitosan encapsulating microscale zero-valent iron: Synthesis, mechanism, and longevity. J. Environ. Sci. 2024, 142, 115–128. [Google Scholar] [CrossRef]
- Qin, H.; Xu, L.; Qin, L.; Kang, B.; Zha, F.; Wang, Q.; Huang, K. Removal of Cu(II) by sodium hexametaphosphate and nano zero-valent iron modified calcium bentonite: Characteristic, adsorption performance and mechanism. J. Environ. Manag. 2024, 358, 120866. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, Z.; Yan, Y.; Feng, M.; Wang, Y.; Wang, J. Preparation of chitosan-loaded zero-valent iron-coated quartz sand and study of its ability to remove Cr(VI) in groundwater. Desalin. Water Treat. 2023, 284, 101–115. [Google Scholar] [CrossRef]
- Kanel, S.R.; Choi, H. Removal of Arsenic from Groundwater by Industrial Byproducts and Its Comparison with Zero-Valent Iron. J. Hazard. Toxic Radioact. Waste 2017, 21, 04016028. [Google Scholar] [CrossRef]
- Anang, E.; Liu, H.; Fan, X. Compositional transformation of Ni2+ and Fe0 during the removal of Ni2+ by nanoscale zero-valent iron and the implications to groundwater remediation. Water Sci. Technol. 2023, 88, 2409–2422. [Google Scholar] [CrossRef]
- Fu, R.; Yang, Y.; Xu, Z.; Zhang, X.; Guo, X.; Bi, D. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 2015, 138, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Azzam, A.M.; El-Wakeel, S.T.; Mostafa, B.B.; El-Shahat, M. Removal of Pb, Cd, Cu and Ni from aqueous solution using nano scale zero valent iron particles. J. Environ. Chem. Eng. 2016, 4, 2196–2206. [Google Scholar] [CrossRef]
- Pullin, H.; Crane, R.; Morgan, D.; Scott, T. The effect of common groundwater anions on the aqueous corrosion of zero-valent iron nanoparticles and associated removal of aqueous copper and zinc. J. Environ. Chem. Eng. 2017, 5, 1166–1173. [Google Scholar] [CrossRef]
- Yin, L.; Liu, L.; Lin, S.; Owens, G.; Chen, Z. Synthesis and characterization of Nanoscale Zero-Valent Iron (nZVI) as an adsorbent for the simultaneous removal of As(III) and As(V) from groundwater. J. Water Process Eng. 2022, 47, 102677. [Google Scholar] [CrossRef]
- Tesnim, D.; Hédi, B.A.; Ridha, D.; Cid-Samamed, A. Green low-cost synthesis of zero-valent iron nanoparticles from Palm Petiole Extract for Cr(VI) removal from water. Environ. Sci. Pollut. Res. 2024, 31, 44272–44288. [Google Scholar] [CrossRef]
- Qasim, G.H.; Lee, S.; Lee, W.; Han, S. Reduction and removal of aqueous Hg(II) using indium-modified zero-valent iron particles. Appl. Catal. B Environ. 2020, 277, 119198. [Google Scholar] [CrossRef]
- He, L.; Huang, D.; He, Z.; Yang, X.; Yue, G.; Zhu, J.; Astruc, D.; Zhao, P. Nanoscale zero-valent iron intercalated 2D titanium carbides for removal of Cr(VI) in aqueous solution and the mechanistic aspect. J. Hazard. Mater. 2020, 388, 121761. [Google Scholar] [CrossRef]
- Jin, G.; Zhang, Z.; Li, R.; Chen, C.; Tang, H.; Li, L.; Barry, D. Transport of zinc ions in the hyporheic zone: Experiments and simulations. Adv. Water Resour. 2020, 146, 103775. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, G.; Zhang, P.; Galindo-Torres, S.; Scheuermann, A.; Li, L. An efficient framework for particle-fluid interaction using Discrete Element Lattice Boltzmann Method: Coupling scheme and periodic boundary condition. Comput. Fluids 2020, 208, 104613. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).