Optimizing Oilfield-Produced Water Reuse for Sustainable Irrigation: Impacts on Soil Quality and Mineral Accumulation in Plants
Abstract
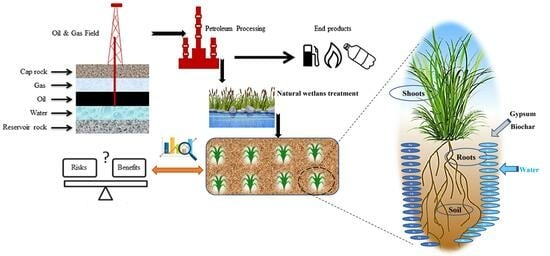
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.1.1. Experiment 1: Pot Trials at Sultan Qaboos University (SQU)
2.1.2. Experiment 2: Field Trials at Nimr Site
2.2. Statistical Analysis and Element Concentration Evaluation
3. Results
3.1. Plant Tissue
3.1.1. Mineral Accumulation in SQU Field Trial
3.1.2. Plant Nutrient Uptake Under Nimr Site Conditions
3.2. Plant Roots
3.2.1. Plant Root Dynamics in SQU Field Trial
3.2.2. Plant Root Dynamics Under Nimr Site Conditions
3.3. Soil Element Composition
3.3.1. Mineral Accumulation in SQU Field Trial Soil
3.3.2. Mineral Accumulation in Nimr Site Trial Soil
3.4. Plant Tissues and Root Mineral Uptake
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Alfa | Alfalfa (Medicago sativa) |
| b | Buffelgrass (Cenchrus ciliaris) |
| DPL | Drip Position Line |
| EC | Electrical Conductivity |
| FW | Farm Groundwater |
| ND | Not Detected |
| NA | Not available |
| N | Normal germination |
| NONE | No soil treatment added |
| p | Panicum (Guinea grass) |
| Pmax | Panicum maximum (Guinea grass) |
| PW | Produced water |
| R/RO | Reverse Osmosis |
| TDS | Total Dissolved Solids |
| W/Well | Ground well water |
References
- Asch, F.; Dingkuhn, M.; Wittstock, C.; Doerffling, K. Leaf K/Na Ratio Predicts Salinity Induced Yield Loss in Irrigated Rice. Euphytica 2000, 113, 109–118. [Google Scholar] [CrossRef]
- Suyama, H.; Benes, S.E.; Robinson, P.H.; Getachew, G.; Grattan, S.R. Forage Yield and Quality under Irrigation with Saline-Sodic Drainage Water: Greenhouse Evaluation. Agric. Water Manag. 2007, 88, 159–172. [Google Scholar] [CrossRef]
- Keating, B.A.; Carberry, P.S.; Bindraban, P.S.; Asseng, S.; Meinke, H.; Dixon, J. Eco-Efficient Agriculture: Concepts, Challenges, and Opportunities. Crop Sci. 2010, 50, S109–S119. [Google Scholar] [CrossRef]
- Wasim, M.A.; Naz, N.; Zehra, S.S. Anatomical Characteristic, Ionic Contents and Nutritional Potential of Buffel Grass (Cenchrus ciliaris L.) under High Salinity. S. Afr. J. Bot. 2022, 144, 471–479. [Google Scholar] [CrossRef]
- Ward, J.P.; Smith, S.E.; McClaran, M.P. Water Requirements for Emergence of Buffelgrass (Pennisetum ciliare). Weed Sci. 2006, 54, 720–725. [Google Scholar] [CrossRef]
- Marshall, V.M.; Lewis, M.M.; Ostendorf, B. Buffel Grass (Cenchrus ciliaris) as an Invader and Threat to Biodiversity in Arid Environments: A Review. J. Arid Environ. 2012, 78, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yu, H.; Shen, C. Development of a Novel Bio-Organic Fertilizer for the Removal of Atrazine in Soil. J. Environ. Manag. 2019, 233, 553–560. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Zhang, L.; Zhu, Y.; Li, J. Effects of Tall Fescue Biochar on the Adsorption and Desorption of Atrazine in Different Types of Soil. Environ. Sci. Pollut. Res. 2021, 28, 4503–4514. [Google Scholar] [CrossRef]
- Pichtel, J. Oil and Gas Production Wastewater: Soil Contamination and Pollution Prevention. Appl. Environ. Soil Sci. 2016, 2016, 2707989. [Google Scholar] [CrossRef]
- Regkouzas, P.; Katie, N.; Bontiotis, K.; Stefanakis, A. Effect of compost and compost-derived biochar on the growth of lettuce irrigated with water and treated wastewater. Nat.-Based Solut. 2025, 7, 100220. [Google Scholar] [CrossRef]
- Stefanakis, A.I. Constructed Wetlands for Wastewater Treatment in Hot and Arid Climates; Springer Nature: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Echchelh, A.; Hess, T.; Sakrabani, R.; Prigent, S.; Stefanakis, A.I. Towards agro-environmentally sustainable irrigation with treated produced water in hyper-arid environments. Agric. Water Manag. 2021, 243, 106449. [Google Scholar] [CrossRef]
- Schultze-Nobre, L.; Wiessner, A.; Bartsch, C.; Paschke, H.; Stefanakis, A.I.; Aylward, L.A.; Kuschk, P. Removal of dimethylphenols and ammonium in laboratory-scale horizontal subsurface flow Constructed Wetlands. Eng. Life Sci. 2017, 17, 1224–1233. [Google Scholar] [CrossRef]
- Al-Ajmi, D.N.; Idrees, A.M.; Al-Hatrushi, S.M. Spatial and Temporal Analysis of Recurrence Time of Rainfall in the Sultanate of Oman. Br. J. Arts Soc. Sci. 2013, 12, 118–126. [Google Scholar]
- Al-Ajmi, D.N. Climate Aridity: The Sultanate of Oman as a Case Study. Int. J. Earth Sci. Geol. 2018, 1, 1–3. [Google Scholar] [CrossRef]
- Atherton, J.G. Health and Environmental Aspects of Recycled Water. In Biotechnology X, Encyclopedia of Life Support Systems (EOLSS); Eolss Publishers: Oxford, UK, 2011; p. 138. [Google Scholar]
- Eddy, C.A.C.C.; Engel, B.A.; Shirmohammadi, A.; Chaubey, I. Water Reuse: Issues, Technologies, and Applications; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Ogundiran, M.B.; Fadiya, O.O.; Osibanjo, O.; Akinjokun, O.I. Heavy Metals Levels in Forage Grasses, Leachate and Lactating Cows Reared around Lead Slag Dumpsites in Nigeria. Int. J. Environ. Res. 2012, 6, 695–702. [Google Scholar]
- ISO 9001:2015; Quality Management Systems—Requirements. International Organization for Standardization: Geneva, Switzerland, 2015. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:9001:ed-5:v1:en (accessed on 9 March 2025).
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. Biochar Adsorbed Ammonia Is Bioavailable. Plant Soil 2012, 350, 57–69. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. A Wood-Based Low-Temperature Biochar Captures NH3–N Generated from Ruminant Urine-N, Retaining Its Bioavailability. Plant Soil 2012, 353, 73–84. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, J.; Yao, R.; Wang, X.; Xie, W.; Zhu, W.; Tao, J. Interactive Effects of Soil Amendments (Biochar and Gypsum) and Salinity on Ammonia Volatilization in Coastal Saline Soil. Catena 2020, 190, 104527. [Google Scholar] [CrossRef]
- Corwin, D.L.; Yemoto, K. Measurement of Soil Salinity: Electrical Conductivity and Total Dissolved Solids. Soil Sci. Soc. Am. J. 2019, 83, 1–2. [Google Scholar] [CrossRef]
- Alloway, B.J. Soil Processes and the Behaviour of Metals. In Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 1995; pp. 11–37. [Google Scholar]
- Abbas, M.T.; Wadaan, M.A.; Ullah, H.; Farooq, M.; Fozia, F.; Ahmad, I.; Khan, M.F.; Baabbad, A.; Ullah, Z. Bioaccumulation and Mobility of Heavy Metals in the Soil-Plant System and Health Risk Assessment of Vegetables Irrigated by Wastewater. Sustainability 2023, 15, 15321. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Kramer, I.; Mau, Y.; Levy, G.J. Soil Degradation Risks Assessed by the SOTE Model for Salinity and Sodicity. Water Resour. Res. 2020, 56, e2020WR027456. [Google Scholar] [CrossRef]
- Rengasamy, P. World Salinization with Emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.A.; Zaman, M.; Heng, L. Guidelines for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer and International Atomic Energy Agency: Vienna, Austria, 2018. [Google Scholar] [CrossRef]
- Göçmen Taşkın, B.; Özbek, Ö.; Keskin Şan, S.; Nachit, M.M.; Kaya, Z. The Assessment of Boron Toxicity Tolerance in F6 RILs of Durum Wheat [Triticum turgidum (L.) Tell. convar. durum (Desf.) Mackey]. Cereal Res. Commun. 2022, 50, 227–235. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, G.; Li, L.; Hu, L.; Wang, J.; Zhang, X. Biochar as an Amendment for Saline Soils: Effects on Soil Properties and Plant Growth. Sci. Total Environ. 2023, 856, 159867. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). Salt-Affected Soils: Guidelines for Sustainable Management. In FAO Soils Bulletin; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M.; Gojon, A.; Busch, F.A.; Sharma, S.; Tester, M.; Plett, D.C.; Roy, S.J. Energy Costs of Salt Tolerance in Crop Plants. New Phytol. 2020, 225, 1072–1090. [Google Scholar] [CrossRef]
- Qadir, M.; Drechsel, P.; Cisneros, B.J.; Kim, Y.; Pramanik, A.; Mahjoub, O. Wastewater Reuse in Agriculture: Global Status and Trends. Water Int. 2020, 45, 573–586. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innov. 2020, 1, 100017. [Google Scholar] [CrossRef]










| Soil Characteristics | Nimr Soil | Control Soil |
|---|---|---|
| EC (mS/cm) | 12.39 | 1.96 |
| * TDS (g/L) | 8.88 | 1.28 |
| pH | 7.5 | 8.1 |
| * MC % | 2.91 | 1.145 |
| Silt % | 6.17 | 0.0 |
| Clay % | 0.0 | 0.0 |
| Sand % | 93.8 | 100 |
| Texture | Sand | Sand |
| Elemental Contents (mg/kg) | Nimr Soil | Control Soil |
|---|---|---|
| Zn | 0.05 | 0.05 |
| Fe | 0.013 | 0.023 |
| B | 0.03 | 0.013 |
| Al | 0.087 | 0.043 |
| Na | 678.43 | 108.09 |
| K | 359.24 | 66.98 |
| Ca | 446.24 | 54.41 |
| Mg | 116.07 | 24.99 |
| As-Pb-Co-Cd-Ni-Hg-Mn-Cr-Cu | * ND | ND |
| Element (mg/L) | Produced Water | Treated Wastewater | Groundwater |
|---|---|---|---|
| B | 6.95 | 1.17 | 0.23 |
| Zn | 0.02 | * ND | ND |
| Ni | 0.03 | ND | ND |
| Fe | 0.04 | 0.01 | 0.01 |
| Mn | 0.02 | ND | ND |
| Cu | 0.01 | ND | ND |
| Al | 0.16 | 0.07 | 0.09 |
| Mg | 39.87 | 9.36 | 84.33 |
| Ca | 117.76 | 37.67 | 45.27 |
| Na | 5022.68 | 188.09 | 222.27 |
| K | 47.56 | 10.86 | 8.01 |
| Elemental Concentrations (mg/L) | Produced Water | Groundwater |
|---|---|---|
| B | 6.95 | 1.84 |
| Zn | 0.02 | 0.04 |
| Ni | 0.03 | 0.03 |
| Fe | 0.04 | 0.03 |
| Mn | 0.02 | 0.02 |
| Cu | 0.01 | 0.01 |
| Al | 0.16 | 0.28 |
| Mg | 39.87 | 305.44 |
| Ca | 117.76 | 579.3 |
| Na | 5022.6 | 1404.0 |
| K | 47.56 | 43.21 |
| Elements | Critical Soil Limit (mg/kg) * | Critical Plant Concentrations (mg/kg) * |
|---|---|---|
| Ag | 2 | - |
| As | 20–50 | 5–20 |
| Cd | 0.1–2.4 | 5–30 |
| Cr | 75–100 | 5–30 |
| Cu | 60–125 | 20–100 |
| Hg | 0.3–5 | 1–3 |
| Mn | 1500–3000 | 300–500 |
| Mo | 2–10 | 10–50 |
| Ni | 100 | 10–100 |
| Pb | 10–400 | 30–300 |
| Zn | 70–400 | 100–400 |
| B | 100 | 283–333 (>150 animal feeding) |
| Al | - | 50–3410 |
| Fe | - | >3080 |
| Tissue Minerals/Factors | B | Zn | Fe | Mn | Na | Mg | K | Ca | Al | |
|---|---|---|---|---|---|---|---|---|---|---|
| Harvest 1 | 60.4 B * | 8.9 B | 182.9 B | 47.9 B | 6864.4 B | 525.9 B | 519.1 B | 516.3 B | 67.6 B | |
| Harvest 2 | 114.6 AB | 29.7 A | 402.1 A | 90.9 A | NA * | NA | NA | NA | 179.4 A | |
| Harvest 3 | 126.1 A | 24.7 A | 215.3 B | 98.5 A | 24,634.8 A | 2660.2 A | 14,790.4 A | 8618.1 A | 110.6 B | |
| p-value | 0.018 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.00 | |
| Wastewater | 94.5 AB | 19.9 A | 260.0 A | 69.0 A | 12,894.3 A | 337.2 B | 7693.3 A | 5024.5 A | 116.7 A | |
| Produced Water | 150.8 A | 24.6 A | 270.9 A | 78.0 A | 23,726.9 A | 848.8 AB | 6086.1 A | 3917.6 A | 125.5 A | |
| Groundwater | 55.9 B | 18.8 A | 269.3 A | 90.2 A | 10,627.7 A | 2015.4 A | 7627.5 A | 4759.5 A | 115.5 A | |
| p-value | 0.004 | 0.335 | 0.977 | 0.092 | 0.005 | 0.647 | 0.725 | 0.299 | 0.91 | |
| Panicum maximum | 105.5 AB | 17.6 A | 264.5 A | 96.4 A | 21,991.5 A | 1041.5 A | 8167.7 AB | 8596.3 B | 111.1 A | |
| Panicum | 98.1 AB | 20.9 A | 258.5 A | 106.4 A | 14,209.3 A | 858.5 A | 6956.3 AB | 2615.5 B | 107.9 A | |
| Buffelgrass | 51.6 B | 23.1 A | 210.6 A | 47.9 B | 13,114.0 A | 551.2 A | 10,004.0 A | 2396.9 B | 102.5 A | |
| Alfalfa | 146.6 A | 22.7 A | 333.4 A | 65.6 AB | 13,683.7 A | 1817.4 A | 3414.5 B | 9659.9 A | 155.1 A | |
| p-value | 0.034 | 0.631 | 0.297 | 0.588 | 0.165 | 0.045 | 0.001 | 0.000 | 0.3 | |
| Gypsum | 118.0 A | 18.0 A | 338.2 A | 93.4 A | 15,469.5 A | 685.9 A | 5540.8 A | 4602.9 A | 143.1 A | |
| Biochar | 103.3 A | 24.8 A | 208.7 A | 63.8 A | 19,453.3 A | 842.8 A | 7974.4 A | 4727.4 A | 90.9 A | |
| None | 79.9 A | 20.3 A | 253.4 A | 80.1 A | 12,326.0 A | 1672.6 A | 7891.6 A | 4371.1 A | 123.4 A | |
| p-value | 0.373 | 0.229 | 0.065 | 0.513 | 0.101 | 0.374 | 0.969 | 0.101 | 0.13 | |
| Control Soil | 103.2 A | 23.6 A | 238.5 A | 74.8 A | 11,173.1 A | 1530.2 A | 8802.5 A | 5115.5 A | 117.3 A | |
| Nimr Soil | 97.6 A | 18.6 A | 295.1 A | 83.3 A | 20,326.2 A | 604.0 A | 5468.7 A | 4018.8 A | 121.0 A | |
| p-value | 0.837 | 0.215 | 0.305 | 0.147 | 0.062 | 0.097 | 0.452 | 0.532 | 0.88 | |
| Drip position | ||||||||||
| DPL 1 | 82.4 A | 19.7 A | 279.2 A | 75.8 A | 7925.3 A | 1225.1 A | 5064.4 A | 3605.8 A | 132.2 A | |
| DPL 2 | 107.8 A | 17.1 A | 275.9 A | 86.2 A | 13,235.4 A | 846.5 A | 7056.8 A | 3554.0 A | 112.6 A | |
| DPL 3 | 104.0 A | 23.3 A | 235.6 A | 77.9 A | 18,294.2 A | 1179.9 A | 8946.0 A | 5731.2 A | 109.6 A | |
| DPL 4 | 107.5 A | 24.2 A | 276.6 A | 76.3 A | 23,543.6 A | 1016.8 A | 7475.2 A | 5377.6 A | 122.1 A | |
| p-value | 0.749 | 0.267 | 0.829 | 0.091 | 0.849 | 0.275 | 0.302 | 0.856 | 0.81 | |
| Interaction p-values | ||||||||||
| Harvest—Species | 0.255 | 0.797 | 0.113 | 0.113 | 0.820 | 0.091 | 0.345 | 0.000 | 0.120 | |
| Harvest—Water | 0.338 | 0.531 | 0.776 | 0.155 | 0.001 | 0.001 | 0.055 | 0.307 | 0.695 | |
| Species—Water | 0.764 | 0.383 | 0.855 | 0.685 | 0.964 | 0.761 | 0.484 | 0.916 | 0.549 | |
| Tissue Minerals/Factors | Zn | Fe | B | Mn | Mg | Ca | Na | K | Al | |
|---|---|---|---|---|---|---|---|---|---|---|
| Harvest 1 | 23.6 C * | 161.5 B | 51.1 C | 62.7 A | NA * | NA | NA | NA | 75.5 A | |
| Harvest 2 | 43.7 A | 260.8 A | 147.7 AB | 70.6 A | NA | NA | NA | NA | 112.9 A | |
| Harvest 3 | 30.9 BC | 160.4 B | 211.0 A | 59.8 A | 4543.4 A | 9097.1 A | 26,780.2 A | 21,328.1 AB | 110.4 A | |
| Harvest 4 | 33.2 ABC | 117.5 B | 183.5 AB | 53.9 A | 5657.3 A | 9228.3 A | 19,745.4 A | 26,955.6 A | 95.0 A | |
| Harvest 5 | 38.2 AB | 152.1 B | 100.2 BC | 74.7 A | 4654.9 A | 10,590.9 A | 28,497.8 A | 15,866.4 B | 113.0 A | |
| p-value | 0.000 | 0.000 | 0.000 | 0.175 | 0.587 | 0.667 | 0.005 | 0.137 | 0.104 | |
| Produced water | 35.1 A | 176.1 A | 186.7 A | 73.4 A | 5360.1 A | 11,037.0 A | 32,412.8 A | 19,083.5 A | 117.1 A | |
| Groundwater | 32.7 A | 164.8 A | 90.7 B | 55.3 B | 4543.6 A | 8240.5 B | 17,602.8 A | 23,683.2 A | 85.6 B | |
| p-value | 0.327 | 0.493 | 0.000 | 0.127 | 0.045 | 0.093 | 0.068 | 0.002 | 0.004 | |
| Panicum maximum | 28.2 B | 158.8 A | 142.5 A | 73.3 A | 5313.0 AB | 9038.9 A | 21,558.6 A | 18,568.4 AB | 88.6 A | |
| Panicum | 31.6 B | 193.1 A | 192.8 A | 78.1 A | 5892.7 A | 11,200.8 A | 30,933.8 A | 19,568.7 B | 112.2 A | |
| buffelgrass | 41.9 A | 159.5 A | 80.8 B | 41.6 B | 3649.9 B | 8676.6 A | 22,531.0 A | 26,013.0 A | 103.3 A | |
| p-value | 0.000 | 0.119 | 0.000 | 0.002 | 0.184 | 0.564 | 0.028 | 0.000 | 0.210 | |
| Germination: | ||||||||||
| Reverse osmosis | 34.2 AB | 179.6 A | 126.4 A | 55.2 A | 4784.58 A | 9340.7 A | 19,266.6 A | 24,109.9 A | 106.832 A | |
| Groundwater | 39.3 A | 173.0 A | 147.2 A | 64.5 A | 4758.13 A | 10,536.6 A | 34,198.2 A | 21,471.7 A | 108.725 A | |
| None | 28.2 B | 158.8 A | 142.5 A | 73.3 A | 5313.08 A | 9038.9 A | 21,558.6 A | 18,568.4 A | 88.675 A | |
| p-value | 0.021 | 0.621 | 0.728 | 0.796 | 0.578 | 0.147 | 0.261 | 0.167 | 0.341 | |
| Interaction p-values | ||||||||||
| Harvest—Species | 0.064 | 0.671 | 0.438 | 0.799 | 0.313 | 0.860 | 0.715 | 0.458 | 0.119 | |
| Harvest—Water | 0.122 | 0.002 | 0.096 | 0.000 | 0.327 | 0.019 | 0.182 | 0.208 | 0.002 | |
| Species—Water | 0.462 | 0.650 | 0.302 | 0.487 | 0.892 | 0.415 | 0.827 | 0.622 | 0.346 | |
| Root Minerals/Factors | B | Fe | Mn | Ca | |
|---|---|---|---|---|---|
| Wastewater | 40.7 A * | 1767.9 A | 325.1 A | 15,933.4 A | |
| Produced water | 71.1 A | 633.4 B | 97.6 B | 14,064.8 AB | |
| Groundwater | 40.1 A | 953.4 B | 140.9 B | 13,279.4 B | |
| p-value | 0.081 | 0.007 | 0.024 | 0.036 | |
| Panicum maximum | 4.1 B | 714.5 A | 101.7 A | 3814.6 C | |
| Panicum | 24.2 B | 1314.9 A | 220.3 A | 6983.5 C | |
| Buffelgrass | 3.4 B | 1447.3 A | 329.7 A | 14,538.1 B | |
| Alfalfa | 171.0 A | 996.3 A | 99.7 A | 32,367.4 A | |
| p-value | 0.005 | 0.078 | 0.073 | 0.000 | |
| Gypsum | 51.1 A | 1235.3 A | 215.3 A | 16,248.0 A | |
| Biochar | 47.8 A | 1058.5 A | 186.8 A | 12,781.0 B | |
| None | 53.1 A | 1060.9 A | 161.3 A | 14,248.7 AB | |
| p-value | 0.871 | 0.404 | 0.830 | 0.018 | |
| Control Soil | 15.0 B | 1091.1 A | 178.4 A | 8067.0 B | |
| Nimr Soil | 86.3 A | 1145.4 A | 197.3 A | 20,784.8 A | |
| p-value | 0.005 | 0.701 | 0.683 | 0.000 | |
| Drip position: | |||||
| DPL 1 | 78.8 A | 1289.8 A | 199.5 A | 16,324.0 A | |
| DPL 2 | 50.8 A | 938.7 A | 221.3 A | 13,739.1 AB | |
| DPL 3 | 34.7 A | 1038.6 A | 165.7 A | 12,911.1 B | |
| DPL 4 | 38.3 A | 1205.9 A | 164.9 A | 14,729.3 AB | |
| p-value | 0.098 | 0.287 | 0.669 | 0.049 | |
| Interaction p-values | |||||
| Soil type—Species | 0.005 | 0.455 | 0.617 | 0.000 | |
| Species—Water | 0.297 | 0.061 | 0.053 | 0.013 | |
| Experiment 2 Root Factors/Elements | Mn | Al | Mg | Ca | K | Na | |
|---|---|---|---|---|---|---|---|
| Produced Water | 20.4 A * | 145.5 A | 1573.4 A | 2926.5 A | 1020.4 A | 20,579.9 A | |
| Groundwater | 35.5 A | 173.1 A | 2592.1 A | 3435.4 A | 2210.9 A | 10,313.9 A | |
| p-value | 0.31 | 0.793 | 0.214 | 0.829 | 0.065 | 0.125 | |
| Panicum max | 22.5 A | 82.2 A | 2012.0 A | 1706.2 A | 1370.2 A | 14,606.8 A | |
| Panicum | 34.1 A | 171.7 A | 2441.3 A | 2822.0 A | 1934.5 A | 21,350.1 A | |
| Buffelgrass | 27.3 A | 224.0 A | 1795.0 A | 5014.7 A | 1542.2 A | 10,383.9 A | |
| p-value | 0.79 | 0.585 | 0.696 | 0.510 | 0.627 | 0.260 | |
| p-values of interaction | |||||||
| Species—Water | 0.522 | 0.425 | 0.486 | 0.568 | 0.281 | 0.345 | |
| Soil Factors/Elements | Fe | B | Al | K | Ca | Mg | Na | |
|---|---|---|---|---|---|---|---|---|
| Irrigated Water: | ||||||||
| Wastewater | 0.05 AB * | 1.6 B | 0.13 B | 53.3 B | 383.1 B | 21.9 C | 6611.7 B | |
| Produced Water | 0.1 A | 5.9 A | 0.24 A | 265.0 A | 640.7 A | 131.7 B | 32,256.3 A | |
| Groundwater | 0.0 B | 1.3 B | 0.10 B | 44.4 B | 380.2 B | 188.5 A | 6886.9 B | |
| p-value | 0.039 | 0.001 | 0.000 | 0.008 | 0.000 | 0.000 | 0.000 | |
| Panicum max | 0.07 A | 3.2 A | 0.17 A | 90.3 A | 585.2 A | 152.6 A | 15,264.8 A | |
| Panicum | 0.03 A | 1.7 A | 0.17 A | 122.2 A | 481.2 A | 134.1 A | 17,180.9 A | |
| Buffelgrass | 0.10 A | 3.5 A | 0.12 A | 57.0 A | 377.2 A | 51.6 B | 10,808.3 A | |
| Alfalfa | 0.08 A | 3.3 A | 0.15 A | 214.1 A | 428.4 A | 117.8 AB | 17,752.4 A | |
| p-value | 0.592 | 0.791 | 0.180 | 0.446 | 0.002 | 0.050 | 0.189 | |
| Soil addition: | ||||||||
| Gypsum | 0.019 B | 3.29 A | 0.16 A | 53.8 A | 761.6 A | 106.4 A | 17,720.4 A | |
| Biochar | 0.12 A | 3.36 A | 0.16 A | 164.1 A | 407.3 B | 124.8 A | 14,532.9 A | |
| None | 0.08 AB | 2.32 A | 0.14 A | 144.7 A | 235.1 B | 110.9 A | 13,501.6 A | |
| p-value | 0.017 | 0.626 | 0.060 | 0.000 | 0.441 | 0.441 | 0.138 | |
| Soil Type: | ||||||||
| Control Soil | 0.04 A | 2.55 A | 0.16 A | 65.6 B | 501.1 A | 133.0 A | 10,826.6 B | |
| Nimr Soil | 0.10 A | 3.43 A | 0.15 A | 176.2 A | 434.9 A | 95.0 B | 19,676.6 A | |
| p-value | 0.078 | 0.472 | 0.027 | 0.445 | 0.018 | 0.322 | 0.000 | |
| Soil depth: | ||||||||
| Top | 0.07 A | 3.21 A | 0.14 B | 121.4 A | 442.6 A | 122.4 A | 17,087.9 A | |
| Bottom | 0.07 A | 2.77 A | 0.16 A | 120.3 A | 493.5 A | 105.7 A | 13,415.3 B | |
| p-value | 0.842 | 0.612 | 0.973 | 0.407 | 0.124 | 0.039 | 0.023 | |
| Drip position: | ||||||||
| DPL 1 | 0.07 A | 2.7 A | 0.15 A | 67.3 B | 477.9 A | 128.1 A | 16,507.9 A | |
| DPL 2 | 0.08 A | 3.4 A | 0.15 A | 62.5 B | 415.4 A | 103.3 A | 19,339.1 A | |
| DPL 3 | 0.06 A | 2.3 A | 0.16 A | 77.9 B | 454.5 A | 98.4 A | 13,620.4 AB | |
| DPL 4 | 0.07 A | 3.5 A | 0.15 A | 275.9 A | 524.1 A | 126.2 A | 11,539.1 B | |
| p-value | 0.921 | 0.807 | 0.002 | 0.768 | 0.258 | 0.995 | 0.034 | |
| Interaction p-values | ||||||||
| Soil type—species | 0.168 | 0.704 | 0.928 | 0.132 | 0.491 | 0.140 | 0.055 | |
| Species—Water | 0.977 | 0.986 | 0.003 | 0.264 | 0.348 | 0.000 | 0.402 | |
| Species—Soil depth | 0.386 | 0.920 | 0.575 | 0.949 | 0.938 | 0.202 | 0.256 | |
| Water—Soil depth | 0.842 | 0.796 | 0.482 | 0.900 | 0.646 | 0.000 | 0.002 | |
| Soil Factors/Elements | Zn | Ni | Fe | B | Cu | Al | Mn | |
|---|---|---|---|---|---|---|---|---|
| Produced Water | 0.46 A * | 0.04 A | 0.14 A | 5.0 A | 0.07 A | 0.31 A | 0.70 A | |
| Groundwater | 0.53 A | 0.045 A | 0.10 A | 1.9 B | 0.05 A | 0.30 A | 0.62 A | |
| p-value | 0.697 | 0.561 | 0.163 | 0.222 | 0.155 | 0.001 | 0.500 | |
| Top | 0.53 A | 0.04 A | 0.17 A | 4.2 A | 0.07 A | 0.32 A | 0.91 A | |
| Bottom | 0.46 A | 0.04 A | 0.07 B | 2.7 A | 0.05 A | 0.28 B | 0.42 B | |
| p-value | 0.697 | 1.000 | 0.002 | 0.094 | 0.002 | 0.060 | 0.002 | |
| Panicum max | 0.37 A | 0.042 A | 0.10 A | 2.94 A | 0.05 A | 0.30 A | 0.78 A | |
| Panicum | 0.64 A | 0.045 A | 0.14 A | 3.66 A | 0.06 A | 0.31 A | 0.68 A | |
| Buffelgrass | 0.48 A | 0.045 A | 0.13 A | 3.91 A | 0.06 A | 0.30 A | 0.53 A | |
| p-value | 0.463 | 0.829 | 0.624 | 0.543 | 0.834 | 0.758 | 0.309 | |
| Germination: | ||||||||
| Reverse Osmosis | 0.50 A | 0.043 A | 0.10 B | 2.83 A | 0.05 A | 0.29 A | 0.49 A | |
| Groundwater | 0.62 A | 0.04 A | 0.17 A | 4.74 A | 0.08 A | 0.32 A | 0.72 A | |
| None | 0.37 A | 0.04 A | 0.10 AB | 2.94 A | 0.05 A | 0.30 A | 0.78 A | |
| p-value | 0.595 | 0.542 | 0.037 | 0.104 | 0.126 | 0.060 | 0.139 | |
| Interaction p-values | ||||||||
| Germination—Water | 0.702 | 0.068 | 0.159 | 0.044 | 0.36 | 0.323 | 0.735 | |
| Germination—Depth | 0.717 | 0.542 | 0.729 | 0.429 | 0.951 | 0.241 | 0.140 | |
| Water—Depth | 0.692 | 0.711 | 0.934 | 0.967 | 0.363 | 0.381 | 0.986 | |
| Species—Water | 0.902 | 0.829 | 0.832 | 0.792 | 0.642 | 0.547 | 0.894 | |
| Species—Depth | 0.344 | 0.532 | 0.972 | 0.628 | 0.364 | 0.375 | 0.201 | |
| Sample | TDS (g/L) | EC (mS/cm) | pH |
|---|---|---|---|
| PW-Pmax-gypsum-Top | 18.27 | 21.8 | 7.4 |
| PW-Pmax-gypsum-Bottom | 12.67 | 15.95 | 7.4 |
| PW-P-gypsum-Top | 22.2 | 24.1 | 6.9 |
| PW-P-gypsum-Bottom | 14.7 | 15.42 | 7.2 |
| PW-Buffel-gypsum-Top | 20.0 | 20.8 | 6.9 |
| PW-Buffel-gypsum-Bottom | 13.63 | 14.0 | 7.0 |
| PW-Alfa-gypsum-Top | 19.78 | 22.9 | 7.4 |
| PW-Alfa-gypsum-Bottom | 13.75 | 16.77 | 7.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Jabri, K.; Al-Busaidi, A.; Ahmed, M.; Janke, R.R.; Stefanakis, A. Optimizing Oilfield-Produced Water Reuse for Sustainable Irrigation: Impacts on Soil Quality and Mineral Accumulation in Plants. Water 2025, 17, 1497. https://doi.org/10.3390/w17101497
Al-Jabri K, Al-Busaidi A, Ahmed M, Janke RR, Stefanakis A. Optimizing Oilfield-Produced Water Reuse for Sustainable Irrigation: Impacts on Soil Quality and Mineral Accumulation in Plants. Water. 2025; 17(10):1497. https://doi.org/10.3390/w17101497
Chicago/Turabian StyleAl-Jabri, Khaled, Ahmed Al-Busaidi, Mushtaque Ahmed, Rhonda R. Janke, and Alexandros Stefanakis. 2025. "Optimizing Oilfield-Produced Water Reuse for Sustainable Irrigation: Impacts on Soil Quality and Mineral Accumulation in Plants" Water 17, no. 10: 1497. https://doi.org/10.3390/w17101497
APA StyleAl-Jabri, K., Al-Busaidi, A., Ahmed, M., Janke, R. R., & Stefanakis, A. (2025). Optimizing Oilfield-Produced Water Reuse for Sustainable Irrigation: Impacts on Soil Quality and Mineral Accumulation in Plants. Water, 17(10), 1497. https://doi.org/10.3390/w17101497