Abstract
Diclofenac, a nonsteroidal anti-inflammatory drug widely used worldwide, has been detected in waterbodies at concentrations ranging from ng L−1 to µg L−1. Although diclofenac is not a persistent compound, aquatic organisms may be exposed to this drug for extended periods due to its incorporation into the environment by continuous release from hospitals and municipal discharges. This study aimed to evaluate the toxic effects of diclofenac on the microalga Pseudokirchneriella subcapitata, the cladoceran Daphnia curvirostris, and zebrafish embryos (Danio rerio). Toxicity bioassays for the microalga were performed according to the OECD 201 protocol with diclofenac concentrations of 0, 6.25, 12.5, 25, 50, 75, and 100 mg L−1. For the determination of acute toxicity in the cladoceran (48 h), concentrations of 0, 10, 20, 30, 40, 50, and 60 mg L−1 were tested; in subchronic bioassays, the effect of the drug on the reproductive parameters of D. curvirostris was determined for 21 days with sublethal concentrations of 10.3, 14.4, 17.2, and 21.3 mg L−1. Toxicity bioassays on zebrafish embryos were performed according to the OECD 236 protocol, using concentrations of 0, 1, 2, 4, 6, 8, and 10 mg L−1 of diclofenac. The results confirmed the toxic effects of the drug. The IC50 for the microalga was 16.57 mg L−1, while the LC50 for D. curvirostris and D. rerio was 32.29 and 6.27 mg L−1, respectively. In the microalga, chlorophyll-a and carotenoids increased at a concentration of 3.62 mg L−1 of diclofenac; however, chlorophyll-b decreased at the highest drug concentration (13.51 mg L−1). Protein and lipid concentrations in P. subcapitata exposed to all concentrations were higher than in the control. Chronic diclofenac exposure did not affect the survival of D. curvirostris; however, the cumulative progeny and number of clutches significantly decreased, and the age of first reproduction was delayed at all drug concentrations. Protein concentration in D. curvirostris hatchlings was higher at all diclofenac concentrations; in contrast, the amount of lipids and carbohydrates decreased significantly. In D. rerio, the hatching rate decreased by 40, 51.6, and 80% at concentrations of 6, 8, and 10 mg L−1 diclofenac, respectively, and exposure to the drug caused lethal effects such as coagulation at 24 and 48 hpf; sublethal effects such as edema and curved tail were also observed at concentrations of 2 to 10 mg L−1, and the effects increased with increasing concentration up to 144 hpf. The results demonstrate the vulnerability of aquatic organisms to the toxic effects of diclofenac, suggesting that discharging it into water bodies should be regulated to prevent potential ecological impacts on the various trophic levels of freshwater biota.
1. Introduction
The pharmaceutical industry is one of the largest and economically most important industries nowadays. Large quantities of pharmaceuticals are produced and used annually in human medicine, veterinary medicine, and agriculture to prevent and treat diseases [1]. Despite their importance, pharmaceuticals are considered a relevant category of emerging pollutants of concern that enter freshwater bodies through municipal effluents, discharges from agricultural and aquaculture activities, wastewater treatment facilities, landfill leachates, and contaminated groundwater [2,3]. According to Mezzelani et al. [4], the reported global concentrations of pharmaceuticals in aquatic environments range from a few nanograms per liter to hundreds of micrograms per liter, with higher concentrations in freshwater environments. Wada and Olawade [5] demonstrated the prevalent presence of pharmaceuticals in ground and surface water in the world, with levels of concern in African countries.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are pharmaceuticals widely used worldwide. As NSAIDs are over-the-counter medicines, their unrestricted consumption has increased, and, therefore, their presence in the environment has increased [1]. Diclofenac, an NSAID, is a monocarboxylic acid composed of a phenylacetic acid possessing an amino group at position two (2,6-chlorophenyl). It acts as an antipyretic, non-narcotic analgesic, and primarily as a nonsteroidal anti-inflammatory agent, prescribed to reduce inflammation and pain caused by arthritis, rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis, among other inflammatory diseases [6,7]. According to Lara-Pérez et al. [8], diclofenac ranks 12th in the pharmaceutical sales market, with an estimated annual consumption of 1443 ± 58 tons worldwide, making it the most widely consumed nonsteroidal anti-inflammatory drug globally. In Mexico, diclofenac consumption ranks fourth in medical centers in the State of México [9].
Like most pharmaceuticals, diclofenac is typically not completely removed through traditional wastewater treatment and can persist in aquatic environments due to its physicochemical properties [10,11]. Due to this, diclofenac can be detected in wastewater sludge, rivers, and sediments [12,13]. Also, diclofenac concentrations have been reported in drinking water sources [14,15,16]. For example, diclofenac has been detected in surface water in various countries such as China (11–356 ng L−1; 0.0058–843 ng L−1), India (0.36–278.9 ng L−1), Jordan (4.4 ng L−1), Malaysia (3–40 ng L−1), Singapore (0.8–38 ng L−1), and Spain (46 ng L−1) [17,18], as well as Pakistan (4900 ng L−1), and Taiwan (24–62 ngL−1) [1,19]. In Mexico, the diclofenac had been detected in state of Hidalgo (2.052–4.824 μg L−1), in the Madín dam located in the State of México (0.20–0.31 μg L−1), in the Lerma-Cutzamala waterway (0.028–0.032 μg L−1), and in untreated wastewaters (0.55 ng L−1) [20,21,22]. The toxic impact of diclofenac has been documented in various aquatic and terrestrial organisms, as well as in humans [23,24].
In the laboratory, the toxic effects of contaminants in aquatic environments are assessed by exposing organisms representing different trophic levels to controlled concentrations of the toxicant [25]. Because of their importance as primary producers, their rapid growth rate, their strong sensitivity to toxic substances, and their short response times, microalgae are considered a valuable and important bioindicator for assessing the impact of pollutants in aquatic environments and for inferring potential adverse effects at higher trophic levels [25,26,27]. Pseudokirchneriella subcapitata is one of the most frequently used microalgae in toxicity bioassays [28]. In recent years, this species has been utilized to assess the toxicity of certain pharmaceuticals [29,30].
Zooplankton is a key component of aquatic communities, linking phytoplankton and secondary consumers; this includes rotifers, copepods, and cladocerans. The latter group is the best-known and most extensively studied zooplankton group [31]. Daphnia curvirostris is a European cladoceran, but it was recorded as an exotic species in Canada, the United States, and México several years ago [32]. We hypothesize that the invasive character of this cladoceran to subtropical latitudes in America could be explained by its tolerance to environmental stressors, including pollutants. On these bases, we have conducted several studies in our laboratory using this cladoceran as a test organism. In this process, we have observed some advantages of this species, including its sensitivity to toxicants, which could make it an adequate species in aquatic toxicology. It could be a good option for Daphnia magna, which is not naturally distributed in Mexico, and for D. pulex, which is difficult to accurately identify because it is part of a species cluster with several hybrid morphotypes that are taxonomically difficult to distinguish. Dalla et al. [33] also conclude that D. curvirostris could be a suitable model for toxicological assays. Furthermore, data on the sensitivity of zooplankton species to diverse pollutants are scarce.
Zebrafish in their early life stages (ELS) are commonly used as a suitable and sensitive test organism in toxicity studies of various substances because of their affordable and easy rearing, oviparous condition, high fecundity, and rapid development. Additionally, due to the transparency of the chorion, it is possible to observe alterations during embryogenesis and teratogenesis, as well as morphological modifications that toxicants can cause [34,35]. Recently, the early life stages of zebrafish have been utilized to assess the toxicity of various pharmaceuticals [36,37,38].
When aquatic organisms representing different trophic levels are simultaneously used to test the toxic effects of pollutants or chemical stressors, this can be classified as a battery of bioassays. Its aim is to determine if differential effects of toxicant exposure occur in organisms with different response mechanisms and ecological functions; this is important to avoid generalizations regarding the potential impact of chemical pollution in the aquatic environment.
The toxicity to aquatic biota resulting from the release of diclofenac into water bodies has not been fully documented. Often, available studies on the ecotoxicity of diclofenac are conducted with a single species, which can lead to trophic level bias. Therefore, testing with species of different trophic levels could supply valuable information for a more comprehensive understanding of the impact of pharmaceuticals on aquatic biota and the environment. For this reason, the toxic effect of diclofenac was studied in a green microalga (P. subcapitata), a cladoceran (D. curvirostris), and a fish (D. rerio). Different stress responses were evaluated to better understand the possible adverse effects of this drug in these three trophic levels. The diclofenac toxic assessment with different organisms would enable us to document how these test organisms respond to this pharmaceutical and their comparative sensitivity by evaluating different endpoints.
2. Materials and Methods
Reagent grade, high purity (99%) diclofenac (molecular weight 318.3 g mol−1, CAS No. 15307-79-6) was obtained from Sigma Aldrich (St. Louise, MO, USA).
2.1. Test Organisms
The green microalga Pseudokirchneriella subcapitata, the cladoceran Daphnia curvirostris, and the embryos of zebrafish (Danio rerio) were obtained from the collections of the Laboratorio de Hidrobiología Experimental at the Instituto Politécnico Nacional, Escuela Nacional de Ciencias Biológicas, México.
The microalga was cultured in autoclaved Bold’s Basal Medium [39] in 250 mL Erlenmeyer glass flasks with a test volume of 165 mL. Cultures were incubated for 10 days at 25 °C ± 1 °C, with constant illumination of 100 µmol m−2 s−1 and continuous aeration. When the cultures were in the exponential growth phase, they were used as inocula for toxicity bioassays.
Parthenogenetic females of known age of Daphnia curvirostris were maintained in 1 L glass beakers with EPA medium [40] and fed with the microalga P. subcapitata (1 × 106 cells mL−1). Incubation conditions comprised a temperature of 25 °C and a photoperiod of 16:8 h (light/dark). All neonates used for acute and chronic toxicity bioassays were obtained from the third clutch and afterward of female propagation cultures.
Adult Danio rerio fish (females and males) were maintained in 40 L capacity glass aquaria in a water recirculation system at 26 °C and a 16:8 h photoperiod (light/dark), monitoring oxygen saturation (>80%), hardness (30–35 mg L−1 CaCO3), and pH (7.5–8.5). The fish were fed three times daily with Azoo® Plus Ultra Fresh (Madrid, Spain) micro pellets and live food, including Artemia and small cladocerans such as Ceriodaphnia dubia.
Figure 1 shows a general outline of the different studies conducted with the selected test organisms.
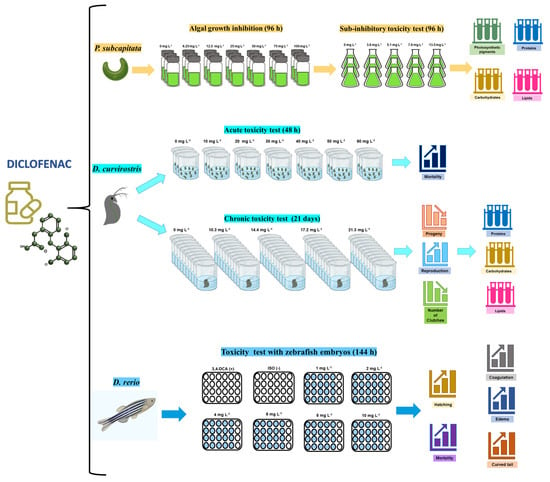
Figure 1.
Outline of the procedures applied in the evaluation of the toxic effects of diclofenac in microalgae, cladocerans, and fish.
2.2. Microalga
2.2.1. Toxicity of Diclofenac on the Growth of the Microalga P. subcapitata
Toxicity bioassays were performed according to OECD protocol 201 [41]. Glass vials of 50 mL were used, adding sterile OECD medium, 1 × 104 cells mL−1 as the initial inoculum, and different concentrations of diclofenac (0, 6.25, 12.5, 25, 50, 50, 75, and 100 mg L−1); these were determined from previous range-finding tests to calculate the IC50 value. Each treatment was performed in triplicate. Vials were incubated at 25 °C with constant illumination of 100 µmol m−2 s−1. The growth of P. subcapitata was assessed every 24 h for 4 days by counting cell density in the different drug concentrations using a Neubauer chamber. The mean inhibitory concentration (IC50) was calculated with the Probit method.
2.2.2. Effect of Diclofenac on Photosynthetic Pigments and Macromolecule Concentrations
Bioassays were performed in 250 mL Erlenmeyer flasks containing 165 mL test volume. Bold’s Basal Medium was used as the exposure medium. The algal inoculum was 5 × 104 cells mL−1. Diclofenac subinhibitory tested concentrations were 0, 3.6, 5.1, 7.6, and 13.5 mg L−1; these values were based on the determined IC50 and corresponded to the IC5, IC10, IC20, and IC40, respectively. Incubation conditions were 25 °C, constant fluorescent “daylight” illumination of 100 µmoles m−2s−1, and continuous aeration of 200 mL min−1. After 96 h, the obtained algal biomass was used to determine, in triplicate, the concentrations of photosynthetic pigments and macromolecules (proteins, carbohydrates, and lipids).
Photosynthetic pigments were extracted according to the procedure proposed by Hernández-Zamora and Martínez-Jerónimo [42]. Briefly, aliquots of 500 µL of algal culture from the control group and each diclofenac treatment were centrifuged at 3,500 rpm for 5 min to obtain a biomass pellet, which was then resuspended in 600 µL of DMSO. After 3 min, the absorbances of the samples were read at 665, 649, and 480 nm. The equations of Wellburn [43] were used for the quantification of chlorophyll-a, chlorophyll-b, and carotenoids:
- Chlorophyll-a = 12.19 A665 − 3.45 A649
- Chlorophyll-b = 21.99 A649 − 5.32 A665
- Carotenoids = (1000 A480 − 2.14 Chlorophyl-a − 70.16 Chlorophyl-b)/220
For the extraction of soluble proteins, aliquots of 500 µL were taken from the control group and the different concentrations of diclofenac treatments. The samples were centrifuged at 14,000 rpm for 5 min, and the supernatant was discarded. The algal pellets were added 300 µL of a 1 N NaOH solution and immediately heated in a thermoblock at 90 °C for 15 min. Afterward, the samples were centrifuged again at 14,000 rpm for 5 min. Protein quantification was performed according to Bradford’s method [44]. For each sample, 100 µL of the supernatant was taken, and 1000 µL of Bradford’s reagent was added. The absorbance was then read at 595 nm. A calibration curve was prepared using a 1% albumin solution.
Carbohydrate extraction was performed with the pellet from the control group and the different concentrations of the drug. The amount of 400 µL of a 2 N HCl solution was added to the samples, which were then heated in a thermoblock at 90 °C for 1 h, followed by centrifugation at 14,000 rpm for 5 min. Finally, 100 µL of the supernatant, 100 µL of a 5% phenol solution, and 500 µL of concentrated H2SO4 were added. The processed samples were shaken carefully and allowed to stand for 20 min. For quantification, absorbance at 490 nm was determined using a 1% glucose solution for calibration [45].
For lipid extraction, 500 µL of each sample, previously exposed to diclofenac and the control, was used. The samples were centrifuged at 14,000 rpm for 5 min. Then, 400 µL of chloroform, 200 µL of methanol, and 500 µL of deionized water were added. The samples were shaken for 20 s and then centrifuged at 14,000 rpm for 5 min. From the organic phase, 200 µL was taken to quantify the total lipids, according to the sulfophosphovanillin technique of Zöllner and Kich [46]. The samples and the 1% cholesterol calibration curve were read at 530 nm.
2.3. Toxicity of Diclofenac in Daphnia curvirostris
2.3.1. Acute and Chronic Toxicity
The acute toxicity was determined according to the USEPA [40]. Reconstituted hard water (EPA medium) was used for water dilutions and as the exposure medium for the controls. The test volume was 30 mL. Neonates (less than 24 h old) were exposed to 0, 10, 20, 30, 40, 50, and 60 mg L−1 of diclofenac, values based on the range-finding test to determine the LC50 value. The bioassays were incubated in a bioclimatic chamber at 25 °C with a 16:8 light/dark photoperiod. The number of affected and dead individuals was counted at 24 and 48 h. Four bioassays were implemented, and the average LC50 was calculated using the Probit method.
The chronic toxicity of diclofenac was evaluated following protocol 1002.0 of the USEPA [40]. For the bioassays, 100 mL beakers were used with a test volume of 80 mL. Reconstituted hard water (EPA medium) was used for water dilutions and as the exposure medium for the controls. The green microalga P. subcapitata (800,000 cells mL−1) was used as food. Four sublethal concentrations of diclofenac were assessed (10.3, 14.4, 17.2, and 21.3 mg L−1, equivalent to the previously determined LC1, LC5, LC10, and LC20, in the acute toxicity test). Each treatment had ten replicates; experiments were started with one neonate in each test vessel. The medium, the diclofenac concentration, and the food were renewed every 48 h. Experiments were incubated in an environmental chamber at 25 °C and a photoperiod of 16:8 (light/dark) for 21 days. Survival of reproducers was recorded daily. Once reproduction commenced, the neonates were separated, counted, and recorded daily. These records determined the total progeny, the number of clutches, and the age at first reproduction in each treatment.
2.3.2. Toxic Effects of Diclofenac on the Biomolecule Concentrations of Progeny
The content of proteins, carbohydrates, and lipids was evaluated in the progeny of the different diclofenac treatments and the control. Thirty neonates of D. curvirostris were used for each treatment and each macromolecule. These samples were then added 1 mL of phosphate buffer solution at pH 7.2 and subsequently macerated with a tissue homogenizer at medium speed for 2 min. The samples were ultra-frozen until processed for the macromolecule content, following the methods previously described.
2.4. Lethal and Sublethal Effects of Diclofenac on Danio rerio Embryos
The OECD 236 protocol [47] was followed to evaluate the effect of diclofenac on zebrafish embryos. Fertilized eggs were obtained under controlled conditions. Embryos (less than 2 h post-fertilization, hpf) were exposed in 24-well plates at 2 mL per well. Tested diclofenac concentrations were 1, 2, 4, 6, 8, and 10 mg L−1; these were based on a range-finding test. A solution of 4 mg L−1 of 3,4-dichloroaniline was used as the positive control, and the ISO medium served as the negative control. Samples were incubated in an environmental chamber at 25 °C with a photoperiod of 16:8 (light/dark) for 144 h. All the exposure solutions were renewed entirely at 24, 72, and 120 h. The effects of diclofenac on the early life stages of D. rerio were evaluated daily. Coagulation, lack of somite formation, absence of tail detachment, and absence of heartbeat were considered lethal responses. The edema and curved tail were considered sublethal effects [42,47]. Photographs of the zebrafish embryos in the control group and at the minimum and maximum concentrations of diclofenac were obtained using a stereomicroscope and analyzed with CellSens Standard ver. 1.
2.5. Statistical Analysis
The median inhibitory concentration (IC50) for P. subcapitata and the median lethal concentration (LC50) for D. curvirostris and Danio rerio were determined through the Probit method with the RA software (Risk Assessment, Hazard Assessment Tools, v. 1.0).
Data normality in the P. subcapitata and D. curvirostris data was confirmed with Shapiro–Wilk tests, and then a one-way analysis of variance (ANOVA) was used to compare the effect of diclofenac on photosynthetic pigments and macromolecule content in the microalga, and reproduction parameters (total progeny, number of litters, and age of first reproduction) in the cladoceran. A post hoc multiple comparison analysis was performed using Tukey’s test (p < 0.05). To determine differences among the treatments and the control group, the Dunnett’s test was conducted.
The Kruskal–Wallis test was used to analyze the effect of the different concentrations of diclofenac on the survival and mortality of D. rerio embryos. For all analyses, the software SigmaPlot ver. 11.0 and Statistica ver.10.0 were used. All graphs were created using GraphPad Prism version 10 software.
3. Results
3.1. Effect of Diclofenac on P. subcapitata: Growth, Photosynthetic Pigments, and Macromolecules
Figure 2A shows that as the drug concentration and exposure time increase, the cell density of the algae decreases significantly. In the control, the cell density was 357,500 cells mL−1. For the concentrations of 6.25, 12.5, 25, 50, 75, and 100 mg L−1 of diclofenac, growth decreased by 19, 41, 49, 91, 97, and 99%, respectively, compared to the control. The determined mean inhibitory concentration (IC50) was 16.57 mg L−1 (95% confidence limits: 10.0–24.15 mg L−1).
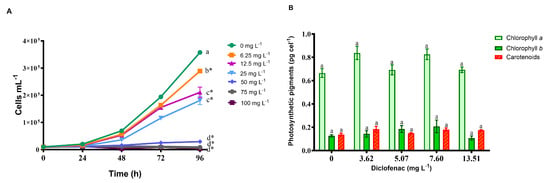
Figure 2.
Effect of diclofenac on population growth of P. subcapitata (A) and photosynthetic pigments (B), after 96 h of exposure. Mean values ± standard error. The asterisks indicate significant differences compared with the control series (Dunnett’s test, p < 0.05). Different letters indicate significant differences for each pigment (Tukey’s pairwise comparisons, p < 0.05).
Figure 2B shows the quantification of chlorophyll-a, -b, and carotenoids for each of the tested concentrations of diclofenac. In the case of chlorophyll-a, increases of 38.3 and 36.6% were found at concentrations of 3.62 and 7.6 mg L−1, respectively; however, no significant differences were determined compared to the control.
For chlorophyll-b, a 15.8% decrease was observed at the highest concentration of diclofenac (13.51 mg L−1), but no significant differences were observed at all the concentrations compared to the control. In the case of carotenoids, there was only an increase in the concentration of diclofenac of 3.62 mg L−1, but it was not significant.
Figure 3 illustrates the concentration of macromolecules. The protein content increased significantly across all concentrations. However, there were no significant differences among the diclofenac concentrations (Figure 3A). The lipid concentration increased significantly at all concentrations compared with the control (Figure 3B), reaching a triplicated value at 5.07 mg L−1 of diclofenac. Figure 3C shows that only the carbohydrates significantly increased at the highest concentration of the drug (13.51 mg L−1).
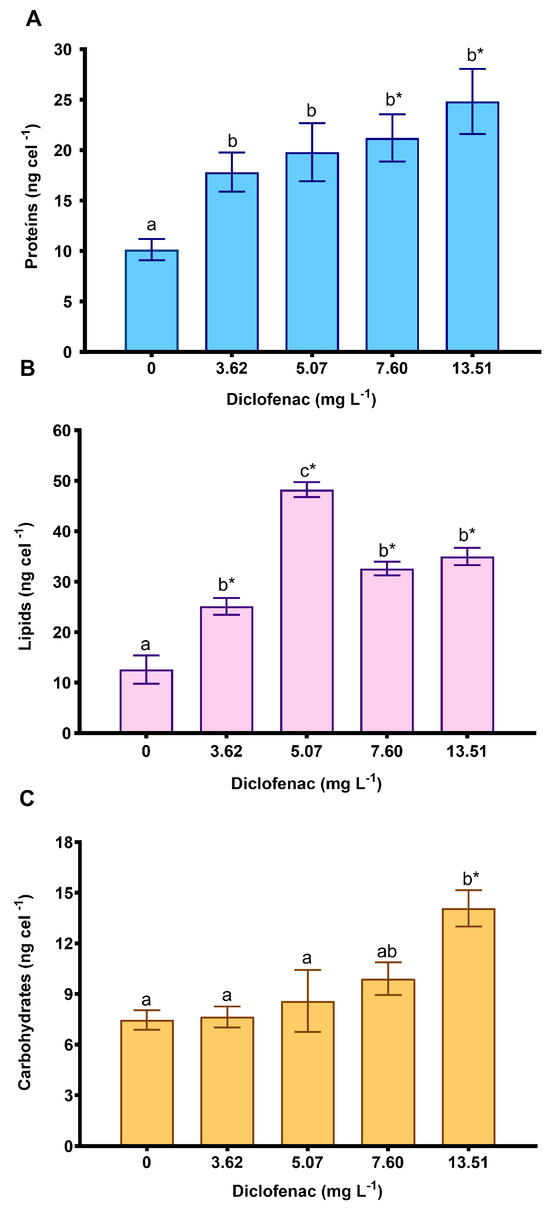
Figure 3.
Biomarkers in cells of P. subcapitata exposed to subinhibitory concentrations of diclofenac during 96 h: (A) proteins, (B) lipids and (C) carbohydrates. Mean values ± standard error. The asterisks indicate significant differences compared with the control (Dunnett’s test, p < 0.05). Different letters above the bars indicate significant differences (Tukey’s pairwise comparisons, p < 0.05).
3.2. Effect of Diclofenac on the Cladoceran D. curvirostris
The determined median lethal concentration (LC50) for diclofenac is 32.29 mg L−1 (95% confidence limits: 25.58–38.32 mg L−1).
The survival of D. curvirostris exposed to diclofenac for 21 days was 100% in the control and in all the diclofenac concentrations. Figure 4A–C show the reproductive parameters of the cladoceran observed in the chronic assay. The accumulated progeny was significantly reduced at all concentrations of diclofenac; however, the age of the first reproduction increased significantly at all concentrations, indicating a delay in the first reproduction. The number of clutches decreased by 6.8, 14.8, and 10.2% at concentrations of 10.3, 14.4, and 21.3 mg L−1 of diclofenac, respectively, compared to the control.
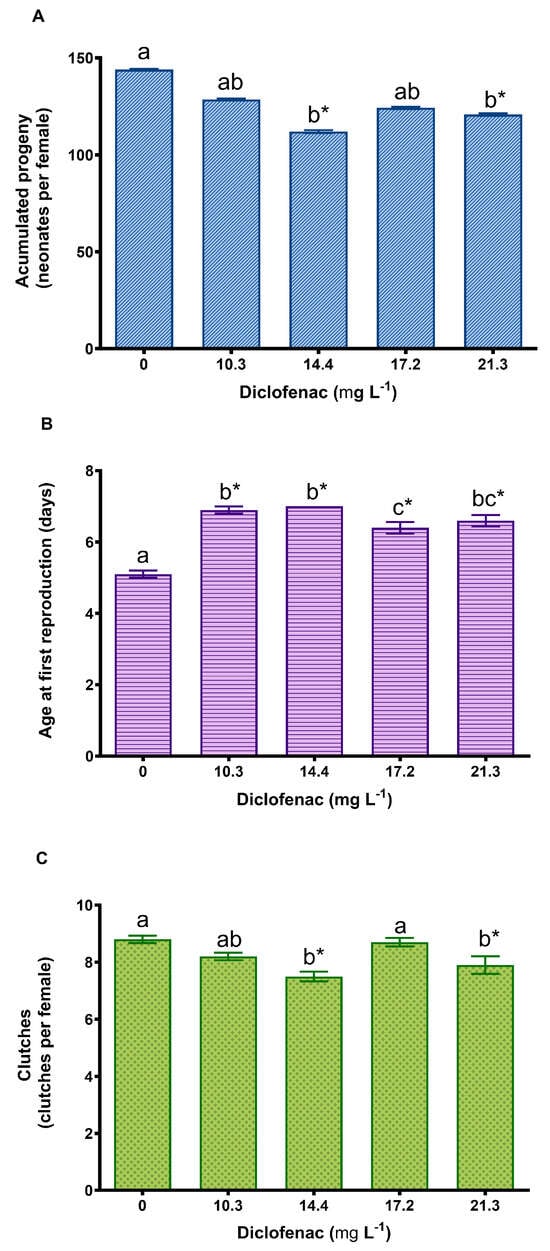
Figure 4.
Effect of diclofenac on Daphnia curvirostris after exposure for 21 days: (A) accumulated progeny, (B) age at first reproduction, and (C) number of clutches. Mean values ± standard error. The asterisk indicates significant differences from the control (Dunnett’s test, p < 0.05). Different letters above the bars indicate significant differences (Tukey’s pairwise comparisons, p < 0.05).
Regarding the effects on the macromolecule content, the protein concentration increased by 3.3, 4.6, 5, and 5.2 times (Figure 5A), and the carbohydrate content decreased by 32, 55.5, 46.9, and 58.2% (Figure 5B) at the diclofenac concentrations of 10.3, 14.4, 17.2, and 21.3 mg L−1, respectively. The lipid concentration decreased significantly as the diclofenac concentration increased (Figure 5C).
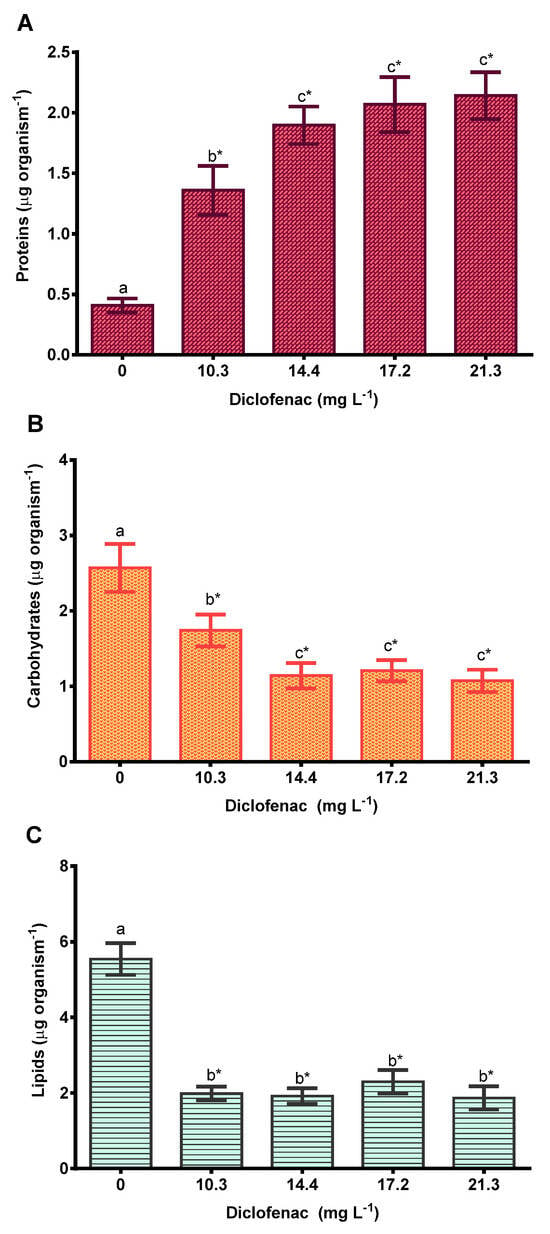
Figure 5.
Biomarkers in neonates of Daphnia curvirostris exposed to diclofenac for 21 days: (A) proteins, (B) carbohydrates, and (C) lipids. Mean values ± standard error. The asterisk indicates significant differences with reference to the control (Dunnett’s test, p < 0.05). Different letters above the bars indicate differences (Tukey’s pairwise comparisons, p < 0.05).
3.3. Effect of Diclofenac on D. rerio Embryos
Figure 6A shows that the hatching rate of D. rerio embryos decreased by 40, 51.6, and 80% at concentrations of 6, 8, and 10 mg L−1 of diclofenac, respectively, compared with the control. In contrast, the mortality of embryos and larvae increased as the concentration of diclofenac and the exposure time increased (Figure 6B). The determined LC50 of diclofenac in zebrafish embryos was 6.27 mg L−1 (confidence limits: 4.72 to 7.91 mg L−1). Diclofenac caused lethal effects in embryos, such as coagulation at 24 and 48 h exposure. Figure 7A shows that the percentage of coagulation of embryos was more significant at 24 h (16.6 and 35%, at 8 and 10 mg L−1 of diclofenac, respectively) than at 48 h. In addition, sublethal effects, such as edema and curved tails, were observed in D. rerio embryos at nearly all tested drug concentrations from 48 to 144 hpf. Figure 7B,C shows that the adverse effects of diclofenac (edema and curved tail) in D. rerio embryos increased as the concentration of the drug and the exposure time increased.
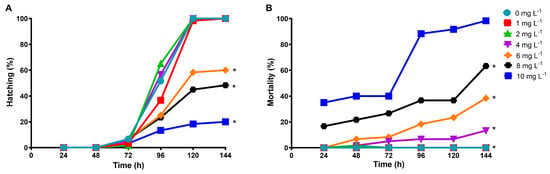
Figure 6.
Effect of diclofenac on embryos of Danio rerio after 144 h of exposure: (A) hatching; (B) mortality. The asterisks indicate differences compared with the control (Kruskal–Wallis test).
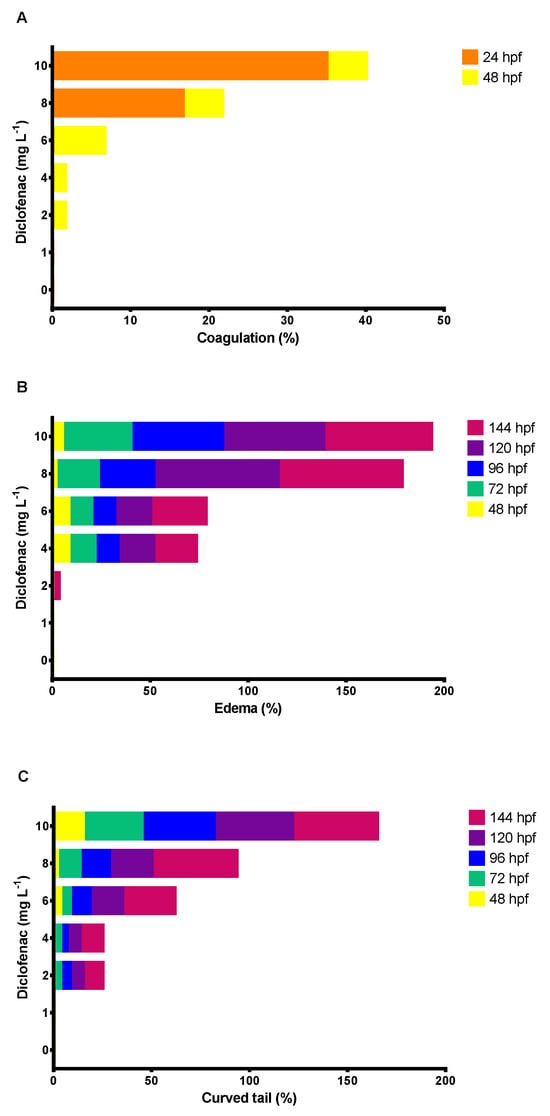
Figure 7.
Lethal and sublethal effects of diclofenac on embryos of Danio rerio after 144 h exposure: (A) coagulation; (B) edema; (C) curved tail.
Figure 8 illustrates the development of embryos in the control group and those exposed to diclofenac from 24 to 120 h post-fertilization (hpf). Organisms in the control and at a concentration of 1 mg L−1 did not exhibit lethal or sublethal effects. However, the embryos exhibited edema at the highest concentration of diclofenac (10 mg L−1). In embryos, deformities and curved tails in larvae were observed at 96 and 120 hpf.
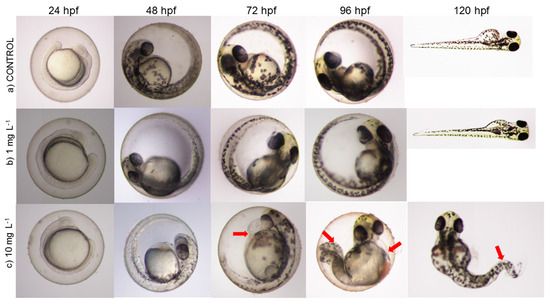
Figure 8.
Toxic effects of diclofenac on the development of zebrafish embryos after 120 h exposure. (a) Normal embryo and larva in the control; (b) normal embryo and larva in 1 mg L−1 of diclofenac; (c) exposure to 10 mg L−1 produced edema in the embryo at 72 and 96 hpf, and curved tail in the 120 hpf larva. The red arrows in the figure indicate sublethal effects.
4. Discussion
Table 1 shows the toxicity values reported for microalgae, cladocerans, and fish in other studies, for comparison with those obtained here.
The growth of P. subcapitata was decreased by exposure to diclofenac (Figure 2A). The mean inhibitory concentration (IC50) determined (16.57 mg L−1) is similar to the value reported by Ferrari et al. [48] (IC50 = 16.3 mg L−1) and is lower than that reported by Quinn et al. [49] (IC50 = 21.3 mg L−1) for this microalga. According to these values, it can be established that diclofenac is a slightly toxic substance for P. subcapitata, as per the criteria established by the USEPA [40]. Zind et al. [50] evaluated the effects of hydroxy-diclofenac and 2-[(2-chlorophenyl) amino] benzaldehyde, both products of the photodegradation of diclofenac, in P. subcapitata, determining the IC50 values of 3.42 and 8.62 mg L−1, respectively (Table 1). Therefore, it was determined that the toxicity of this drug’s metabolites is higher in microalgae, thereby increasing the potential risk associated with this emerging contaminant.
The evaluation of photosynthetic pigments allows for the assessment of alterations in photosynthesis. Diclofenac stimulated chlorophyll-a content in P. subcapitata, and chlorophyll-b decreased at the highest concentration of this drug (Figure 2B). This is consistent with the findings of Zhang et al. [51], who reported an increase in chlorophyll concentration in Chlorella pyrenoidosa exposed to low concentrations of diclofenac (<30 mg L−1), inferring that the drug could provide carbon and nitrogen sources that favored the synthesis of photosynthetic pigments. Gomaa et al. [52] reported that chlorophyll-a levels increased with increasing diclofenac concentration in Chlorella sp.; however, these authors consider this increase in the pigment to be a protective response against reactive oxygen species that could accumulate in the chloroplast. The change in the proportions of chlorophyll-a, -b, and carotenoids among the tested diclofenac concentrations we evaluated could be related to the light-harvesting activity of photosystem II, indicating the beginning of an antioxidant response, as pointed out by Wang et al. [53].
Exposure to the drug significantly modified the concentrations of proteins and lipids (Figure 3A,B). However, only the highest concentration of diclofenac produced a significant increase in carbohydrates (Figure 3C). The increase in protein concentration may be associated with a defense mechanism against environmental stress [54,55]. The proteins and polysaccharides in microalgae can interact with free radicals, protecting cells from oxidative damage [56]. Therefore, the increase in protein in P. subcapitata could be considered a response to the stress induced by diclofenac.
Mou et al. [57] noted that various factors, including nutrients, growth phase, and physical and chemical conditions, can influence the lipid content in microalgal cells. Zhang et al. [51] reported that concentrations lower than 30 mg L−1 of diclofenac promoted lipid accumulation in C. pyrenoidosa, a finding similar to the results observed in our study.
Escher et al. [58] and Corcoll et al. [59] indicated that in microalgae, the toxicity of diclofenac may be related to its effects on the membrane due to its high solubility in water, but also its lipophilicity. The chemical stress produced by diclofenac in P. subcapitata stimulated lipid production, similar to that observed by Zhang et al. [51].
Zhang et al. [51] found that diclofenac concentrations lower than 10 mg L−1 significantly promoted carbohydrate synthesis in the green alga C. pyrenoidosa. The increase in carbohydrates at low diclofenac concentrations is directly related to environmental stress, as carbohydrates play a role in removing reactive oxygen species [60]. This may have occurred in our case when exposing P. subcapitata to diclofenac.
The 48 h LC50 of diclofenac determined here in D. curvirostris (32.29 mg L−1) is similar to the value reported by Ferrari et al. [48] and Haap et al. [61] in Daphnia magna (EC50 = 22.4 and 44.7 mg L−1, respectively). For D. similis, Junqueira de Castro et al. [62] determined an EC50 value of 46 mg L−1. These results indicate similar sensitivity to diclofenac in different cladocerans (Table 1). However, Barbosa et al. [63] reported an LC50 for diclofenac of 24.6 g L−1 in D. magna, which is a very high value, suggesting that this drug may not produce significant acute toxicity in this cladoceran; it is possible that the strain or test conditions could have influenced the low sensitivity in that study. Different studies in laboratory conditions (Table 1) have shown that the toxicity values of diclofenac in aquatic organisms range from 480 μg L−1 to 24.6 g L−1. Despite the concentrations of diclofenac in the environment being lower than experimentally reported values (0.0058 ng L−1 to 606.1 ng L−1), the environmental concern of this compound is due to its increasing presence in different bodies of water on all the continents, its bioaccumulation potential, and the transformation into toxic byproducts [5]. In this respect, Schmitt-Jansen et al. [64] demonstrated that 50 mg L−1 of diclofenac did not produce toxicity in aquatic organisms under laboratory conditions. Still, these authors demonstrated that the photo-transformation products of diclofenac increased the toxicity sixfold after 53 h of natural sunlight exposure.
In chronic exposure, the survival of D. curvirostris was not affected by diclofenac. Similar results were observed by Lee et al. [65] in D. magna and Moina macrocopa, who reported that concentrations lower than 75 and 150 mg L−1, respectively, did not affect survival. According to Dodson and Hanazato [66], under stress conditions, organisms’ energy is primarily allocated to ensuring survival, although at the cost of diverting energy resources from reproduction.
Our study showed that both the number of clutches and the accumulated progeny decreased significantly (Figure 4A,C) in D. curvirostris exposed to subacute diclofenac concentrations, similar to that reported by Lee et al. [65], who reported that the number of clutches in D. magna and M. macrocopa decreased significantly as diclofenac concentrations increased. Smirnov [67] reported that cladocerans exhibit a plastic response, allowing them to adjust their reproductive rates according to the environmental conditions in which they develop. In this respect, Edward and Chapman [68] also note that exposure to an environmental stressor causes organisms to allocate more energy toward survival, thereby decreasing their reproductive capacity. Beyers and Odum [69] indicated that modifications in the homeostasis trigger compensatory mechanisms in the organisms, demanding a significant amount of energy, leading to a redistribution of energy and a subsequent reduction in the allocated energy available for growth and reproduction. What we observed in D. curvirostris exposed chronically to diclofenac confirms what several authors have reported regarding the tradeoff that cladocerans make in the face of environmental stress.
Our results also demonstrated that the age at first reproduction significantly increased in D. curvirostris at different concentrations of diclofenac (Figure 4C). Similar results were reported by Liu et al. [70] for D. magna, who found that the age at first reproduction increased significantly at 50 mg L−1. However, Lee et al. [65] determined that in the cladoceran M. macrocopa, the age at first reproduction decreased at this same concentration of diclofenac. Barbosa et al. [63] demonstrated that this medicine did not affect the reproduction of D. magna. However, LfW [71] reported that diclofenac affected the reproduction of D. magna during a 21-day exposure period, which is consistent with our study’s findings.
Liu et al. [70] suggest that diclofenac is an endocrine disruptor in D. magna, promoting vitellogenin expression at high diclofenac concentrations (500 and 5000 µg L−1). However, Le et al. [72] noted that vitellogenin expression could have positive effects on the reproductive capacity of D. magna in 21-day bioassays; a similar phenomenon may have occurred in D. curvirostris, but further gene expression studies are required to confirm this hypothesis.
Cladocerans can store energy in macromolecules such as carbohydrates, lipids, and proteins, although this depends on their age and physiological and reproductive condition. Some macromolecules constitute the body’s caloric reserve and can be modified in response to physical and nutritional factors, environmental stress, and exposure to toxic compounds [73]. Chronic exposure of D. curvirostris to diclofenac resulted in variations in the concentration of macromolecules (Figure 5A–C), with proteins increasing significantly in all treatments. At the same time, lipids and carbohydrates were inhibited compared to the control at all concentrations of the drug tested. Results similar to ours were observed in D. magna during exposure to glyphosate (Roundup®) [74]. The author mentioned that this herbicide inhibits carbohydrases and stimulates proteases, resulting in a reduction in carbohydrates and an increase in protein levels, as observed in the results of our research. Calow and Silby [75] note that the exposure of cladocerans to toxic chemical stressors can result in adverse metabolic effects, decreasing energy reserves, and impairing growth and reproduction. Sancho et al. [76] report that carbohydrates constitute a vital energy reserve that can be efficiently utilized to obtain energy and thus cope with the harmful effects of toxic compounds. This observation aligns with our findings in D. curvirostris, where exposure to the drug resulted in decreased reproduction and reduced amounts of lipids and carbohydrates.
Lipids provide a significant portion of the energy required for growth, locomotion, and reproduction, in addition to their importance in cellular structure and membranes in aquatic organisms [77]. Arts and Wainmann [78] emphasize the significance of lipids for zooplankton organisms and their responses to natural and anthropogenic stressors and environmental parameters. During the early stages of development, parthenogenetic females allocate lipid reserves to egg formation and embryo development before being released as juveniles from the brood chamber. Lipid concentration is also related to the energy investment in offspring, which the mother provides as reserves for neonates [79]. Therefore, a change in lipid and carbohydrate content, as the primary energy sources, would suggest that organisms under stress conditions, due to exposure to diclofenac, use more energy to survive but reduce their reproduction, as observed in our results.
The here-determined LC50 of diclofenac for the early-life stages of D. rerio was 6.27 mg L−1. This value is higher than that reported by Chabchoubi et al. [80], who determined an LC50 of 2.93 mg L−1 for ELS. Praskova et al. [81] reported LC50 values of 6.11 mg L−1 and 166.6 mg L−1, respectively, for D. rerio embryos and juveniles, indicating a higher sensitivity in embryos. In their study, van den Brandhof and Montforts [82] determined an EC50 of 5.3 mg L−1. Nevertheless, Dietrich and Prietz [83] reported an LC50 of 480 µg L−1 in zebrafish embryos (Table 1). In our study, concentrations of diclofenac from 6 to 10 mg L−1 caused a decrease in the hatching percentage of D. rerio embryos (Figure 6A), similar to that observed by Hallare et al. [84], who mentioned that hatching time was affected at 96 h at diclofenac concentrations higher than 2 mg L−1. Xia et al. [85] reported that exposure of zebrafish embryos to 500 µg L−1 of diclofenac caused a decrease in the hatching percentage of 58, 33, and 19% at 55, 57, and 59 hpf, respectively. D. rerio embryos exposed to 2.95 and 12.5 mg L−1 of diclofenac showed a significant decrease in hatching rate compared to lower concentrations, as reported by van den Brandhof and Montforts [82] and Ribeiro et al. [86], respectively. In contrast, Horie et al. [87] reported no significant differences in hatching time at diclofenac concentrations ranging from 0.4 to 7 mg L−1; however, organisms exposed to 7 mg L−1 died after hatching. Escapa et al. [88] reported that 81% of unhatched embryos died at 12.5 mg L−1 of diclofenac. In our research, D. rerio larval mortality was greater than 50% at a concentration of 10 mg L−1 of diclofenac after 96 hpf (Figure 6B). Similar results were described by Yadav et al. [89] with a concentration of 11.25 mg L−1; however, Zhou et al. [90] mention that the 48 h LC50 in D. rerio larvae was 14.15 mg L−1. Escapa et al. [88] reported that after 80 hpf, zebrafish embryo mortality increased dramatically at diclofenac concentrations equal to or greater than 2.5 mg L−1, and at a concentration of 25 mg L−1 all embryos died before hatching. Ribeiro et al. [86] reported that concentrations of 1.25 and 12.5 mg L−1 caused 21.3 and 18.8% mortality in D. rerio, respectively. van den Brandhof and Montforts [82] demonstrated that the mortality of zebrafish embryos increased from 50 to 100% at diclofenac concentrations of 11.6 and 23.3 mg L−1, respectively. In other studies, Glaberman et al. [91] and Bereketoglu et al. [92] mention that the variation in D. rerio mortality can be attributed to the experimental conditions, the exposure period, the purity of the tested product, and the particular susceptibility of the test organism batches, so it is essential to describe these factors accurately to enable a more appropriate comparison of results.
Regarding the lethal effects of diclofenac on D. rerio embryos, our results show that drug concentrations of 2 to 10 mg L−1 caused embryo coagulation at 24 and 48 hpf (Figure 7A). Similar results were reported by van den Brandhof and Montforts [82] at nominal concentrations of 5.8 and 23.3 mg L−1 of the anti-inflammatory (Table 1). A diclofenac concentration of 1 mg L−1 did not produce mortality or malformations; similar results were reported by Hallare et al. [84] with D. rerio embryos exposed to diclofenac concentrations of 1 to 2 mg L−1 for 96 h. In contrast, our results demonstrate that concentrations greater than 2 mg L−1 produced sublethal effects, such as tail curling and edema (Figure 7B,C), as observed in other studies [81,82].

Table 1.
Reported toxicity data of diclofenac and some diclofenac metabolites for different aquatic organisms.
Table 1.
Reported toxicity data of diclofenac and some diclofenac metabolites for different aquatic organisms.
| Species | Chemical Compound | Exposure Time | IC50/EC50/LC50 | Reference |
|---|---|---|---|---|
| Pseudokircheneriella subcapitata | Diclofenac | 96 h | IC50: 16.3 mg L−1 | Ferrari et al. [48] |
| P. subcapitata | Diclofenac | 72 h | IC50: 64.8 mg L−1 | Quinn et al. [49] |
| P. subcapitata | Diclofenac | 96 h | EC50: 6 mg L−1 | Zind et al. [50] |
| P. subcapitata | Hydroxy-diclofenac | 96 h | IC50: 3.42 mg L−1 | Zind et al. [50] |
| P. subcapitata | 2-[(2-chlorophenyl) amino] benzaldehyde | 96 h | IC50: 8.62mg L−1 | Zind et al. [50] |
| P. subcapitata | Diclofenac | 96 h | IC50: 16.57 mg L−1 | This study |
| Daphnia magna | Diclofenac | 48 h | EC50: 22.4 mg L−1 | Ferrari et al. [48] |
| D. magna | Diclofenac | 48 h | EC50: 39.9-44.7 mg L−1 | Haap et al. [61] |
| D. magna | Diclofenac | 48 h | LC50: 80.1 mg L−1 | Zind et al. [50] |
| D. magna | Diclofenac | 48 h | LC50: 24.6 g L−1 | Barbosa et al. [63] |
| D. similis | Diclofenac | 48 h | EC50: 46 mg L−1 | Junqueira de Castro et al. [62] |
| D. curvirostris | Diclofenac | 48 h | LC50: 32.29 mg L−1 | This study |
| Danio rerio | Diclofenac | 120 h | LC50: 37.7 mg L−1 | Chabchoubi et al. [80] |
| D. rerio | Diclofenac | 144 h | LC50: 6.11 mg L−1 | Praskova et al. [81] |
| D. rerio | Diclofenac | 96 h | LC50: 166.6 mg L−1 | Praskova et al. [81] |
| D. rerio | Diclofenac | 72 h | LC50: 7.8 mg L−1 | van den Brandhof and Montforts [82] |
| D. rerio | Diclofenac | 72 h | EC50: 5.3 mg L−1 | van den Brandhof and Montforts [82] |
| D. rerio | Diclofenac | 96 h | LC50: 480 μg L−1 | Dietrich and Prietz [83] |
| D. rerio | Diclofenac | 144 h | LC50: 6.27 mgL−1 | This study |
Escapa et al. [88] reported that diclofenac concentrations of 6.5, 12.5, and 25 mg L−1 caused yolk sac deformities, pericardial edema, and lack of pigmentation in D. rerio embryos at 32 hpf. These authors also noted that embryos exposed for 144 h to a low concentration of diclofenac (2.5 mg L−1) had a 25% mortality rate, and live embryos exhibited excessive pigmentation, reduced motility, and abnormalities in the yolk sac. Chabchoubi et al. [80] reported that diclofenac concentrations of 0.86 and 2.8 mg L−1 caused deformities, including scoliosis and lordosis, respectively. They also observed that the drug exposure induced skin depigmentation and edema in the embryos, findings consistent with our study.
Diclofenac has been reported to act as a cytotoxic agent due to membrane permeabilization [93], which could be related to the observed effects. Less than 5% of diclofenac was associated with the embryo of D. rerio, and more than 95% remained in the aqueous solution. Of the drug linked to the fish embryo, roughly 53% was retained on the membranes, while the remaining is internalized into the cytosol of the cells, causing various abnormalities, diminishing embryo mobility, and reducing their hatching potential [94]; this effect was also observed in our study. Exposure of D. rerio embryos to high concentrations of the drug (10 mg L−1) produced edema and tail curvature (Figure 8). These results are similar to those described by Horie et al. [87] during exposure to 7 mg L−1 of diclofenac; these authors reported that larvae with abnormal spine development had also body curvature, suggesting that this deformity is caused by abnormalities in the vertebrae, which cause the organisms to die a week later. In other studies, Buchan et al. [95] and Gray et al. [96] have noted that several genes are associated with the development of the spine during embryogenesis, including kinesin family member 6 (KIF6) and recessive mutations in collagen type VIII, alpha 1a (col8a1a). Based on the above, we can conclude that the combination of sublethal effects, such as edema and tail curling in D. rerio embryos, could drive lethal effects in the larval stages.
The Commission of the European Communities [97] classifies chemical pollutants into risk classes based on their EC50 values. This legislation establishes that for aquatic organisms, an EC50 < 1 mg L−1 is for a highly toxic compound, values between 1 and 10 mg L−1 are for toxic chemicals, and values between 11 and 100 mg L−1 are for pollutants classified as harmful to aquatic organisms. Based on this classification and the results of our research, diclofenac could be considered a highly toxic compound for D. rerio and toxic for P. subcapitata and D. curvirostris.
Although diclofenac is not considered a persistent compound, aquatic organisms such as microalgae, cladocerans, and fish may be exposed to it for prolonged periods due to its incorporation into the aquatic environment through continuous release from hospital and municipal wastewaters, where drug concentrations tend to be higher [98]. Despite the rapid photodegradation of diclofenac, its metabolites are oxidized to benzoquinonimine intermediates, which are more persistent in the environment and more toxic to aquatic organisms [99].
In an integrative analysis of the toxic effects of diclofenac on microalgae, cladocerans, and fish embryos, we determined that the response is species-specific and could depend on the position of organisms in the food web and the complexity of the mechanisms of response to environmental stressors they have. Physiological and significant damages in vital processes could explain the differences in their sensitivity to toxicants. Our results demonstrated that D. rerio embryos were the most sensitive species to diclofenac, followed by the green microalga P. subcapitata. Finally, the cladoceran D. curvirostris was the most resistant species. The high sensitivity of fish to diclofenac could be attributed to a greater incorporation of this pharmaceutical through the chorion of the eggs and the interference of this chemical in the embryonic development.
In this research, toxicological bioassays with different aquatic organisms allow us to construct a risk scenario in a broader context. Although the concentrations of diclofenac here tested could be higher than those detected in aquatic ecosystems, pharmaceuticals, as pollutants of emerging concern, could have a significant contribution to water pollution in the following years, as these are products of mass consumption, and diclofenac, particularly, is a popular drug for pain relief, used without prescription.
5. Conclusions
Diclofenac caused significant toxicity on the population growth of P. subcapitata and in the reproductive parameters of D. curvirostris; it also caused lethal and sublethal effects in embryos and larvae of D. rerio. Our results warn about the toxic effects of this medical product on the food web structure in aquatic communities, especially in primary producers (microalgae), filter-feeding consumers (such as zooplankton), and predators (fish) in the aquatic biota. Therefore, different endpoints should be determined in aquatic organisms for a comprehensive assessment of the toxicity of drugs that can be classified as contaminants of emerging concern. This will enable the establishment of maximum permissible limits for these compounds before they are released into aquatic ecosystems, including hospital and domestic discharges and effluents discharged from treatment plants.
Author Contributions
Conceptualization, M.H.-Z.; Methodology, L.M.C.-C., L.M.-J. and F.M.-J.; Validation, F.M.-J.; Formal analysis, M.H.-Z., L.M.-J. and F.M.-J.; Investigation, L.M.C.-C. and F.M.-J.; Data curation, L.M.C.-C. and L.M.-J.; Writing—review & editing, M.H.-Z. and F.M.-J.; Supervision, F.M.-J.; Funding acquisition, F.M.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Instituto Politécnico Nacional, SIP-20231333.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity-a review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.B. Pharmaceutical and personal care products (PPCPs) in urban receiving waters. Environ. Pollut. 2006, 144, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Abdulrazaq, Y.; Abdulsalam, A.; Rotimi, A.L.; Abdulbasit, A.A.; Clifford, O.; Abdulsalam, O.A.; Bilal, M. Classification, Potential Routes and Risk of Emerging Pollutants/Contaminant. In Emerging Contaminants; Nuro, L., Ed.; IntechOpen: London, UK, 2020; pp. 1–12. [Google Scholar]
- Mezzelani, M.; Gorbi, S.; Regoli, F. Pharmaceuticals in the aquatic environments: Evidence of emerged threat and future challenges for marine organisms. Mar. Environ. Res. 2018, 140, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Wada, O.Z.; Olawade, D.B. Recent occurrence of pharmaceuticals in freshwater, emerging treatment technologies, and future considerations: A review. Chemosphere 2025, 374, 144153. [Google Scholar] [CrossRef]
- Parolini, M. Toxicity of the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) acetylsalicylic acid, paracetamol, diclofenac, ibuprofen and naproxen towards freshwater invertebrates: A review. Sci. Total Environ. 2020, 740, 140043. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information NCBI. 2023; PubChem Compound Summary for CID 3033, Diclofenac. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diclofenac (accessed on 13 June 2024).
- Lara-Pérez, C.; Leyva, E.; Zermeño, B.; Osorio, I.; Montalvo, C.; Moctezuma, E. Photocatalytic degradation of diclofenac sodium salt: Adsorption and reaction kinetic studies. Environ. Earth Sci. 2020, 79, 277. [Google Scholar] [CrossRef]
- Gómez-Oliván, L.; Carmona-Zepeda, F.; Galar-Martínez, M.; Téllez-López, A.; Amaya Chávez, A. Estudio de automedicación en una farmacia comunitaria de la ciudad de Toluca. Rev. Mex. Cienc. Farma 2009, 40, 5–11. [Google Scholar]
- Luongo, G.; Guida, M.; Siciliano, A.; Libralato, G.; Saviano, L.; Amoresano, A.; Zarrelli, A. Oxidation of diclofenac in water by sodium hypochlorite: Identification of new degradation by-products and their ecotoxicological evaluation. J. Pharm. Biomed. Anal. 2021, 194, 113762. [Google Scholar] [CrossRef]
- Mohan, H.; Rajput, S.S.; Jadhav, E.B.; Sankhla, M.S.; Sonone, S.S.; Jadhav, S.; Kumar, R. Ecotoxicity, Occurrence, and Removal of Pharmaceuticals and Illicit Drugs from Aquatic Systems. Bio. Res. Appl. Chem 2021, 11, 12530–12546. [Google Scholar]
- Langford, K.H.; Reid, M.; Thomas, K.V. Multi-residue screening of prioritised human pharmaceuticals, illicit drugs and bactericides in sediments and sludge. J. Environ. Monit. 2011, 13, 2284–2291. [Google Scholar] [CrossRef]
- Kunkel, U.; Radke, M. Fate of pharmaceuticals in rivers: Deriving a benchmark dataset at favorable attenuation conditions. Water Res. 2012, 46, 5551–5565. [Google Scholar] [CrossRef] [PubMed]
- Carmona, E.; Andreu, V.; Picó, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River basin: From waste to drinking water. Sci. Total Environ. 2014, 484, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Simazaki, D.; Kubota, R.; Suzuki, T.; Akiba, M.; Nishimura, T.; Kunikane, S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015, 76, 187–200. [Google Scholar] [CrossRef]
- Tröger, R.; Klöckner, P.; Ahrens, L.; Wiberg, K. Micropollutants in drinking water from source to tap—Method development and application of a multiresidue screening method. Sci. Total Environ. 2018, 627, 1404–1432. [Google Scholar] [CrossRef]
- Shamsudin, M.S.; Farhan, S.A.; Suzylawati, I. A review of diclofenac occurrences, toxicology, and potential adsorption of clay-based materials with surfactant modifier. J. Environ. Chem. Eng. 2022, 10, 107541. [Google Scholar] [CrossRef]
- Iglesias, A.; Nebot, C.; Vázquez, B.I.; Coronel-Olivares, C.; Abuín, C.M.F.; Cepeda, A. Monitoring the presence of 13 active compounds in surface water collected from rural areas in Northwestern Spain. Int. J. Environ. Res. Public Health 2014, 11, 5251–5272. [Google Scholar]
- Hashim, N.H.; Nasir, M.H.; Ramlee, M.S. Emerging pollutant of concern: Occurrence of pharmaceutical compounds in Asia with particular preference to Southeast Asia countries. MATEC Web Conf. 2016, 47, 05026. [Google Scholar] [CrossRef]
- Gibson, R.; Durán-Álvarez, J.C.; Estrada, K.L.; Chávez, A.; Jiménez Cisneros, B. Accumulation and leaching potential of some pharmaceuticals and potential endocrine disruptors in soils irrigated with wastewater in the Tula Valley, Mexico. Chemosphere 2010, 81, 1437–1445. [Google Scholar] [CrossRef]
- González-González, E.; Gómez-Oliván, L.; Galar-Martínez, M.; Vieyra-Reyes, P.; Islas-Flores, H.; García-Medina, S.; Jiménez-Vargas, J.; Razo-Estrada, C.; Pérez-Pastén, R. Metals and nonsteroidal anti-inflammatory pharmaceuticals drugs present in water from Madín Reservoir (Mexico) induce oxidative stress in gill, blood, and muscle of common carp (Cyprinus carpio). Arch. Environ. Contam. Toxicol. 2014, 67, 281–295. [Google Scholar] [CrossRef]
- Siemens, J.; Huschek, G.; Siebe, C.; Kaupenjohann, M. Concentrations and mobility of human pharmaceuticals in the world’s largest wastewater irrigation system, Mexico City-Mezquital Valley. Water Res. 2008, 42, 2124–2134. [Google Scholar] [CrossRef]
- Schwaiger, J.; Ferling, H.; Mallow, U.; Wintermayr, H.; Negele, R.D. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: Part I: Histopathological alterations and bioaccumulation in rainbow trout. Aquat. Toxicol. 2004, 68, 141–150. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Meena, R.A.A.; Palanisami, T.; Ashokkumar, V.; Palvannan, T.; Gu, F.L. Occurrence, interactive effects, and ecological risk of diclofenac in environmental compartments and biota-a review. Sci. Total Environ. 2020, 698, 134057. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, S.; An, S.A.; Khim, J.S. Methodological advances and future directions of microalgal bioassays for evaluation of potential toxicity in environmental samples: A review. Environ. Int. 2023, 173, 107869. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.; Mally, A.; Liedtke, D. Zebrafish embryos and larvae as alternative animal models for toxicity testing. Int. J. Mol. Sci. 2021, 22, 13417. [Google Scholar] [CrossRef]
- Nestler, H.; Groh, K.J.; Schönenberger, R.; Behra, R.; Schirmer, K.; Eggen, R.I.; Suter, M.J.F. Multiple-endpoint assay provides a detailed mechanistic view of responses to herbicide exposure in Chlamydomonas reinhardtii. Aquat. Toxicol. 2012, 110, 214–224. [Google Scholar] [CrossRef]
- Martínez-Jerónimo, F.F. Conceptos Generales. In Ensayos Toxicológicos y Métodos de Evaluación de Calidad de Aguas; En Castillo, G., Ed.; IMTA: Jiutepec, Mexico, 2004; pp. 89–98. [Google Scholar]
- Mofeed, J. Effects of three commonly used pharmaceutical products on biochemical parameters of the Micro-alga Pseudokirchneriella subcapitata (Under Laboratory Conditions). Catrina Inter. J. Environ. Sci. 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Udebuani, A.C.; Pereao, O.; Akharame, M.O.; Fatoki, O.S.; Opeolu, B.O. The potential ecological risk of veterinary pharmaceuticals from swine wastewater on freshwater aquatic environment. Water Environ. Res. 2023, 95, e10833. [Google Scholar] [CrossRef]
- Conde-Porcuna, J.M.; Ramos-Rodríguez, E.; Morales-Baquero, R. El zooplancton como integrante de la estructura trófica de los ecosistemas lénticos. Ecosistemas 2004, 13, 23–29. [Google Scholar]
- Nandini, S.; Silva-Briano, M.; García, G.G.; Sarma, S.S.S.; Adabache-Ortiz, A.; de la Rosa, R.G. First record of the temperate species Daphnia curvirostris Eylmann, 1887 emend. Johnson, 1952 (Cladocera: Daphniidae) in Mexico and its demographic characteristics in relation to algal food density. Limnology 2009, 10, 87–94. [Google Scholar] [CrossRef]
- Dalla Bona, M.; Di Leva, V.; De Liguoro, M. The sensitivity of Daphnia magna and Daphnia curvirostris to 10 veterinary antibacterials and to some of their binary mixtures. Chemosphere 2014, 115, 67–74. [Google Scholar] [CrossRef]
- Zhang, C.; Willett, C.; Fremgen, T. Zebrafish: An animal model for toxicological studies. Curr. Protoc. Toxicol. 2003, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.E.; Conforti, V.; Ruiz, L.; Gomez, N. An in situ test to explore the responses of Scenedesmus acutus and Lepocinclis acus as indicators of the changes in water quality in lowland streams. Ecotoxicol. Environ. Saf. 2012, 77, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Cunha, V.; Oliva-Teles, L.; Ferreira, M.; Guimarães, L. Norfluoxetine and venlafaxine in zebrafish larvae: Single and combined toxicity of two pharmaceutical products relevant for risk assessment. J. Hazard. Mater. 2020, 400, 123171. [Google Scholar] [CrossRef]
- Zhang, K.; Yuan, G.; Werdich, A.A.; Zhao, Y. Ibuprofen and diclofenac impair the cardiovascular development of zebrafish (Danio rerio) at low concentrations. Environ. Pollut. 2020, 258, 113613. [Google Scholar] [CrossRef]
- Porretti, M.; Arrigo, F.; Di Bella, G.; Faggio, C. Impact of pharmaceutical products on zebrafish: An effective tool to assess aquatic pollution. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 261, 109439. [Google Scholar] [CrossRef]
- Stein, J.R. Handbook of Phycological Methods. Culture Methods and Growth Measurements; Cambridge University Press: London, UK, 1973; pp. 7–24. [Google Scholar]
- US Environmental Protection Agency USEPA. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organism, 5th ed.; EPA-821-R-02-012; Office of Research and Development: Cincinnati, OH, USA, 2002.
- Organisation for Economic Co-Operation and Development (OECD). Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2011. [Google Scholar]
- Hernández-Zamora, M.; Martínez-Jerónimo, F. Congo red diversely affects organisms of different trophic levels: A comparative study with microalgae, cladocerans and zebrafish embryos. Environ. Sci. Pollut. Res. Int. 2019, 26, 11743–11755. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zöllner, N.; Kirsch, K. Über die quantitative Bestimmung von Lipoiden (Mikromethode) mittels der vielen natürlichen Lipoiden (allen bekannten Plasmalipoiden) gemeinsamen Sulfophosphovanillin-Reaktion. Z. Für Die Gesamte Exp. Med. 1962, 135, 545–561. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Test No. 236: Fish Embryo Acute Toxicity (FET) Test. OECD Guidelines for the Testing of Chemicals; OECD: Paris, France, 2013. [Google Scholar]
- Ferrari, B.; Mons, R.; Vollat, B.; Fraysse, B.; Paxéus, N.; Lo Giudice, R.; Pollio, A.; Garric, J. Environmental risk assessment of six human pharmaceuticals: Are the current environmental risk assessment procedures sufficient for the protection of the aquatic environment? Environ. Toxicol. Chem. 2004, 23, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.; Schmidt, W.; O’Rourke, K.; Hernan, R. Effects of the pharmaceuticals gemfibrozil and diclofenac on biomarker expression in the zebra mussel (Dreissena polymorpha) and their comparison with standardised toxicity tests. Chemosphere 2011, 84, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Zind, H.; Mondamert, L.; Remaury, Q.B.; Cleon, A.; Leitner, N.K.V.; Labanowski, J. Occurrence of carbamazepine, diclofenac, and their related metabolites and transformation products in a French aquatic environment and preliminary risk assessment. Water Res. 2021, 196, 117052. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Yao, T.; Zhang, Y.; Zhou, X.; Chu, H. The influence of four pharmaceuticals on Chlorella pyrenoidosa culture. Sci. Rep. 2019, 9, 1624. [Google Scholar] [CrossRef]
- Gomaa, M.; Zien-Elabdeen, A.; Hifney, A.F.; Adam, M.S. Phycotoxicity of antibiotics and non-steroidal anti-inflammatory drugs to green algae Chlorella sp. and Desmodesmus spinosus: Assessment of combined toxicity by Box–Behnken experimental design. Environ. Technol. Innov. 2021, 23, 101586. [Google Scholar] [CrossRef]
- Wang, H.; Jin, M.; Mao, W.; Chen, C.; Fu, L.; Li, Z.; Du, S.; Liu, H. Photosynthetic toxicity of non-steroidal anti-inflammatory drugs (NSAIDs) on green algae Scenedesmus obliquus. Sci. Total Environ. 2020, 707, 136176. [Google Scholar] [CrossRef]
- Tang, J.; Wu, Y.; Esquivel-Elizondo, S.; Sørensen, S.J.; Rittmann, B.E. How microbial aggregates protect against nanoparticle toxicity. Trends Biotechnol. 2018, 36, 1171–1182. [Google Scholar] [CrossRef]
- You, X.; Xu, N.; Yang, X.; Sun, W. Pollutants affect algae-bacteria interactions: A critical review. Environ. Pollut. 2021, 276, 116723. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, S.; Tang, C.; Duan, P.; Yao, L.; Tang, J.; Wong, P.K.; Dionnysiou, D.D.; Wu, Y. Protection mechanisms of periphytic biofilm to photocatalytic nanoparticle exposure. Environ. Sci. Technol. 2019, 53, 1585–1594. [Google Scholar] [CrossRef]
- Mou, S.; Xu, D.; Ye, N.; Zhang, X.; Liang, C.; Liang, Q.; Zheng, Z.; Zhuang, Z.; Miao, J. Rapid estimation of lipid content in an Antarctic ice alga (Chlamydomonas sp.) using the lipophilic fluorescent dye BODIPY505/515. J. Appl. Phycol. 2012, 24, 1169–1176. [Google Scholar] [CrossRef]
- Escher, B.; Bramaz, N.; Eggen, R.; Richter, M. In vitro assessment of modes of toxic action of pharmaceuticals in aquatic life. Environ. Sci. Technol. 2005, 39, 3090–3100. [Google Scholar] [CrossRef] [PubMed]
- Corcoll, N.; Acuna, V.; Barcelo, D.; Casellas, M.; Guasch, H.; Huerta, B.; Petrovic, M.; Ponsati, L.; Rodriguez-Mozaz, S.; Sabater, S. Pollution-induced community tolerance to non-steroidal anti-inflammatory drugs (NSAIDs) in fluvial biofilm communities affected by WWTP effluents. Chemosphere 2014, 112, 185–193. [Google Scholar] [CrossRef]
- Liang, S.X.T.; Wong, L.S.; Dhanapal, A.C.T.A.; Djearamane, S. Toxicity of metals and metallic nanoparticles on nutritional properties of microalgae. Water Air Soil. Pollut. 2020, 231, 52. [Google Scholar] [CrossRef]
- Happ, T.; Triebskorn, R.; Köhler, H.R. Acute effects of diclofenac and DMSO to Daphnia magna: Immobilisation and hsp70-induction. Chemosphere 2008, 73, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Junqueira de Castro, F.; Alves dos Santos, D.R.; Picolomini, B.C.R.; Sanzi, C.F.; Brasil, C.R.; Cesar, A. Ecotoxicological assessment of four pharmaceuticals compounds through acute toxicity test. O Mundo Saúde Sao Paulo 2014, 38, 51–55. [Google Scholar] [CrossRef]
- Barbosa, I.R.; Martins, R.M.; Sá e Melo, M.L.; Soares, A.M.V.M. Acute and chronic toxicity of dimethyl sulfoxide to Daphnia magna. Bull. Environ. Contam. Toxicol. 2003, 70, 1264–1268. [Google Scholar] [CrossRef]
- Smith-Jansen, M.; Bartels, P.; Adler, N.; Altenburger, R. Phytotoxicity assessment of diclofenac and its phototransformation products. Anal. Bioanal. Chem. 2007, 387, 1389–1396. [Google Scholar] [CrossRef]
- Lee, J.; Ji, K.; Kho, Y.L.; Kim, P.; Choi, K. Chronic exposure to diclofenac on two freshwater cladocerans and Japanese medaka. Ecotoxicol. Environ. Saf. 2011, 74, 1216–1225. [Google Scholar] [CrossRef]
- Dodson, S.; Hanazato, T. Commentary on effects of anthropogenic and natural organic chemicals on development, swimming behavior, and reproduction of Daphnia, a key member of aquatic ecosystems. Environ. Health. Perspect. 1995, 103, 7–11. [Google Scholar]
- Smirnov, N.N. Physiology of the Cladocera, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017; p. 402. [Google Scholar]
- Edward, D.A.; Chapman, T. Mechanisms underlying costs of reproduction. In Mechanisms of Life History Evolution: The Genetics and Physiology of Life History Traits and Trade-Offs; En Flatt, T., Heyland, A., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 137–152. [Google Scholar]
- Beyers, R.J.; Odum, H.T. Metabolism and Homeostasis. In Ecological Microcosms; Springer Advanced Texts in Life Sciences; Springer: New York, NY, USA, 1993; pp. 11–40. [Google Scholar]
- Liu, Y.; Wang, L.; Pan, B.; Wang, C.; Bao, S.; Nie, X. Toxic effects of diclofenac on life history parameters and the expression of detoxification-related genes in Daphnia magna. Aquat. Toxicol. 2017, 183, 104–113. [Google Scholar] [CrossRef]
- LfW (Landesamt für Wasserwirtschaft). Arzneimittel in der Umwelt. Abschlussbericht des Bayerischen Landesamtes für Wasserwirtschaft zum Forschungs-und Entwicklungsvorhaben 2000–2003; LfW: Augsburg, Germany, 2003. [Google Scholar]
- Le, T.-H.; Lim, E.-S.; Lee, S.-K.; Park, J.-S.; Kim, Y.-H.; Min, J. Toxicity evaluation of verapamil and tramadol based on toxicity assay and expression patterns of Dhb, Vtg, Arnt, CYP4, and CYP314 in Daphnia magna. Environ. Toxicol. 2011, 26, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Arzate-Cárdenas, M.; Martínez-Jerónimo, F. Energy resource reallocation in Daphnia schodleri (Anomopoda: Daphniidae) reproduction induced by exposure to hexavalent chromium. Chemosphere 2012, 87, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Papchenkova, G.A.; Golovanova, I.L.; Shakova, N.V.U. The parameters of reproduction, sizes, and activities of hydrolases in Daphnia magna Straus of successive generations affected by Roundup Herbicide. Inland. Water Biol. 2009, 2, 286–291. [Google Scholar] [CrossRef]
- Calow, P.; Silby, M.M. A physiological basis of population processes: Ecotoxicological implications. Funct. Ecol. 1990, 4, 283–288. [Google Scholar] [CrossRef]
- Sancho, E.; Ferrando, M.D.; Andreu, E. Physiological stress responses of Anguilla anguilla to fenitrothion. J. Environ. Sci. Health B 1996, 31, 87–98. [Google Scholar] [CrossRef]
- Ferain, A.; De Saeyer, N.; Larondelle, Y.; Rees, J.-F.; Debier, C.; De Schamphelaere, K.A.C. Body lipid composition modulates acute cadmium toxicity in Daphnia magna adults and juveniles. Chemosphere 2018, 205, 328–338. [Google Scholar] [CrossRef]
- Arts, M.T.; Wainmann, B.C. Lipids in Freshwater Ecosystems; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; 319p. [Google Scholar]
- Tessier, A.J.; Goulden, C.E. Estimating food limitation in cladoceran populations. Limnol. Oceanogr. 1982, 27, 707–717. [Google Scholar] [CrossRef]
- Chabchoubi, I.B.; Bouchhima, R.A.; Louhichi, N.; Baanannou, A.; Masmoudi, S.; Hentati, O. Short-term effects of various non-steroidal anti-inflammatory drugs (NSAIDs) on Danio rerio embryos. MethodsX 2023, 11, 102215. [Google Scholar] [CrossRef]
- Praskova, E.; Voslarova, E.; Siroka, Z.; Plhalova, L.; Macova, S.; Marsalek, P.; Pistekova, V.; Svobodova, Z. Assessment of diclofenac LC50 reference values in juvenile and embryonic stages of the zebrafish (Danio rerio). Pol. J. Vet. Sci. 2011, 14, 545–549. [Google Scholar] [CrossRef]
- Van den Brandhof, E.J.; Montforts, M. Fish embryo toxicity of carbamazepine, diclofenac and metoprolol. Ecotoxicol. Environ. Saf. 2010, 73, 1862–1866. [Google Scholar] [CrossRef]
- Dietrich, D.R.; Prietz, A. Fish embryo toxicity and teratogenicity of pharmaceuticals, detergents and pesticides regularly detected in sewage treatment plant effluents and surface waters. Toxicologist 1999, 48, 151. [Google Scholar]
- Hallare, A.V.; Köhler, H.-R.; Triebskorn, R. Developmental toxicity and stress protein responses in zebrafish embryos after exposure to diclofenac and its solvent, DMSO. Chemosphere 2004, 56, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zheng, L.; Zhou, J.L. Effects of ibuprofen, diclofenac and paracetamol on hatch and motor behavior in developing zebrafish (Danio rerio). Chemosphere 2017, 182, 416–425. [Google Scholar] [CrossRef]
- Ribeiro, S.; Torres, T.; Martins, R.; Santos, M.M. Toxicity screening of diclofenac, propranolol, sertraline and simvastatin using Danio rerio and Paracentrotus lividus embryo bioassays. Ecotoxicol. Environ. Saf. 2015, 114, 67–74. [Google Scholar] [CrossRef]
- Horie, Y.; Yamagishi, T.; Yagi, A.; Shintaku, Y.; Iguchi, T.; Tatarazako, N. The non-steroidal anti-inflammatory drug diclofenac sodium induces abnormal embryogenesis and delayed lethal effects in early life stage zebrafish (Danio rerio). J. Appl. Toxicol. 2018, 39, 622–629. [Google Scholar] [CrossRef]
- Escapa, C.; Torres, T.; Neuparth, T.; Coimbra, R.N.; García, A.I.; Santos, M.M.; Otero, M. Zebrafissh embryo bioassays for a comprehensive evaluation of microalgae efficiency in the removal of diclofenac from water. Sci. Total Environ. 2018, 640–641, 1024–1033. [Google Scholar] [CrossRef]
- Yadav, P.; Verma, M.; Ahmed, S.; Singh, A.; Yadav, S.; Zahra, K. Risk assessment of diclofenac sodium on Zebra Fish, Danio rerio: Protein estimation in tissues. Res. J. Pharm. Technol. 2019, 12, 4635–4638. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, Q.; Di Paolo, C.; Shao, Y.; Hollert, H.; Seiler, T.B. Behavioral profile alterations in zebrafish larvae exposed to environmentally relevant concentrations of eight priority pharmaceuticals. Sci. Tot. Environ. 2019, 664, 89–98. [Google Scholar] [CrossRef]
- Glaberman, S.; Padilla, S.; Barron, M.G. Evaluating the zebrafish embryo toxicity test for pesticide hazard screening. Environ. Toxicol. Chem. 2017, 36, 1221–1226. [Google Scholar] [CrossRef]
- Bereketoglu, C.; Pradhan, A.; Olsson, P.E. Nonsteroidal anti-inflammatory drugs (NSAIDs) cause male-biased sex differentiation in zebrafish. Aquat. Toxicol. 2020, 223, 105476. [Google Scholar] [CrossRef]
- Tomisato, W.; Tsutsumi, S.; Hoshino, T.; Hwang, H.J.; Mio, M.; Tsuchiya, T.; Mizushima, T. Role of direct cytotoxic effects of NSAIDs in the induction of gastric lesions. Biochem. Pharmacol 2004, 67, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.B.; Gao, H.W.; Zhang, Y.L.; Zhang, Y.; Zhou, X.F.; Li, C.Q.; Gao, H.P. Developmental toxicity of diclofenac and elucidation of gene regulation in zebrafish (Danio rerio). Sci. Rep. 2014, 4, 4841. [Google Scholar] [CrossRef] [PubMed]
- Buchan, J.G.; Gray, R.S.; Gansner, J.M.; Alvarado, D.M.; Burgert, L.; Gitlin, J.D.; Gurnett, C.A.; Goldsmith, M.I. Kinesin family member 6 (kif6) is necessary for spine development in zebrafish. Dev. Dyn. 2014, 243, 1646–1657. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.S.; Wilm, T.P.; Smith, J.; Bagnat, M.; Dale, R.M.; Topczewski, J.; Johnson, S.L.; Solnica-Krezel, L. Loss of col8a1a function during zebrafish embryogenesis results in congenital vertebral malformations. Dev. Biol. 2014, 386, 72–85. [Google Scholar] [CrossRef]
- Commission of the European Communities. Technical Guidance Document in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances and Commission Regulation (EC) No. 1488/94 on Risk Assessment for Existing Substances. Part II: Environmental Risk Assessment; Office for Official Publications of the European Communities: Luxembourg, 1996. [Google Scholar]
- Heberer, T.; Feldmann, D. Contribution of effluents from hospitals and private households to the total loads of diclofenac and carbamazepine in municipal sewage effluents--modeling versus measurements. J. Hazard. Mater. 2005, 122, 211. [Google Scholar] [CrossRef]
- Oviedo-Gómez, D.G.; Galar-Martínez, M.; García-Medina, S.; Razo-Estrada, C.; Gómez-Oliván, L.M. Diclofenac-enriched artificial sediment induces oxidative stress in Hyalella azteca. Environ. Toxicol. Pharmacol. 2010, 29, 39–43. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).