Abstract
Abandoned, lost, or discarded fishing gear (ALDFG) poses significant environmental threats, namely contributing to microplastic (MP) pollution. However, the release of MPs from ALDFG remains poorly studied, despite its crucial role in understanding plastic pollution in marine ecosystems. This study is, to the best of our knowledge, the first to assess the environmental impact of ALDFG as a source of MPs, using an integrated approach combining laboratory experiments, in situ field trials, and environmental surveys. Laboratory tests showed that in the presence of light and sediment, braided polyethylene net released 1 fibre after incubation, demonstrating that the studied plastic fishing nets had the potential to release MPs. In situ experiments in a semi-enclosed marine environment did not show a clear influence of submerged fishing nets on water MPs, due to the high MP contamination in the selected location (5322 ± 4936 MP m−3). Nonetheless, at ALDFG hotspots off northwest Portugal, an increased presence of MPs in water samples compared to locations without ALDFG suggested potential MP release. These findings demonstrate the potential of ALDFG to act as a source of MPs and showcase the need for further studies, in order to comprehensively investigate the degradation of different plastic fishing nets in the field. Reducing ALDFG pollution is critical to mitigating its environmental impact and preserving marine ecosystems.
1. Introduction
Abandoned, lost, or otherwise discarded fishing gear (ALDFG), such as buoys, lines, nets, traps, and other related fishing gear, is an important source of marine litter. ALDFG has been present in the marine environment for as long as fishing activities have taken place and has been identified as a major problem since the 1980s [1,2]. Before the large-scale production of plastic began in the 1950s, fishing gear was produced using non-synthetic resources such as cotton, flax, or hemp fibres [3]. However, due to rapid evolution and industrialization, the exploitation of synthetic fbres increased substantially, leading to their use in the manufacturing of fishing gear. These synthetic fbres have characteristics that make them competitive and attractive to fishers, namely resistance to breakage, long life cycle, ease of handling, and low associated cost [4]. In fact, a significant amount of fishing gear is nowadays made of various synthetic polymers, including nylon, polyethylene, and polypropylene [5], which contribute significantly to plastic waste.
Despite ALDFG’s confirmed presence in marine environments, concrete data regarding it, both at a regional and global scale, are still scarce and in some cases contradictory. In most cases, it is difficult to monitor and retrieve ALDFG, and fishers may be reluctant to report lost gear [6]. The Food and Agriculture Organization of the United Nations reports that ALDFG represents 10% of all plastic in the oceans and makes up 70% (by weight) of all macroplastic marine litter in the oceans [7]. Richardson et al. [8] reported that 2% of all fishing gear is lost in the ocean every year, translating to an annual loss of 2963 km2 of gillnets, 75,049 km2 of purse seine nets, 218 km2 of trawl nets, 739,583 km of longline mainlines, and more than 25 million pots and traps. The European Union (EU) [9] reported that a minimum of 20% of EU fishing gear is lost or discarded at sea, representing up to 11,000 tons.
Although fishing gear is mostly composed of plastic [4,10], namely specific plastic polymers such as nylon or polyethylene, it can differ in terms of physical characteristics, such as thickness (e.g., monofilament, multifilament) and braided or twisted construction. Therefore, different types of fishing gear may have different potential to release MPs and consequently pose different risks to the marine environment when abandoned, lost, or discarded. In addition to MP release, ALDFG can damage habitats and continuously trap and entangle marine animals, possibly resulting in death or injury, the so-called ghost fishing [6,11,12]. Some ghost gear may continue to trap and injure marine species for months or years after their initial discarding, raising both direct and long-term negative consequences [13]. This gear can also cause the transfer and spread of invasive alien species and harmful microalgae [14,15], habitat degradation [16], and obstruction of navigation [17]. Together, these negative consequences may lead to a severe impact on water quality, ecosystems and biota, and even human health [14]. Environmental dynamics can induce modifications on plastics’ surface, making plastic fishing gear more prone to degradation or suitable to adsorb pollutants. Thus, the gear can start to release microplastics (MPs, plastic < 5 mm) into the water [18,19], transforming ALDFG into a source of MP contamination in the environment. Through a series of environmental factors such as UV photooxidation, biodegradation, and abrasion, plastic fragments turn into progressively smaller pieces, which may increase the risk of ingestion by different marine species as the size decreases [20].
MPs have been detected in numerous habitats, such as oceans [21], rivers [22], and estuaries [23], all around the world, including the Portuguese coast [24] and in Portuguese estuaries [25,26,27]. In addition, MPs have also been found in different aquatic species, from zooplankton [28] to mussels and bivalves [29,30] and large migratory fish [31]. Nevertheless, despite recent studies regarding MP contamination, the potential for ALDFG to release MPs, to the best of our knowledge, has not yet been sufficiently explored. It is still poorly understood whether the potential to release MPs varies with the plastic polymer and the physical characteristics of fishing nets. A deeper understanding of the ecological impacts of fishing-related debris is crucial for guiding the selection of polymers and materials toward more environmentally sustainable alternatives. In this context, the present work aimed to evaluate the release of MPs from different types (i.e., different plastic polymers and different physical properties) of fishing nets by combining laboratory assays, in situ experiments in quasi-real environmental scenarios, and environmental surveys of ALDFG hotspots.
2. Materials and Methods
To assess the environmental harmfulness of ALDFG as a new pollutant regarding the release of MPs, a combined approach was followed, namely: (i) laboratory assessment of MP release from fishing nets; (ii) in situ experiments to expose fishing nets to quasi-real environmental conditions in a semi-enclosed area; and (iii) environmental surveys of MPs in ALDFG hotspots. New fishing nets of different polymers and thicknesses were used in the laboratory (see Section 2.1) and in situ experiments (see Section 2.2). Four different types of new fishing nets were used (Figure 1):

Figure 1.
Fishing nets used in the laboratory experiments: N1 (thin nylon); N2 (braided nylon); N3 (braided polyethylene (PE)); N4 (twisted PE).
- N1—thin nylon—nylon net, thin (seine net, 0.30 mm, mesh 100 mm), used in seine fisheries;
- N2—braided nylon—nylon net, thick (“braided” type, 2.3 mm, mesh 53 mm), used in seine fisheries;
- N3—braided polyethylene—polyethylene net, thin (“braided” type, 1.5 mm, mesh 50 mm), used in trawling fisheries;
- N4—twisted polyethylene—polyethylene net, thin (“twisted” type 10/24, 1.8 mm, mesh 80 mm FM), used in trawling fisheries.
The selected fishing nets are used in relevant fishing gear, and pieces of these gear are typically found among ALDFG [14], namely purse seine for small pelagics (N1—thin nylon) and for tuna (N2—braided nylon) and trawl nets (N3—braided polyethylene; N4—twisted polyethylene).
2.1. Laboratory Assessment of MP Release from Fishing Nets
2.1.1. Experimental Setup
A laboratory experimental assay was assembled to evaluate the potential of fishing nets to release MPs, under two experimental conditions: (i) presence or absence of light (L: photoperiod regime (12 h of light followed by 12 h of dark); D: 24 h of dark); and (ii) presence or absence of sediment (W: only water, without sediment; S: water + sediment) (Table 1 and Figure 2). These conditions were selected to investigate the isolated effect of light and abrasion (sediment) as the main drivers responsible for the release of MPs. The experiments were conducted in 500 mL glass flasks using 450 mL of synthetic seawater prepared with deionized water (41.9 g sea salts (a commercial mixture of salts to mimic seawater composition) in 1 L of deionized water: salinity of 33‰). Synthetic seawater was used to avoid any possible MP contamination, normally present in natural seawater, and to guarantee suitable salinity and stable medium composition. In the W condition, only 400 mL of synthetic seawater was used. In the S condition, gravel previously washed with deionized water and inspected under a stereomicroscope to guarantee that it was not contaminated with MPs was added to the bottom as a proxy of sediment (ca. 100 mL).

Table 1.
Codes of experimental flasks and tested conditions. * without fishing nets.
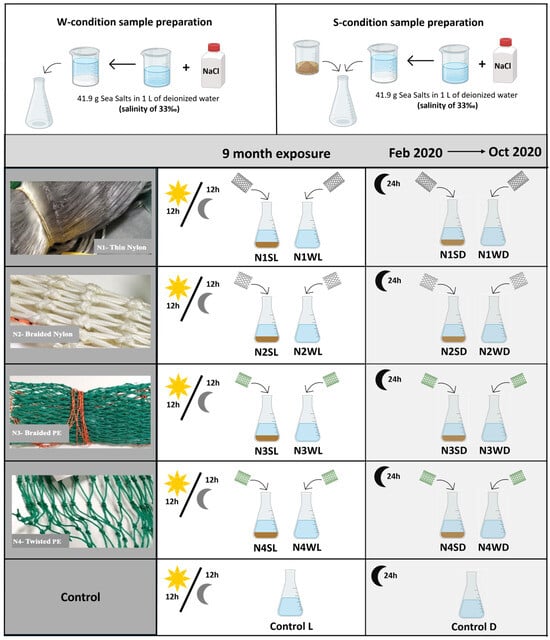
Figure 2.
Experimental conditions in laboratory settings for assessment of microplastic release from fishing nets.
Since each net has a specific weight, pieces with similar areas of each net type were used to guarantee a similar quantity of net in each flask. Thus, for the N1 (thin nylon net), a piece with more than 20 nodes of length was used (ca. 0.70 g per flask), for the N2 (braided nylon net), a piece with 4 nodes of length (ca. 7.4 g per flask), while pieces with 6 nodes of length for the N3 (braided PE net) (ca. 1.6 g per flask) and N4 (twisted PE net) (ca. 2.0 g per flask) were used. All the pieces of nets used in each flask were weighted and carefully rinsed with deionized water before being added to their flask. Two controls were assembled, one for the light and another for the dark condition, with seawater only. No controls were assembled for the S condition, as controls were set up to control any MP contamination that could be introduced in the seawater by any source other than the fishing nets. As the gravel was thoroughly cleaned before being used, it was considered that it would not contribute to MP contamination (Table 1 and Figure 2). The flasks were labeled following the codes displayed in Table 1, e.g., N1WL—fishing net N1 (thin nylon) in a flask with only water (W) and the presence of light (L).
Experiments were conducted in a chamber with a controlled temperature (16 °C, similar to NW Portuguese seawater) for 9 months between February 2020 and October 2020 (Figure 2). During the experiment, flasks were manually agitated once every two weeks and opened to prevent the formation of anoxic environments. During these procedures, control flasks with synthetic seawater were also opened to account for possible aerial MP contaminations.
2.1.2. Microplastic Analysis
At the end of the experiment, the synthetic seawater from each flask was filtered through a 0.03 mm-mesh polyester filter (previously rinsed with deionized water, completely dried, and weighted). All the water samples in all the experiments were filtered using the same type of filter (0.03 mm-mesh polyester filter) to maintain methodological consistency and ensure data comparability across experiments. Afterwards, each experimental glass flask was rinsed three times with deionized water, and all washing water was filtered through the same filter to ensure that all solid residues were retained in the filter. This washing step was also carried out with the gravel. The filters and pieces of nets were stored in different clean closed glass Petri dishes, dried at 30 °C, and afterwards weighed. All particles collected in the filters were visually inspected under a stereomicroscope and classified in terms of shape, color, and size. All retrieved MPs were afterward analyzed by FTIR (Fourier transform infrared spectroscopy) to identify the polymer and to evaluate if the collected particles belonged to the fishing nets used in the experiments. Polymer spectra were registered in a Perkin Elmer (Waltham, MA, USA) FT-IR Spectrum 2 instrument, coupled with attenuated total reflectance (ATR). The obtained spectra were compared with reference library spectra, and matches with confidence levels of ≥75% were accepted after the visual confirmation of each spectrum.
All laboratory procedures were complemented with blank controls to consider any contaminations by aerial MPs. In order to prevent MP contamination, during laboratory procedures, cotton lab coats were always used, and all materials were carefully rinsed with distilled water to prevent contamination of the samples. A petri dish with deionized water was placed near the stereomicroscope during the visual inspection for contamination control. No contamination was found in any of the controls used.
2.2. In Situ Experimental Exposure of Fishing Nets to Quasi-Real Environmental Conditions
2.2.1. Experimental Setup
An in situ experiment was carried out to evaluate the potential of fishing nets to release MPs under natural conditions. For that, fishing nets were exposed to quasi-real environmental conditions in a semi-enclosed bay within the Marina of Leixões, NW Portugal. In this experiment, fishing nets were exposed to natural conditions of light, currents, tides, temperature fluctuations, and biological interactions. Three fishing nets were selected (N1—thin nylon, N2—braided nylon, and N3—braided PE; Figure 1) and placed in water 50 cm apart from each other (Figure 3). The N4—twisted PE net used in the laboratory experiments was not selected, as its fishing activity (trawling), polymer, and thickness were similar to N3—braided PE net. To ensure that the nets were submerged to about 50 cm below the surface, each net was held open transversely by placing a weight on the bottom of the net, as shown in Figure 3. The in situ experiment was carried out for 12 months between February 2020 and February 2021. Water samples were collected every 2 or 3 months to monitor the presence of MPs in water near the nets, namely in February 2020 (considered the baseline for initial conditions), March, May, July, and October 2020 and February 2021. Physicochemical water parameters such as salinity, water temperature (°C), pH, and turbidity (FNU) in each site were recorded and obtained using a multiparameter probe (YSI EXO1 Sonde) (Supplementary Material Table S1).
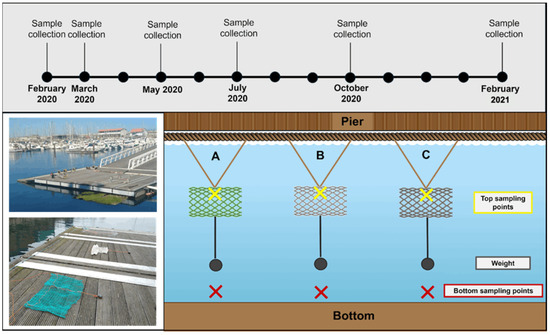
Figure 3.
Experimental assembly of in situ experimental exposure of fishing nets at Marina of Leixões, NW Portugal. Site A (N3—braided PE), Site B (N2—braided nylon), and Site C (N1—thin nylon).
2.2.2. Microplastic Analysis
Water samples (500 mL) were collected using a water sample bottle at two depths: (i) at the surface (near each net), corresponding to fishing net samples; and (ii) at the bottom (below each net), considered the control samples (no net) (Figure 3). A total of 6 samples were collected in each survey and properly stored in decontaminated glass flasks for further laboratory analysis. In the laboratory, MPs were quantified using the methodology described by Rodrigues et al. [32]. The organic content of water samples was degraded with 30% hydrogen peroxide (H2O2), after which MPs were separated from other inorganic material using a saturated sodium chloride (NaCl) solution, and afterwards collected in a 0.03 mm-mesh polyester filter. MPs isolated from the samples were then counted, weighted, and classified into size, type and color classes using a stereomicroscope (Nikon SMZ800). Afterwards, MPs were characterized by FTIR as described in Section 2.1. All laboratory procedures were complemented with blank controls to consider any contaminations by aerial MPs. Laboratory procedures to prevent MP contamination were the same as described in Section 2.1. No contamination was found in any of the controls used. MP contamination in water from February 2020, March, May, and July has been already reported by Rodrigues et al. [27].
2.3. Environmental Surveys of MPs in ALDFG Hotspots
2.3.1. Sampling Strategy
Two ALDFG hotspots with intensive fishing activity located along the NW Portuguese coast were surveyed, namely a coastal marine protected area (Parque Natural do Litoral Norte (PNLN) in Ofir/Esposende) and a submarine wreck in Matosinhos (Figure 4). The first hotspot is a natural rocky reef area, while the second hotspot is an artificial reef composed of the wreck of a German submarine from World War 2.
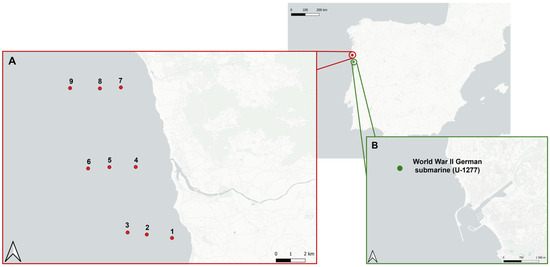
Figure 4.
Location of the two ALDFG hotspots: PNLN (sites 1–6 were within the ALDFG hotspot; sites 7–9 were outside the hotspot and were used as controls) and the submarine wreck in Matosinhos surveyed for MP contamination.
To survey the ALDFG hotspot located in the PNLN, a total of nine sampling sites were selected for water collection (Figure 4): six sites located within the hotspot (sites W1, W2, and W3 at the southern limit, and sites W4, W5, and W6 at the northern limit of the Cavalos de Fão area), and three sites (sites W7, W8, and W9) located outside the area, to be used as control sampling sites. For sediment samples, only three sites (S1, S2, and S3) within the hotspot (Cavalos de Fão) were sampled. Due to the presence of many rocky reefs that prevent the use of sediment grab, it was not possible to sample for sediment sites 4, 5, or 6. Three more sampling sites (S7, S8, and S9) were surveyed in the control zone.
Three seasonal sampling campaigns were carried out to collect water (with planktonic nets) and sediment (with a grab) samples to investigate MP contamination in January (considered winter), March (spring) and June (summer) 2019. Due to a lack of good weather and navigability conditions, performing the autumn campaign was impossible. Sediment samples were only collected during winter and summer campaigns, as sediments tend to have less seasonal variations. Subsurface (1–2 m depth) planktonic trawls were performed with a 500 µm-mesh net (1 m diameter and 4 m long) and the volume of water sampled was determined by a flowmeter attached to the net. After the collection, water samples were immediately fixed with 96% alcohol and stored for laboratory MP analyses. Sediment samples were collected with a Van Veen grab, and from each sampling site, three replicates were stored in aluminum foil for further MP analyses. Physicochemical water parameters such as salinity, water temperature (°C), pH, and turbidity (FNU) at each site were recorded and obtained using a multiparameter probe (YSI EXO1 Sonde) (Supplementary Material Table S2). MP contamination in water samples was previously published by Rodrigues et al. [27].
The second ALDFG hotspot was the wreck of a World War 2 German submarine (U-1277) that sank off the Matosinhos coast (near Porto city) at ca. 30 m depth. The area of the wreck has a sandy bottom without rocks nearby, and the wreck is the only hard structure in the area, and consequently is where the pieces of lost fishing nets and ropes (less than 1 m long) are trapped and concentrated. For this hotspot, three sampling sites were selected, namely one close to the torpedo exit (site M1), another close to the periscope of the submarine (site M2), and the other 50 m distant from the submarine to be used as a control sampling site (site M3) (Figure 5). Due to the difficulties in sampling water and sediment close to the wreck (30 depth), the sampling was carried out by recreational divers from the diving school Submersus. Two sampling campaigns were conducted, the first in June 2019 (corresponding to summer) and the second in September 2019 (autumn). Water and sediment samples were collected from each sampling site in triplicate (when possible) or duplicate. Several factors constrained the number of collected samples, namely the weather and navigability conditions, boat availability, and the difficulty of properly collecting and storing water and sediment samples at 30 m depth (leading to several samples being lost).
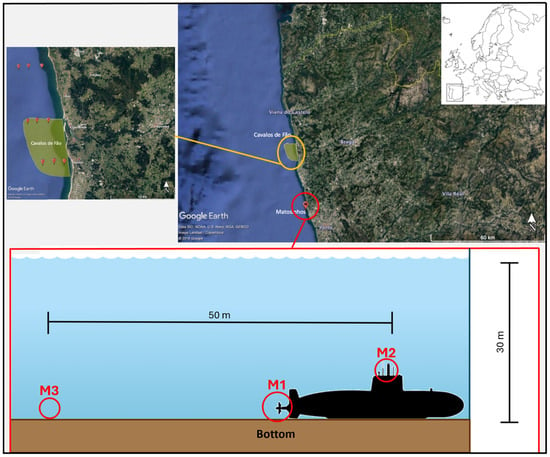
Figure 5.
Location of sampling sites M1, M2, and M3 of the lost gear hotspot, the wreck of the World War 2 German submarine (U-1277) on Matosinhos coast (near Porto city).
2.3.2. Microplastic Analysis
For MP analysis, the three (or two) water samples collected at each sampling site (ca. 500 mL) of the Matosinhos hotspot were combined into a single water sample per site (M1, M2, and M3), to increase the volume of water sampling/site and thus increase the representativeness of the samples. Water samples from both hotspots were filtered through a 0.03 mm-mesh filter that was afterwards washed with deionized water and dried at 90 °C. Afterwards, MPs were determined following the procedure described in Section 2.1.
All sediment samples were lyophilized and then sieved through a 2 mm net until further processing. A previously optimized procedure [33] was then used to determine MPs, in which two different density separation steps and one oxidation step were carried out. First, dry sediments were mixed with a saturated NaCl solution for density separation of any MPs (50–100 g of sediment for ca. 100 mL of saturated NaCl solution). The solution was left to settle until the water phase was transparent, and afterwards, samples were filtered through a 0.03 mm-mesh polyester filter. The filter was then dried and subjected to the procedure described for water samples.
All MPs isolated from the samples were visually inspected, counted, weighed, and classified into size, type, and color classes using a stereomicroscope (Nikon SMZ800). MPs were characterized by FTIR analysis for polymer identification as described in Section 2.1. Lab procedures to prevent MP contamination were the same as described in Section 2.1. No contamination was found in any of the controls used. MP contamination in water samples was previously published by Rodrigues et al. [27].
3. Results and Discussion
3.1. Laboratory Assessment of MP Release from Fishing Nets
At the end of the experiment (9 months), visual inspection of the flasks revealed that some types of nets, namely N3 and N4, that were in contact with the gravel/sediment seemed to be thinner and looked more damaged than the ones in the flasks containing only water, either in the light (Figure 6a) or dark condition (Figure 6b).
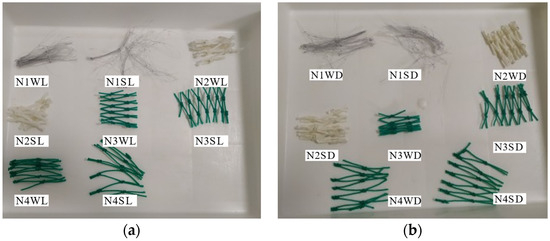
Figure 6.
Fishing nets at the end of the experiment: (a) in the light condition (L) and (b) in the dark condition (D). Codes for treatments are those reported in Table 1 (N1—thin nylon; N2—braided nylon, N3—braided PE, and N4—twisted PE. W—water; S—water + sediment).
After the experiment, all tested fishing nets weighed less, indicating a potential loss of polymer in the nets. The nets presented an average mass variation of ca. 0.020 ± 0.003 g for N1—thin nylon, 0.15 ± 0.12 g for N2—braided nylon, 0.05 ± 0.07 g for N3—braided PE and 0.040 ± 0.003 g for N4—twisted PE. The N3—braided nylon net presented the greatest decrease in weight of ca. 15% and 30% for light and dark conditions, respectively (Figure 7).
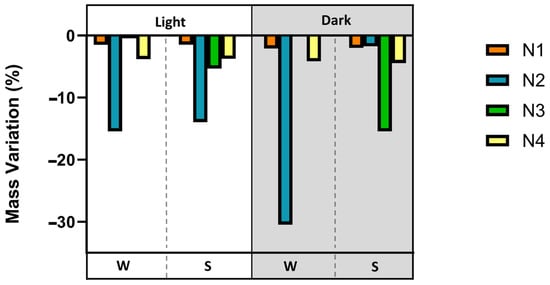
Figure 7.
Mass variation (%) in the four tested nets, N1—thin nylon, N2—braided nylon, N3—braided PE, and N4—twisted PE, at the end of the laboratory experiments under the conditions W (water) or S (water + sediment), and L (light) or D (dark).
The visual inspection of the filters under a stereomicroscope (Figure 8) indicated the presence of MPs in the following treatments: (a) one blue particle mixed with blue fibres in the N1SD flask; (b) one white fibre in the N2SD flask; (c) one green fre in the N3SL flask; and (d) several blue particles in the N4WL flask.
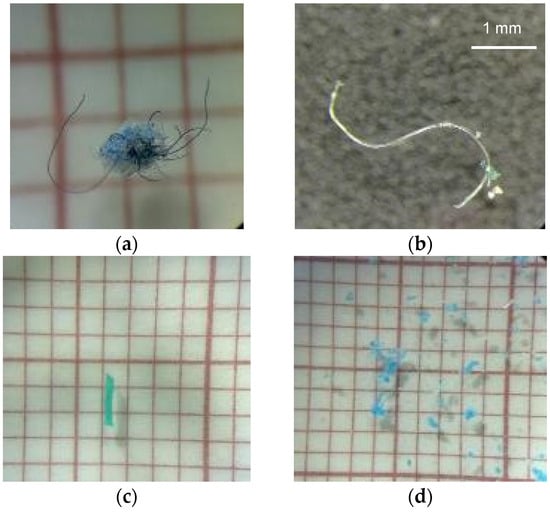
Figure 8.
Pictures of the filters under a stereomicroscope selected for the presence of fibres and/or microplastics; (a) N1SD, with 1 blue particle mixed with blue fibres; (b) N2SD, with 1 white fibre; (c) N3SL with 1 green fibre; and (d) N4WL, with several blue particles. Light condition (L) and dark condition (D); 1—thin nylon; 2—braided nylon, 3—braided PE and 4—twisted PE; W—water; S—water + sediment.
Afterwards, all MPs were then analyzed by FTIR, and the identified polymers are shown in Table 2.

Table 2.
FTIR analysis of the microplastics found in flasks N1SD, N2SD, N3SL and N4WL of the laboratory experiment. N1—thin nylon; N2—braided nylon; N3—braided PE; N4—twisted PE; W—water; S—water + sediment; L—light condition; D—dark condition.
Based on the polymer analysis of the collected MPs, only the fibre collected in treatment N3SL was of the same polymer as the fishing net, PE. In treatment N2SD, FTIR analysis showed that the fibre was not plastic. In treatments N1SD and N4WL, the other plastic particles detected were of polymers distinct from the tested fishing net, indicating that those MPs were probably a contamination, for example, from aerial MPs that were not detected in the blank contamination controls.
A study conducted by Nelms et al. [5] that registered the loss of mass in fishing nets exposed to benthic conditions reported similar results, with nylon fishing nets the ones with the most decrease in weight, as well as faster fragmentation. The loss of mass observed in our results may indicate that the fishing nets tested have the potential to release polymers or other substances used in the production of plastic fishing nets, namely additives, that might have been dissolved in the water. These results raise additional environmental concerns about ALDFG, as it could potentially leach chemical components into marine ecosystems.
3.2. In Situ Experimental Exposure of Fishing Nets to Quasi-Real Environmental Conditions
During the six sampling campaigns, several particles and fibres were retrieved from the water samples collected. A total of 206 MPs were collected, including fbres, fragments, films, and beads, with an average concentration of 5322 ± 4936 MP m−3 varying according to temporal patterns (two-way ANOVA: F = 4.53; p < 0.05), with higher contamination registered in October 2020. MP concentration did not vary between sampling stations A, B, or C (two-way ANOVA: F = 0.086; p ≥ 0.05) nor was there significant interaction between factor sampling stations and sampling months (two-way ANOVA: F = 0.985; p ≥ 0.05) (Figure 9). There were no significant differences between samples collected from the surface (near the net) and from the bottom (below each net) (two-way ANOVA: F = 3.24; p ≥ 0.05), nor were there any significant interactions between stations (A, B or C) and surface or bottom samples (two-way ANOVA: F = 0.273; p ≥ 0.05). The presence of MPs at the beginning of the in situ experiments in February 2020 indicated preexisting MP contamination of the marina waters. Thus, it was not possible to clearly distinguish between previous MP contamination between sites and sampling months for this experiment.
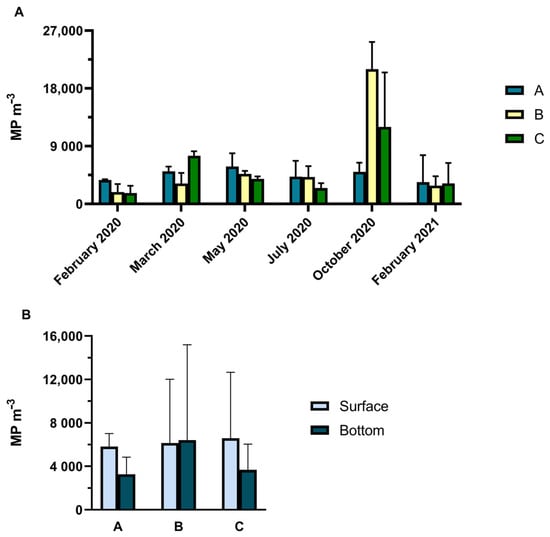
Figure 9.
(A) Temporal variation in MP concentration (MP m−3) at three sampling sites in water samples and (B) MP concentration (MP m−3) on the surface and at the bottom of sampling sites A, B, and C.
MPs collected were mostly composed of fibres, with the most predominant colors being black, transparent, and white. Regarding size, MPs less than 1 mm (0.03–1 mm) represented 62% of the MPs collected (Figure 10).
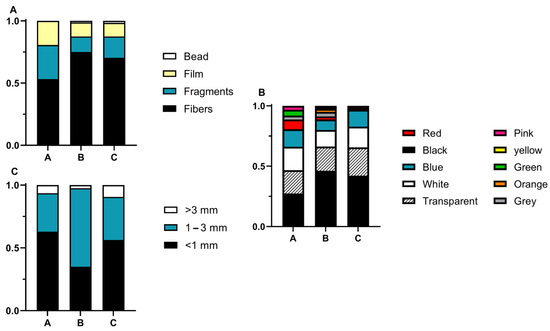
Figure 10.
Characterization of MPs by (A) shape, (B) color, and (C) size in sampling sites A (N3—braided PE), B (N2—braided nylon), and C (N1—thin nylon).
A subsample of the most representative MPs was selected for further FTIR analysis for polymer identification (Table 3). Polyethylene (PE), polypropylene (PP), and nylon were the main types of plastic polymers identified. Also, two non-plastic particles were identified as semi-synthetic-rayon (fbre) and polytetrafluoroethylene (particle) and were not included in the MP contamination results. FTIR results showed that PE was the most common polymer identified in the subsample of MPs from the Marina of Leixões. In fact, PE was the polymer of the fishing net used in Site A (Braided PE). However, PE is one of the most common plastic polymers found in aquatic environments [34], and therefore it is not possible to associate these PE MPs with the fishing net tested.

Table 3.
FTIR analysis of the representative subsample of MPs from water samples collected during in situ experiments near Site A (N3—braided PE), Site B (N2—braided nylon), and Site C (N1—thin nylon) at the surface (S) and bottom (F). PP—polypropylene, PE—polyethylene. The search score indicates the confidence level of the match when the MP spectrum was compared with the reference library spectra.
In these in situ experiments, at the Marina of Leixões, MPs were found to be present in water samples from all sampling campaigns, with the highest MP concentrations found in March and October 2020. MP contamination can be a consequence of the shipping and fishery activities of the Marina of Leixões. In fact, at the beginning of the experiment (February 2020), MPs were also found in the marina water. This indicated preexisting water contamination by MPs independently of the presence of the testing fishing nets. Regarding the variability of MP contamination across different sampling months, a study exploring seasonal MP contamination in Ría de Vigo, an area of great human impact and Europe’s main landing point for fishing, also revealed its highest contamination value in October. Several reasons could explain this, such as the intensity of the fishing activity in the area, the start of the rainy season, and the input of rivers and winds [35].
Furthermore, PE, PP, and nylon were the main types of plastic polymers identified at the marina. As such, no clear influence of fishing nets was observed on the marina water regarding MP contamination. This recreational marina is situated in an enclosed area, bordered by the north, west, and east quays. This enclosed layout acts as a retention area for MPs released by maritime activities in the commercial harbor. These activities, including commercial shipping, fisheries, and recreational vessels [23], can differ by time of year, potentially affecting the concentration of MPs in the study area. Hence, these results highlight the need for further research, including in situ experiments in different environments with low levels of MP contamination, to fully understand the impact of fishing gear on the release of MPs into the environment. Furthermore, our results emphasize the importance of implementing efficient waste management strategies and developing MP pollution monitoring for commercial and fishing ports to prevent and reduce plastic pollution in marine environments.
3.3. Environmental Surveys of Microplastics in ALDFG Hotspots
In the PNLN hotspot, the concentration of MPs was relatively low (average concentration of 0.015 ± 0.014 MP m−3) (Figure 11A), similar to other Portuguese coastal waters [36] and other marine protected areas and their vicinities [37]. Despite low contamination levels, we verified a tendency for more MPs at sites within the hotspot was observed (Figure 11). March 2019 presented the highest concentration, with a higher amount of irregular plastic particles (fragments and film), which may indicate that a relevant input of MPs occurred during this season. Films were the most frequent MPs, representing 49% of the total MPs collected, followed by fragments (29%), and fibres (22%) (Figure 11B). MPs of eleven different colors were detected: transparent was predominant (37%), followed by white (17%) and blue (12%) (Figure 11C). Overall, most MPs measured between 1 and 3 mm (53%) (Figure 11D). Five polymers were identified: PE, polyethylene terephthalate (PET), polyamide (PA), polypropylene (PP), and nylon. PE and PA were the main polymer types identified. PE is the most common polymer found in different monitoring studies. Many of the fbres found were identified as PA, often used in fishing nets, and therefore commonly associated with fishery activities [38,39].
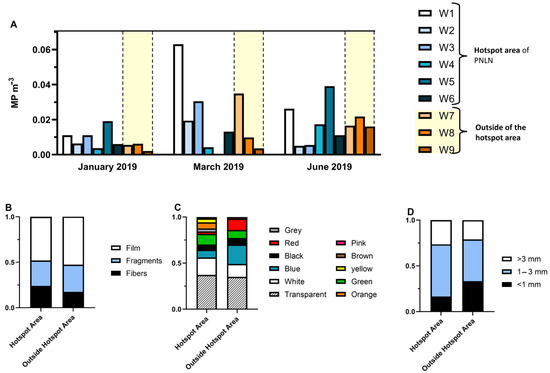
Figure 11.
(A) MP concentration (MP m−3) across three sampling months, sampling sites within and outside the hotspot in the PNLN, and characterization of MPs within and outside the hotspot in the PNLN by (B) shape, (C) color, and (D) size.
MPs were also found in sediments (Figure 12). Higher MP levels were observed in June (two-way ANOVA: F = 11.56; p < 0.05) and in general at sites outside the hotspots, with no clear influence of fishing nets (two-way ANOVA: F = 5.58; p < 0.05). Fibres were found in higher amounts than particles, being mostly of blue or white color with a size of 1–3 mm.
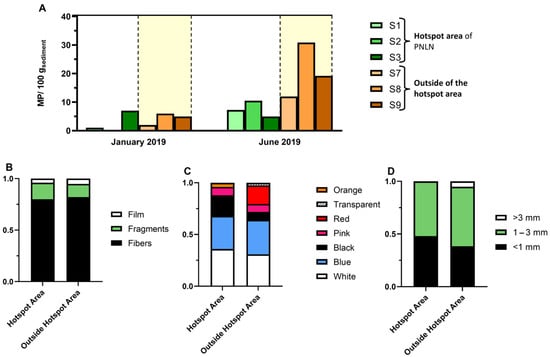
Figure 12.
(A) MP concentration (MP/100 g sediment) in two sampling months, and sampling sites within and outside the hotspot of Cavalos de Fão. (B) Characterization of MPs within and outside the hotspot of Cavalos de Fão by shape, (C) color, and (D) size.
MP concentrations in water samples for the Matosinhos submarine wreck are shown in Figure 13. The average MP concentration was 2748 ± 2617 MP m−3, with a trend for higher MP contamination in the second sampling campaign in September 2019 (two-way ANOVA: F = 0.56; p ≥ 0.05). The highest MP concentrations were detected at site M2 during the September campaign, which may derive from the proximity of the ALDFG attached to the submarine structure. Overall, fragments were the most common MP type retrieved, although at site M1, only fibres were detected on the two sampling campaigns (Figure 13B). Blue was the most common color at sites M2 and M3, while at site M1, white was the only color found. Transparent, black, and pink MPs were also found in our samples (Figure 13C). Regarding size, MPs less than 1 mm (0.03–1 mm) were the most common at sites M2 and M3 (representing more than 60% of the total MPs collected, while at site M1, MPs between 1 and 3 mm were the most common (representing more than 50% of the total collected).
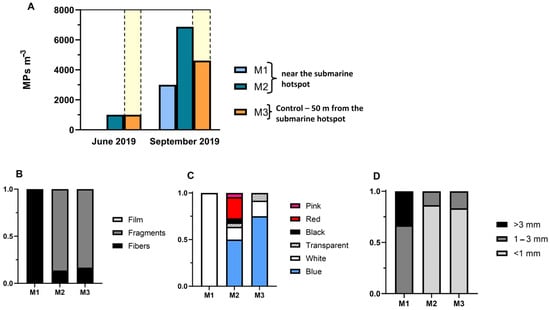
Figure 13.
(A) MP concentration (MP m−3) in two sampling months and sampling sites within and outside the submarine wreck in Matosinhos hotspot. (B) Characterization of MPs within and outside the submarine hotspot by shape, (C) color, and (D) size.
Overall, MPs found at site M1 (near the torpedo exit) seem to be quite different from MPs at site M2 and control site M3, possibly indicating the presence of ALDFG in this location. All particles and fbres found were of PE, a commonly found polymer, which can be present in fishing gear. In addition, a large number (more than 100) of other fbres were found at all sites and identified by FTIR as cellulose and therefore not included in the total of MPs collected [27]. This large quantity of cellulose fibers may derive from the abrasion and breakdown of old fishing nets and ropes, which before the 1970s were typically made with natural fibres [3], and have accumulated in this hotspot.
Regarding sediment samples, only microfibers were observed, similar to those found in water samples at this location and different from those observed in the PNLN. These fibers were identified as cellulose. No MPs were observed in these sediments despite their presence in the water.
At the PNLN hotspot, the main human impact comes from fisheries, small-scale agriculture, a small city nearby (Esposende), and some maritime tourist activities. Hence, severe contamination by urban, agricultural, and industrial effluents is unlikely to occur. However, the Cávado estuary is close to the hotspot of the PNLN, and its plume can constitute a contamination source of the adjacent coastal area, including the PNLN [40]. Regarding the possibility of ALDFG acting as an MP source, MP concentrations in water samples showed a slight tendency to increase within the hotspot of the PNLN comparatively to an area outside.
In contrast with the PNLN area, the Matosinhos submarine hotspot is located near a highly urbanized area, hosting the second major city of Portugal (Porto) and supporting several human activities, with tourism exponentially growing over the last decade. There is an oil-refining industry and two maritime harbors (located at Leixões, and Viana do Castelo), high population density, and the development of several industrial and urban activities. The hotspot is directly located near the oil refinery and commercial harbor mentioned above, both possibly being an MP contamination source [41]. This area is also under the influence of the Douro estuary, an ecosystem highly impacted by several pressures, namely high levels of MP contamination [26,28,42].
Overall, MP concentrations observed in the PNLN were in general lower in comparison with other coastal areas along the NW coast of Portugal [36]. At the Matosinhos submarine hotspot, MP contamination observed was substantially higher in comparison with the PNLN and even higher than in other locations known to be highly impacted [43,44,45], which could be associated with the high amount of ALDFG present in this location. These contamination levels highlight the need for concise, regular monitoring of ALDFG hotspots. Moreover, it also stresses the urgency of strategies to reduce ALDFG, namely preventing the loss and incentivizing the recovery of fishing gear, promoting the recycling of end-of-life gear, and providing fishing ports with proper waste management facilities [9,46,47].
4. Conclusions
The present work investigated the potential of plastic fishing nets to release microplastics to uncover the harmful potential of ALDFG as a new pollutant. The potential of ALDFG to release MPs was investigated for the first time in controlled laboratory conditions, quasi-real environmental conditions, and natural conditions of ALDFG hotspots. The results obtained gave an initial indication that fishing nets have the potential to release MPs. Laboratory experiments showed a weight decrease in the three fishing net types tested, which indicates a potential loss of polymer. Furthermore, the presence of gravel/sediment seemed to be an aggravating factor in the deterioration of the fishing nets. In quasi-real environmental conditions (Marina of Leixões), in situ experiments were inconclusive regarding the influence of plastic fishing nets on MP contamination, since it was not possible to discriminate between MPs already existing in the marina from those potentially released by the testing plastic fishing nets. This could be associated with the high level of MP contamination of the marina, which might have compromised the identification of the MPs potentially released by the testing fishing nets. Thus, we believe a potential next step would be more thorough in situ studies on this topic, exploring other locations with a lower baseline of MP contamination. The environmental monitoring of the two ALDFG hotspots revealed a slight tendency for higher levels of MP contamination in sampling sites within the hotspots when compared to control areas outside the ALDFG hotspots. These contamination levels emphasize the need for the monitoring of ALDFG hotspots, as well as strategies to promote the prevention and mitigation of ALDFG. Overall, this study showed the potential for plastic fishing gear to release MPs, highlighting the role of fishing gear as an MP contamination source when abandoned, lost, or discarded in the ocean. Moreover, this study showed that the potential of plastic fishing gear to release MPs varies with the polymer and physical characteristics of the nets, emphasizing the need for future research to further investigate this hazard of fishing gear. These findings support the fact that ALDFG can pose an additional environmental hazard by acting as an additional source of MPs in the ocean and compromising the environmental health of marine ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17101439/s1, Table S1: Physicochemical water parameters of the three sites surveyed in situ experiment at the Marina of Leixões, NW Portugal in February, March, May, July and October of 2022 and February 2021. A (N3—Braided PE), site B (N2—Braided Nylon) and site C (N1—Thin Nylon). Values represent average ± SD. Table S2: Physicochemical water parameters of the nine sampling sites (1–9) surveyed in coastal Marine Protected Area (Parque Natural do Litoral Norte (PNLN) in Ofir/Esposende) in January (winter), March (spring) and June (summer) 2019. Sites 1–6 are within the lost gear hotspot area and sites 7–9 are outside.
Author Contributions
S.R.: writing—review and editing, validation, supervision, resources, investigation, funding acquisition, conceptualization. F.E.: writing—review and editing, validation. S.M.R.: writing—original draft, investigation, formal analysis, methodology. D.S.: investigation, writing—review and editing. R.P. (Rúben Pereira): investigation, writing—review and editing. L.R.: validation, formal analysis. R.P. (Rafaela Perdigão): writing—original draft, methodology. C.M.R.A.: writing—review and editing, supervision, resources, investigation, funding acquisition, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by NETTAG+ project, funded by the European Union under the Horizon Europe Program (grant agreement 101112812).
Data Availability Statement
The data will be provided upon request.
Acknowledgments
This research was also partially supported by the NetTag project financed by EMFF-EASME (EASME/EMFF/2017/1.2.1.12/S2/02/S12.789121). This study was also supported by the OMARE project (POSEUR-15-2016-54) and by national funds through FCT—Foundation for Science and Technology within the scope of UIDB/04423/2020 and UIDP/04423/2020, and PhD fellowships attributed to Diogo Silva (2020.06088.BD), to Sabrina Rodrigues (SFRH/BD/145736/2019), to Rafaela Perdigão (2020.04689.BD), and to Rúben Pereira (2021.04850.BD). The authors acknowledge the FCUP|DQB—Lab&Services for the FTIR analysis, the Submersus school divers for water sample collection, and all people involved in sampling. Views and opinions expressed are, however, those of the author(s) only and do not necessarily reflect those of the European Union or the European Climate, Infrastructure, and Environment Executive Agency (CINEA). Neither the European Union nor the granting authority can be held responsible for them.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ALDFG | abandoned, lost, or otherwise discarded fishing gear |
| EU | European Union |
| FTIR | Fourier transform infrared spectroscopy |
| MP | microplastic |
| PNLN | Parque Natural do Litoral Norte |
| PA | polyamide |
| PE | polyethylene |
| PET | polyethylene terephthalate |
| PP | polypropylene |
References
- Giskes, I.B., Jr.; Pragnell-Raasch, H.; Perez Roda, A. Report on Good Practices to Prevent and Reduce Marine Plastic Litter from Fishing Activities; FAO and IMO: Rome, Italy; London, UK, 2022. [Google Scholar]
- Richardson, K.; Asmutis-Silvia, R.; Drinkwin, J.; Gilardi, K.V.; Giskes, I.; Jones, G.; O’Brien, K.; Pragnell-Raasch, H.; Ludwig, L.; Antonelis, K.; et al. Building evidence around ghost gear: Global trends and analysis for sustainable solutions at scale. Mar. Pollut. Bull. 2019, 138, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Sahrhage, D.; Lundbeck, J. A History of Fishing; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Kim, S.H.; Kim, P.; Jeong, S.-J.; Lee, K.; Oh, W. Physical Properties of Biodegradable Fishing Net in Accordance with Heat-Treatment Conditions for Reducing Ghost Fishing. Turk. J. Fish. Aquat. Sci. 2019, 20, 127–135. [Google Scholar]
- Nelms, S.E.; Duncan, E.M.; Patel, S.; Badola, R.; Bhola, S.; Chakma, S.; Chowdhury, G.W.; Godley, B.J.; Haque, A.B.; Johnson, J.A.; et al. Riverine plastic pollution from fisheries: Insights from the Ganges River system. Sci. Total Environ. 2021, 756, 143305. [Google Scholar] [CrossRef]
- Lively, J.A.; Good, T.P. Chapter 10—Ghost Fishing. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Academic Press: New York, NY, USA, 2019; pp. 183–196. [Google Scholar]
- Drinkwin, J. Reporting and Retrieval of Lost Fishing Gear: Recommendations for Developing Effective Programs; Food and Agriculture Organization: Rome, Italy, 2022. [Google Scholar]
- Richardson, K.; Hardesty, B.D.; Vince, J.; Wilcox, C. Global estimates of fishing gear lost to the ocean each year. Sci. Adv. 2022, 8, eabq0135. [Google Scholar] [CrossRef] [PubMed]
- European Union. New Proposal Will Tackle Marine Litter and “Ghost Fishing”. 2018. Available online: https://ec.europa.eu/newsroom/mare/items/628060/en (accessed on 22 January 2024).
- Wataniyakun, W.; Le Gall, M.; El Rakwe, M.; Karl, C.W.; Larsen, R.B. Biodegradable fishing gears: A potential solution to ghost fishing and marine plastic pollution. Mar. Pollut. Bull. 2025, 212, 117607. [Google Scholar] [CrossRef]
- Barry, J.; Rindorf, A.; Gago, J.; Silburn, B.; McGoran, A.; Russell, J. Top 10 marine litter items on the seafloor in European seas from 2012 to 2020. Sci. Total Environ. 2023, 902, 165997. [Google Scholar] [CrossRef]
- Karli Thomas, C.D.; Obaidullah, F. Ghost Gear: The Abandoned Fishing Nets Haunting Our Oceans; Greenpeace e.V.: Hamburg, Germany, 2019. [Google Scholar]
- Wasave, S.; Kamble, S.; Kazi, T.; Wasave, S.; B., S.G.; Sharma, A. A bibliometric review on ghost fishing: Impacts on marine environment and governing measures. Mar. Pollut. Bull. 2025, 212, 117604. [Google Scholar] [CrossRef]
- Gilman, E.; Antonelis, K.; Drinkwin, J.; Gorgin, S.; Suuronen, P.; Thomas, S.N.; Wilson, J. Introduction to the Marine Policy special issue on abandoned, lost and discarded fishing gear: Causes, magnitude, impacts, mitigation methods and priorities for monitoring and evidence-informed management. Mar. Policy 2023, 155, 105738. [Google Scholar] [CrossRef]
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic pollution in the marine environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef]
- Do, H.-L.; Armstrong, C.W. Ghost fishing gear and their effect on ecosystem services—Identification and knowledge gaps. Mar. Policy 2023, 150, 105528. [Google Scholar] [CrossRef]
- Royer, S.-J.; Corniuk, R.N.; McWhirter, A.; Lynch, H.W.; Pollock, K.; O’Brien, K.; Escalle, L.; Stevens, K.A.; Moreno, G.; Lynch, J.M. Large floating abandoned, lost or discarded fishing gear (ALDFG) is frequent marine pollution in the Hawaiian Islands and Palmyra Atoll. Mar. Pollut. Bull. 2023, 196, 115585. [Google Scholar] [CrossRef] [PubMed]
- Rafa, N.; Ahmed, B.; Zohora, F.; Bakya, J.; Ahmed, S.; Ahmed, S.F.; Mofijur, M.; Chowdhury, A.A.; Almomani, F. Microplastics as carriers of toxic pollutants: Source, transport, and toxicological effects. Environ. Pollut. 2024, 343, 123190. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Liu, Y.; Yu, K.; Zhang, H.; Yu, H.; Jiang, J. Neglected microplastics pollution in the nearshore surface waters derived from coastal fishery activities in Weihai, China. Sci. Total Environ. 2021, 768, 144484. [Google Scholar] [CrossRef]
- Dimassi, S.N.; Hahladakis, J.N.; Yahia, M.N.D.; Ahmad, M.I.; Sayadi, S.; Al-Ghouti, M.A. Degradation-fragmentation of marine plastic waste and their environmental implications: A critical review. Arab. J. Chem. 2022, 15, 104262. [Google Scholar] [CrossRef]
- Coyle, R.; Hardiman, G.; Driscoll, K.O. Microplastics in the marine environment: A review of their sources, distribution processes, uptake and exchange in ecosystems. Case Stud. Chem. Environ. Eng. 2020, 2, 100010. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Defontaine, S.; Sous, D.; Tesan, J.; Monperrus, M.; Lenoble, V.; Lanceleur, L. Microplastics in a salt-wedge estuary: Vertical structure and tidal dynamics. Mar. Pollut. Bull. 2020, 160, 111688. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.; Frias, J.; Sobral, P. Microplastics on the Portuguese coast. Mar. Pollut. Bull. 2018, 131, 294–302. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Sáez-Zamacona, I.; Silva, D.M.; Rodrigues, S.M.; Pereira, R.; Ramos, S. The Role of Estuarine Wetlands (Saltmarshes) in Sediment Microplastics Retention. Water 2023, 15, 1382. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Almeida, C.M.R.; Silva, D.; Cunha, J.; Antunes, C.; Freitas, V.; Ramos, S. Microplastic contamination in an urban estuary: Abundance and distribution of microplastics and fish larvae in the Douro estuary. Sci. Total Environ. 2019, 659, 1071–1081. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Almeida, C.M.R.; Ramos, S. Microplastics contamination along the coastal waters of NW Portugal. Case Stud. Chem. Environ. Eng. 2020, 2, 100056. [Google Scholar] [CrossRef]
- Espincho, F.; Pereira, R.; Rodrigues, S.M.; Silva, D.M.; Almeida, C.M.R.; Ramos, S. Assessing Microplastic Contamination in Zooplanktonic Organisms from Two River Estuaries. Water 2024, 16, 992. [Google Scholar] [CrossRef]
- Bom, F.C.; Sá, F. Concentration of microplastics in bivalves of the environment: A systematic review. Environ. Monit. Assess. 2021, 193, 846. [Google Scholar] [CrossRef]
- Gérigny, O.; Pedrotti, M.L.; El Rakwe, M.; Brun, M.; Pavec, M.; Henry, M.; Mazeas, F.; Maury, J.; Garreau, P.; Galgani, F. Characterization of floating microplastic contamination in the bay of Marseille (French Mediterranean Sea) and its impact on zooplankton and mussels. Mar. Pollut. Bull. 2022, 175, 113353. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Rodrigues, S.M.; Silva, D.; Freitas, V.; Almeida, C.M.R.; Ramos, S. Microplastic contamination in large migratory fishes collected in the open Atlantic Ocean. Mar. Pollut. Bull. 2023, 186, 114454. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; Almeida, C.M.R.; Ramos, S. Adaptation of a laboratory protocol to quantity microplastics contamination in estuarine waters. MethodsX 2019, 6, 740–749. [Google Scholar] [CrossRef]
- Castiglioni, M.; Rodrigues, S.M.; Freitas, V.; Rivoira, L.; Bruzzoniti, M.C.; Almeida, C.M.; Ramos, S. Microplastic in marine environment: Reworking and optimization of two analytical protocols for the extraction of microplastics from sediments and oysters. MethodsX 2020, 7, 101116. [Google Scholar]
- Erni-Cassola, G.; Zadjelovic, V.; Gibson, M.I.; Christie-Oleza, J.A. Distribution of plastic polymer types in the marine environment; A meta-analysis. J. Hazard. Mater. 2019, 369, 691–698. [Google Scholar] [CrossRef]
- Carretero, O.; Gago, J.; Filgueiras, A.V.; Viñas, L. The seasonal cycle of micro and meso-plastics in surface waters in a coastal environment (Ría de Vigo, NW Spain). Sci. Total Environ. 2022, 803, 150021. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental status of (micro)plastics contamination in Portugal. Ecotoxicol. Environ. Saf. 2020, 200, 110753. [Google Scholar] [CrossRef]
- Nunes, B.Z.; Huang, Y.; Ribeiro, V.V.; Wu, S.; Holbech, H.; Moreira, L.B.; Xu, E.G.; Castro, I.B. Microplastic contamination in seawater across global marine protected areas boundaries. Environ. Pollut. 2023, 316, 120692. [Google Scholar] [CrossRef] [PubMed]
- GESAMP. Sea-Based Sources of Marine Litter. 2021. Available online: http://www.gesamp.org/publications/sea-based-sources-of-marine-litter (accessed on 19 February 2025).
- Napper, I.E.; Wright, L.S.; Barrett, A.C.; Parker-Jurd, F.N.F.; Thompson, R.C. Potential microplastic release from the maritime industry: Abrasion of rope. Sci. Total Environ. 2022, 804, 150155. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Rodrigues, S.M.; Silva, D.M. Ramos Assessing Environmental Control on Temporal and Spatial Patterns of Larval Fish Assemblages in a Marine Protected Area. Ecologies 2023, 4, 288–309. [Google Scholar] [CrossRef]
- Preston-Whyte, F.; Silburn, B.; Meakins, B.; Bakir, A.; Pillay, K.; Worship, M.; Paruk, S.; Mdazuka, Y.; Mooi, G.; Harmer, R.; et al. Meso- and microplastics monitoring in harbour environments: A case study for the Port of Durban, South Africa. Mar. Pollut. Bull. 2021, 163, 111948. [Google Scholar] [CrossRef]
- Prata, J.C.; Godoy, V.; da Costa, J.P.; Calero, M.; Martín-Lara, M.A.; Duarte, A.C.; Rocha-Santos, T. Microplastics and fibers from three areas under different anthropogenic pressures in Douro river. Sci. Total Environ. 2021, 776, 145999. [Google Scholar] [CrossRef]
- Nakakuni, M.; Nishida, M.; Nishibata, R.; Kishimoto, K.; Yamaguchi, H.; Ichimi, K.; Ishizuka, M.; Suenaga, Y.; Tada, K. Convergence zones of coastal waters as hotspots for floating microplastic accumulation. Mar. Pollut. Bull. 2024, 206, 116691. [Google Scholar] [CrossRef]
- Taha, Z.D.; Amin, R.M.; Anuar, S.T.; Nasser, A.A.A.; Sohaimi, E.S. Microplastics in seawater and zooplankton: A case study from Terengganu estuary and offshore waters, Malaysia. Sci. Total Environ. 2021, 786, 147466. [Google Scholar] [CrossRef]
- Ourmieres, Y.; Arnaud, M.; Deixonne, P.; Ghiglione, J.-F.; Albignac, M.; Poulain-Zarcos, M.; Mercier, M.; Halle, A.T. Inferring microplastics origins in the Mediterranean Sea by coupling modelling and in-situ measurements. Mar. Pollut. Bull. 2023, 195, 115333. [Google Scholar] [CrossRef]
- European Commission and Joint Research Centre. Guidance on the Monitoring of Marine Litter in European Seas—An Update to Improve the Harmonised Monitoring of Marine Litter Under the Marine Strategy Framework Directive; Publications Office of the European Union: Brussels, Luxembourg, 2023. [Google Scholar]
- Grimstad, S.M.F.; Ottosen, L.M.; James, N.A. Marine Plastics: Innovative Solutions to Tackling Waste; Springer Nature: Cham, Switzerland, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).