Particulate and Dissolved Metals in the Pearl River Estuary, China—Part 2: Partitioning Characteristics and Influencing Factors
Abstract
:1. Introduction
2. Materials and Methods
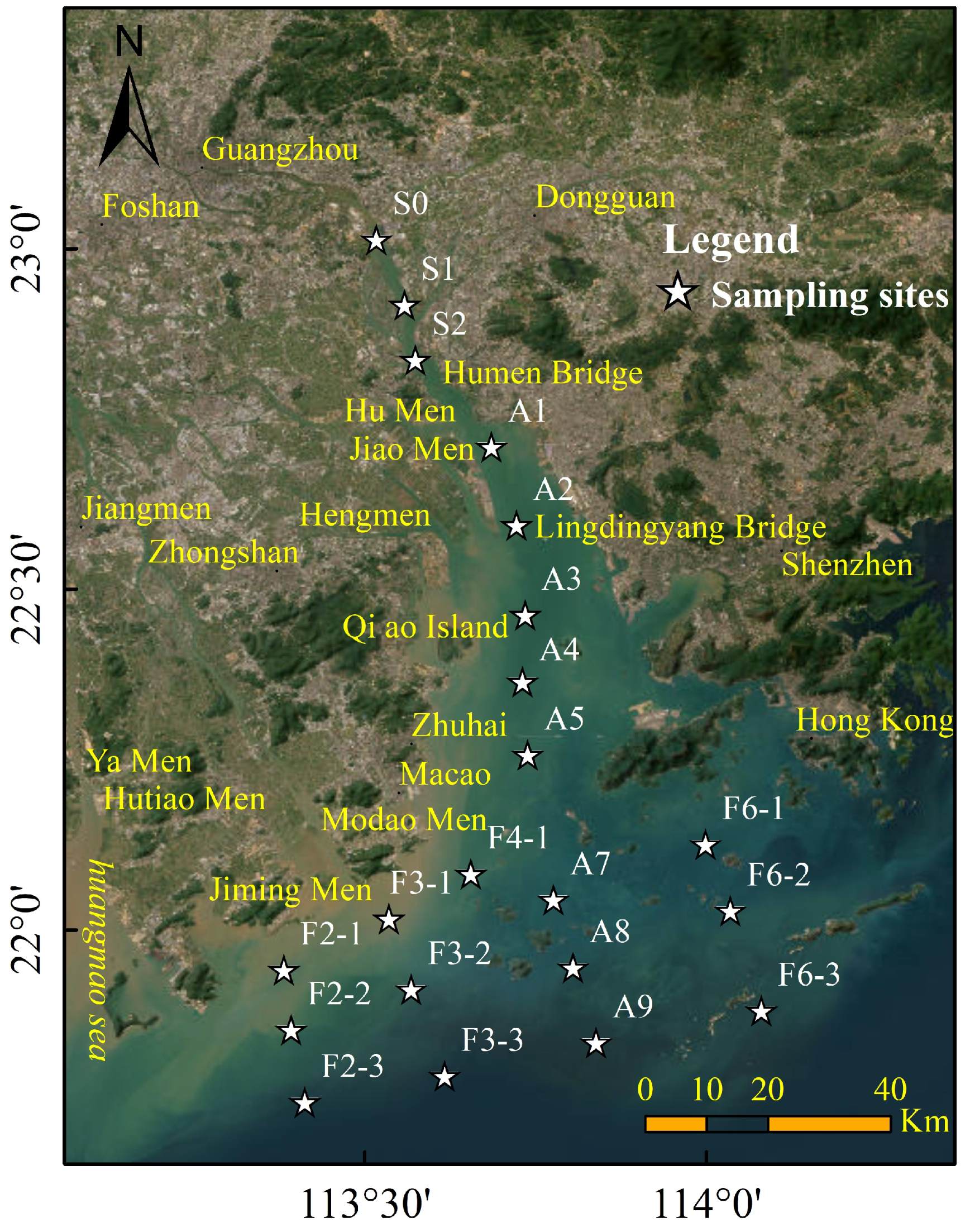
2.1. Study Area, Sample Collection, and Analysis
2.2. Statistical and Spatial Methods
2.3. Partition Coefficient
3. Results and Discussion
3.1. Comparison of Dissolved and Suspended Particulate Metal Contents
3.2. Comparison of Spatial Distribution of Dissolved and Particulate Metals
3.3. Comparison of Correlation Between Dissolved and Particulate Metals
3.4. Metal Partition Coefficients
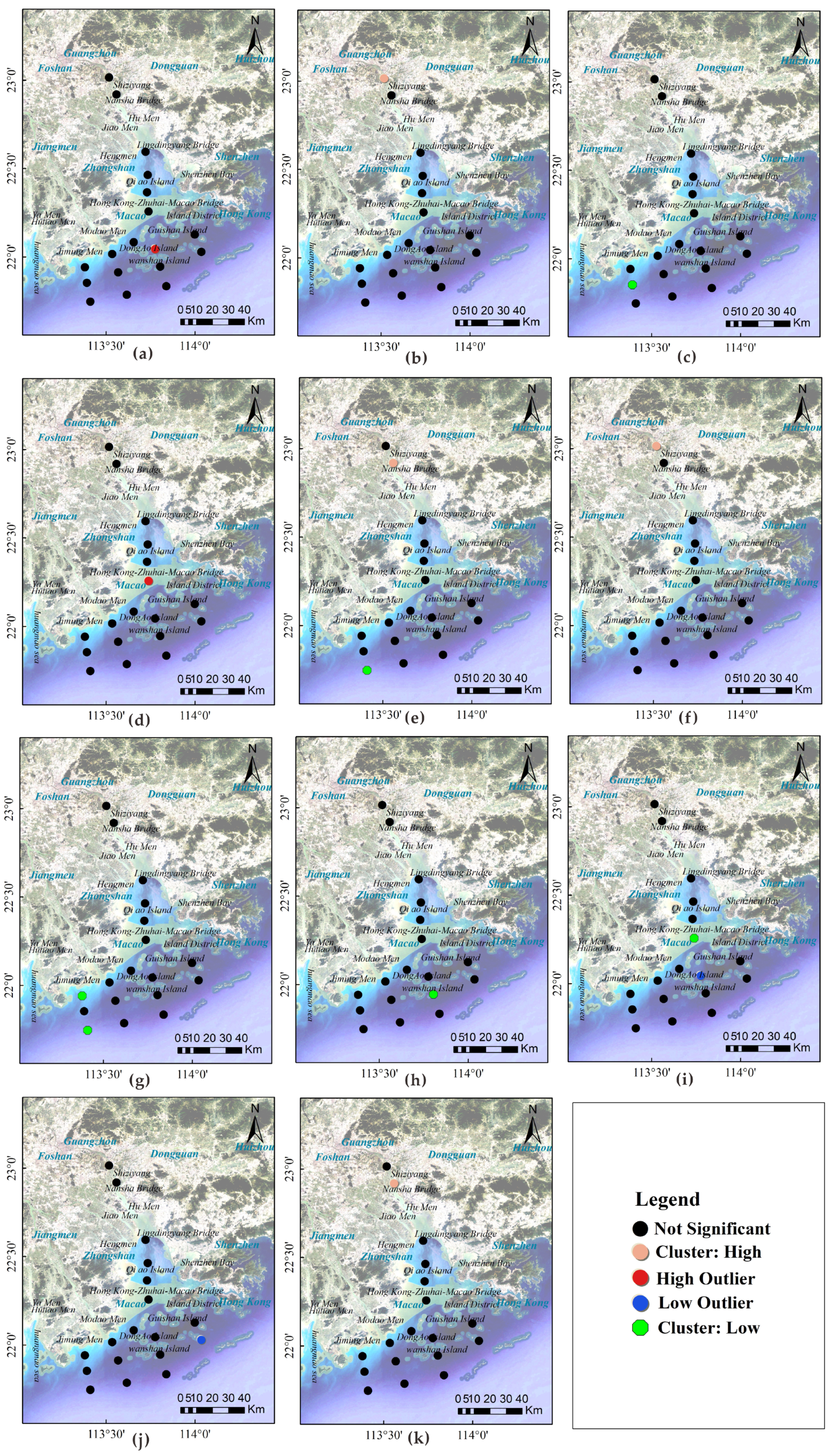
3.5. Spatial Distribution Patterns of Metal Partitioning
3.6. Correlation Analysis of the Metal Partition Coefficient
3.7. Multiple Regression Analysis
4. Conclusions
- (1)
- Elevated concentrations of particulate metals are predominantly found towards the Humen area, and particulate metals tend to exhibit higher concentrations near the Hong Kong-Zhuhai-Macao Bridge. The distribution of dissolved metals is hardly affected by the bridge, although high concentrations of certain metals, including Fe, Mo, and Ni, are observed not only in the Humen direction, but also towards the ocean.
- (2)
- Significant correlations between metals and environmental factors are observed more frequently in the particulate form.
- (3)
- The metals in the Pearl River Estuary are predominantly present in their dissolved form, with the except of Mn, whose concentration in suspended particulate matter is higher than that in filtered water.
- (4)
- The spatial distribution of metal partition coefficients shows a distinct clustering pattern: In the Pearl River Estuary, the partition coefficients of metals such as Fe, Mo, Mn, Ni, Co, and Zn show a clear spatial clustering, possibly related to local geochemical processes or human activities. In particular, a high-value clustering of certain metals (such as Fe, Mo, Mn, and Zn) is observed in the vicinity of Humen and the Hong Kong-Zhuhai-Macao Bridge, while some metals (such as Tl and Pb) are mainly concentrated in the oceanic direction, further away from land.
- (5)
- The partition coefficients of metals (Mn, Fe, Co, Ni, Zn, and Mo) show significant correlations with those of other metals (≥5 metals) and are strongly influenced by environmental factors, indicating a strong sensitivity to these factors. In contrast, the partition coefficients of Cr, Cu, Cd, Tl, and Pb show lower correlations with both the partition coefficients of other metals and environmental factors.
- (6)
- Temperature, oxygen content, and water depth are the most important determinants of metal distribution dynamics in the Pearl River Estuary, while the effects of pH, salinity, and suspended solids concentration on metal partitioning are relatively small.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, H.; Wang, Y.; Chen, C.; Zhang, Y. Particulate and Dissolved Metals in the Pearl River Estuary, China—Part 1: Spatial Distributions and Influencing Factors. Water 2025, 17, 1019. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, X.; Jian, H.; Chen, H.; Yu, Z. Characterization of the Particle Size Fraction associated with Heavy Metals in Suspended Sediments of the Yellow River. Int. J. Environ. Res. Public Health 2015, 12, 6725–6744. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Huang, X.; Zhang, D.; Tian, L.; Zeng, Y. Distribution of heavy metals in sediments of the Pearl River Estuary, Southern China: Implications for sources and historical changes. J. Environ. Sci. 2012, 24, 579–588. [Google Scholar] [CrossRef]
- Strady, E.; Quoc Tuc, D.; Nemery, J.; Thanh Nho, N.; Guedron, S.; Nhu Sang, N.; Denis, H.; Phuoc Dan, N. Spatial variation and risk assessment of trace metals in water and sediment of the Mekong Delta. Chemosphere 2017, 179, 367–378. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, W.W.; Wang, J.H. Trace-metal chemistry of the Huanghe (Yellow-River), China—Examination of the data from in-situ measurements and laboratory approach. Chem. Geol. 1994, 114, 83–94. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, C.; Wang, D.; Liu, Y.; Shen, Z. Salinity increases the mobility of Cd, Cu, Mn, and Pb in the sediments of Yangtze Estuary: Relative role of sediments’ properties and metal speciation. Chemosphere 2013, 91, 977–984. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Strady, E.; Tran Thi, N.T.; David, F.; Marchand, C. Trace metals partitioning between particulate and dissolved phases along a tropical mangrove estuary (Can Gio, Vietnam). Chemosphere 2018, 196, 311–322. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Q.; Chen, J.; Lin, C.; Ji, W. Heavy metals process in water and pollution risk assessment in surface sediments of the Zhujiang River Estuary. Acta Oceanol. Sin. 2013, 35, 178–186. [Google Scholar]
- Hatje, V.; Payne, T.E.; Hill, D.M.; McOrist, G.; Birch, G.F.; Szymczak, R. Kinetics of trace element uptake and release by particles in estuarine waters: Effects of pH, salinity, and particle loading. Environ. Int. 2003, 29, 619–629. [Google Scholar] [CrossRef]
- Kuang, Z.; Fan, Z.; Wang, H.; Gu, Y.; Zhang, W.; Wang, S.; Huang, H. Heavy metal(loid)s in multiple media within a mussel mariculture area of Shangchuan Island, China: Partition, transfer and health risks. Environ. Res. 2022, 211, 113100. [Google Scholar] [CrossRef]
- Ashayeri, N.Y.; Keshavarzi, B. Geochemical characteristics, partitioning, quantitative source apportionment, and ecological and health risk of heavy metals in sediments and water: A case study in Shadegan Wetland, Iran. Mar. Pollut. Bull. 2019, 149, 110495. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, G.; Jiang, M.; Wang, R.; Zheng, L. Partition characteristics and correlation of heavy metal between sediment and surface water from Chaohu Lake. J. Univ. Sci. Technol. China 2011, 41, 9–15. [Google Scholar]
- Wang, Y.; Zhang, J.; Yan, Q.; Guo, J.; Liu, G.; Hu, H.; Zhao, Y. Spatial distribution, sediment-water partitioning, risk assessment and source apportionment of heavy metals in the Golmud River-Dabson Salt Lake ecosystem. Environ. Res. 2025, 268, 120792. [Google Scholar] [CrossRef]
- Jakomin, L.M.; Marban, L.; Grondona, S.; Glok Galli, M.; Martinez, D.E. Mobility of Heavy Metals (Pb, Cd, Zn) in the Pampeano and Puelche Aquifers, Argentina: Partition and Retardation Coefficients. Bull. Environ. Contam. Toxicol. 2015, 95, 325–331. [Google Scholar] [CrossRef]
- Xu, X.; Huang, R.; Liu, J.; Shu, Y. Fractionation and release of Cd, Cu, Pb, Mn, and Zn from historically contaminated river sediment in Southern China: Effect of time and pH. Environ. Toxicol. Chem. 2019, 38, 464–473. [Google Scholar] [CrossRef]
- Trinh Anh, D.; Vu Duc, L.; Ta Thi, T. Partition of heavy metals in a tropical river system impacted by municipal waste. Environ. Monit. Assess. 2013, 185, 1907–1925. [Google Scholar] [CrossRef]
- Liao, B.; Liu, Q.; Jia, Z.; Li, S.; Hu, J. Heavy metal distribution patterns and their influence factors in Modaomen estuary of the Pearl River. Mar. Environ. Sci. 2021, 40, 8–15. [Google Scholar]
- Khadhar, S.; Mlayah, A.; Chekirben, A.; Charef, A.; Methammam, M.; Nouha, S.; Khemais, Z. Transport of heavy metal pollution from the Wadi El Bey basin toward the Tunisian Gulf. Hydrol. Sci. J. 2013, 58, 1803–1812. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, F.; Chen, C.; Sun, X.; Shi, Y.; Zhao, H.; Chen, F. Spatial distribution and correlation characteristics of heavy metals in the seawater, suspended particulate matter and sediments in Zhanjiang Bay, China. PLoS ONE 2018, 13, e0201414. [Google Scholar] [CrossRef]
- Feng, C.; Guo, X.; Yin, S.; Tian, C.; Li, Y.; Shen, Z. Heavy metal partitioning of suspended particulate matter-water and sediment-water in the Yangtze Estuary. Chemosphere 2017, 185, 717–725. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, K.; Yi, Q.; Zhang, T.; Shi, W.; Zhou, X. Transport and partitioning of metals in river networks of a plain area with sedimentary resuspension and implications for downstream lakes. Environ. Pollut. 2022, 294, 118668. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Li, S.; Liu, Q.; Jiang, F.; Hu, J. Distribution and partitioning of heavy metals in water and sediments of typical estuary (Modaomen, South China): The effect of water density stratification associated with salinity. Environ. Pollut. 2021, 287, 117277. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yan, C.; Yang, C.; Li, R.; Wu, Q.; Tian, D.; Ouyang, L. Phases partitioning and occurrence forms of arsenic, chromium, and vanadium in a tidal reach of the Pearl river estuary, South China. Environ. Pollut. 2025, 368, 125745. [Google Scholar] [CrossRef]
- Li, X.; Shen, Z.; Wai, O.W.; Li, Y.S. Chemical partitioning of heavy metal contaminants in sediments of the Pearl River Estuary. Chem. Speciat. Bioavailab. 2000, 12, 17–25. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Ni, Z.; Li, J.; Ren, Y.; Lin, J.; Huang, X. Saltwater intrusion regulates the distribution and partitioning of heavy metals in water in a dynamic estuary, South China. Mar. Environ. Res. 2023, 186, 105943. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Sediment Distribution Coefficients and Concentration Factors for Biota in the Marine Environment; International Atomic Energy Agency: Vienna, Austria, 2004. [Google Scholar]
- Jianhui, Y. Suspended Solid and its Influence on Interphasepartitions of Heavy Metals in Zhanjiang Bay. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2015. [Google Scholar]
- Yu, L. Study on the Distribution and Characteristics of Main Heavy Metalsand Organic Pollutants of Jiaozhou Bay. Ph.D.Thesis, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China, 2005. [Google Scholar]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef]
- Strawn, D.G.; Baker, L.L. Speciation of cu in a contaminated agricultural soil measured by XAFS, μ-XAFS, and μ-XRF. Environ. Sci. Technol. 2008, 42, 37–42. [Google Scholar] [CrossRef]
- Komarek, M.; Ettler, V.; Chrastny, V.; Mihaljevic, M. Lead isotopes in environmental sciences: A review. Environ. Int. 2008, 34, 562–577. [Google Scholar] [CrossRef]
- Raiswell, R.; Canfield, D.E. The iron biogeochemical cycle past and present. Geochem. Perspect. 2012, 1, 1–220. [Google Scholar] [CrossRef]
- Kotas, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef]
- Shiller, A.M.; Boyle, E. Dissolved zinc in rivers. Nature 1985, 317, 49–52. [Google Scholar] [CrossRef]
- Turner, A.; Millward, G.E. Suspended particles: Their role in estuarine biogeochemical cycles. Estuar. Coast. Shelf Sci. 2002, 55, 857–883. [Google Scholar] [CrossRef]
- Sholkovitz, E.R.; Boyle, E.A.; Price, N.B. Removal of dissolved humic acids and iron during estuarine mixing. Earth Planet. Sci. Lett. 1978, 40, 130–136. [Google Scholar] [CrossRef]
- Xu, P.A.; Zeng, G.M.; Huang, D.L.; Lai, C.; Zhao, M.H.; Wei, Z.; Li, N.J.; Huang, C.; Xie, G.X. Adsorption of Pb(II) by iron oxide nanoparticles immobilized Phanerochaete chrysosporium: Equilibrium, kinetic, thermodynamic and mechanisms analysis. Chem. Eng. J. 2012, 203, 423–431. [Google Scholar] [CrossRef]
- Kucuksezgin, F.; Uluturhan, E.; Batki, H. Distribution of heavy metals in water, particulate matter and sediments of Gediz River (Eastern Aegean). Environ. Monit. Assess. 2008, 141, 213–225. [Google Scholar] [CrossRef]
- Li, R.; Tang, C.; Cao, Y.; Jiang, T.; Chen, J. The distribution and partitioning of trace metals (Pb, Cd, Cu, and Zn) and metalloid (As) in the Beijiang River. Environ. Monit. Assess. 2018, 190, 399. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.M.; Song, X.X.; Hua, C.X. Influence of point sources on heavy metal accumulation in different apartments of Jiaozhou Bay. Acta Ecol. Sin. 2009, 29, 5592–5599. [Google Scholar]
- Baeyens, W.; Elskens, M.; Gillain, G.; Goeyens, L. Biogeochemical behaviour of Cd, Cu, Pb and Zn in the Scheldt estuary during the period 1981–1983. Hydrobiologia 1998, 366, 15–44. [Google Scholar] [CrossRef]
- Liu, Y.; Du, Q.; Wang, Q.; Yu, H.; Liu, J.; Tian, Y.; Chang, C.; Lei, J. Causal inference between bioavailability of heavy metals and environmental factors in a large-scale region. Environ. Pollut. 2017, 226, 370–378. [Google Scholar] [CrossRef]
- Balls, P.W. The control of trace-metal concentrations in coastal seawater through partition onto suspended particulate matter. Neth. J. Sea Res. 1988, 22, 213–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, J.; Zhang, W.; Wu, J. Importance of salinity-induced stratification on flocculation in tidal estuaries. J. Hydrol. 2021, 596, 126063. [Google Scholar] [CrossRef]
- Noh, S.; Choi, M.; Kim, E.; Dan, N.P.; Thanh, B.X.; Van Ha, N.T.; Sthiannopkao, S.; Han, S. Influence of salinity intrusion on the speciation and partitioning of mercury in the Mekong River Delta. Geochim. Cosmochim. Acta 2013, 106, 379–390. [Google Scholar] [CrossRef]
- Briant, N.; Chiffoleau, J.-F.; Knoery, J.; Araujo, D.F.; Ponzevera, E.; Crochet, S.; Thomas, B.; Brach-Papa, C. Seasonal trace metal distribution, partition and fluxes in the temperate macrotidal Loire Estuary (France). Estuar. Coast. Shelf Sci. 2021, 262, 107616. [Google Scholar] [CrossRef]
- Miranda, L.S.; Wijesiri, B.; Ayoko, G.A.; Egodawatta, P.; Goonetilleke, A. Water-sediment interactions and mobility of heavy metals in aquatic environments. Water Res. 2021, 202, 117386. [Google Scholar] [CrossRef]
- Zwolsman, J.J.G.; Berger, G.W.; Van Eck, G.T.M. Sediment accumulation rates, historical input, postdepositional mobility and retention of major elements and trace metals in salt marsh sediments of the Scheldt estuary, SW Netherlands. Mar. Chem. 1993, 44, 73–94. [Google Scholar] [CrossRef]
- Du Laing, G.; Meers, E.; Dewispelaere, M.; Rinklebe, J.; Vandecasteele, B.; Verloo, M.G.; Tack, F.M.G. Effect of Water Table Level on Metal Mobility at Different Depths in Wetland Soils of the Scheldt Estuary (Belgium). Water Air Soil Pollut. 2009, 202, 353–367. [Google Scholar] [CrossRef]
- Sholkovitz, E.R. Flocculation of dissolved organic and inorganic matter during the mixing of river water and seawater. Geochim. Cosmochim. Acta 1976, 40, 831–845. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Spencer, K.; Kloas, W.; Toffolon, M.; Zarfl, C. Metal fate and effects in estuaries: A review and conceptual model for better understanding of toxicity. Sci. Total Environ. 2016, 541, 268–281. [Google Scholar] [CrossRef]
- Thorpe, A.; Harrison, R.M. Sources and properties of non-exhaust particulate matter from road traffic: A review. Sci. Total Environ. 2008, 400, 270–282. [Google Scholar] [CrossRef]
- Adachi, K.; Tainosho, Y. Characterization of heavy metal particles embedded in tire dust. Environ. Int. 2004, 30, 1009–1017. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Li, R.; Tian, D.; Wu, Q.; Yang, Z.; Liang, Z.; Gao, L.; Lian, J.; Chen, J. Spatial distribution and quantitative source identification of heavy metals in sediment cores of Jiaomen Waterway. China Environ. Sci. 2023, 43, 4819–4827. [Google Scholar]
- Lu, X.; Zhang, Y.; Liu, H.; Xing, M.; Shao, X.; Zhao, F.; Li, X.; Liu, Q.; Yu, D.; Yuan, X.; et al. Influence of early diagenesis on the vertical distribution of metal forms in sediments of Bohai Bay, China. Mar. Pollut. Bull. 2014, 88, 155–161. [Google Scholar] [CrossRef]
- Wu, Y.; Falconer, R.; Lin, B. Modelling trace metal concentration distributions in estuarine waters. Estuar. Coast. Shelf Sci. 2005, 64, 699–709. [Google Scholar] [CrossRef]

| Particulate | Dissolved | |||
|---|---|---|---|---|
| Metals | Range | Mean | Range | Mean |
| Cr | 0.055–1.284 | 0.59 | 1.01–10.25 | 4.86 |
| Mn | 0.19–57.15 | 10.26 | 0.78–1.54 | 0.98 |
| Fe | 16.24–659.60 | 227.31 | 190.9–472.09 | 381.94 |
| Co | 0.0024–0.3168 | 0.12 | 0.12–0.21 | 0.18 |
| Ni | 0.017–1.886 | 0.46 | 3.49–24.2 | 18.29 |
| Cu | 0.020–0.836 | 0.33 | 1.08–3.55 | 2.10 |
| Zn | 0.20–3.31 | 1.37 | 14.13–48.28 | 21.78 |
| Mo | 0.0013–0.0246 | 0.0090 | 4.61–10.63 | 8.74 |
| Cd | 0.0002–0.0171 | 0.0036 | 0.006–0.062 | 0.026 |
| Tl | 0.0001–0.0106 | 0.0040 | 0.015–0.045 | 0.025 |
| Pb | 0.078–3.353 | 0.65 | 2.43–9.64 | 4.66 |
| Metals | Range | Mean | CV |
|---|---|---|---|
| Cr | 3.73–4.77 | 4.18 | 0.06 |
| Mn | 5.31–6.70 | 5.83 | 0.07 |
| Fe | 4.52–5.30 | 4.81 | 0.04 |
| Co | 4.33–5.24 | 4.79 | 0.05 |
| Ni | 2.98–4.19 | 3.37 | 0.11 |
| Cu | 3.73–4.65 | 4.25 | 0.04 |
| Zn | 3.62–4.24 | 3.9 | 0.04 |
| Mo | 1.77–2.73 | 2.06 | 0.12 |
| Cd | 3.36–4.84 | 4.2 | 0.09 |
| Tl | 3.74–4.61 | 4.26 | 0.04 |
| Pb | 3.76–4.73 | 4.19 | 0.06 |
| Areas | Metric | Fe | Mn | Cr | Ni | Cu | Zn | Cd | Pb | Co |
|---|---|---|---|---|---|---|---|---|---|---|
| our results | Mean | 4.81 | 5.83 | 4.18 | 3.37 | 4.25 | 3.90 | 4.20 | 4.19 | 4.79 |
| Zhanjiang Bay [19] | Mean | 5.32 | 5.21 | 4.73 | 4.69 | 3.67 | 4.40 | 5.14 | 5.60 | |
| Day River [16] | Mean | 5.8 | 5.0 | 5.5 | 5.3 | 5.4 | 5.1 | 5.7 | 5.3 | 5.6 |
| our results | Range | 4.52–5.30 | 5.31–6.70 | 3.73–4.77 | 2.98–4.19 | 3.73–4.65 | 3.62–4.24 | 3.36–4.84 | 3.76–4.73 | 4.33–5.24 |
| Jiaozhou Bay [40] | Range | 3.8–4.9 | 1.9–4.7 | 2.2–5.0 | 2.8–5.4 | |||||
| Scheldt Estuary [41] | Range | 4.3–5.4 | 4.5–5.0 | 5.5–6.4 | 5.4–6.0 | |||||
| Six Texas Estuaries [42] | Range | 4.7–7.2 | 3.0–5.1 | 3.8–6.0 | 3.8–6.8 | |||||
| North Australian [43] | Range | 5.7–8.8 | 3.8–5.3 | 3.7–5.4 | 4.4–6.7 | 3.3–6.3 | 5.5–7.2 |
| Metals | Moran’s I Index | z-Score | p-Value |
|---|---|---|---|
| Cr | 0.212 | 1.083 | 0.279 |
| Mn | 0.523 | 2.271 | 0.023 |
| Fe | 0.609 | 2.600 | 0.009 |
| Co | 0.401 | 1.760 | 0.078 |
| Ni | 0.559 | 2.347 | 0.019 |
| Cu | 0.257 | 1.364 | 0.173 |
| Zn | 0.418 | 1.785 | 0.074 |
| Mo | 0.731 | 3.307 | 0.001 |
| Cd | 0.269 | 1.230 | 0.219 |
| Tl | −0.067 | −0.037 | 0.971 |
| Pb | −0.251 | −0.720 | 0.471 |
| Depth of Water | Temperature | Salinity | Oxygen | pH | Suspended Solids | R2 | |
|---|---|---|---|---|---|---|---|
| Cr | −0.37 | 0.31 | −0.03 | 0.16 | −0.39 | 0.19 | 0.266 |
| Mn | −0.07 | −0.33 | −0.52 | −0.42 | 0.24 | −0.15 | 0.838 *** |
| Fe | −0.45 * | −1.08 ** | 0.78 | −1.58 *** | 0.89 | −0.23 | 0.870 *** |
| Co | −0.45 * | −1.07 ** | 0.87 | −1.35 ** | 0.63 | −0.18 | 0.849 *** |
| Ni | 0.01 | 0.43 | −0.65 | 0.77 | −1.17 | 0.13 | 0.708 ** |
| Cu | −0.14 | −0.74 | 1.61 | −0.94 | −0.51 | −0.41 | 0.235 |
| Zn | −0.18 | 0.08 | −0.01 | −1.07 | 0.32 | −0.04 | 0.642 ** |
| Mo | 0.06 | −0.40 | −0.55 | −0.90 ** | 0.72 | −0.16 | 0.921 *** |
| Cd | 0.44 | 0.96 | −1.31 | 1.01 | −0.83 | 0.01 | 0.263 |
| Tl | −0.34 | −1.47 * | 1.97 | −1.63 | 0.80 | −0.03 | 0.334 |
| Pb | −0.26 | −0.11 | −0.27 | −0.88 | 1.12 | −0.34 | 0.185 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Wang, Y.; Chen, C.; Zhang, Y. Particulate and Dissolved Metals in the Pearl River Estuary, China—Part 2: Partitioning Characteristics and Influencing Factors. Water 2025, 17, 1436. https://doi.org/10.3390/w17101436
Ma H, Wang Y, Chen C, Zhang Y. Particulate and Dissolved Metals in the Pearl River Estuary, China—Part 2: Partitioning Characteristics and Influencing Factors. Water. 2025; 17(10):1436. https://doi.org/10.3390/w17101436
Chicago/Turabian StyleMa, Hongyan, Yunpeng Wang, Chuqun Chen, and Yuanzhi Zhang. 2025. "Particulate and Dissolved Metals in the Pearl River Estuary, China—Part 2: Partitioning Characteristics and Influencing Factors" Water 17, no. 10: 1436. https://doi.org/10.3390/w17101436
APA StyleMa, H., Wang, Y., Chen, C., & Zhang, Y. (2025). Particulate and Dissolved Metals in the Pearl River Estuary, China—Part 2: Partitioning Characteristics and Influencing Factors. Water, 17(10), 1436. https://doi.org/10.3390/w17101436








