Denitrification Performance and Microbiological Mechanisms Using Polyglycolic Acid as a Carbon Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Experimental Water and Inoculated Sludge
2.3. Single-Factor Experimental Design
2.4. Analytical Items and Methods
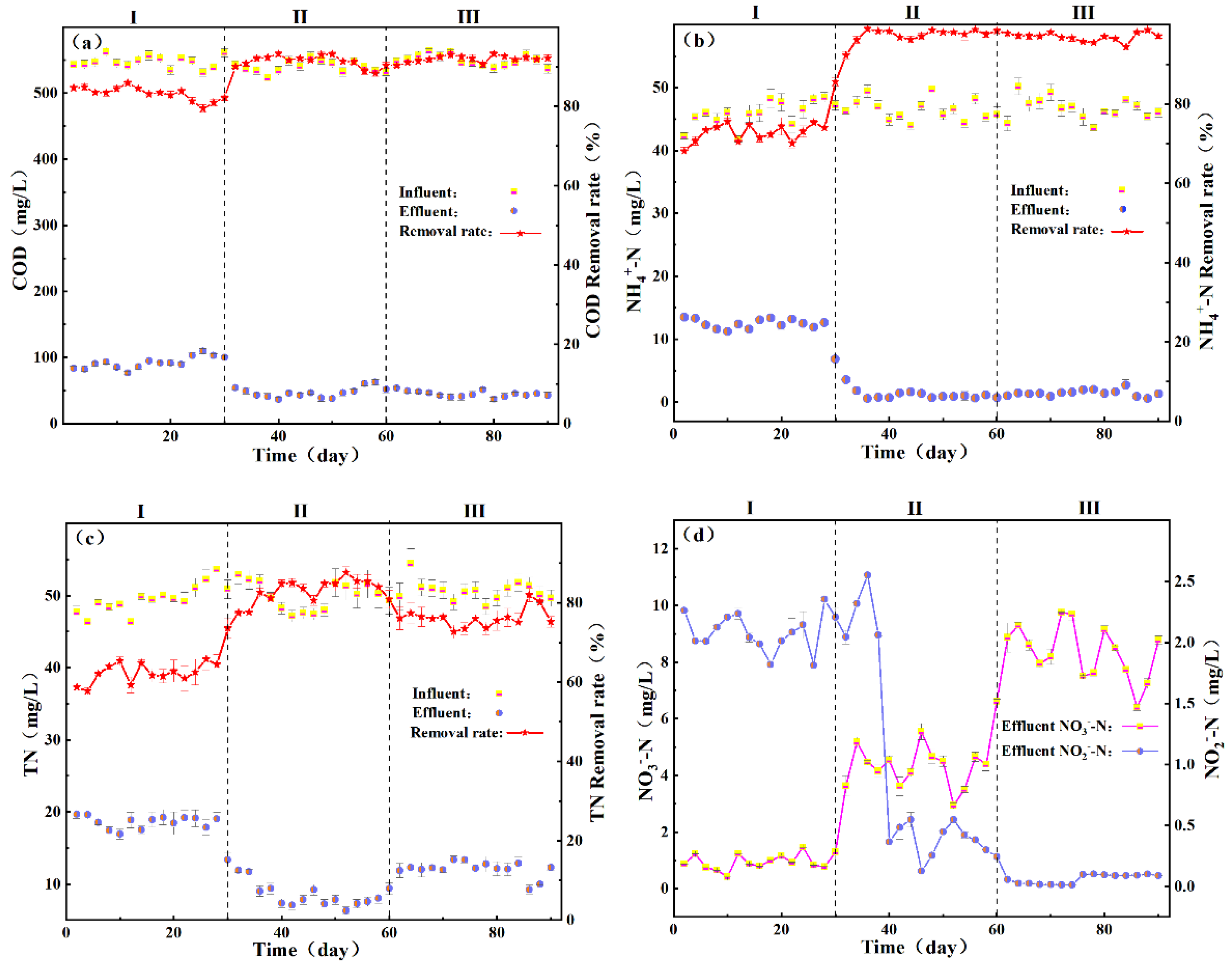
2.4.1. Conventional Indicators
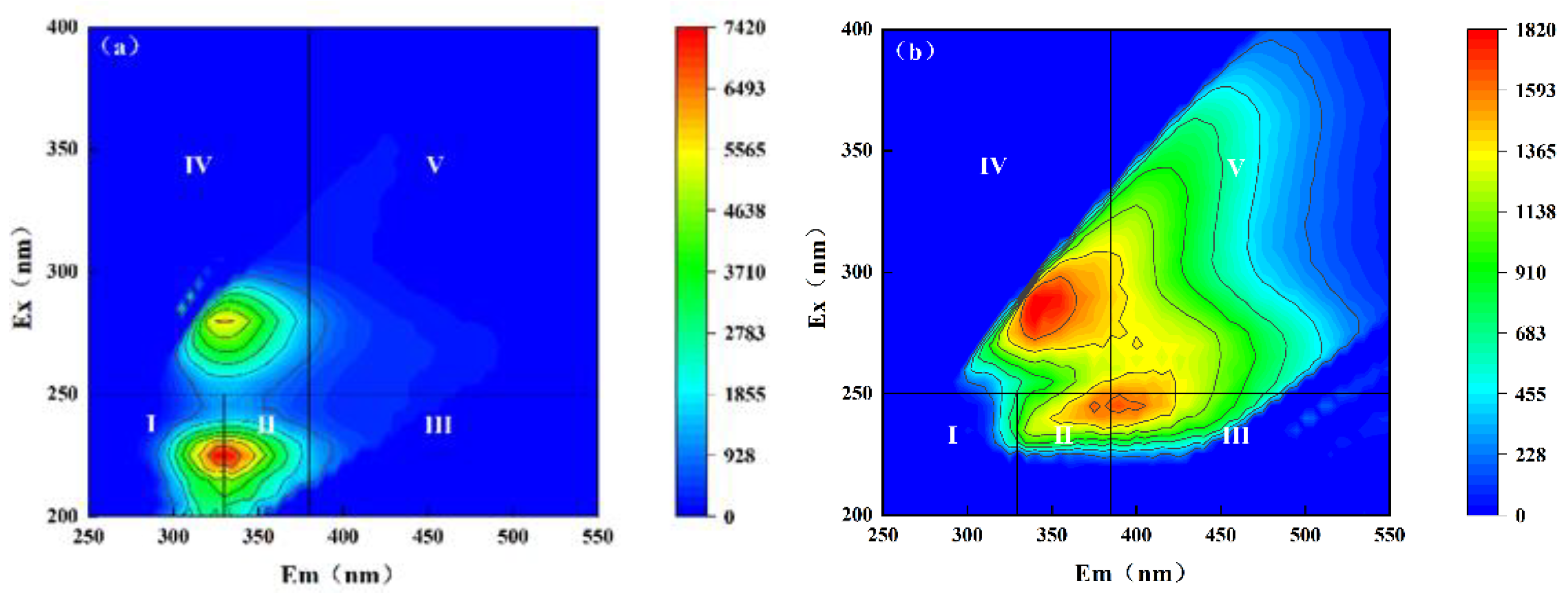
2.4.2. Three-Dimensional Fluorescence Spectroscopy
2.4.3. DNA Extraction, Polymerase Chain Reaction (PCR) Amplification, and High-throughput Sequencing
3. Results and Discussion
3.1. Investigation of the Optimum Dosage of PGA
3.1.1. Effect of PGA Dosage on Pollutant-Removal Effect
3.1.2. DOM Analysis of Influent and Effluent Water
3.2. Exploration of the Optimum External Conditions for the Use of PGA as a Carbon Source
3.2.1. The Optimum pH of PGA as a Carbon Source
3.2.2. The Optimum DO of PGA
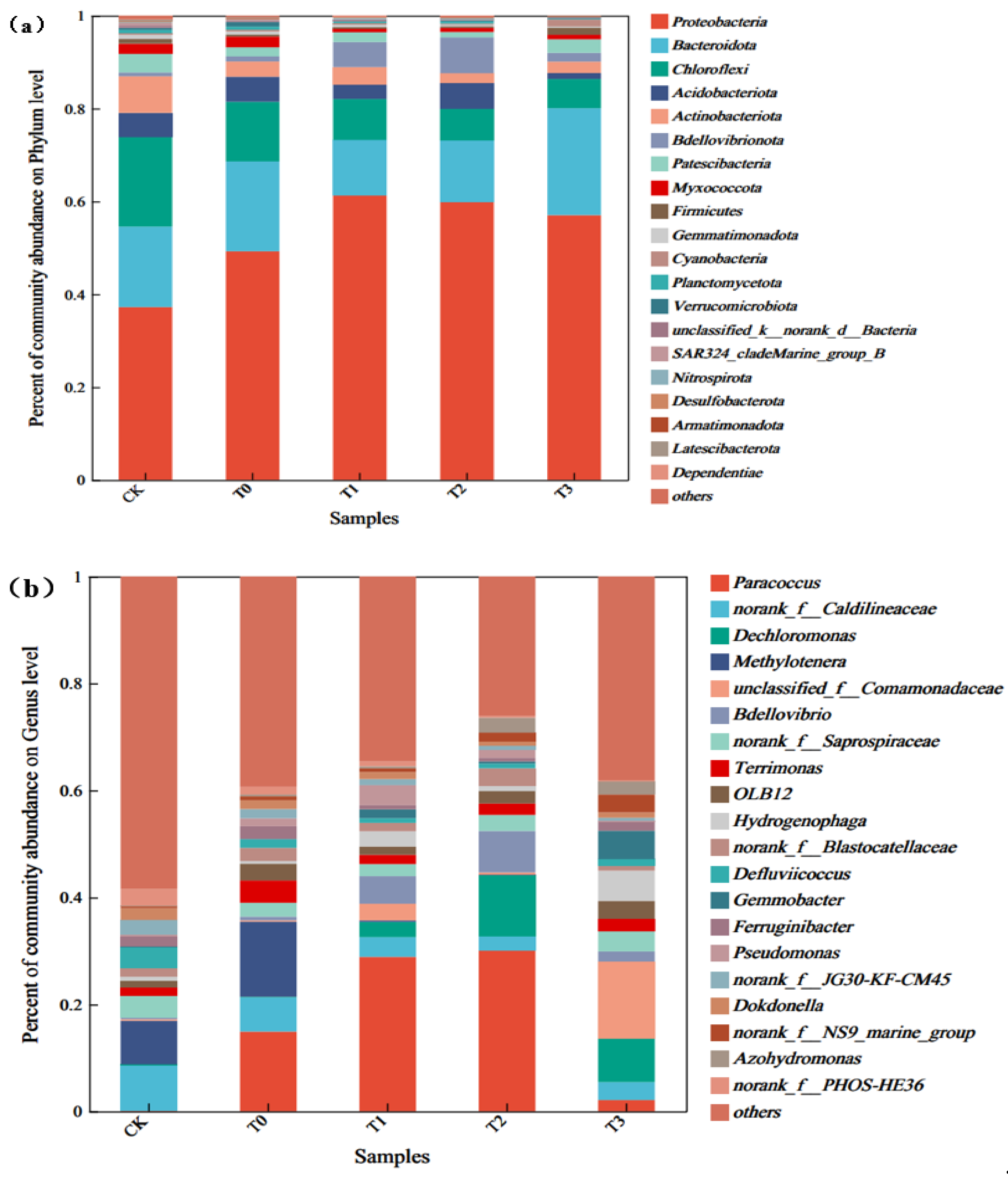
3.3. Analysis of Microbial-Community Structure and Diversity
3.3.1. Microbial Alpha Diversity
3.3.2. Microbial-Community Structure
3.3.3. Microbial Species Diversity
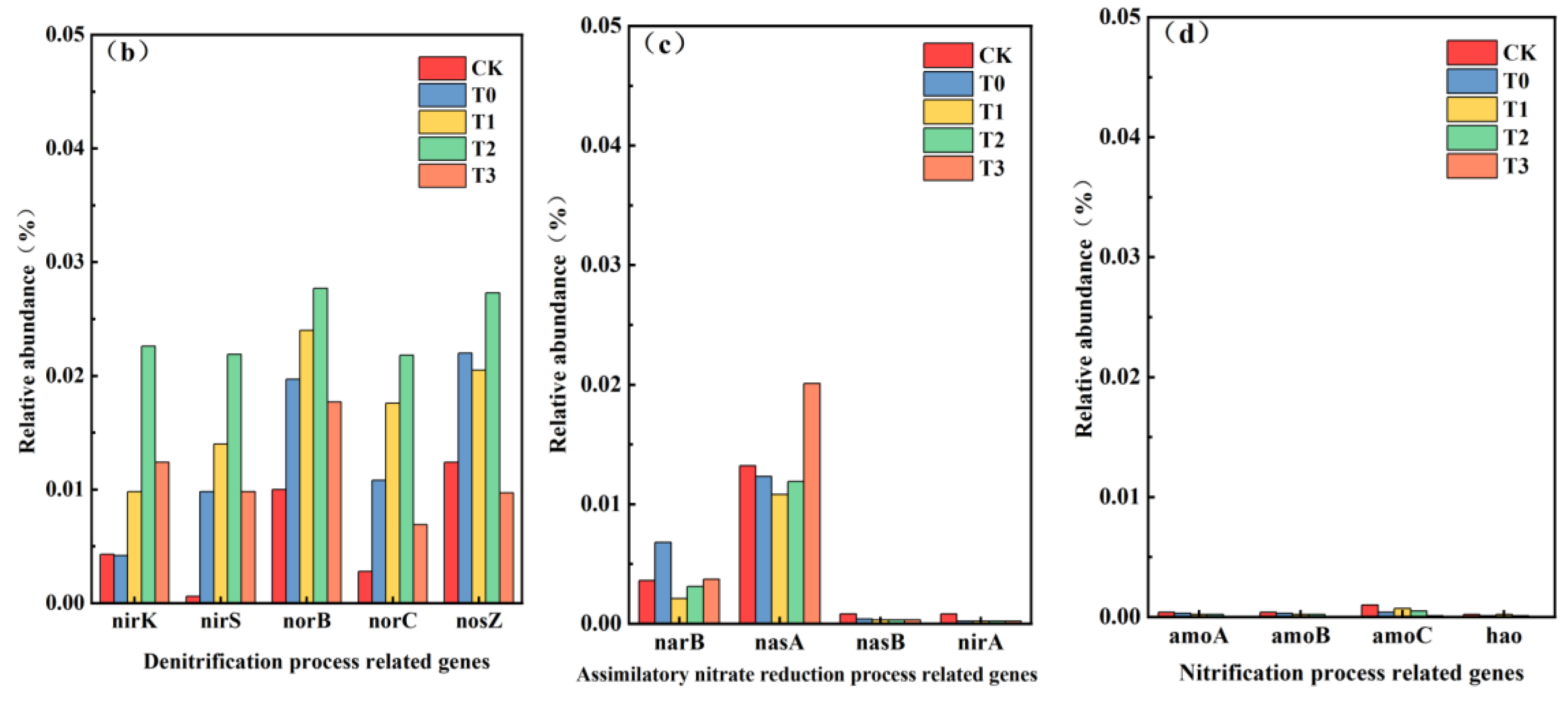
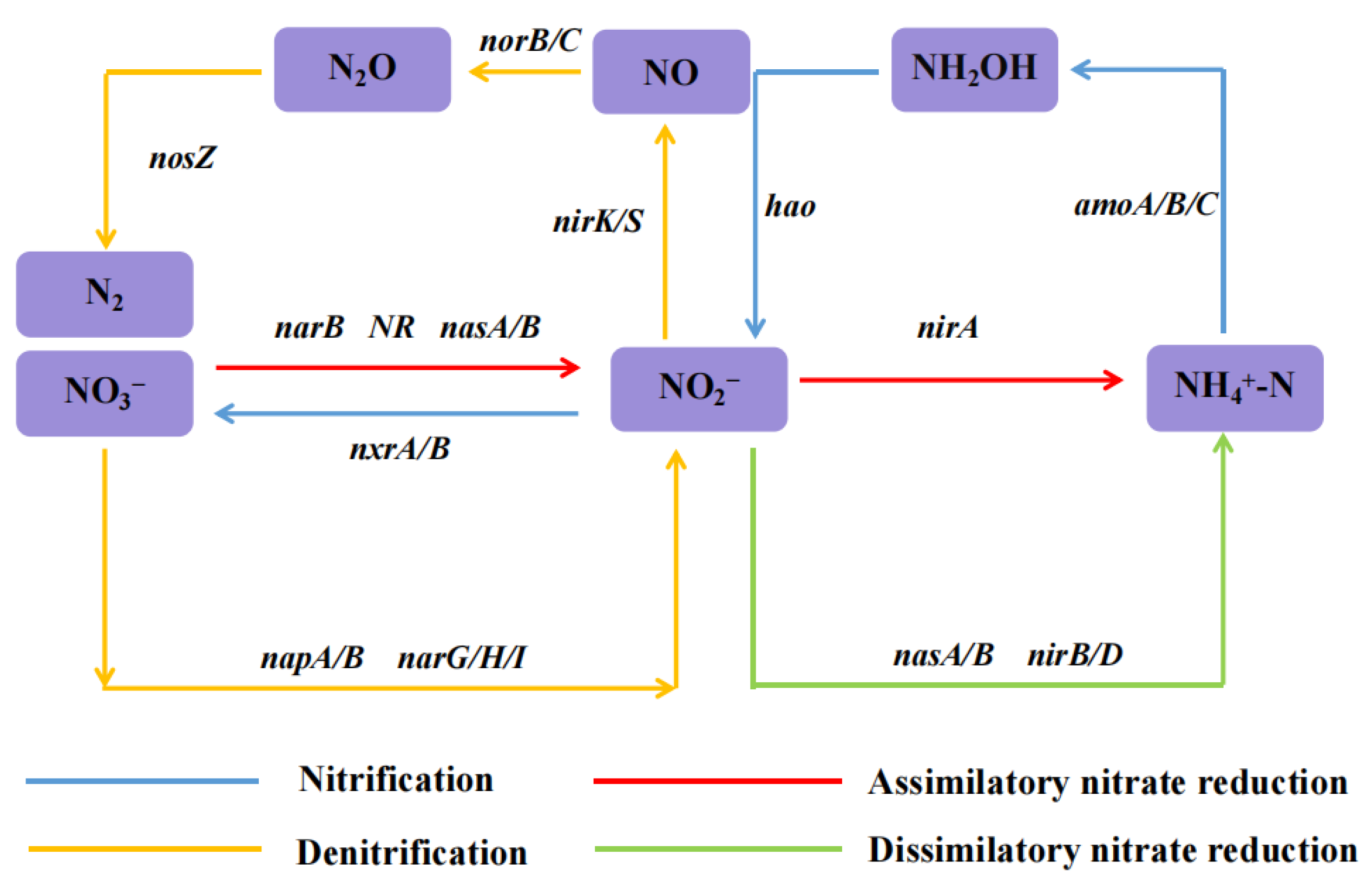
3.4. Functional Genes for Nitrogen Metabolism
4. Domestic and International Research Status about Carbon Source
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.C.; Zhang, C.M. Analysis and prospect of spatial and temporal distribution of urban sewage in China. City Town Water Supply 2020, 73–78. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Fan, J. Preparation of a new efficient composite carbon source and its application in denitrification and nitrogen removal. Water Wastewater Eng. 2019, 55, 153–155, 158. [Google Scholar] [CrossRef]
- Li, Z.L.; Zhang, K.H. Treatment of pharmaceutical wastewater by Fenton Pre-oxidation and MBR process. China Water & Wastewater 2022, 38, 160–165. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Z.Y.; Zhang, X.; You, Z.P.; Huang, Z.J.; Peng, Y.Z. Effects of external carbon sources on ultimate nitrogen removal performance and microbial community in secondary effluent treating process. Environ. Sci. 2022, 43, 4717–4726. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Dai, X.; Chai, X. Effect of different carbon sources on denitrification performance, microbial community structure and denitrification genes. Sci. Total Environ. 2018, 634, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zhou, M.; Luo, Z.; Zhang, H.; Yang, Z.; Cheng, H.; Li, R.; He, Q.; Ai, H. Selection and synthesization of multi–carbon source composites to enhance simultaneous nitrification–denitrification in treating low C/N wastewater. Chemosphere 2022, 288, 132567. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Lavagnolo, M.C.; Concheri, G.; Stevanato, P.; Squartini, A.; Spagni, A. Application of anaerobic dynamic membrane bioreactor (AnDMBR) for the successful enrichment of Anammox bacteria using mixed anaerobic and aerobic seed sludge. Bioresour. Technol. 2018, 266, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.C.; Li, J.C.; Yang, M.X.; Guo, Z.Z.; Lin, S.J.; Hu, Q. Environment and economic benefit analysis of external carbon source under double carbon target and management suggestions. In Proceedings of the 2022 Annual Scientific and Technical Conference of the Chinese Society of Environmental Sciences—Environmental Engineering Technology Innovation and Application Session (III), Nanchang, China, 20 August 2022; pp. 824–829. [Google Scholar]
- Chen, M.F.; Zheng, K.K.; Wang, Y.; Gao, J.X.; Wang, S.; Li, J. Optimized operation of a wastewater treatment plant with high nitrate nitrogen concentration influent based on whole process analysis method. China Water Wastewate 2019, 35, 118–122+128. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Yin, L.; Ruan, X. The characteristics of carbon sources for denitrification in groundwater from ten kinds of agricultural wastes. Acta Sci. Circumstant. 2017, 37, 1787–1797. [Google Scholar] [CrossRef]
- Fu, X.; Hou, R.; Yang, P.; Qian, S.; Feng, Z.; Chen, Z.; Wang, F.; Yuan, R.; Chen, H.; Zhou, B. Application of external carbon source in heterotrophic denitrification of domestic sewage: A review. Sci. Total Environ. 2022, 817, 153061. [Google Scholar] [CrossRef]
- Xu, C.; Yin, Z.; Li, C.; Bi, X.; Gu, R. Nitrogen removal enhancement with landfill leachate as supplemental carbon source for denitrification. Chin. J. Environ. Eng. 2019, 13, 1106–1112. [Google Scholar]
- Wang, X.; Zhang, Y.; Zhang, T.; Zhou, J.; Chen, M. Waste activated sludge fermentation liquid as carbon source for biological treatment of sulfide and nitrate in microaerobic conditions. Chem. Eng. J. 2016, 283, 167–174. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, Z.; Sun, F.; Miao, M.; Cui, M.-H.; Liu, H.; Zhang, H.; Zhang, C.; Hu, Z.; Liu, H. Valorization of citric acid production wastewater as alternative carbon source for biological nutrients removal: A pilot-scale case study. J. Clean. Prod. 2020, 258, 120576. [Google Scholar] [CrossRef]
- Li, X.; Zhi, Y.; Jia, M.; Wang, X.; Tao, M.; Wang, Z.; Xing, B. Properties and photosynthetic promotion mechanisms of artificial humic acid are feedstock-dependent. Carbon Res. 2024, 3, 4. [Google Scholar] [CrossRef]
- Dong, L.Q.; Xu, F.; Wen, Y.X. Research progress in modification and application of poly(glycolic acid). China Plast. 2022, 36, 166–174. [Google Scholar]
- Matsuda, Y.; Karino, M.; Okui, T.; Kanno, T. Complications of poly-l-lactic acid and polyglycolic acid (PLLA/PGA) osteosynthesis systems for maxillofacial surgery: A retrospective clinical investigation. Polymers 2021, 13, 889. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wen, L.; Sun, Z.Y.; Ji, Y. Properties and applications of PGA/PBAT composites. China Plast. 2020, 34, 36–40. [Google Scholar] [CrossRef]
- Zhong, W.M.; Liu, L.P.; Wei, Z.Y. Synthesis and industrialization of biodegradable plastics PGA. China Synth. Resin Plast. 2021, 38, 80–84. [Google Scholar] [CrossRef]
- Gao, S.; He, W.; Wang, S.K.; Qin, W. Demonstration for application of denitrifying phosphorus removal process in rural domestic sewage treatment. China Water Wastewater 2023, 39, 114–119. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Qiang, Z. Start-up of solid-phase denitrification process for treatment of nitrate-rich water in recirculating mariculture system: Carbon source selection and nitrate removal mechanism. Chemosphere 2023, 338, 139568. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, M.; He, C.; Hu, Z.; Yu, Y.; Wang, W. Enhanced treatment of low-temperature and low carbon/nitrogen ratio wastewater by corncob-based fixed bed bioreactor coupled sequencing batch reactor. Bioresour. Technol. 2022, 351, 126975. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wang, F.; Geng, H.; Liu, H.; Pu, S.; Tian, Z.; Chen, H.; Zhou, B.; Yuan, R.; Yao, J. Integrating high-throughput sequencing and metagenome analysis to reveal the characteristic and resistance mechanism of microbial community in metal contaminated sediments. Sci. Total Environ. 2020, 707, 136116. [Google Scholar] [CrossRef]
- Liu, C.; Li, Q.; Song, Z.Y.; Hu, P.; Jing, S.Y.; Li, W.P. Analysis of microbial community characteristics and function prediction of MBBR with magnetic biocarriers at low temperature. Environ. Sci. 2023, 44, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, Z.; Wei, Q.; Luo, F.; Jiang, Z.; Ao, Z.; Chen, H.; Niu, X.; Liu, G.-h.; Qi, L. Exploratory study on the metabolic similarity of denitrifying carbon sources. Environ. Sci. Pollut. Res. 2024, 31, 19961–19973. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Alengebawy, A.; Zhu, X.; Raza, A.F.; Chen, L.; Chen, W.; Guo, J.; Ai, P.; Li, D. Performance of Paracoccus pantotrophus MA3 in heterotrophic nitrification–anaerobic denitrification using formic acid as a carbon source. Bioprocess Biosyst. Eng. 2022, 45, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, M.Y.; Hao, Z.N.; Zhong, H.; He, H.; Lei, P. Dynamic changes of dissolved organic matter derived from algal decomposition and the environmental effects in eu-trophiclakes. Environ. Sci. 2024, 45, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sun, R.Z.; Wang, Y.; Chen, G.L.; Fu, Y.Y.; Yu, H.Q. Carbon source shaped microbial ecology, metabolism and performance in denitrification systems. Water Res. 2023, 243, 120330. [Google Scholar] [CrossRef]
- Leng, J.; Lu, J.; Hai, C.; Liu, X.; Wu, P.; Sun, Y.; Yuan, C.; Zhao, J.; Hu, B. Exploring influence mechanism of small-molecule carbon source on heterotrophic nitrification-aerobic denitrification process from carbon metabolism, nitrogen metabolism and electron transport process. Bioresour. Technol. 2023, 387, 129681. [Google Scholar] [CrossRef]
- Zhu, K.; Zhou, J.; He, Q.; Zhou, J.; Meng, H.; He, X.J. Comprehensive impact of carbon to nitrogen ratio, dissolved oxygen and temperature on advanced phosphorus and nitrogen removal efficiencies in sequencing batch biofilm reactor(SBBR) system. Environ. Eng. 2020, 38, 45–50. [Google Scholar] [CrossRef]
- Yi, K.; Wang, D.; Li, X.; Chen, H.; Sun, J.; An, H.; Wang, L.; Deng, Y.; Liu, J.; Zeng, G. Effect of ciprofloxacin on biological nitrogen and phosphorus removal from wastewater. Sci. Total Environ. 2017, 605, 368–375. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, D.; Cai, L.; Xie, Y.; Wang, L.; Li, Q.; Hua, Y. An innovative double-layer microsphere used as slow-release carbon source for biological denitrification. Water Air Soil Pollut. 2020, 231, 1–12. [Google Scholar] [CrossRef]
- Loh, Z.Z.; Zaidi, N.S.; Yong, E.L.; Bahrodin, M.B.; Aris, A. Simultaneous nitrification and denitrification of real domestic wastewater using novel biodegradable Cocos Nucifera fibre as biofilter media and carbon source: Performances and microbial community composition. J. Water Process Eng. 2024, 57, 104592. [Google Scholar] [CrossRef]
- Tian, Z.; Li, G.; Xiong, Y.; Cao, X.; Pang, H.; Tang, W.; Liu, Y.; Bai, M.; Zhu, Q.; Du, C. Step-feeding food waste fermentation liquid as supplementary carbon source for low C/N municipal wastewater treatment: Bench scale performance and response of microbial community. J. Environ. Manag. 2023, 345, 118434. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Z.; Shen, J.; Kang, J.; Zhang, X.; Li, J.; Zhao, X. Effect of carbon source on pollutant removal and microbial community dynamics in treatment of swine wastewater containing antibiotics by aerobic granular sludge. Chemosphere 2020, 260, 127544. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, F.; Xu, S.; Sun, W.; Wang, Y.; Ji, M. Woodchips bioretention column for stormwater treatment: Nitrogen removal performance, carbon source and microbial community analysis. Chemosphere 2021, 285, 131519. [Google Scholar] [CrossRef]
- An, R.; Guan, J.; Li, G.; Li, Z.; Sheng, L.; Bian, H.; Lu, N. The electrode strategy and its coordination mechanism in constructed wetland-microbial fuel cell (CW-MFC): A review. Carbon Res. 2024, 3, 17. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Y.; Shen, Q.; Mao, Y.; Zhang, Q.; Ji, F. The metabolic patterns of the complete nitrates removal in the biofilm denitrification systems supported by polymer and water-soluble carbon sources as the electron donors. Bioresour. Technol. 2021, 342, 126002. [Google Scholar] [CrossRef] [PubMed]
- Matar, G.K.; Bagchi, S.; Zhang, K.; Oerther, D.B.; Saikaly, P.E. Membrane biofilm communities in full-scale membrane bioreactors are not randomly assembled and consist of a core microbiome. Water Res. 2017, 123, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Gao, J.; Song, J.; Jia, X.; Liu, Y.; Niu, J.; Yuan, X.; Zhao, Y. Performance and mechanism of chromium reduction in denitrification biofilm system with different carbon sources. Sci. Total Environ. 2024, 906, 167191. [Google Scholar] [CrossRef]
- Long, Y.; Ma, Y.; Wan, J.; Wang, Y.; Tang, M.; Zheng, Q.; Ma, Y. Hydrolysate from the enzymatic treatment of corn cob as a carbon source for heterotrophic denitrification process. J. Water Process Eng. 2023, 51, 103473. [Google Scholar] [CrossRef]
- Miao, H.; Zeng, W.; Li, J.; Liu, H.; Zhan, M.; Dai, H.; Peng, Y. Simultaneous nitrate and phosphate removal based on thiosulfate-driven autotrophic denitrification biofilter filled with volcanic rock and sponge iron. Bioresour. Technol. 2022, 366, 128207. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, S.; Li, S.; Zeng, H.; Zhang, J. Aerobic granular sludge operation and nutrients removal mechanism in a novel configuration reactor combined sequencing batch reactor and continuous-flow reactor. Bioresour. Technol. 2019, 292, 122024. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.Y.; Song, Z.Y.; Liu, C.; Li, W.P.; Li, Q.; Zhang, T.J. Start-up and performance study on the simultaneous nitrification endogenous denitrification phosphorus remov-al (SNEDPR)in the biological process of the moving bed biofilm reactor (MBBR). China Environ. Sci. 2022, 42, 3121–3129. [Google Scholar] [CrossRef]
- Li, J.; Gong, B.Z.; Zhang, Y.; Wei, W.Q.; Cao, M.; Zhou, J. Performance of two-stage constructed wetland for advanced nitrogen and phosphorus removal from wastewater treatment plant effluent. China Water Wastewater 2023, 39, 75–81. [Google Scholar] [CrossRef]
- Niu, T.; Zhu, H.; Shutes, B.; He, C. Gaseous carbon and nitrogen emissions from microbial fuel cell-constructed wetlands with different carbon sources: Microbiota-driven mechanisms. J. Clean. Prod. 2024, 435, 140404. [Google Scholar] [CrossRef]
- Liang, D.; He, W.; Li, C.; Wang, F.; Crittenden, J.C.; Feng, Y. Remediation of nitrate contamination by membrane hydrogenotrophic denitrifying biofilm integrated in microbial electrolysis cell. Water Res. 2021, 188, 116498. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Shi, W.; Cai, C.; Ge, S.; Ma, B.; Li, X.; Ding, J. Responses of microbial structures, functions, metabolic pathways and community interactions to different C/N ratios in aerobic nitrification. Bioresour. Technol. 2020, 311, 123422. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, Z.H.; Ding, Y.N. Effect of low temperature on nitrogen removal in a CANON-type sequencing batch biofilm reactor. China Environ. Sci. 2019, 39, 1533–1541. [Google Scholar] [CrossRef]
- Chen, Z.W.; Zuo, Q.Y.; Liu, C.H.; Li, L.; Katherine, Y.; He, Q. Insights into solid phase denitrification in wastewater tertiary treatment: The role of solid carbon source in carbon biodegradation and heterotrophic denitrification. Bioresour. Technol. 2023, 376, 128838. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Gong, B.Z.; Wang, Y.M.; Lin, Z.Y.; He, L.; Zhou, J.; He, Q. Metagenomic analysis reveals enhanced nutrients removal from low C/N municipal wastewater in a pilot-scale modified AAO system coupling electrolysis. Water Research. 2020, 173, 115530. [Google Scholar] [CrossRef]
- Li, J.; Feng, Y.; Qiu, Y.; Chen, D.; Liang, D.; Zhou, J.; Liu, G. Recovery of electron and carbon source from agricultural waste corncob by microbial electrochemical system to enhance wastewater denitrification. Sci. Total Environ. 2023, 878, 162926. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, H.; İbici, H.N.; Erdoğan, E.M.; Türedi, Z.; Ergenekon, P.; Özkan, M. Nitrite is reduced by nitrite reductase NirB without small subunit NirD in Escherichia coli. J. Biosci. Bioeng. 2022, 134, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Ma, Y.; Wan, J.; Wang, Y.; Tang, M.; Fu, H.; Cao, J. Denitrification efficiency, microbial communities and metabolic mechanisms of corn cob hydrolysate as denitrifying carbon source. Environ. Res. 2023, 221, 115315. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Li, C.; Li, N.; Zou, P.; Gao, X.; Cao, Q. Nitrogen removal performances and metabolic mechanisms of denitrification systems using different volatile fatty acids as external carbon sources. Chem. Eng. J. 2023, 474, 145998. [Google Scholar] [CrossRef]
- Han, L.; Han, Y.; Dai, Y.; Zhang, J.; Zhong, C.; Zhuo, Y. Performance of sludge thermal hydrolyzed liquor as carbon source for sewage denitrification. Zhongguo Huanjing Kexue/China Environ. Sci. 2021, 41, 3653–3659. [Google Scholar]





| Serial Number | Carbon Source | Dosage (mL/L) | Serial Number | Carbon Source | pH | Serial Number | Carbon Source | DO (mg/L) |
|---|---|---|---|---|---|---|---|---|
| CK | / | / | P1 | PGA | 5–6 | D1 | PGA | 1 ± 0.5 |
| T0 | methanol | 0.45 | P2 | 6–7 | ||||
| T1 | PGA | 0.5 | P3 | 7–8 | D2 | 3 ± 0.5 | ||
| T2 | 1.2 | P4 | 8–9 | |||||
| T3 | 1.9 | D3 | 5 ± 0.5 |
| Specimens | OTUs | Shannon | Chao1 | Ace | Simpson | Coverage | Number of Original Sequences |
|---|---|---|---|---|---|---|---|
| T1 | 647 | 3.919 | 1347.735 | 1398.332 | 0.0804 | 0.9955 | 80,133 |
| T2 | 722 | 4.486 | 1353.320 | 1417.281 | 0.0698 | 0.9953 | 89,153 |
| T3 | 746 | 4.599 | 1556.727 | 1627.018 | 0.0711 | 0.9956 | 91,248 |
| T4 | 753 | 4.788 | 1627.013 | 1697.362 | 0.0388 | 0.9962 | 89,561 |
| T5 | 787 | 5.622 | 1725.522 | 1746.811 | 0.0141 | 0.9957 | 84,138 |
| Serial Number | Title | Research Contents | Finding | Reference |
|---|---|---|---|---|
| 1 | Denitrification efficiency, microbial communities, and metabolic mechanisms of corn cob hydrolysate as denitrifying carbon source | In this study, the denitrification efficacy of corn cob hydrolysate (CCH) was compared and analyzed with that of glucose and acetate to determine its feasibility as an additional carbon source, and its metabolic mechanism as a denitrification carbon source was investigated in depth. | By constructing a denitrification reactor, it was found that the TN-removal rate exceeded 97% and the effluent COD remained below 70 mg/L during the stable operation with CCH as the carbon source | [54] |
| 2 | The nitrogen-removal performances and metabolic mechanisms of denitrification systems using different volatile fatty acids as external carbon sources | In this study, denitrification using acetate, propionate, or butyrate as a sole carbon source was compared. | Propionate and butyrate systems had obviously higher denitrification efficiencies than the acetate system (the maximum nitrate-removal rates were 122.58, 110.67, and 80.79 mg N/(L · h) in propionate, butyrate, and acetate systems, respectively). | [55] |
| 3 | Performance of sludge thermal hydrolyzed liquor as a carbon source for sewage denitrification. | The denitrification performance of sludge thermal hydrolyzed liquor was investigated using sludge thermal hydrolyzed liquor as an external carbon source and compared with that of sodium acetate, a conventional carbon source. | The total nitrogen (TN) concentrations of the effluent were reduced from 27.64 mg/L to 12.05 mg/L with the dosage of TH liquor and 7.98 mg/L with the dosage of sodium acetate, respectively, indicating that the nitrogen-removal ability could be improved by them. | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, C.; Yang, W.; Wei, Y.; Li, W. Denitrification Performance and Microbiological Mechanisms Using Polyglycolic Acid as a Carbon Source. Water 2024, 16, 1277. https://doi.org/10.3390/w16091277
Wang Z, Li C, Yang W, Wei Y, Li W. Denitrification Performance and Microbiological Mechanisms Using Polyglycolic Acid as a Carbon Source. Water. 2024; 16(9):1277. https://doi.org/10.3390/w16091277
Chicago/Turabian StyleWang, Zhichao, Chenxi Li, Wenhuan Yang, Yuxia Wei, and Weiping Li. 2024. "Denitrification Performance and Microbiological Mechanisms Using Polyglycolic Acid as a Carbon Source" Water 16, no. 9: 1277. https://doi.org/10.3390/w16091277
APA StyleWang, Z., Li, C., Yang, W., Wei, Y., & Li, W. (2024). Denitrification Performance and Microbiological Mechanisms Using Polyglycolic Acid as a Carbon Source. Water, 16(9), 1277. https://doi.org/10.3390/w16091277







