Preparation of CeO2 Supported on Graphite Catalyst and Its Catalytic Performance for Diethyl Phthalate Degradation during Ozonation
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Preparation of Catalysts
2.3. Characterization of Catalysts
2.4. Ozonation Alone and Catalytic Ozonation Procedure
2.5. Analytical Methods
3. Results and Discussion
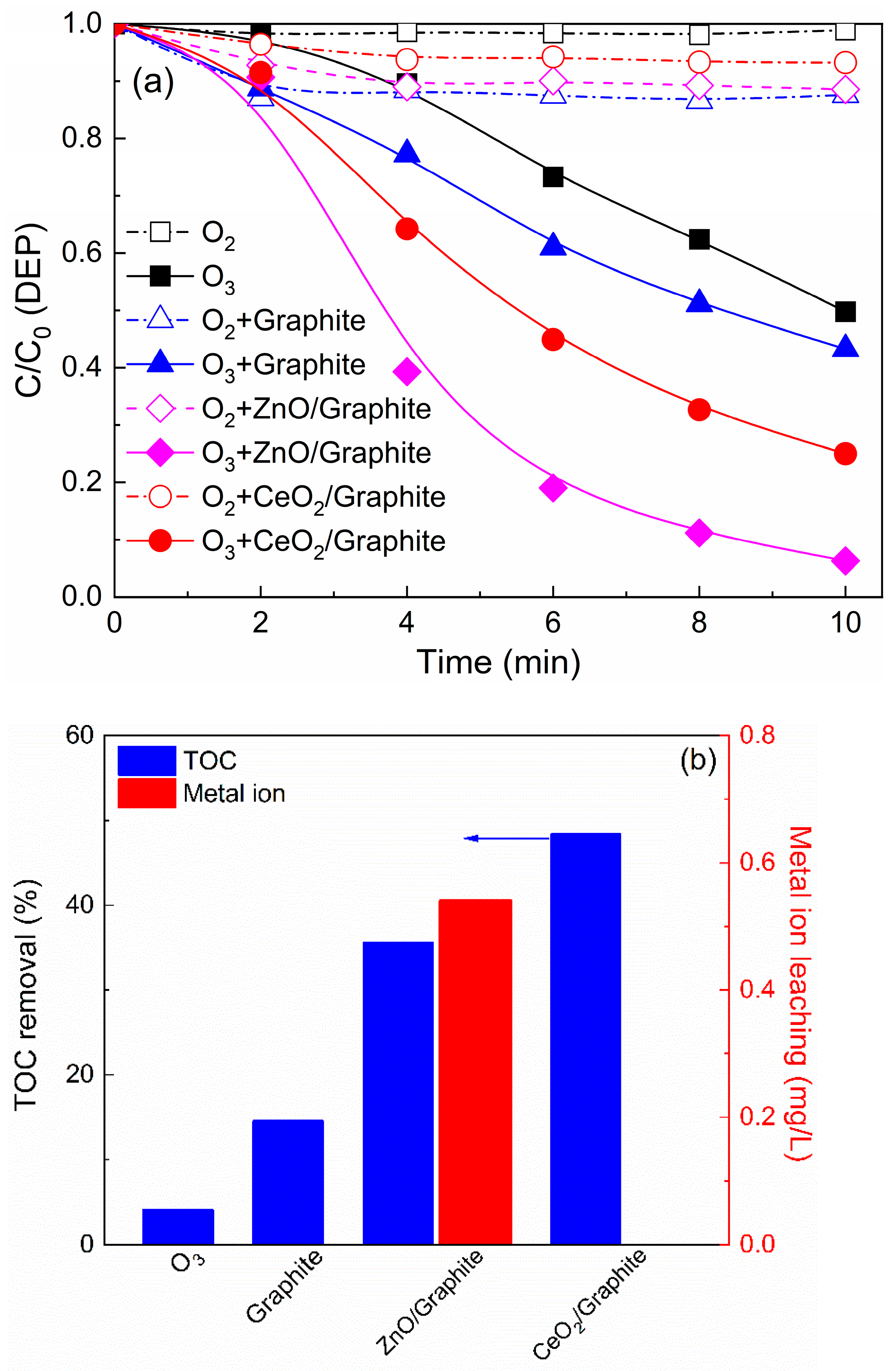
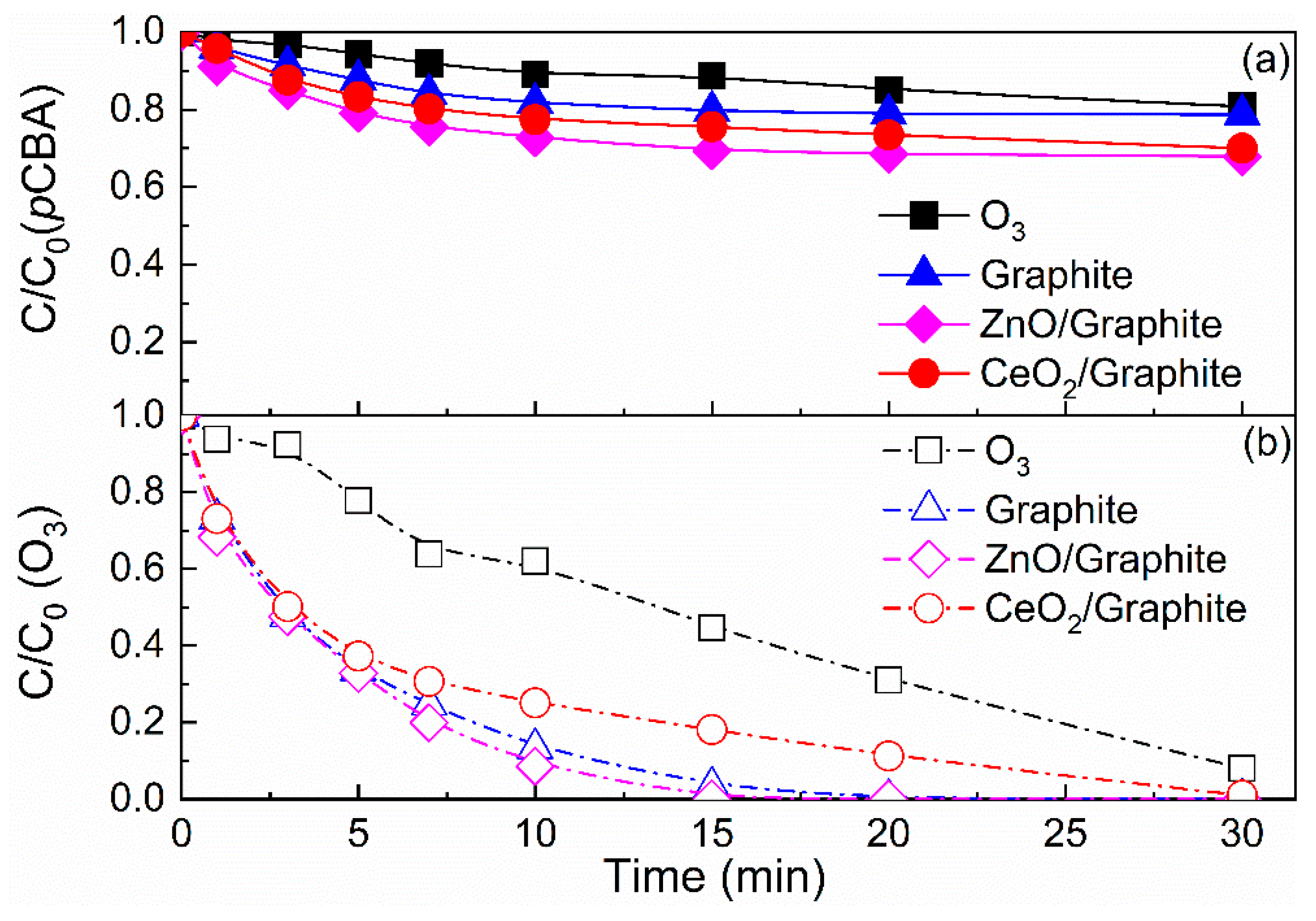
3.1. Catalytic Activity Comparison of CeO2/Graphite Catalyst with Other Catalyst
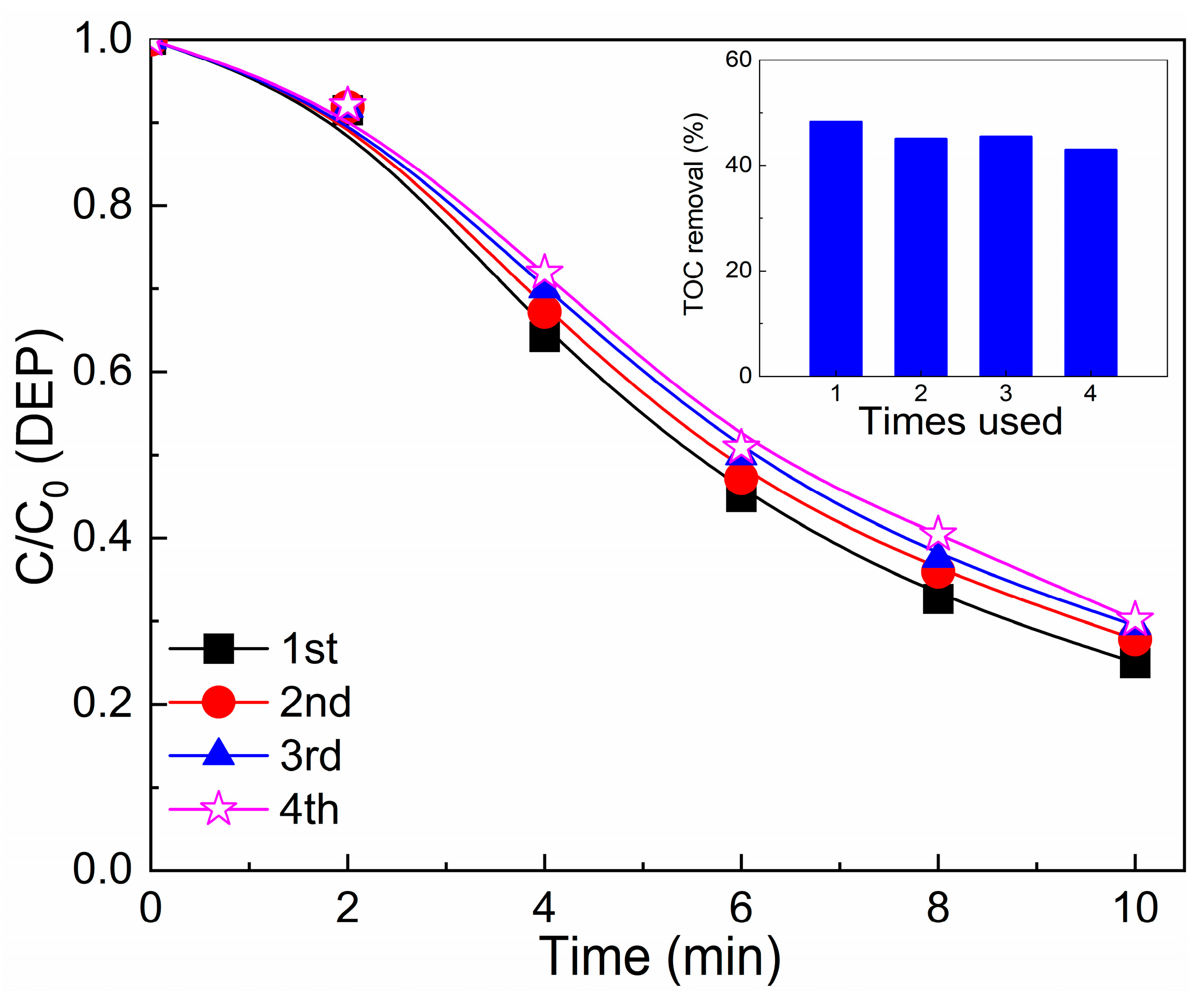
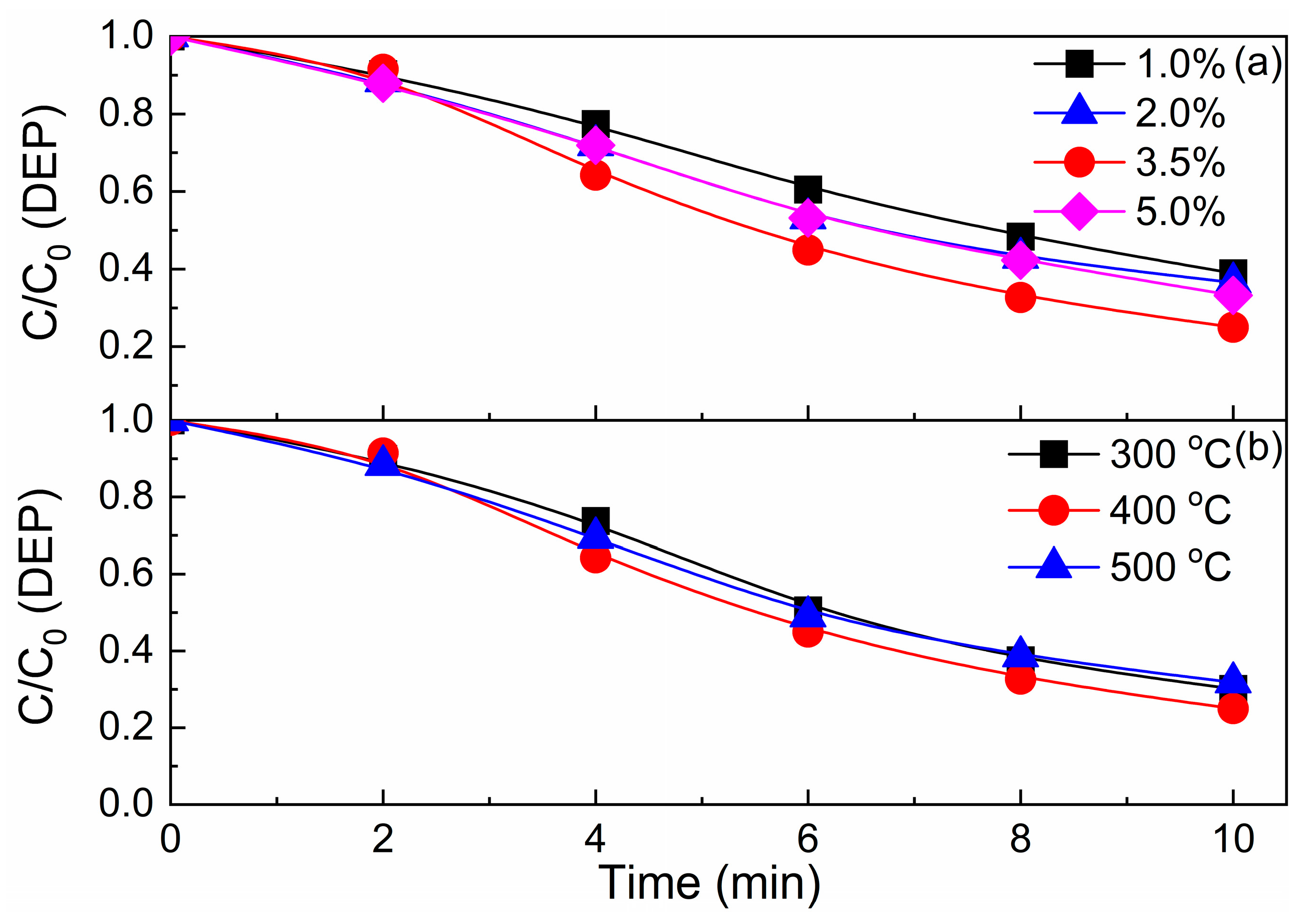
3.2. Optimization of CeO2/Graphite Catalyst
3.3. Characterization of CeO2/Graphite Catalyst
3.4. Effect of Operation Conditions on the Performance of CeO2/Graphite Catalyst
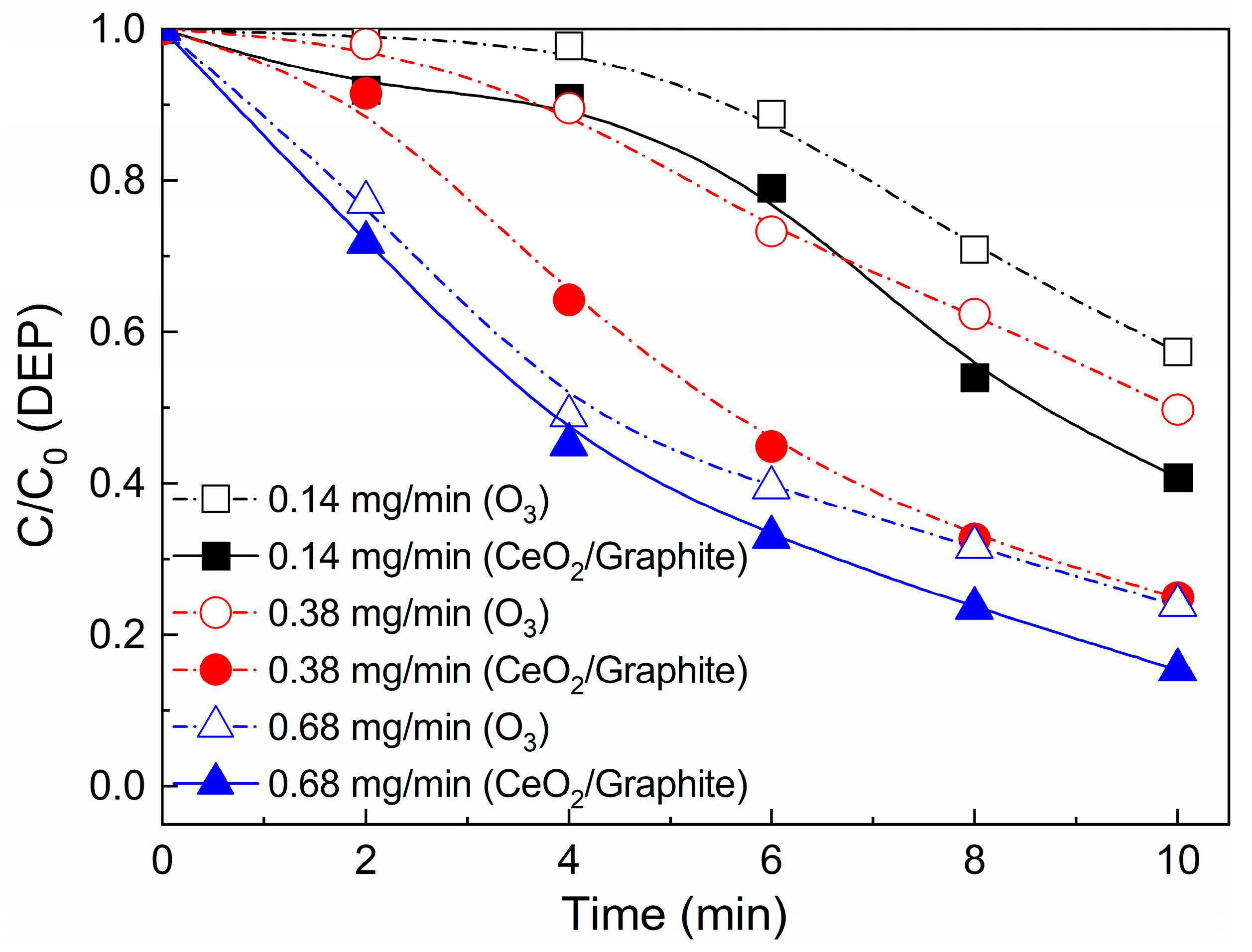
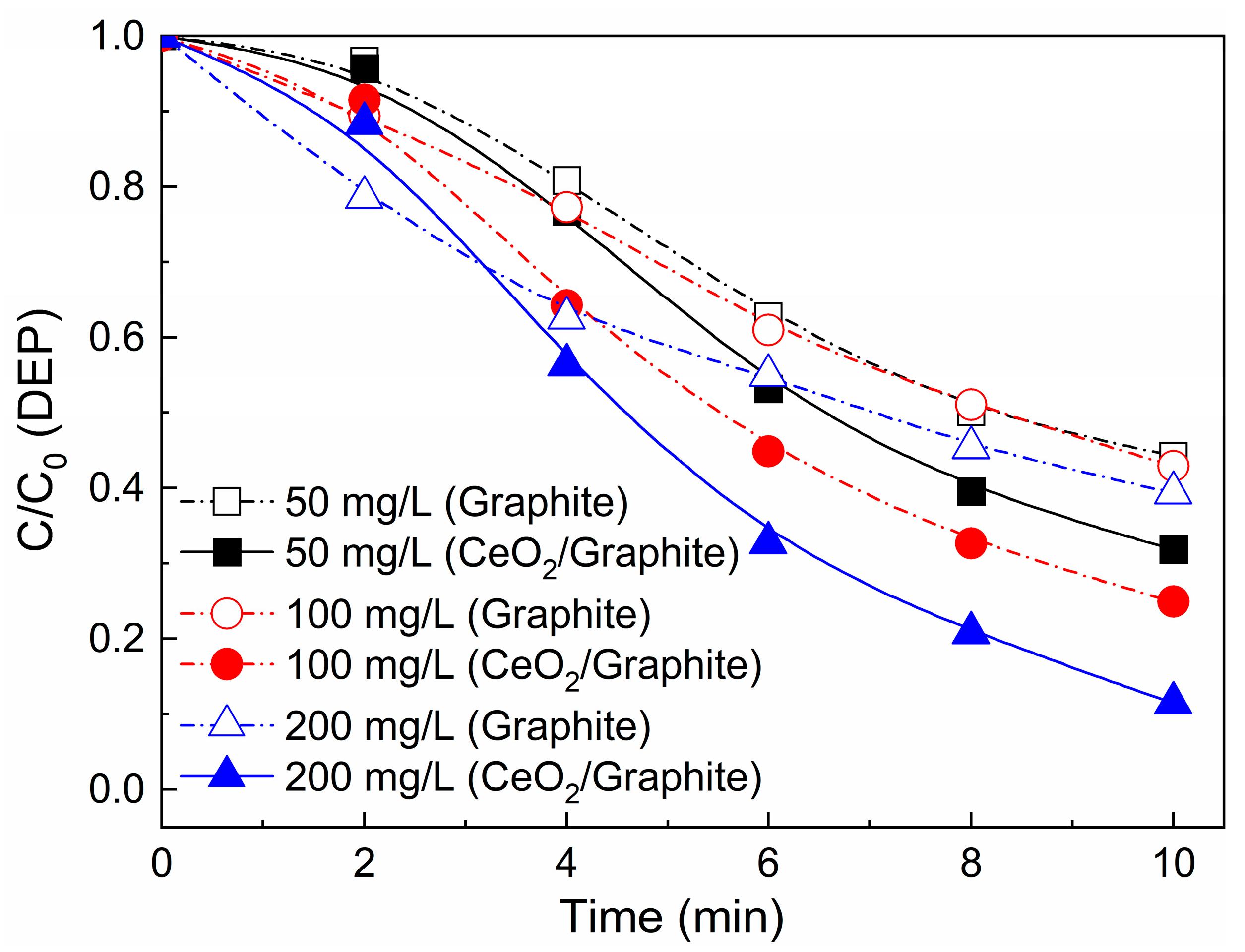
3.4.1. Effect of Ozone Dosage and Catalyst Dosage
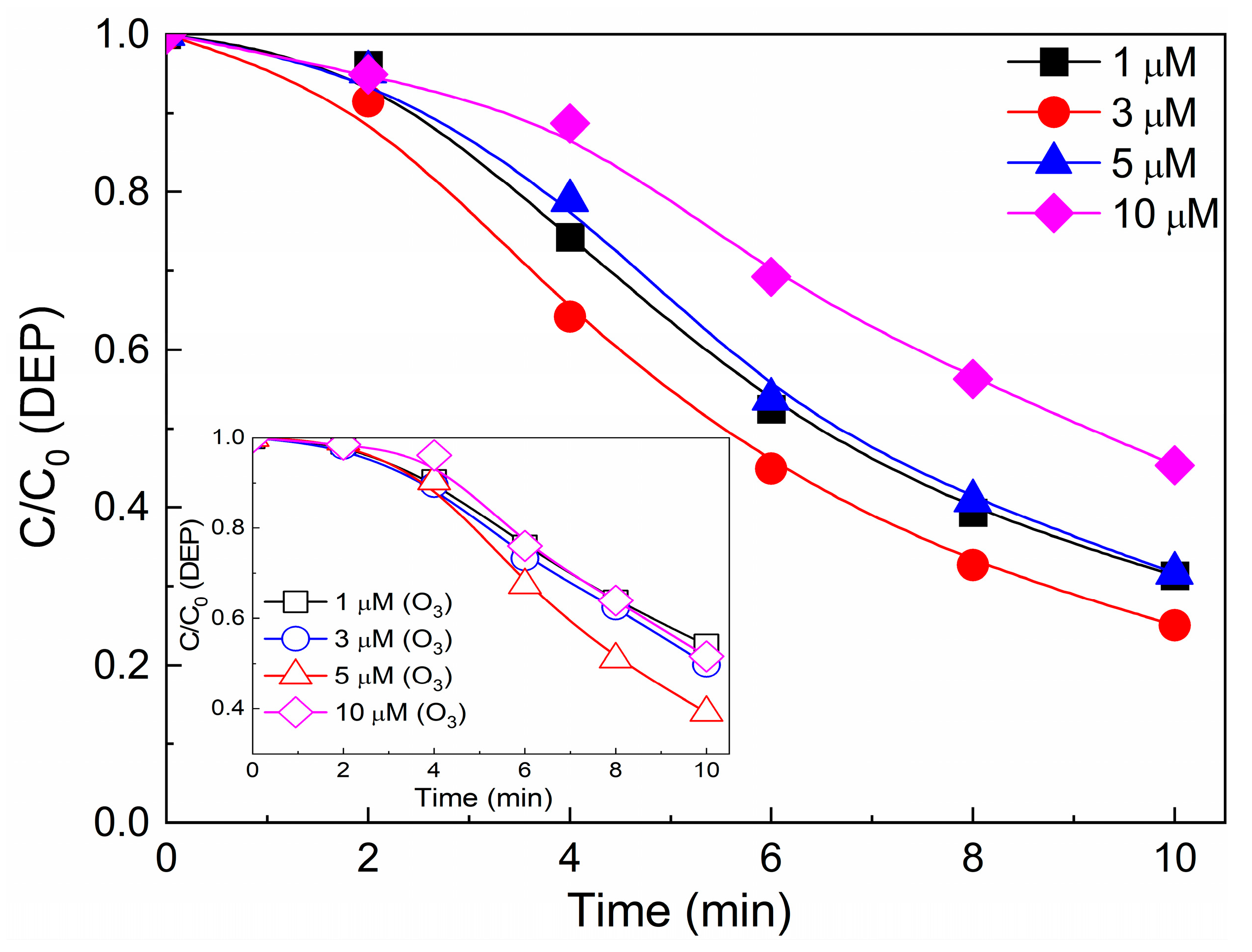
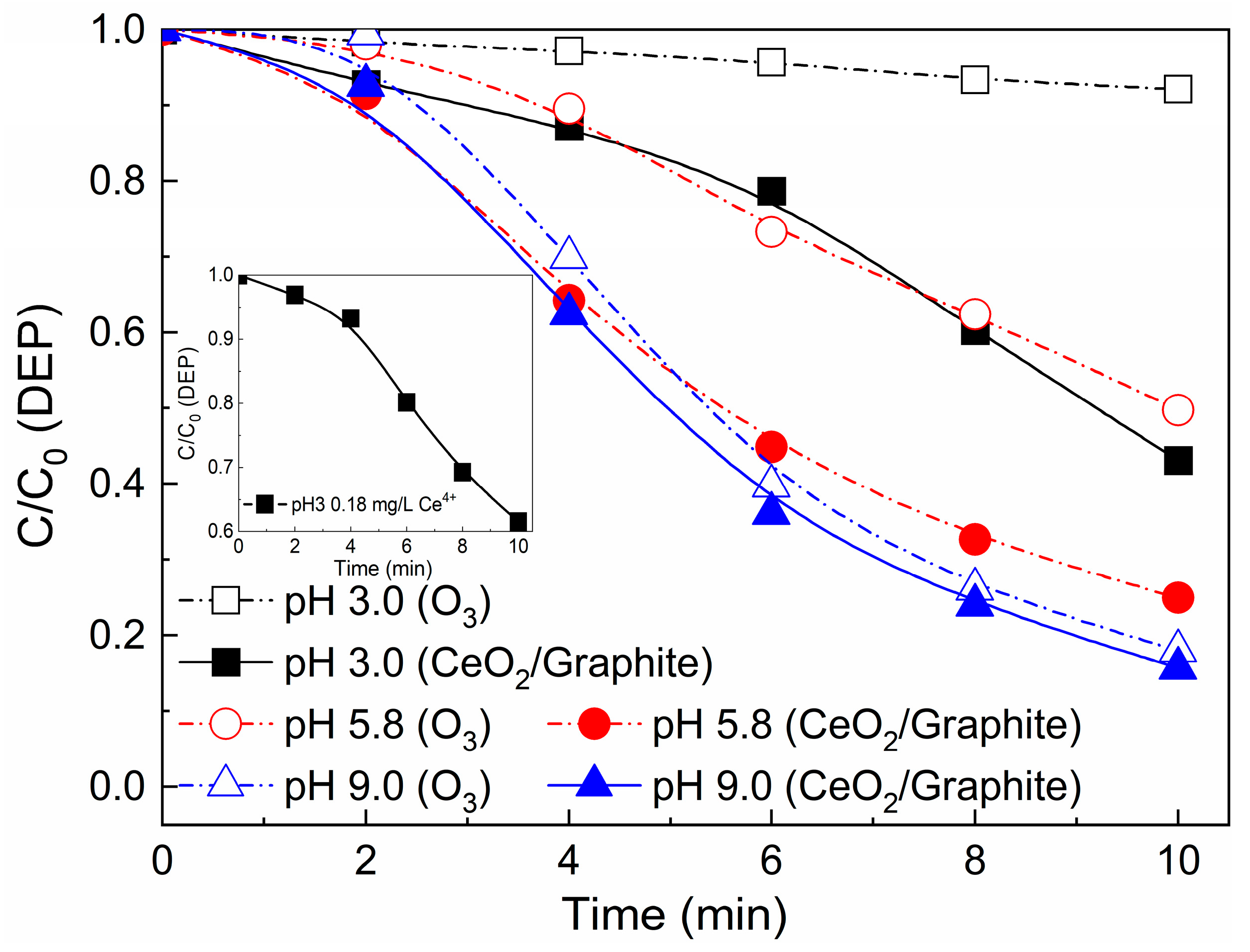
3.4.2. Effect of Initial DEP Concentration and Initial pH
3.4.3. Effect of Reaction Temperature
3.5. Reaction Mechanism of CeO2/Graphite Catalytic Ozonation
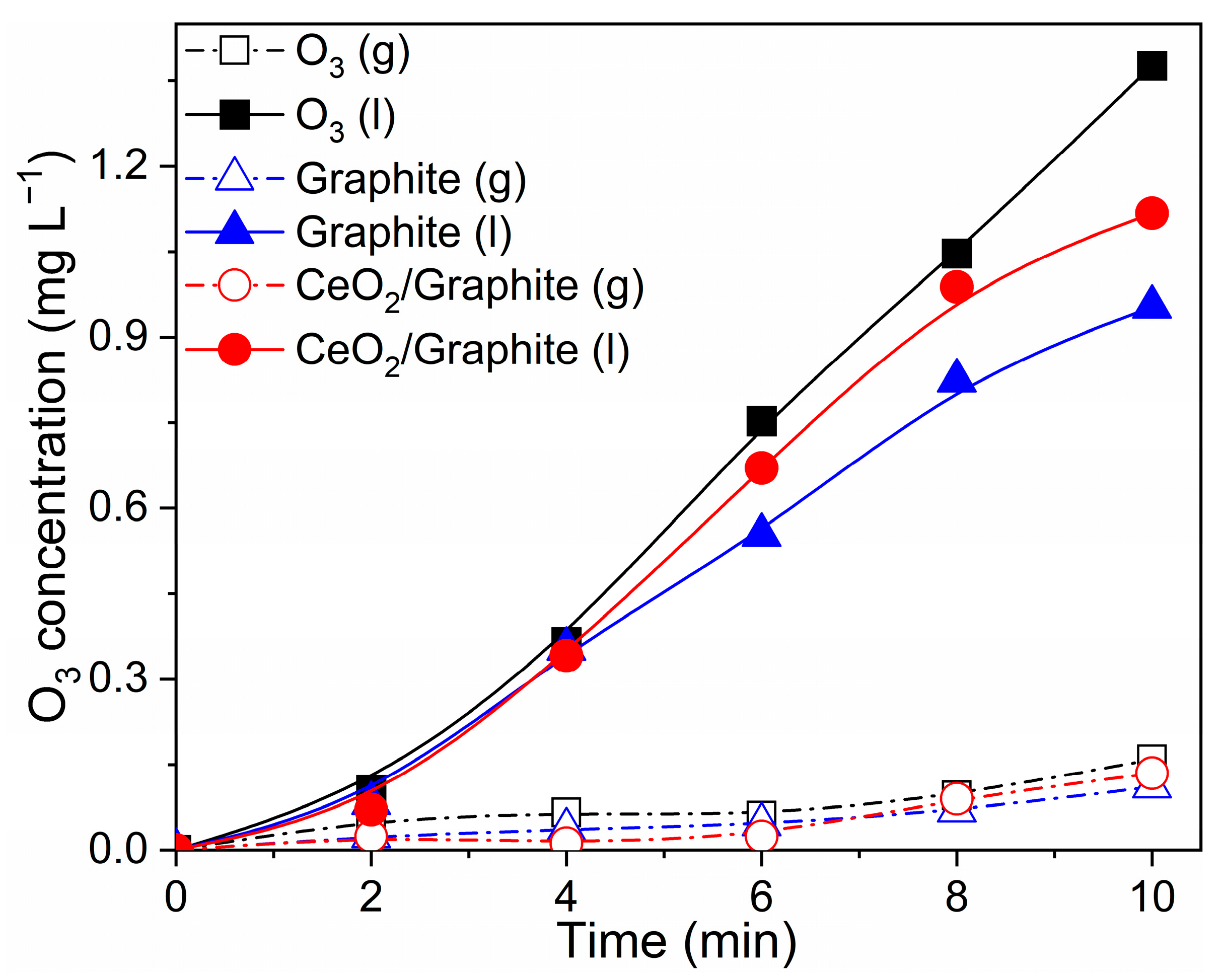
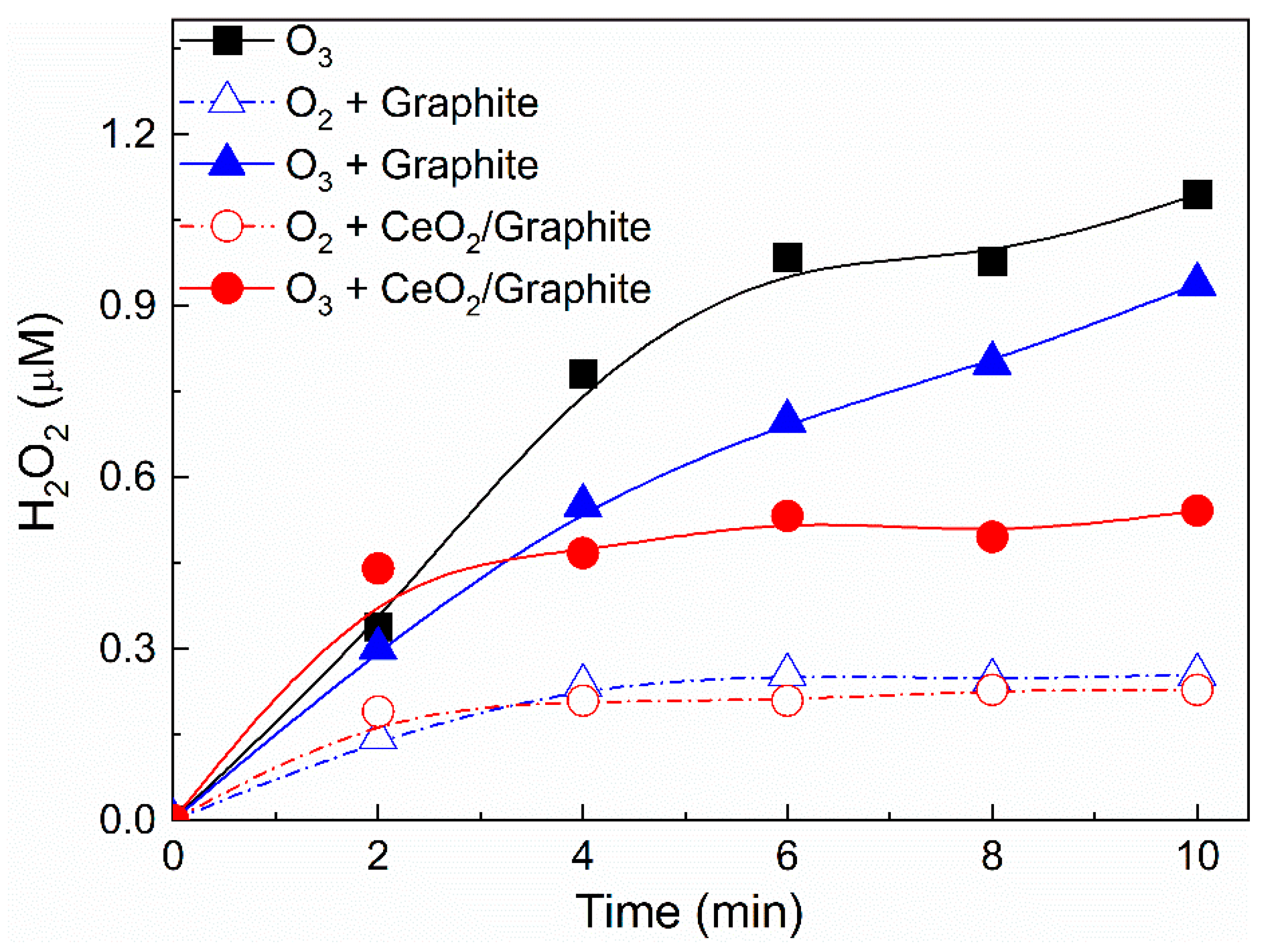
3.5.1. Evolution of Ozone Concentration and Hydrogen Peroxide Concentration
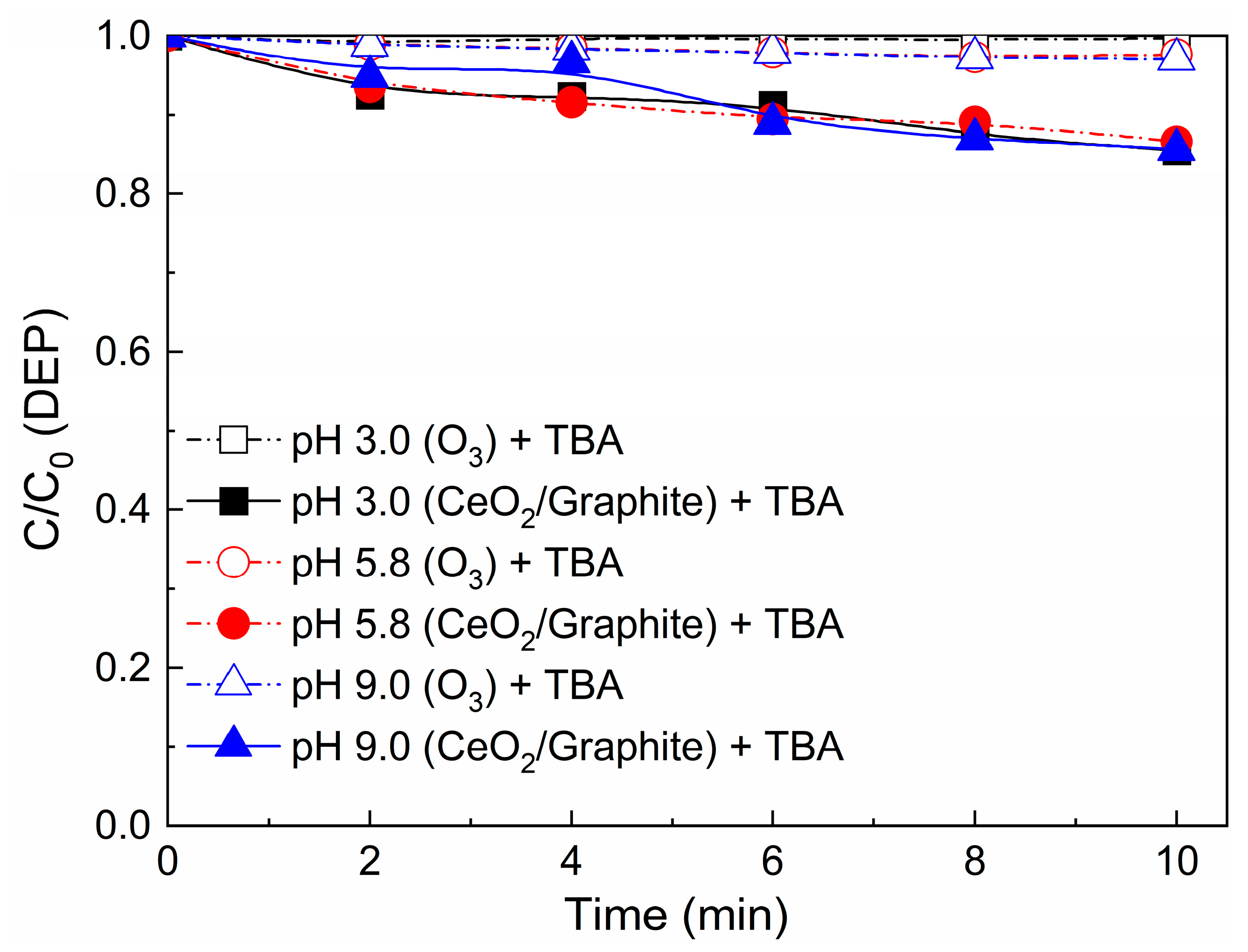
3.5.2. Effect of the Presence of TBA
3.5.3. Discussion of the Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parvulescu, V.I.; Epron, F.; Garcia, H.; Granger, P. Recent Progress and Prospects in Catalytic Water Treatment. Chem. Rev. 2022, 122, 2981–3121. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, S.; Cheng, M.; Lai, C.; Zeng, G.; Qin, L.; Liu, X.; Li, B.; Zhang, W.; Yi, Y.; et al. Improving the Fenton-like catalytic performance of MnOx-Fe3O4/biochar using reducing agents: A comparative study. J. Hazard. Mater. 2021, 406, 124333. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wei, Z.; Duan, X.; Long, M.; Spinney, R.; Dionysiou, D.D.; Xiao, R.; Alvarez, P.J.J. Merits and limitations of radical vs. nonradical pathways in persulfate-based advanced oxidation processes. Environ. Sci. Technol. 2023, 57, 12153–12179. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Han, B.-J.; Wen, G.; Ma, J.; Wang, S.-J.; Zha, R.-G.; Shen, L.-P.; Wang, C. Full-scale application of catalytic ozonation for drinking water treatment: Case study in China. J. Environ. Eng. 2014, 140, A5013002. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Masjedi-Arani, M.; Salavati-Niasari, M. Size controllable synthesis of cobalt vanadate nanostructures with enhanced photocatalytic activity for the degradation of organic dyes. J. Mol. Catal. A Chem. 2016, 425, 31–42. [Google Scholar] [CrossRef]
- Mazloom, F.; Masjedi-Arani, M.; Ghiyasiyan-Arani, M.; Salavati-Niasari, M. Novel sodium dodecyl sulfate-assisted synthesis of Zn3V2O8 nanostructures via a simple route. J. Mol. Liq. 2016, 214, 46–53. [Google Scholar] [CrossRef]
- Cui, Y.H.; Li, X.Y.; Chen, G. Electrochemical degradation of bisphenol A on different anodes. Water Res. 2009, 43, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Yang, S.Q.; Li, J.Y.; Sun, F.; Liu, Z.Q.; Yang, J.; Cui, Y.H.; Zhang, B. Insight into micropollutant abatement during ultraviolet light-emitting diode combined electrochemical process: Reaction mechanism, contributions of reactive species and degradation routes. Sci. Total Environ. 2023, 876, 162798. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fu, L.; Wu, F.; Chen, X.; Wu, C.; Wang, Q. Recent developments in activated carbon catalysts based on pore size regulation in the application of catalytic ozonation. Catalysts 2022, 12, 1085. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Li, J.Y.; Li, S.T. Catalytic Ozonation over Activated Carbon-based Materials. In Advanced Ozonation Processes for Water and Wastewater Treatment: Active Catalysts and Combined Technologies; Cao, H., Xie, Y., Wang, Y., Xiao, J., Eds.; Royal Society of Chemistry: Croydon, UK, 2022; pp. 85–122. [Google Scholar]
- Oh, S.-Y.; Nguyen, T.-H.A. Ozonation of phenol in the presence of biochar and carbonaceous materials: The effect of surface functional groups and graphitic structure on the formation of reactive oxygen species. J. Environ. Chem. Eng. 2022, 10, 107386. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Tu, J.; Wang, Q.; Cui, Y.-H.; Zhang, L.; Wu, X.; Zhang, B.; Ma, J. Catalytic ozonation of diethyl phthalate in aqueous solution using graphite supported zinc oxide. Sep. Purif. Technol. 2018, 200, 51–58. [Google Scholar] [CrossRef]
- Jiménez-López, M.A.; Rey, A.; Rivas, F.J.; Beltrán, F.J. Water ozone decomposition in graphitic and graphene based catalytic materials: Kinetics of catalyst deactivation. Catal. Today 2024, 430, 114541. [Google Scholar] [CrossRef]
- Bernat-Quesada, F.; Espinosa, J.C.; Barbera, V.; Álvaro, M.; Galimberti, M.; Navalón, S.; García, H. Catalytic ozonation using edge-hydroxylated graphite-based materials. ACS Sustain. Chem. Eng. 2019, 7, 17443–17452. [Google Scholar] [CrossRef]
- Jiménez-López, M.A.; Rey, A.; Montes, V.; Beltrán, F.J. Testing carbon structures for metal-free catalytic/photocatalytic ozonation to remove disinfection by-product formation potential. Sep. Purif. Technol. 2024, 329, 125156. [Google Scholar] [CrossRef]
- He, J.; Song, W.; Gao, Z.; Huang, X. Calcium carbonate/expanded graphite as an efficient catalyst for catalytic ozonation of ethylenediaminetetraacetic acid. Environ. Eng. Sci. 2020, 37, 450–456. [Google Scholar] [CrossRef]
- Song, Y.; Feng, S.; Qin, W.; Ma, J. Mechanism of catalytic ozonation in expanded graphite aqueous suspension for the degradation of organic acids. Environ. Technol. 2023, 44, 739–750. [Google Scholar] [CrossRef]
- He, J.; Song, W.; Huang, X.; Gao, Z. Preparation, characterization, and catalytic activity of a novel MgO/expanded graphite for ozonation of Cu-EDTA. Environ. Sci. Pollut. Res. 2021, 28, 39513–39523. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, T.; Ding, Y.; Tong, S. Graphite felt supported MgO catalytic ozonation of bisphenol A. Ozone Sci. Eng. 2019, 41, 541–550. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Ma, J.; Zhao, L. Preparation, Characterization and catalytic activity of Pt/graphite catalyst. Chin. J. Inorg. Chem. 2006, 22, 2263–2268. (In Chinese) [Google Scholar]
- Liu, Z.Q.; Cui, Y.H.; Wang, M.Y.; Zheng, Y.J.; Zhong, Z.X.; Wu, X.H.; Wang, Z.; Zhang, B.P. Ozonation of oxytetracycline in the presence of activated carbon supported cerium oxide. Environ. Eng. Manag. J. 2016, 15, 2231–2237. [Google Scholar] [CrossRef]
- Orge, C.A.; Órfão, J.J.M.; Pereira, M.F.R. Catalytic ozonation of organic pollutants in the presence of cerium oxide–carbon composites. Appl. Catal. B Environ. 2011, 102, 539–546. [Google Scholar] [CrossRef]
- Yao, C.C.D.; Haag, W.R. Rate constants for direct reactions of ozone with several drinking water contaminants. Water Res. 1991, 25, 761–773. [Google Scholar] [CrossRef]
- Wen, G.; Ma, J.; Liu, Z.-Q.; Zhao, L. Ozonation kinetics for the degradation of phthalate esters in water and the reduction of toxicity in the process of O3/H2O2. J. Hazard. Mater. 2011, 195, 371–377. [Google Scholar] [CrossRef]
- Noh, J.S.; Schwarz, J.A. Effect of HNO3 treatment on the surface acidity of activated carbons. Carbon 1990, 28, 675–682. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Ma, J.; Cui, Y.-H. Carbon nanotube supported platinum catalysts for the ozonation of oxalic acid in aqueous solutions. Carbon 2008, 46, 890–897. [Google Scholar] [CrossRef]
- von Sonntag, C.; von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications; IWA Publishing: London, UK, 2012. [Google Scholar]
- Elovitz, M.S.; von Gunten, U. Hydroxyl radical/ozone ratios during ozonation processes. I. The rct concept. Ozone Sci. Eng. 1999, 21, 239–260. [Google Scholar] [CrossRef]
- Bader, H.; Hoigné, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–456. [Google Scholar] [CrossRef]
- Rakness, K.; Gordon, G.; Langlais, B.; Masschelein, W.; Matsumoto, N.; Richard, Y.; Robson, C.M.; Somiya, I. Guideline for measurement of ozone concentration in the process gas from an ozone generator. Ozone Sci. Eng. 1996, 18, 209–229. [Google Scholar] [CrossRef]
- Bader, H.; Sturzenegger, V.; Hoigné, J. Photometric method for the determination of low concentrations of hydrogen peroxide by the peroxidase catalyzed oxidation of N,N-diethyl-p-phenylenediamine (DPD). Water Res. 1988, 22, 1109–1115. [Google Scholar] [CrossRef]
- Zhang, S.; Quan, X.; Zheng, J.-F.; Wang, D. Probing the interphase “HO• zone” originated by carbon nanotube during catalytic ozonation. Water Res. 2017, 122, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, H.E.; Van Bekkum, H. Preparation of platinum on activated carbon. J. Catal. 1991, 131, 335–349. [Google Scholar] [CrossRef]
- Lu, J.; Do, I.; Drzal, L.T.; Worden, R.M.; Lee, I. Nanometal-decorated exfoliated graphite nanoplatelet based glucose biosensors with high sensitivity and fast response. ACS Nano 2008, 2, 1825–1832. [Google Scholar] [CrossRef]
- Beltran, F.J.; Alvarez, P.M.; Gimeno, O. Graphene-Based catalysts for ozone processes to decontaminate water. Molecules 2019, 24, 3438. [Google Scholar] [CrossRef]
- Ishii, T.; Kaburagi, Y.; Yoshida, A.; Hishiyama, Y.; Oka, H.; Setoyama, N.; Ozaki, J.-I.; Kyotani, T. Analyses of trace amounts of edge sites in natural graphite, synthetic graphite and high-temperature treated coke for the understanding of their carbon molecular structures. Carbon 2017, 125, 146–155. [Google Scholar] [CrossRef]
- Coloma, F.; Sepulveda-Escribano, A.; Fierro, J.L.G. Rodriguez-Reinoso, Preparation of platinum supported on pregraphitized carbon blacks. Langmuir 1994, 10, 750–755. [Google Scholar] [CrossRef]
- Leon, C.A.L.Y.; Solar, J.M.; Calemma, V.; Radovic, L.R. Evidence for the protonation of basal plane sites on carbon. Carbon 1992, 30, 797–811. [Google Scholar] [CrossRef]
- Fijolek, L.; Wolski, L. Bifunctional CePO4/CeO2 nanocomposite as a promising heterogeneous catalyst for the enhancement of the ozonation recovery effect in the presence of chloride ions. Sci. Rep. 2022, 12, 9043. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, B.; Liu, J.; Yang, W.; Ge, S.; He, S. The role of Mn doping on Ce-based γ-Al2O3 catalysts for phenol degradation. Environ. Eng. Sci. 2021, 39, 56–63. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Zhang, S.; Quan, X.; Wang, D. Catalytic ozonation in arrayed zinc oxide nanotubes as highly efficient mini-column catalyst reactors (MCRs): Augmentation of hydroxyl radical exposure. Environ. Sci. Technol. 2018, 52, 8701–8711. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Tryk, D.; Yeager, E. The electrochemistry of graphite and modified graphite surfaces: The reduction of O2. Electrochim. Acta 1989, 34, 1733–1737. [Google Scholar] [CrossRef]
- Ahumada, E.; Lizama, H.; Orellana, F.; Suárez, C.; Huidobro, A.; Sepúlveda-Escribano, A.; Rodrı, F. Catalytic oxidation of Fe(II) by activated carbon in the presence of oxygen: Effect of the surface oxidation degree on the catalytic activity. Carbon 2002, 40, 2827–2834. [Google Scholar] [CrossRef]
- Beltran, F.J.; Pocostales, P.; Alvarez, P.; Oropesa, A.L. Diclofenac removal from water with ozone and activated carbon. J. Hazard. Mater. 2009, 163, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, F.J.; García-Araya, J.F.; Giráldez, I. Gallic acid water ozonation using activated carbon. Appl. Catal. B Environ. 2006, 63, 249–259. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Giráldez, I.; García-Araya, J.F. Kinetics of activated carbon promoted ozonation of polyphenol mixtures in water. Ind. Eng. Chem. Res. 2008, 47, 1058–1065. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, W.; Ma, J.; Qiang, Z. Minimizing bromate formation with cerium dioxide during ozonation of bromide-containing water. Water Res. 2008, 42, 3651–3658. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Mamane, H.; Avisar, D.; Gozlan, I.; Kaplan, A.; Dayalan, G. Treatment of diethyl phthalate leached from plastic products in municipal solid waste using an ozone-based advanced oxidation process. Materials 2019, 12, 4119. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, L.; Tizaoui, C.; Geissen, S.U.; Bousselmi, L. A comparative study on ozone, hydrogen peroxide and UV based advanced oxidation processes for efficient removal of diethyl phthalate in water. J. Hazard. Mater. 2019, 363, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Jabesa, A.; Ghosh, P. Removal of diethyl phthalate from water by ozone microbubbles in a pilot plant. J. Environ. Manag. 2016, 180, 476–484. [Google Scholar] [CrossRef]
- Hoigné, J.; Bader, H. Rate constants of reactions of ozone with organic and inorganic compounds in water—I. Water Res. 1983, 17, 173–183. [Google Scholar] [CrossRef]
- Sánchez-Polo, M.; von Gunten, U.; Rivera-Utrilla, J. Efficiency of activated carbon to transform ozone into OH radicals: Influence of operational parameters. Water Res. 2005, 39, 3189–3198. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Polo, M.; Salhi, E.; Rivera-Utrilla, J.; von Gunten, U. Combination of ozone with activated carbon as an alternative to conventional advanced oxidation processes. Ozone Sci. Eng. 2006, 28, 237–245. [Google Scholar] [CrossRef]
- Oulton, R.; Haase, J.P.; Kaalberg, S.; Redmond, C.T.; Nalbandian, M.J.; Cwiertny, D.M. Hydroxyl radical formation during ozonation of multiwalled carbon nanotubes: Performance optimization and demonstration of a reactive CNT filter. Environ. Sci. Technol. 2015, 49, 3687–3697. [Google Scholar] [CrossRef] [PubMed]
- Jothinathan, L.; Hu, J. Kinetic evaluation of graphene oxide based heterogenous catalytic ozonation for the removal of ibuprofen. Water Res. 2018, 134, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Guo, Y.; Yu, G.; Komarneni, S.; Wang, Y. Evaluation of the effect of catalysts on ozone mass transfer and pollutant abatement during laboratory catalytic ozonation experiments: Implications for practical water and wastewater treatment. ACS EST Eng. 2022, 3, 387–397. [Google Scholar] [CrossRef]
- Kwon, M.; Kye, H.; Jung, Y.; Yoon, Y.; Kang, J.-W. Performance characterization and kinetic modeling of ozonation using a new method: ROH,O3 concept. Water Res. 2017, 122, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C.; Duan, X.; Wang, S.; Wang, Y. Carbocatalytic ozonation toward advanced water purification. J. Mater. Chem. A 2021, 9, 18994–19024. [Google Scholar] [CrossRef]
- Beltran, F.J.; Rivas, F.J.; Fernandez, L.A.; Alvarez, P.M.; Montero-de-Espinosa, R. Kinetics of catalytic ozonation of oxalic acid in water with activated carbon. Ind. Eng. Chem. Res. 2002, 41, 6510–6517. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Activated carbon catalytic ozonation of oxamic and oxalic acids. Appl. Catal. B Environ. 2008, 79, 237–243. [Google Scholar] [CrossRef]
- Werner, S.; Morgan, J.J. Aquatic chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Wiley: New York, NY, USA, 1996. [Google Scholar]


| Process | O3 Exposure (M•s) | •OH Exposure (M•s) | Rct | ROH,O3 (s) |
|---|---|---|---|---|
| O3 | 3.78 × 10−2 | 4.26 × 10−11 | 1.13 × 10−9 | 9.84 × 10−7 |
| O3 + Graphite | 9.38 × 10−3 | 4.75 × 10−11 | 5.21 × 10−9 | 1.63 × 10−6 |
| O3 + ZnO/Graphite | 9.21 × 10−3 | 7.30 × 10−11 | 7.26 × 10−9 | 1.94 × 10−6 |
| O3 + CeO2/Graphite | 1.60 × 10−2 | 7.13 × 10−11 | 4.10 × 10−9 | 2.29 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, X.-Y.; Cui, Y.-H.; Liu, Z.-Q. Preparation of CeO2 Supported on Graphite Catalyst and Its Catalytic Performance for Diethyl Phthalate Degradation during Ozonation. Water 2024, 16, 1274. https://doi.org/10.3390/w16091274
Tao X-Y, Cui Y-H, Liu Z-Q. Preparation of CeO2 Supported on Graphite Catalyst and Its Catalytic Performance for Diethyl Phthalate Degradation during Ozonation. Water. 2024; 16(9):1274. https://doi.org/10.3390/w16091274
Chicago/Turabian StyleTao, Xin-Yi, Yu-Hong Cui, and Zheng-Qian Liu. 2024. "Preparation of CeO2 Supported on Graphite Catalyst and Its Catalytic Performance for Diethyl Phthalate Degradation during Ozonation" Water 16, no. 9: 1274. https://doi.org/10.3390/w16091274
APA StyleTao, X.-Y., Cui, Y.-H., & Liu, Z.-Q. (2024). Preparation of CeO2 Supported on Graphite Catalyst and Its Catalytic Performance for Diethyl Phthalate Degradation during Ozonation. Water, 16(9), 1274. https://doi.org/10.3390/w16091274






