1. Introduction
Antimony (Sb), a metalloid, is increasingly utilized in diverse industrial applications owing to its unique physical and chemical attributes, including as a kind of flame retardant, a component in white pigments, a catalyst in polyethylene terephthalate production, and a medication for Asian cholera [
1,
2,
3,
4]. It has resulted in a heightened demand for antimony. At present extraction rates, it is projected that global extractable antimony resources will be depleted by 2050 [
5]. Consequently, antimony has been classified as one of the most critical strategic reserve resources, and its production has exhibited a strategic decline in recent years. The amount of antimony mined in China has decreased from 150 kt in 2011 to 80 kt in 2020 [
6]. Given the absence of viable substitutes for antimony, the depletion of antimony resources may lead to serious antimony supply risks in the future. Therefore, the research on antimony recovery technology has attracted a lot of attention.
At present, the sources of antimony recovery are mainly concentrated in three aspects: metallurgical residue, scrap materials containing antimony (such as lead-acid batteries, flame retardants, plastic catalysts, etc.), and residual antimony contaminants in soil and water. Commonly employed recovery techniques include adsorption, electrodeposition, extraction, reduction, and selective precipitation [
7,
8,
9].
Wikedzi et al. [
10] recovered antimony during copper metallurgy using precipitation and crystallization techniques. A lead silicate slag was leached in an alkaline sulfide solution to selectively dissolve it, and H
2O
2 was added to precipitate it as sodium thioantimonate and sodium hydroxyl antimonate, achieving a recovery rate of more than 90%. Hernández-Pérez et al. [
11] employed electrodeposition to recover antimony from a secondary waste effluent of copper electrorefining. The near-complete recovery of antimony was achieved without being affected by the concentration of hydrochloric acid. Liang et al. [
12] first used the biochar-loaded ferric oxide (Fe
3O
4/BC) as the adsorbent to treat textile wastewater, then extracted antimony for Sb(III)/Fe
3O
4/BC by a KOH glycol solution and, finally, employed the eluent as the catalyst to synthesize polyethylene terephthalate (PET). Benabdallah et al. [
13] combined the extraction by Cyanex 923 resin and chemical precipitation by NaOH/NaCl or NaOH to recover antimony as SbOCl(s) or Sb
2O
3(s) from copper electrolytes. These studies provided some references for antimony recovery technology but still had little effect. Thus, it is urgent to explore and discover an efficient method for antimony recovery.
In recent years, fluidized-bed granulation technology has attracted lots of attention because it turns waste into valuable products, avoiding sludge disposal. This process achieved crystal granulation by facilitating crystalline growth on the surface of the pre-injected fluidized seed, thus preferring heterogeneous crystallization. It addressed the limitations of traditional chemical precipitation methods, including the excessive sludge generation, high water content in the products, large footprint, and economic inefficiency [
14]. The granulation products obtained through the fluidized bed exhibit a low water content and high purity, enabling direct reutilization [
15]. In principle, the fluidized-bed granulation process can remove all elements that can be precipitated in the form of crystals, rendering it extensively applicable in various domains such as softening [
16], nonmetal recovery [
17], and metal oxide recovery [
18,
19,
20]. Previous studies have shown that antimony present in the solution can also be recovered in the form of crystals. Ye et al. [
21] used the hydrolysis method to selectively separate antimony from an antimony–iron mixture. At a solution pH below 1.9, Sb(III) hydrolyzed to form Sb
4Cl
2O
5 while iron remained in the solution. Then, the generated Sb
4Cl
2O
5 was further converted into Sb
2O
3 with an excellent crystal form and high purity by using Na
2CO
3, NaOH, or NH
4OH as mediating agents. Palden et al. [
22] compared the effects of potassium carbonate, sodium sulfide, and water on the precipitation of antimony from lead dross, and found that Sb(III) in solution can undergo hydrolysis in water to generate Sb
4Cl
2O
5. These investigations presented the potential for utilizing the granulation process in antimony recovery. However, the application of granulation in a fluidized-bed reactor for antimony recovery has never been investigated.
The objective of this study was to recover antimony from a solution using fluidized-bed granulation technology. It aimed to explore the recovery efficiency of antimony and its influencing variables within the fluidized-bed reactor. The removal and granulation efficiency were evaluated under the different conditions of the solution pH, fluidized seed, up-flow velocity, and hydraulic retention time to determine the optimal operational parameters of the reactor. The formed granules were identified by a X-ray polycrystalline diffractometer (XRD) and scanning electron microscopy (SEM). This study exhibited great potential for the recovery of antimony from wastewater or from the extraction water solution.
2. Materials and Methods
2.1. Chemicals
All reagents were analytical grade and purchased from Aladdin, Shanghai, China. All solutions were prepared with deionized water produced by a laboratory-grade RO-ultrapure water system and used without further purification. The antimony solution was prepared from antimony potassium tartrate (C8H4K2O12Sb2). The precipitant solution was potassium sodium tartrate (NaKC4H4O6). Finally, 0.5 M NH4·H2O was used to achieve the desired pH of the solution.
2.2. Fluidized-Bed Reactor
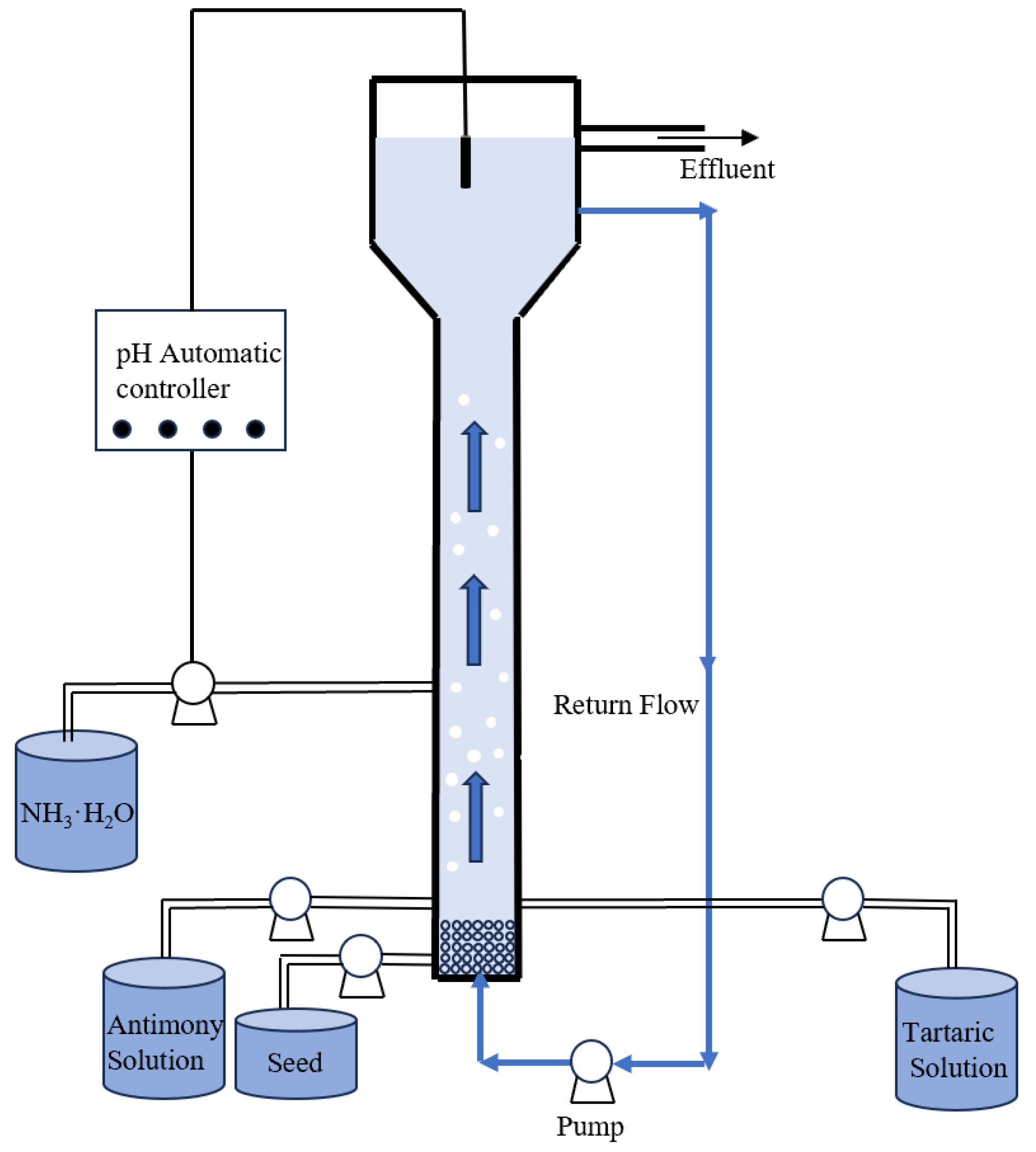
The experiments were conducted in a fluidized-bed reactor with a total volume of 500 mL, as shown in
Figure 1. It included a feeding zone with antimony inlet, tartrate inlet, reflux inlet, crystallization zone with ammonia influent controlled by pH automatic controller, and precipitation zone with effluent and reflux. The feeding zone and the granulation reaction zone were an organic glass cylinder with a height of 80 cm and an inner diameter of 2 cm. The glass beads at the bottom were necessary for supporting the granulation bed, avoiding bubble formation and clogging, and distributing the liquid solution flow inside the reactor uniformly from the three inlets. The precipitation zone had an inner diameter of 4 cm and a height of 20 cm. The sudden-expansion pipe provided a larger volume for the particles in the reactor, reducing the up-flow rate and preventing the discharge of fine particles.
2.3. Experimental Procedure
Before initiating FBR, Sb2O3 as seed crystals were pumped from the bottom of the reactor. Both antimony and tartrate solution were pumped into the reactor by the pumps. The mixed solution in the reactor moved toward the top. Once the mixture level arrived at the reflux outlet, the reflux pump was activated to recirculate the mixture to the bottom of the reactor, to contribute to the up-flow rate, ensuring the fluidization of the seed. At the same time, the NH3·H2O pump began to work according to the signal from controller. The mixture continued to move to the effluent outlet and the clear water was transported out. The ratio of [Tar]/[Sb(III)] was obtained by variation of tartrate at a fixed antimony concentration. The up-flow velocity(U) was varied by adjusting the reflux flow rate.
The typical experiment was conducted as follows. Seed size was 13–38 μm and seed concentration was 6 g/L. Initial antimony and tartrate concentration in the reactor were 500 mg/L and 8.2 mmol/L, respectively, resulting in the ratio of [Tar]/[Sb(III)] being 2.0. The pH in the reactor was kept at pH9.0. U was 42 m/h and hydraulic retention time (HRT) was 40 min.
For single-factor experiments, seed size varied between 2.5–13 μm, 13–38 μm, 38–75 μm, 75–150 μm, and 150–300 μm. Seed concentration varied from 2 g/L to 10 g/L. U varied in the range of 14–70 m/h. The HRT was investigated at 10–80 min by changing the influent flow rate. All experiments were conducted at 25 °C with the solution pH continuously monitored and adjusted in real time using a 0.5 mol/L NH3·H2O solution. The large tartrate concentration and slow dissociation of NH3·H2O resulted in a slow rate of pH increase, and, consequently, led to a longer start-up time (about 10 h). All the samples were collected after successful start-up.
2.4. Analytical Methods
The residual concentration of antimony ion in effluent was determined by atomic fluorescence spectrophotometry (AFS-9700A, Shimadzu, Kyoto, Japan). The standard curve was prepared by diluting 1000 μg/mL of Sb standard solution. The matrix of all standard and experimental solutions consisted of a 5% thiourea-ascorbic acid solution with a carrier solution containing 5% hydrochloric acid. The instrument used a 2% solution of potassium borohydride as a reducing agent, with potassium borohydride dissolved in 0.5% potassium hydroxide. A 10 mL sample of effluent water was divided into two parts: one part was directly determined to test the residual total antimony concentration in the solution and calculate the removal efficiency of antimony using Equation (1), while the other part was filtered through a 0.45 μm filter membrane to determine the dissolved antimony concentration and calculate the granulation efficiency of antimony using Equation (2). The crystallization products were removed from the lower part of the reactor, thoroughly cleaned with ultra-pure water, and dried. Crystal morphology of the crystal products was analyzed by field emission SEM (FEI, San Jose, CA, USA). The crystal phase was investigated by routine scanning with an XRD (D8 advance, Bruker, Ettlingen, Germany) within the range of 10°–90°, while the particle size was determined by the laser particle size analyzer (Mastersizer 2000, Malvern, UK).
where [Sb]
0 was the initial antimony concentration in the solution (mg/L). [Sb]
D was the concentration of dissolved antimony after treatment in the fluidized bed (mg/L), and [Sb]
t represented the total antimony concentration of the effluent in the reactor (mg/L).
3. Results and Discussion
3.1. Optimal pH for Sb(III) Recovery
Antimony (III) exhibits high hydrolytic ability in solution and exists in numerous chemical species. Usually, antimony predominantly exists as the positively charged ion SbO
+ in the pH range of 0–2.0. Within the pH range of 2.0 to 12.0, it primarily exists as Sb(OH)
3, and the concentration of SbO
2− ions begins to rise at pH 12.0–14.0. The equation representing the hydrolysis of antimony in water has been shown in Equations (3)–(5) [
23]. The granulation process of antimony is initiated by the reaction of Sb
3+ with OH
− and is, consequently, dependent on the solution pH. The pH level regulates the degree of supersaturation within a solution, promoting the granulation process. To facilitate the formation of Sb
2O
3, the experiment was conducted within the pH range of 7.0 to 12.0. With addition of tartrate, it will compete with OH
− for Sb(III), and, hence, mediate the crystal structure [
24].
As illustrated in
Figure 2a, the efficiency of Sb(III) removal and granulation at pH 7.0 were relatively low, with rates of 66% and 65%, respectively. Then, both the removal efficiency and the granulation efficiency went through a high-speed increase at a pH of 7.0–9.0 and gradually reached the maximum at pH 9.0, at 94% and 91%, respectively. However, both the removal and granulation efficiency began to decrease at a pH above 9.0. The removal efficiency and granulation efficiency dropped to 90% and 79% at pH 10.0, respectively.
At a pH less than 9.0, the concentration of OH
− was low, resulting in a low supersaturation of the solution, which did not facilitate the antimony granulation [
25]. Thus, as the pH increased from 7.0 to 9.0, more and more OH
− in the solution promoted granulation reactions, which was supported by the increasing crystallization efficiency. However, excessively high pH levels can induce a small amount of Sb
2O
3 to redissolve due to its amphoteric character [
26]. This resulted in a slight reduction in removal efficiency. In addition, the granulation efficiency substantially dropped due to the excessive supersaturation, which resulted in the occurrence of homogeneous nucleation and smaller granules [
27].
Figure 2b showed the XRD analysis of the granules at pH 7.0–10.0. From the XRD pattern, all labeled reflection peaks could be readily recognized as the cubic phase of Sb
2O
3 according to the JCPDS cards (No. 71-0365). The formation of cubic Sb
2O
3 may be related to the addition of tartaric acid to the solution [
28].
Figure 3 showed the morphology of the resulting products at pH 7.0–10.0. At pH 7.0, the products were irregularly shaped with a limited presence of cubic shape agglomerates. The shape of the products became much closer to octahedral, and the number of crystals also increased with the increasing pH, which, finally, achieved the optimum at pH 9.0. However, above pH 9.0, the products enlarged, leading to the gradual disappearance of the octahedral structure. As a result, the optimal pH for Sb(III) granulation was 9.0. This pH was used for subsequent experiments.
3.2. Effect of Ratio of [TA]/[Sb3+] on the Recovery of Sb(III)
Sb
2O
3 exists in two crystallographic forms, orthorhombic and cubic [
29], the cubic form of which has more stable properties and a higher industrial value. Compounds like tartaric acid (TA) and EDTA are known to have an impact on the crystalline structure of antimony trioxide [
24]. Consequently, to directly synthesize cubic Sb
2O
3, tartaric acid was added to the reaction solution as a precipitant. The influence of the tartaric acid dosage on the crystallization of Sb
3+ was further explored below. The precipitant dosage is usually expressed as the molar ratio of the precipitant to the Sb
3+.
As shown in
Figure 4, the granulation and removal efficiency were 72% and 78%, respectively, at a [TA]/[Sb
3+] molar ratio of 1.5. As the molar ratio increased to 2.0–2.5, the removal and granulation efficiency arrived at a peak of 93% and 89%, respectively.
Owing to the crucial role of tartaric acid in modulating the granulation structure of Sb
2O
3, smaller granules might be formed at lower concentrations of tartaric acid and were carried away with the effluent or passed through the filter membrane into the solution during the sampling section, consequently leading to a higher concentration of Sb
3+ in the effluent. With an increasing molar ratio, this phenomenon was notably mitigated, accompanied by an increase in the antimony granulation efficiency. However, in subsequent experiments, it was observed that, by increasing the molar ratio of [TA]/[Sb
3+] to 6, the granulation reaction in the solution no longer occurred. This phenomenon was due to the complexation between the tartrate ion and Sb
3+ [
30]. An excessive concentration of tartrate ions led to a robust complexation with Sb
3+, potentially impeding the complexation of Sb
3+ with OH
−, thereby resulting in a notable decrease in antimony recovery. The same phenomenon occurred in the granulation of Cu
2+ [
31]. Consequently, a [TA]/[Sb
3+] molar ratio of 2 was selected to achieve optimal recovery efficiency.
3.3. The Effect of Seed Size on the Recovery of Sb(III)
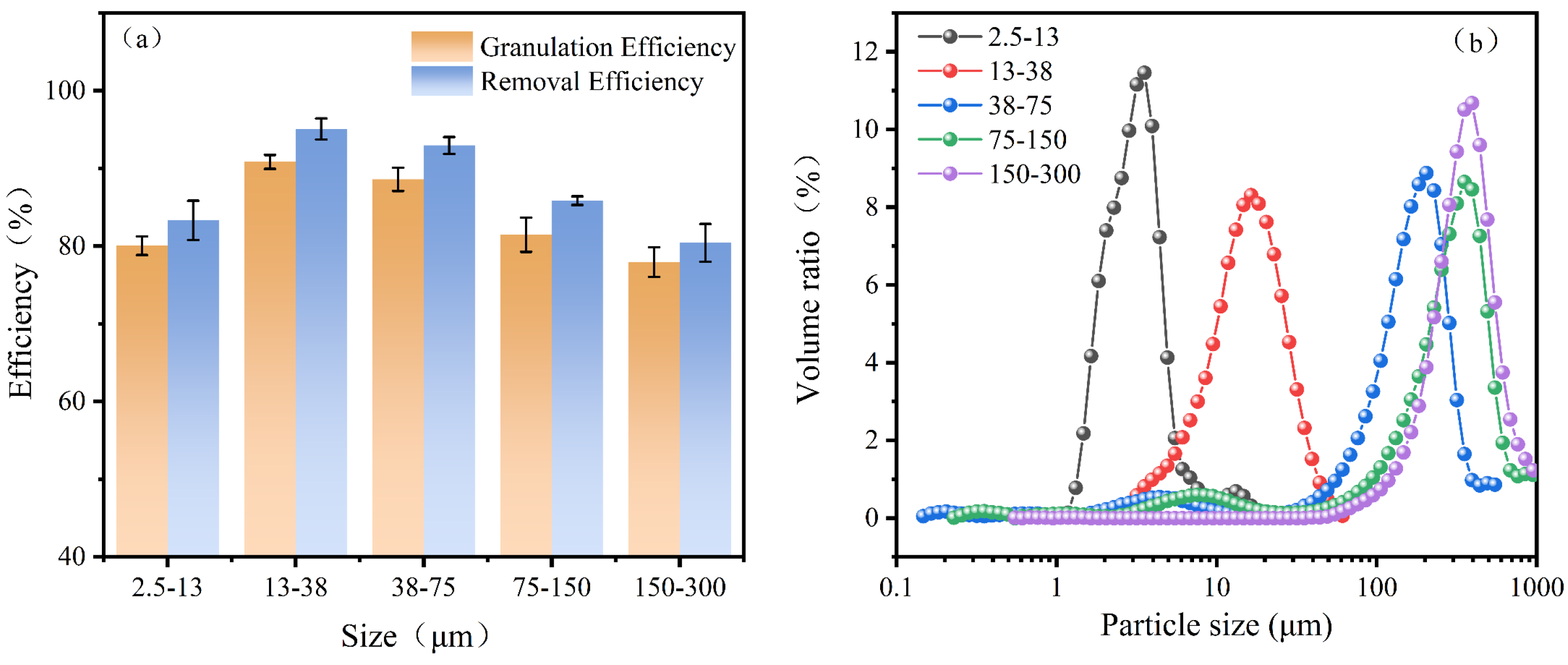
Different seed sizes may result in variations in their specific surface areas and gravities, consequently leading to diverse effects on the granulation process. To investigate this, five kinds of seed sizes, S1 (2.5–13 μm, D50 = 4.6 μm), S2 (13–38 μm, D50 = 15.4 μm), S3 (38–75 μm, D50 = 34.2 μm), S4 (75–150 μm, D50 = 80.2 μm), and S5 (150–300 μm, D50 = 175.2 μm), respectively, were obtained from experiments, where D50 was the median diameter measured by a Malvern particle size analyzer. The seed was cleaned thoroughly with pure water for subsequent experiments.
As shown in
Figure 5a, with a seed size of 2.5–13 μm, the granulation and removal efficiency reached 80% and 83%, respectively. As it increased to 13–38 μm and 38–75 μm, the granulation and removal efficiency were higher at 13–38 μm and reached 93% and 95%. However, continuing to increase the seed size to 75–150 μm and 150–300 μm had a negative effect on the recovery of antimony.
The fluidized seeds with small sizes exhibited inadequate settling properties and were susceptible to being carried out of the reactor by the water flow, resulting in a high concentration of antimony ions in the effluent. Therefore, enlarging the seed sizes could enhance the sedimentation performance, prevent the loss of the seed, and promote the nucleation of Sb
3+ on the seed surface. However, the granulation efficiency did not exhibit a proportional relationship with the grain sizes. There were several possible aspects responsible for this. First, once the surface area provided by the seed was sufficient for the granulation, the smaller the grain size of the crystalline seed, the greater the specific surface area [
32]. Thus, more electrostatic adsorption sites were available for the inhomogeneous nucleation of Sb
2O
3, leading to a higher granulation efficiency. In addition, a larger seed with a bigger mass and a smaller potential for fluidization resulted in an incomplete contact between the seeds and nucleating molecules and, hence, led to a low recovery efficiency of antimony.
Figure 5b showed the granule size distribution after running for 360 min. It can be seen that the D
50 increases compared to the initial seed size. The shift to the larger size was attributed to the accumulation of Sb
2O
3 by the granulation process. Among them, D
50 increased from 4.6 μm to 5.5 μm for seeds with a size of 13–38 μm. For seeds with a larger particle size, D
50 increased even more. This may be due to the fact that seeds with smaller particle sizes have a greater specific surface area resulting in a lower size increase rate. Thus, the bed may soon fail to fluidize if the seeds’ sizes were too large. In summary, combined with the granulation efficiency, a seed size of 13–38 μm was the optimal selection in this experiment.
3.4. The Effect of Seed Concentration on the Recovery of Sb(III)
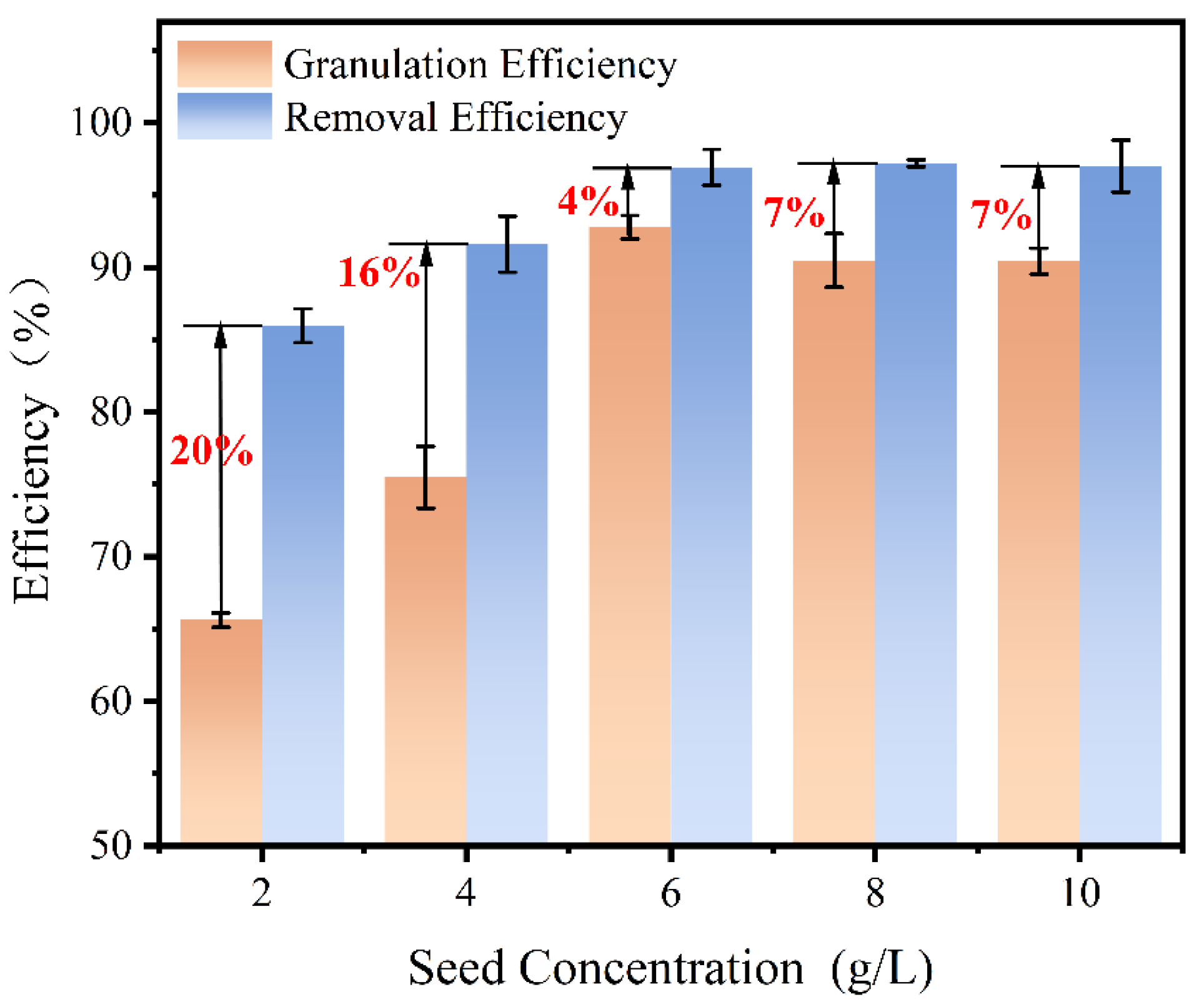
The concentration of the seed is another factor that influences its specific surface area. Thus, seed concentrations of 2 g/L, 4 g/L, 6 g/L, 8 g/L, and 10 g/L were selected to determine the optimal dosage, as shown in
Figure 6. The removal and granulation efficiency increased first, and then decreased. At the seed amounts were 2 g/L, the removal and crystallization efficiency were 85% and 65%, respectively. With the seed amounts increased to 6 g/L, the removal and granulation efficiency reached the maximum values of 96% and 92%. However, further increasing the seed concentration resulted in a deterioration of the granulation efficiency, while the removal efficiency remained relatively stable. The disparity in granulation and removal efficiency resulting from seed concentration warranted our attention.
The concentration of the fluidized seed determined the available reactive surface for adsorption and nucleation. At lower seed concentrations, the limited interaction between Sb
3+, OH
−, and the seed led to a low removal efficiency. Moreover, the majority of Sb
3+ underwent homogeneous nucleation at this dosage. Therefore, the granulation efficiency on the seed surface was lower than Sb’s removal efficiency. As more and more seed were added, both the removal and granulation efficiency increased, and the difference between them became smaller. More seed increased the relative specific surface area and promoted the non-homogeneous granulation of Sb
3+ [
33]. However, an excessive concentration of seed led to diminished bed swelling, thereby restricting the interaction of Sb
3+ and OH
− with the seed. This phenomenon again promoted homogeneous nucleation and reduced the granulation efficiency, which can be improved with a bigger up-flow velocity. In summary, the concentration of 6 g/L was chosen as the optimal dosage of seed.
3.5. The Effect of Up-Flow Velocity on the Recovery of Sb(III)
The up-flow velocity (U), which is essential for granulation, is the ratio of the volumetric flow rate (m
3/h) per unit area (m
2). According to diffusion theory, an interface diffusion boundary layer exists between the crystal surface and the solution, and it will impede the approach of unstable molecules. Nucleation molecules first pass through the interfacial layer by diffusion to the crystal surface. In general, the thickness of the interfacial layer is influenced by the rate of solid–liquid phase interaction in the system. In turbulent systems, the interface layer may be thin, whereas, in static flow systems, it may be thick [
34]. Therefore, an appropriate U can enhance the solid–liquid mass transfer efficiency and promote the adhesion of Sb
3+ and the precipitant to the surface of the seed. In addition, it also facilitates the regulation of supersaturation to avoid homogenous granulation [
35].
Changing reflux ratios is the way to vary the up-flow velocity in the FBR system [
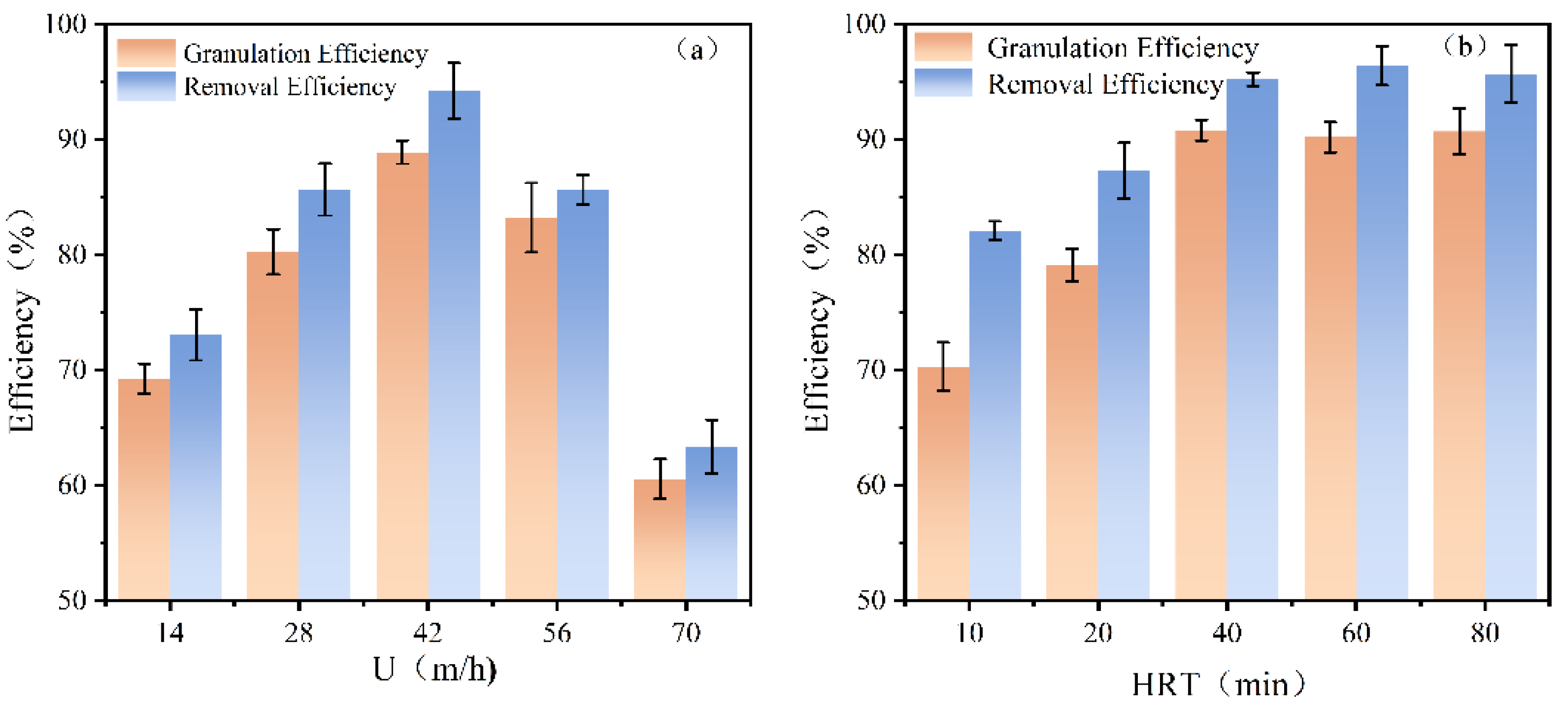
36]. In this study, 14, 28, 42, 56, and 70 m/h were selected for to investigating the optimal up-flow velocity for the granulation of antimony. As depicted in
Figure 7a, the recovery efficiency increased with U in the range of 14–42 m/h, resulting in the removal and granulation efficiency of 94% and 88%, respectively. However, as the U was above 42 m/h, the antimony recovery began to decrease. At a U of 70 m/h, the removal and granulation efficiency decreased to 63% and 60%, respectively.
At a U of l4 m/h, the low U resulted in a minimal hydraulic disturbance in the FBR, and the bed expansion was negligible, which hindered the diffusion of the solute to the seed surface. Consequently, the antimony granulation effect was poor. As the up-flow velocity increased, the hydraulic disturbance became more pronounced, greatly increasing the chance of Sb
3+ and OH
− to access the seed surface, thereby enhancing the crystallization efficiency. However, as the U exceeded 42 m/h, an excessively big up-flow velocity created high shear stress, which broke the granules and caused them to fly away from the reactor [
37,
38]. Furthermore, a high up-flow velocity, which extended the fluidized height, decreased the number of seed per unit volume, and resulted in smaller opportunities for the access of the solute to the seed. In summary, the optimal up-flow velocity identified in this study was 42 m/h.
3.6. The Effect of Hydraulic Retention Time on the Recovery of Sb(III)
HRT is another important hydraulic parameter for a fluidized-bed reactor. During antimony granulation, the induction of granulation reactions and the growth of crystals require a certain amount of time [
39]. Therefore, selecting the appropriate HRT is crucial for ensuring optimal granulation efficiency. In this study, the HRT of 10, 20, 40, 60, and 80 min were selected to investigate their impact on the recovery of antimony in the fluidized bed.
Figure 7b showed that, with the increase of HRT, both the antimony removal efficiency and granulation efficiency exhibited an initial increase, followed by a plateau. When the HRT was 10 min, the removal and granulation efficiency were 82% and 70%, respectively, with a difference of 12%. At an HRT of 40 min, the FBR achieved antimony removal and granulation efficiencies of 95% and 91%, respectively. The disparity is diminishing. As the HRT increased from 40 min to 80 min, the recovery efficiency remained stable and unchanged.
Theoretically, the reaction occurs incompletely if the HRT in the FBR is short. Due to the limited contact time between the reactants, the total removal efficiency decreases. It was evident from the experimental results that the Sb
3+ cannot granulate adequately at an HRT of 10 min or 20 min. Furthermore, the high flow rate of the feed water during a shorter HRT led to supersaturation, thereby fostering extensive homogeneous crystallization. This phenomenon elucidated the significant disparities in the removal and granulation efficiencies observed at an HRT of 10–20 min [
40]. When the HRT was longer than 40 min, the remaining Sb
3+ in the solution did not continue to crystallize. Therefore, the recovery efficiency no longer increased. In addition, an HRT that is too long will result in a smaller water treatment capacity per unit time and lower economic efficiency [
41]. Consequently, the optimal HRT was considered to be 40 min in this experiment in order to achieve both granulation and economic efficiency.
3.7. Effect of Continuous Operation of the FBR at Optimum Parameters
Through the analysis of the above experiments, it was established that the optimal operating conditions for the reactor were an antimony concentration of 500 mg/L, a ratio of [TA]/[Sb3+] of 2, pH 9.0, a concentration of 6 g/L of 13–38 μm Sb2O3 as the seed, an up-flow velocity 42 m/h, and an HRT of 40 min. Based on the above parameters, the reactor was operated continuously for 7 d. The pH of the effluent and the concentration of residual antimony ions in the solution were determined at regular intervals in order to investigate the recovery efficiency of antimony.
The results in the FBR were shown in
Figure 8. At the start-up stage of the reactor, the effluent Sb
3+ concentration decreased with the pH increasing to 9.0, which was regarded as the induced nucleation stage. At this stage, Sb
3+ and OH
− began to granulate on the surface of the seed, accompanied by the formation of a large number of homogeneous nucleated crystals. These crystals were small and may flow with the effluent, thereby leading to a high concentration of Sb
3+ in the effluent. With the increase in time, the granulation and removal efficiency of antimony gradually increased and stabilized. The FBR had a short start-up time and achieved more than 90% removal within 3 h. Subsequently, Sb
3+ granulated in a non-homogeneous phase upon the seed surface. During the following observed period (7 d), the FBR demonstrated a stable performance on the removal and granulation efficiency of 96% and 93%, respectively, suggesting that it had a potential for continuing the granulation process.
3.8. Characterization of the Granules in FBR
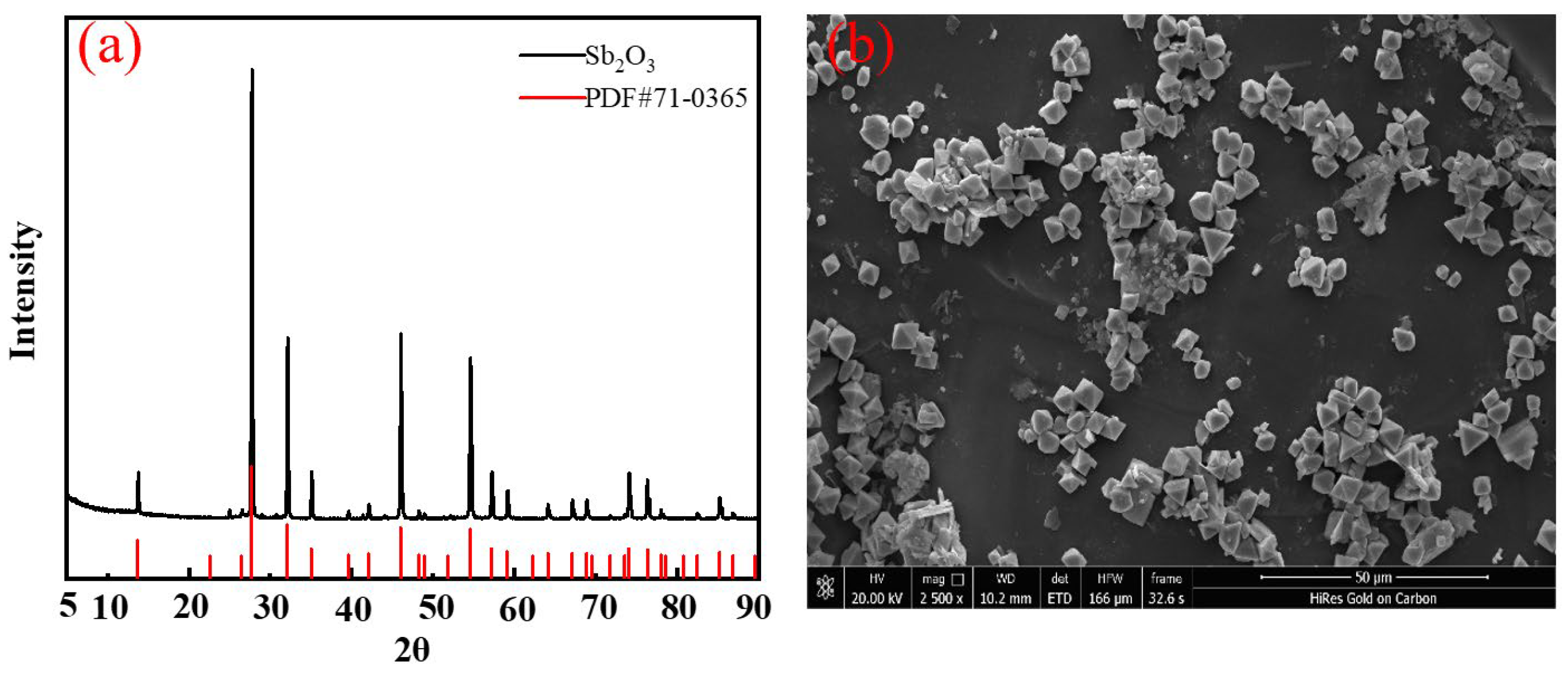
The granular products from the FBR were characterized. The XRD analysis was shown in
Figure 9a: there were strong diffraction peaks in 2θ = 13.50°, and 27.62°, indicating that the granules showed a high crystallinity and were identified as a cubic-phase Sb
2O
3 according to the JCPDS standards of #71-0365. The SEM image in
Figure 9b indicated the crystalline shape of the product is a standard octahedral structure. These characterizations have demonstrated that the crystallization products were cubic Sb
2O
3.
The results of this study demonstrated the possibility of using fluidized-bed granulation technology for antimony recovery from solution. Theoretically, this technology can be directly applied to antimony-containing wastewater such as antimony soil leachate, antimony smelting wastewater, and printing and dyeing wastewater. Although the antimony concentration in this experiment was 500 mg/L, our previous study has demonstrated effective granulation even at a concentration of as low as 25 mg/L. In addition, plastics and other antimony-containing wastes are usually disposed of by incineration. The antimony content in fly ash from the combustion of antimony-containing plastics reported is in the range of 23–179 mg/L [
42]. A potential approach involves leaching the fly ash to extract antimony in a certain solution, and granulating to form cubic Sb
2O
3 in FBR. This technology provides a new idea for the disposal of antimony-containing wastes. However, the actual wastewater has not been treated in this experiment. In addition, the temperature may also be a key factor affecting the crystallization of antimony; further investigations will be carried out in the future.