The Influence of Aqueous Iron on River Sand’s Arsenic Adsorption: Characteristics and Mechanisms
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents and Samples
2.2. Testing and Calculation
2.3. Measurements
3. Results and Discussion
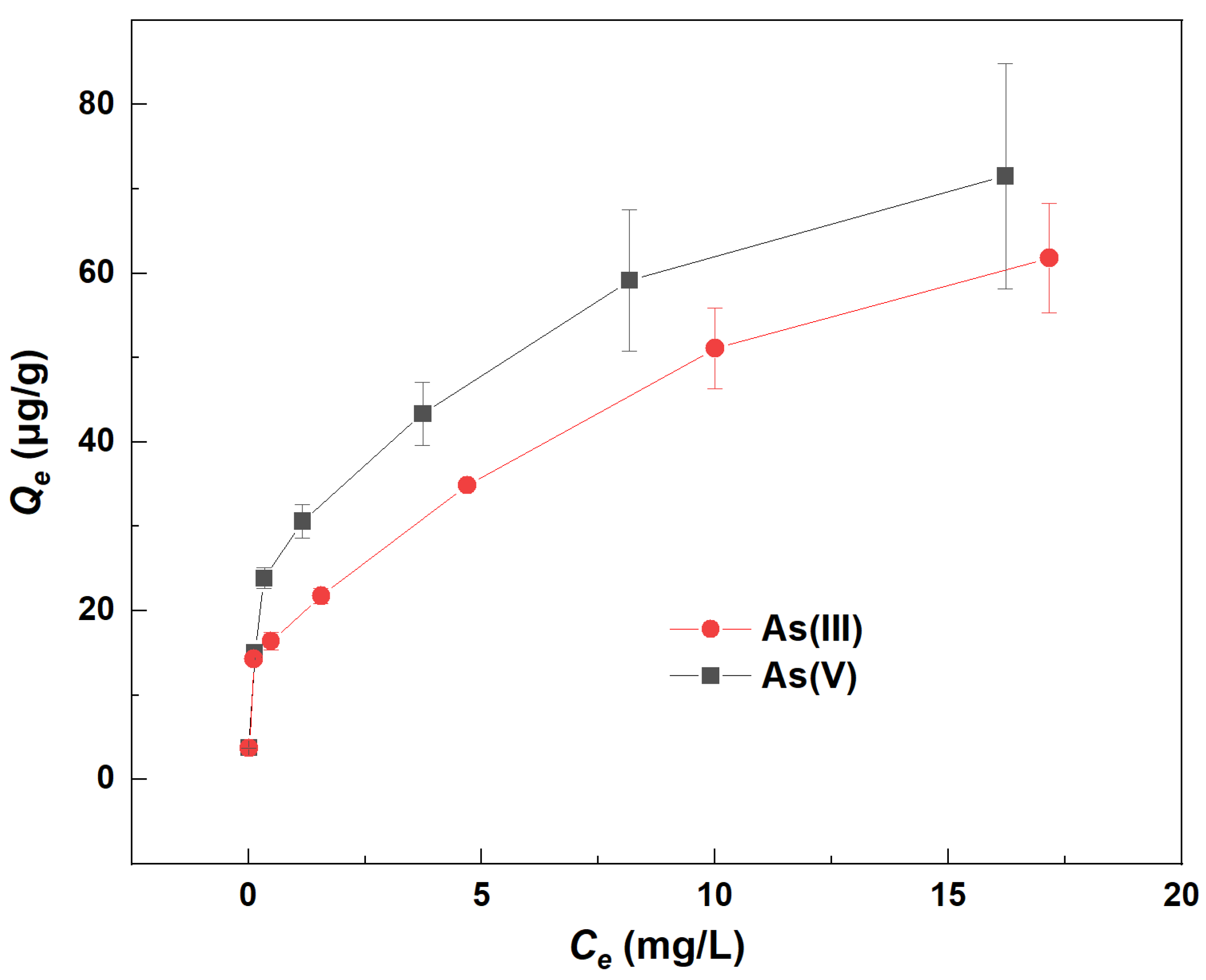
3.1. Adsorption Characteristics of River Sand for As
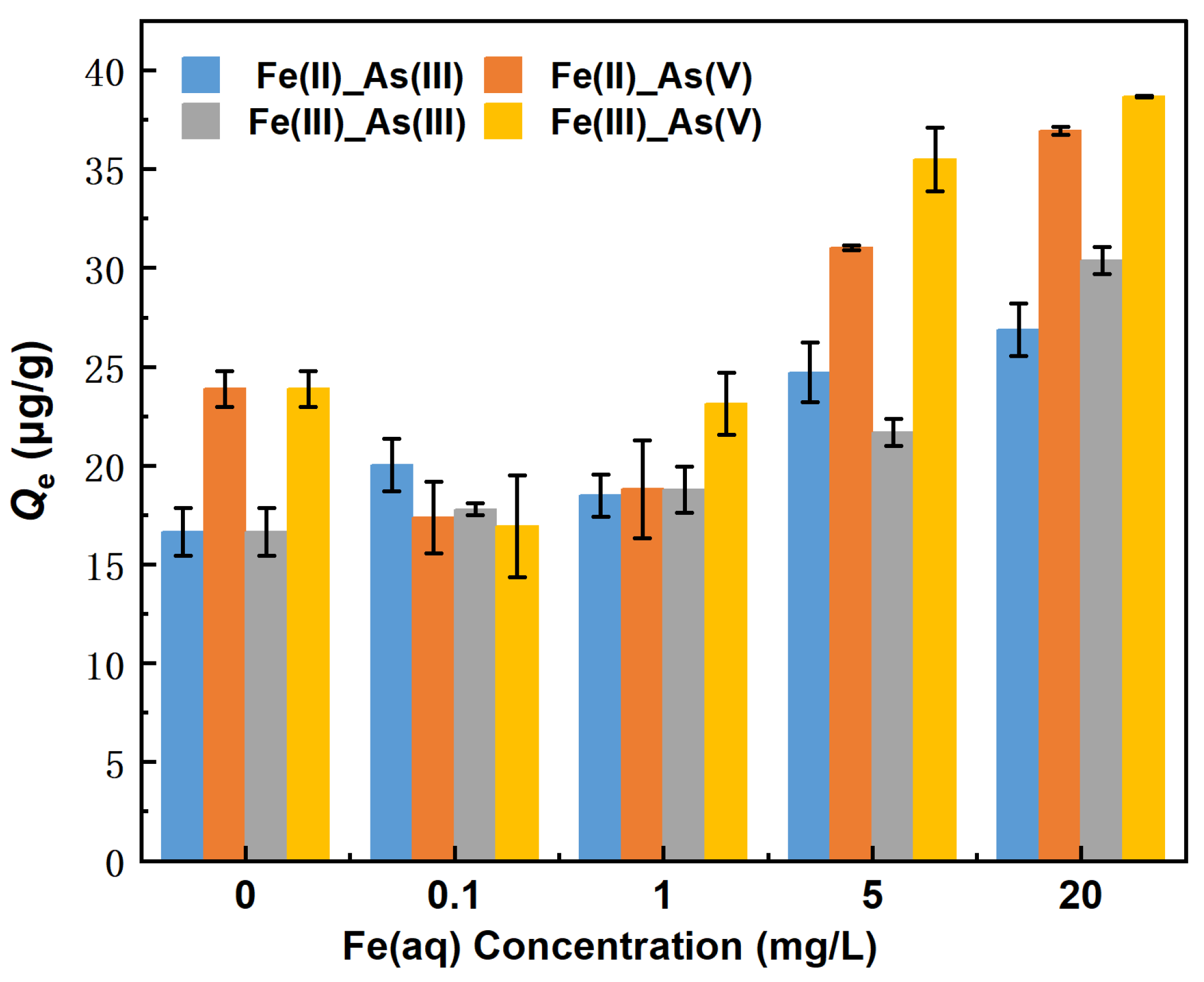
3.2. Impact of Fe(aq) on As Absorption
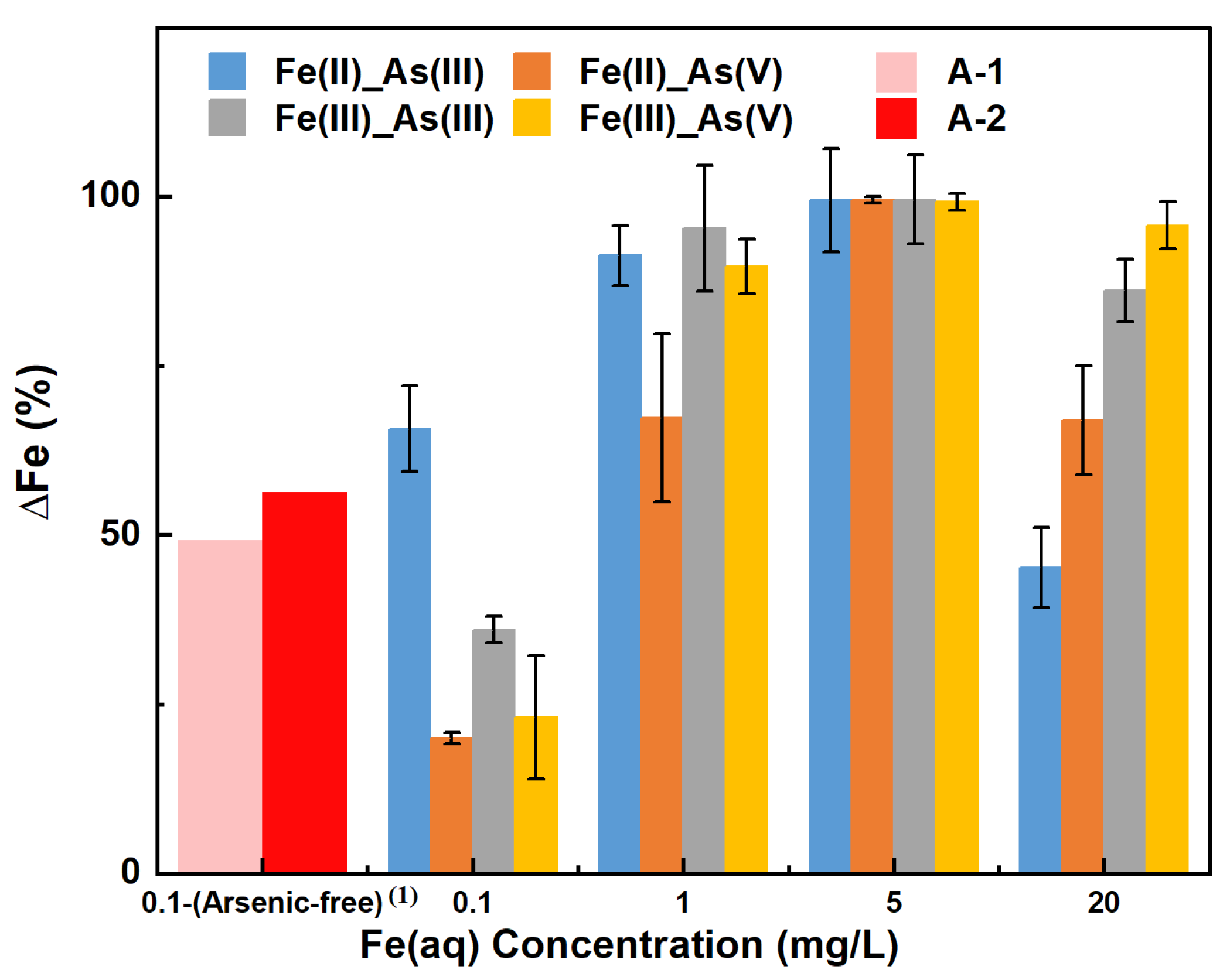
3.3. Changes in Fe(aq) Concentration
3.4. Changes in the Microstructure and Surface Elemental Composition of River Sand
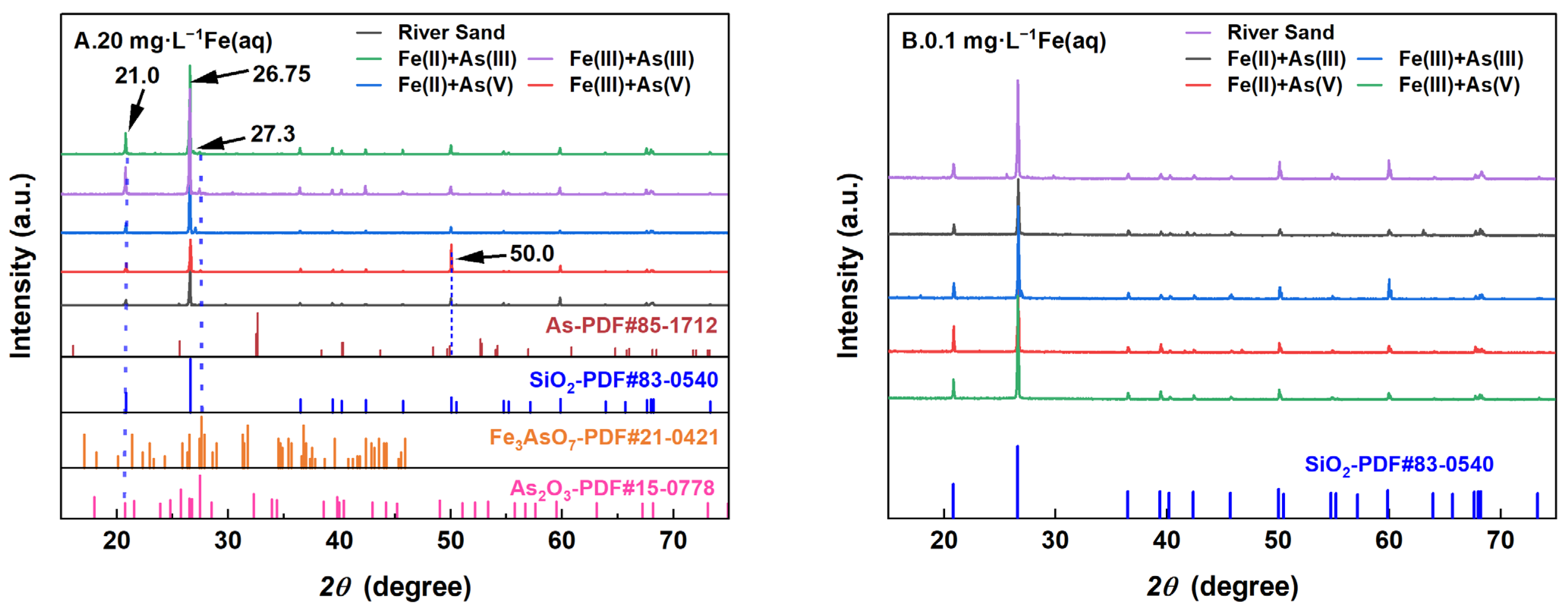
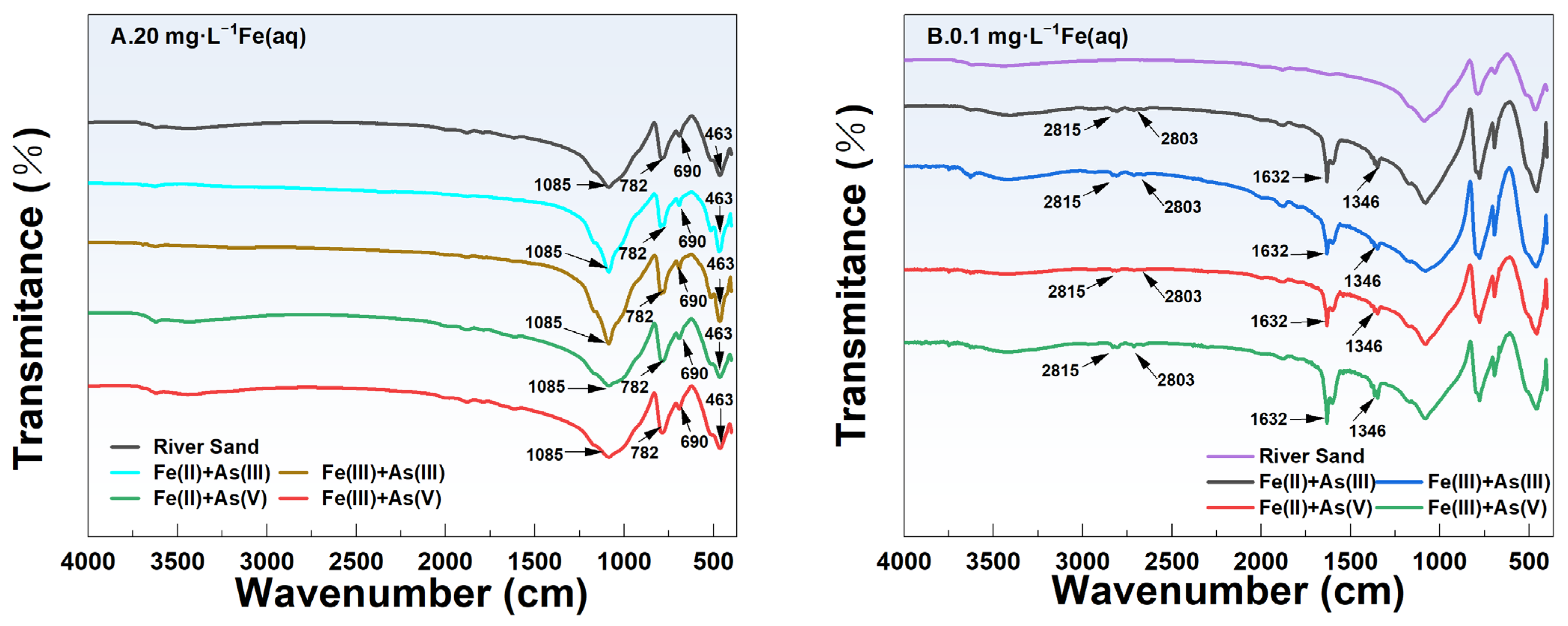
3.5. Changes in the Main Crystalline Phases and Functional Groups of River Sand
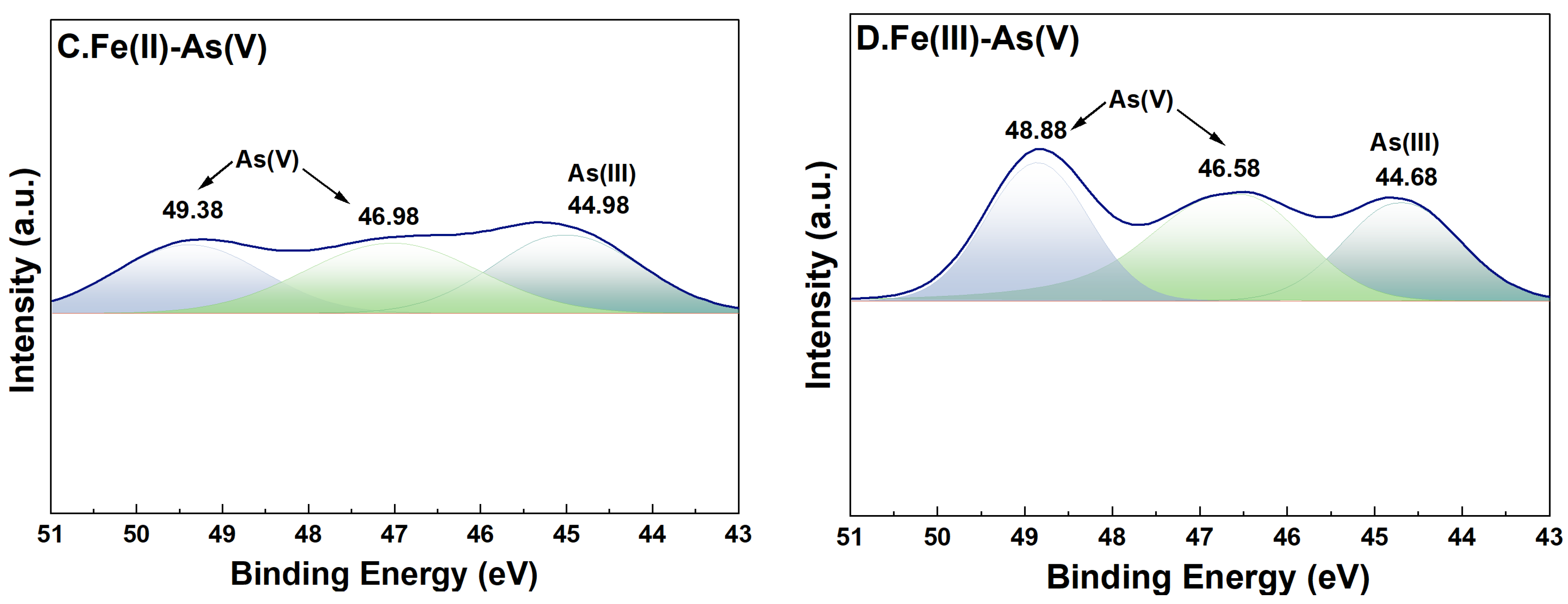
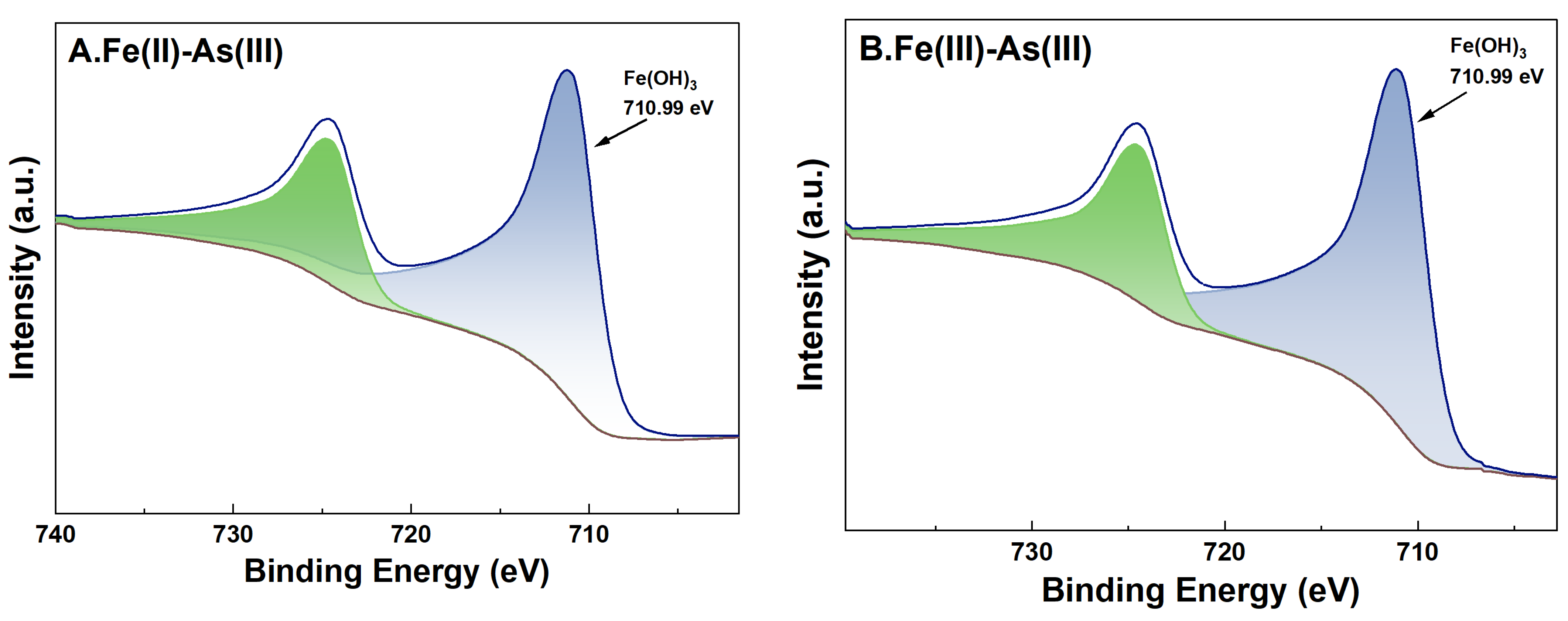
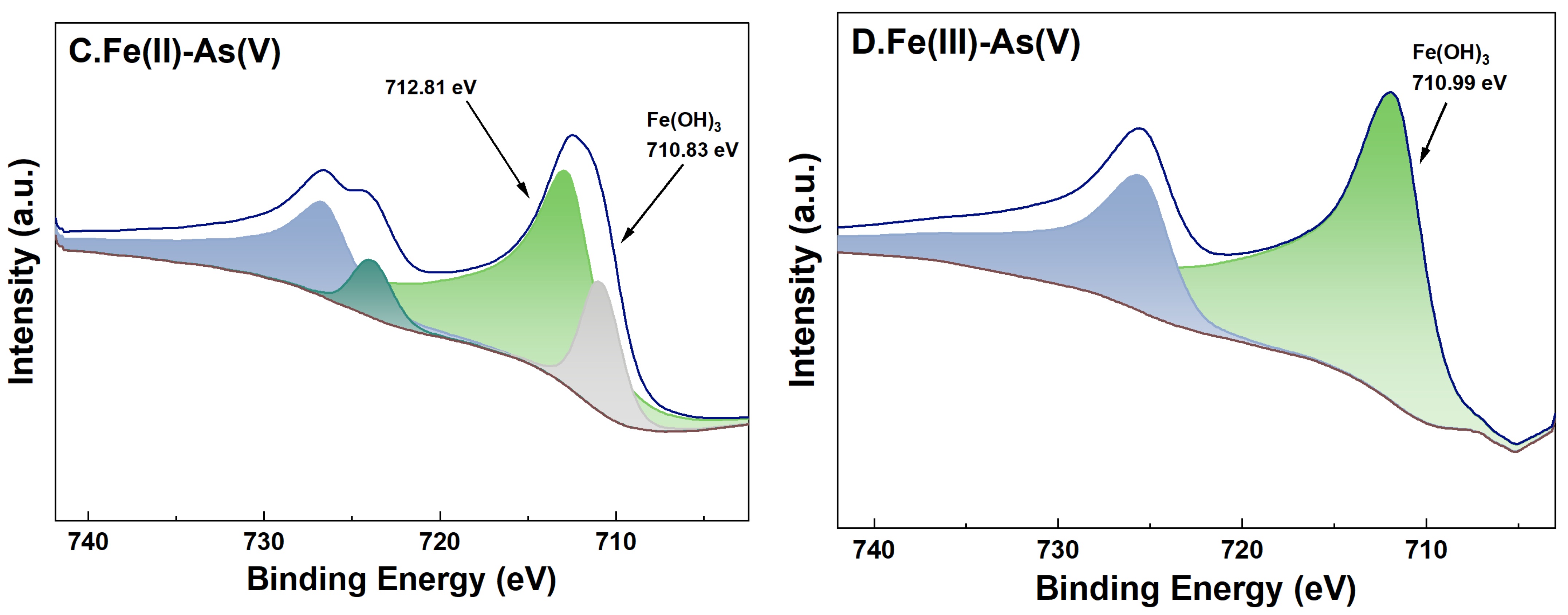
3.6. Changes in the Valence of As and Fe on the Surface of River Sand
3.7. Mechanism Analysis
4. Conclusions
- (1)
- When the initial concentration of Fe(aq) was below 1 mg/L, the adsorption of As(III) by river sand was promoted, while the adsorption of As(V) was inhibited. When the initial concentration of Fe(aq) exceeded 5 mg/L, the adsorption of both As(III) and As(V) was promoted. Hence, Fe(aq) promoted the adsorption of As(III) by river sand, while low-concentration Fe(aq) inhibited the adsorption of As(V) and high-concentration Fe(aq) promoted the adsorption of As(V).
- (2)
- Adsorption kinetics analysis and adsorbent characterization revealed that in the As(V) adsorption systems under low-Fe(aq) conditions (0.1–1 mg/L), Fe(II) reduced As(V) to As(III), with the latter As species demonstrated a lower adsorbed amount at its adsorption equilibrium, thus lessening the removal of dissolved As from the solution. Under the same low-Fe(aq) conditions, Fe(III) transformed As(V) into As(III) through more complex reactions, restricting the adsorption of As(V) by river sand. Conversely, under high-Fe(aq) conditions (5–20 mg/L), Fe(aq) promoted the adsorption of As by river sand through the formation of Fe(OH)3.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, J.; Cao, W.; Lang, G.; Sun, Q.; Nan, T.; Li, X.; Ren, Y.; Li, Z. Worldwide distribution, health risk, treatment technology, and development tendency of geogenic high-arsenic groundwater. Water 2024, 16, 478. [Google Scholar] [CrossRef]
- Sinha, D.; Prasad, P. Health effects inflicted by chronic low-level arsenic contamination in groundwater: A global public health challenge. J. Appl. Toxicol. 2020, 40, 87–131. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Dzeng, S.R.; Yang, M.-H.; Chiu, K.-H.; Shieh, G.-M.; Wai, C.M. Arsenic species in groundwaters of the blackfoot disease Area, Taiwan. Environ. Sci. Technol. 1994, 28, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; García-Montalvo, E.A.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, Y.; Li, H.; Li, H.; Guo, W.; Duan, Y.; Dong, C.; Gan, Y.; Liu, N.; Ding, X. Seasonal variation of arsenic speciation in shallow groundwater from endemic arsenicosis area in Jianghan Plain. Earth Sci. J. China Univ. Geosci. 2015, 40, 1876–1886. [Google Scholar]
- Yan, Y.; Xie, X.; Xiao, Z.; Li, J. Influence of irrigation practices on arsenic mobilization and transformation in the unsaturated zone. Geol. Sci. Technol. Inf. 2018, 37, 206–214. [Google Scholar] [CrossRef]
- Yang, C.; Li, S.; Liu, R.; Sun, P.; Liu, K. Effect of reductive dissolution of iron (hydr)oxides on arsenic behavior in a water–sediment system: First release, then adsorption. Ecol. Eng. 2015, 83, 176–183. [Google Scholar] [CrossRef]
- Bauer, M.; Blodau, C. Mobilization of arsenic by dissolved organic matter from iron oxides, soils and sediments. Sci. Total Environ. 2006, 354, 179–190. [Google Scholar] [CrossRef]
- Winde, F.; Jacobus van der Walt, I. The significance of groundwater–stream interactions and fluctuating stream chemistry on waterborne uranium contamination of streams—A case study from a gold mining site in South Africa. J. Hydrol. 2004, 287, 178–196. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, F.; Meng, Y.; Byrne, P.; Ghomshei, M.; Abbaspour, K.C. Modeling transport and fate of heavy metals at the watershed scale: State-of-the-art and future directions. Sci. Total Environ. 2023, 878, 163087. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.D.; Brikowski, T.H.; Johannesson, K.H. Natural arsenic-rich spring waters discharging from the Austin Chalk, North-Central Texas, USA: Mineral and chemical evidence of pyrite oxidation followed by reductive dissolution of neo-formed Fe (III) oxides/oxyhydroxides. Appl. Geochem. 2023, 150, 105547. [Google Scholar] [CrossRef]
- Bretzler, A.; Nikiema, J.; Lalanne, F.; Hoffmann, L.; Biswakarma, J.; Siebenaller, L.; Demange, D.; Schirmer, M.; Hug, S.J. Arsenic removal with zero-valent iron filters in Burkina Faso: Field and laboratory insights. Sci. Total Environ. 2020, 737, 139466. [Google Scholar] [CrossRef] [PubMed]
- Stolze, L.; Zhang, D.; Guo, H.; Rolle, M. Surface complexation modeling of arsenic mobilization from goethite: Interpretation of an in-situ experiment. Geochim. Cosmochim. Acta 2019, 248, 274–288. [Google Scholar] [CrossRef]
- Mejia-Santillan, M.E.; Pariona, N.; Bravo-C, J.; Herrera-Trejo, M.; Montejo-Alvaro, F.; Zarate, A.; Perry, D.L.; Mtz-Enriquez, A.I. Physical and arsenic adsorption properties of maghemite and magnetite sub-microparticles. J. Magn. Magn. Mater. 2018, 451, 594–601. [Google Scholar] [CrossRef]
- Navarathna, C.M.; Karunanayake, A.G.; Gunatilake, S.R.; Pittman, C.U., Jr.; Perez, F.; Mohan, D.; Mlsna, T. Removal of Arsenic (III) from water using magnetite precipitated onto Douglas fir biochar. J. Environ. Manag. 2019, 250, 109429. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.; Liu, G.; Cai, Y. Adsorption kinetics and isotherms of arsenite and arsenate on hematite nanoparticles and aggregates. J. Environ. Manag. 2017, 186, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ona-Nguema, G.; Morin, G.; Wang, Y.; Foster, A.L.; Juillot, F.; Calas, G.; Brown, G.E. XANES evidence for rapid arsenic(III) oxidation at magnetite and ferrihydrite surfaces by dissolved O2 via Fe2+-mediated reactions. Environ. Sci. Technol. 2010, 44, 5416–5422. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Guo, H.; Xiu, W. Arsenite oxidation and arsenic adsorption on birnessite in the absence and the presence of citrate or EDTA. Environ. Sci. Pollut. Res. 2020, 27, 43769–43785. [Google Scholar] [CrossRef]
- Jiang, X.; Peng, C.; Fu, D.; Chen, Z.; Shen, L.; Li, Q.; Ouyang, T.; Wang, Y. Removal of arsenate by ferrihydrite via surface complexation and surface precipitation. Appl. Surf. Sci. 2015, 353, 1087–1094. [Google Scholar] [CrossRef]
- Jain, A.; Loeppert, R.H. Effect of competing anions on the adsorption of arsenate and arsenite by ferrihydrite. J. Environ. Qual. 2000, 29, 1422–1430. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Xu, H.; Zhao, W.; Qi, W.; Hao, R.; Zhang, J.; Li, D. Adsorption of As(V) and P(V) by magnetic iron-based alginate-chitosan beads: Competitive adsorption and reduction mechanism of As(V) induced by Fe(II). Colloids Surf. A 2023, 675, 132068. [Google Scholar] [CrossRef]
- Cornell, R.M.; Giovanoli, R.; Schneider, W. Review of the hydrolysis of iron(III) and the crystallization of amorphous iron(III) hydroxide hydrate. J. Chem. Technol. Biotechnol. 1989, 46, 115–134. [Google Scholar] [CrossRef]
- Ji, Y.; Luo, W.; Lu, G.; Fan, C.; Tao, X.; Ye, H.; Xie, Y.; Shi, Z.; Yi, X.; Dang, Z. Effect of phosphate on amorphous iron mineral generation and arsenic behavior in paddy soils. Sci. Total Environ. 2019, 657, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Shan, H.; Huang, J.; Zeng, C.; Zhang, J.; Liu, Y. Experiment on the influence of flow velocity and medium particle size on As migration. Earth Sci. 2022, 1–14. Available online: http://kns.cnki.net/kcms/detail/42.1874.P.20220907.1732.004.html (accessed on 14 March 2024).
- Chen, H.; Shan, H.; Peng, S.; Huang, J.; Liao, D.; Yan, Z. Hydrochemical influences on arsenic adsorption by river sand. Acta Sci. Circumstantiae 2021, 41, 2727–2739. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Kang, S.; Li, Y.; Zhang, Q. Formation of active Fe(OH)3 in situ for enhancing arsenic removal from water by the oxidation of Fe (II) in air with the presence of CaCO3. J. Clean. Prod. 2019, 227, 1–9. [Google Scholar] [CrossRef]
- Wajda, A.; Goldmann, W.H.; Detsch, R.; Boccaccini, A.R.; Sitarz, M. Influence of zinc ions on structure, bioactivity, biocompatibility and antibacterial potential of melt-derived and gel-derived glasses from CaO-SiO2 system. J. Non Cryst. Solids 2019, 511, 86–99. [Google Scholar] [CrossRef]
- Sun, S.; Wen, K.; Yang, B.; Zhou, Q.; Dong, F.; Nie, X.; Liu, L.; Fan, S. The preparation and adsorption properties of novel active carbon/diatomite. Acta Petrol. Mineral. 2013, 32, 941–946. [Google Scholar]
- Wang, Y.; Yu, W.; Chang, Z.; Gao, C.; Yang, Y.; Zhang, B.; Wang, Y.; Xing, B. Effects of dissolved organic matter on the adsorption of norfloxacin on a sandy soil (fraction) from the Yellow River of Northern China. Sci. Total Environ. 2022, 848, 157495. [Google Scholar] [CrossRef]
- Zhang, L.; Song, L.; Zheng, X.; Teng, Y.; Wang, J. The remobilization of heavy metals influenced by interaction of DOM and iron oxides. Chin. J. Ecol. 2014, 33, 2193–2198. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Y.; Chen, X.; Xu, S.; Chen, J.; Zhang, Q.; Zhao, B.; Ning, P. Promoting effect of SO42- functionalization on the performance of Fe2O3 catalyst in the selective catalytic reduction of NOx with NH3. J. Fuel Chem. Technol. 2020, 48, 584–593. [Google Scholar] [CrossRef]
- Xie, Q.; Ma, X.; Hadiya, A.; Nurmamat, X.; Zhao, Z. Effect of humic acid on the adsorption of dimethylarsinic acid by magnetite. Environ. Chem. 2023, 42, 658–670. [Google Scholar]
- Zhu, Y.; Peng, L.; Chen, S.; Feng, Y.; Xia, J.; Wang, W.; Chen, L.; Yin, H.; Zhou, M.; Hou, Z. Constructing all-in-one graphene-based supercapacitors for electrochemical energy storage via interface integration strategy. Carbon Lett. 2023, 33, 873–882. [Google Scholar] [CrossRef]
- Xi, Y.; Liao, T.; Li, J.; Zhang, L. Mechanism of zero-valent lead reduction for removing high concentration of arsenic from waste acid of lead smelting system. Process Saf. Environ. Prot. 2021, 156, 244–255. [Google Scholar] [CrossRef]
- Sudhakar, C.; Mukherjee, S.; Kumar, A.A.; Paramasivam, G.; Meena, P.K.; Nonappa; Pradeep, T. Interference of phosphate in adsorption of arsenate and arsenite over confined metastable two-line ferrihydrite and magnetite. J. Phys. Chem. C 2021, 125, 22502–22512. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, G.; Liu, C.; Li, J.; Zheng, T.; Ma, J.; Wang, L.; Jiang, J.; Zhai, X. Enhanced removal of arsenite and arsenate by a multifunctional Fe-Ti-Mn composite oxide: Photooxidation, oxidation and adsorption. Water Res. 2018, 147, 264–275. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, J.; Han, Y.; Li, Y.; Zhu, Y. Effect of natural oxidation on floatability of pyrite and its mechanism. Met. Mine 2019, 111–114. Available online: http://dx.chinadoi.cn/10.19614/j.cnki.jsks.201902021 (accessed on 15 March 2024).
- Tong, J.; Luo, Y.; Liu, C. Effect of sulfide on arsenic-bearing ferrihydrite and arsenic-bearing alumina. Environ. Sci. Technol. 2022, 45, 99–105. [Google Scholar]
- Liu, Z.; Zhang, Q.; Ma, H.; Liu, J. Spectral characteristics of Hangjin2# clay and its mechanism in heterogeneous fenton reaction. Spectrosc. Spectr. Anal. 2021, 41, 3512–3517. [Google Scholar]
- Liu, H.; Wei, Y.; Sun, Y. The Formation of hematite from ferrihydrite using Fe(II) as a catalyst. J. Mol. Catal. A Chem. 2005, 226, 135–140. [Google Scholar] [CrossRef]
- Li, Y.; Gao, X.; Zhang, X.; Luo, W.; Hu, Q. Geochemistry of arsenic in sediments and groundwater in areas with arsenic polluted groundwater in Yuncheng Basin. Saf. Environ. Eng. 2017, 24, 68–74. [Google Scholar]
- Li, Y.; Gao, X.; Zhang, X.; Luo, W.; Hu, Q. Arsenic enrichment mechanism of groundwater in the western Hetao Plain. Bull. Mineral. Petrol. Geochem. 2023, 42, 289–297. [Google Scholar] [CrossRef]



| Components | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | MgO | MnO | Na2O | TiO2 |
|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 90.69 | 5.91 | 1.51 | 0.32 | 1.62 | 0.15 | 0.06 | 0.14 | 0.17 |
| Group | Simulation Buffer Composition | As Concentration (mg/L) | Fe Concentration (mg/L) |
|---|---|---|---|
| A-1 | Fe(II) | 0 | 0.1 |
| A-2 | Fe(III) | 0 | 0.1 |
| B-1 | As(III) | 1 | 0 |
| B-2 | As(V) | 1 | 0 |
| C | As(III)_Fe(II) | 1 | 0.1, 1, 5, 20 |
| D | As(III)_Fe(III) | 1 | 0.1, 1, 5, 20 |
| E | As(V)_Fe(II) | 1 | 0.1, 1, 5, 20 |
| F | As(V)_Fe(III) | 1 | 0.1, 1, 5, 20 |
| Group | Simulation Buffer Composition | pH Value | ||||
|---|---|---|---|---|---|---|
| 0.1 (mg/L) Fe(aq) | 1 (mg/L) Fe(aq) | 5 (mg/L) Fe(aq) | 20 (mg/L) Fe(aq) | |||
| C | As(III)_Fe(II) | before | 6.12 | 6.12 | 5.89 | 5.72 |
| after | 5.56 | 6.81 | 6.38 | 4.50 | ||
| D | As(III)_Fe(III) | before | 6.20 | 6.01 | 5.84 | 5.69 |
| after | 5.40 | 7.48 | 6.38 | 5.27 | ||
| E | As(V)_Fe(II) | before | 5.98 | 4.40 | 3.57 | 3.18 |
| after | 5.53 | 6.77 | 5.47 | 3.04 | ||
| F | As(V)_Fe(III) | before | 6.01 | 4.71 | 3.84 | 3.31 |
| after | 5.56 | 6.99 | 6.48 | 3.48 | ||
| Condition | C | O | Si | Al | Fe | As |
|---|---|---|---|---|---|---|
| Before reaction | 4.47 | 52.14 | 42.45 | 0.21 | 0.73 | 0 |
| 0 mg/L Fe(aq) + As(III) | 3.17 | 58.82 | 22.79 | 1.12 | 0 | 0.16 |
| 0 mg/L Fe(aq) + As(V) | 0.47 | 41.48 | 57.34 | 0.60 | 0 | 0.11 |
| 0.1 mg/L Fe(II) + As(III) | 4.35 | 54.35 | 40.12 | 0.08 | 1.06 | 0.05 |
| 0.1 mg/L Fe(III) + As(III) | 13.65 | 38.56 | 46.07 | 0.19 | 1.44 | 0.10 |
| 0.1 mg/L Fe(II) + As(V) | 7.52 | 60.13 | 32.22 | 0.07 | 0.07 | 0 |
| 0.1 mg/L Fe(III) + As(V) | 8.42 | 36.00 | 55.29 | 0.18 | 0.11 | 0 |
| 20 mg/L Fe(II) + As(III) | 6.40 | 56.88 | 33.62 | 0.68 | 2.20 | 0.22 |
| 20 mg/L Fe(II) + As(V) | 11.80 | 55.76 | 31.39 | 0.30 | 0.66 | 0.09 |
| 20 mg/L Fe(III) + As(III) | 5.60 | 55.35 | 33.29 | 2.69 | 2.80 | 0.27 |
| 20 mg/L Fe(III) + As(V) | 8.20 | 56.32 | 31.65 | 1.19 | 2.22 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Peng, S.; Shan, H.; Liao, Q.; Zhou, H.; Zhao, Z. The Influence of Aqueous Iron on River Sand’s Arsenic Adsorption: Characteristics and Mechanisms. Water 2024, 16, 1107. https://doi.org/10.3390/w16081107
Li Z, Peng S, Shan H, Liao Q, Zhou H, Zhao Z. The Influence of Aqueous Iron on River Sand’s Arsenic Adsorption: Characteristics and Mechanisms. Water. 2024; 16(8):1107. https://doi.org/10.3390/w16081107
Chicago/Turabian StyleLi, Zheying, Sanxi Peng, Huimei Shan, Qian Liao, Hai Zhou, and Zhicheng Zhao. 2024. "The Influence of Aqueous Iron on River Sand’s Arsenic Adsorption: Characteristics and Mechanisms" Water 16, no. 8: 1107. https://doi.org/10.3390/w16081107
APA StyleLi, Z., Peng, S., Shan, H., Liao, Q., Zhou, H., & Zhao, Z. (2024). The Influence of Aqueous Iron on River Sand’s Arsenic Adsorption: Characteristics and Mechanisms. Water, 16(8), 1107. https://doi.org/10.3390/w16081107





