Transport of Floating Plastics through the Fluvial Vector: The Impact of Riparian Zones
Abstract
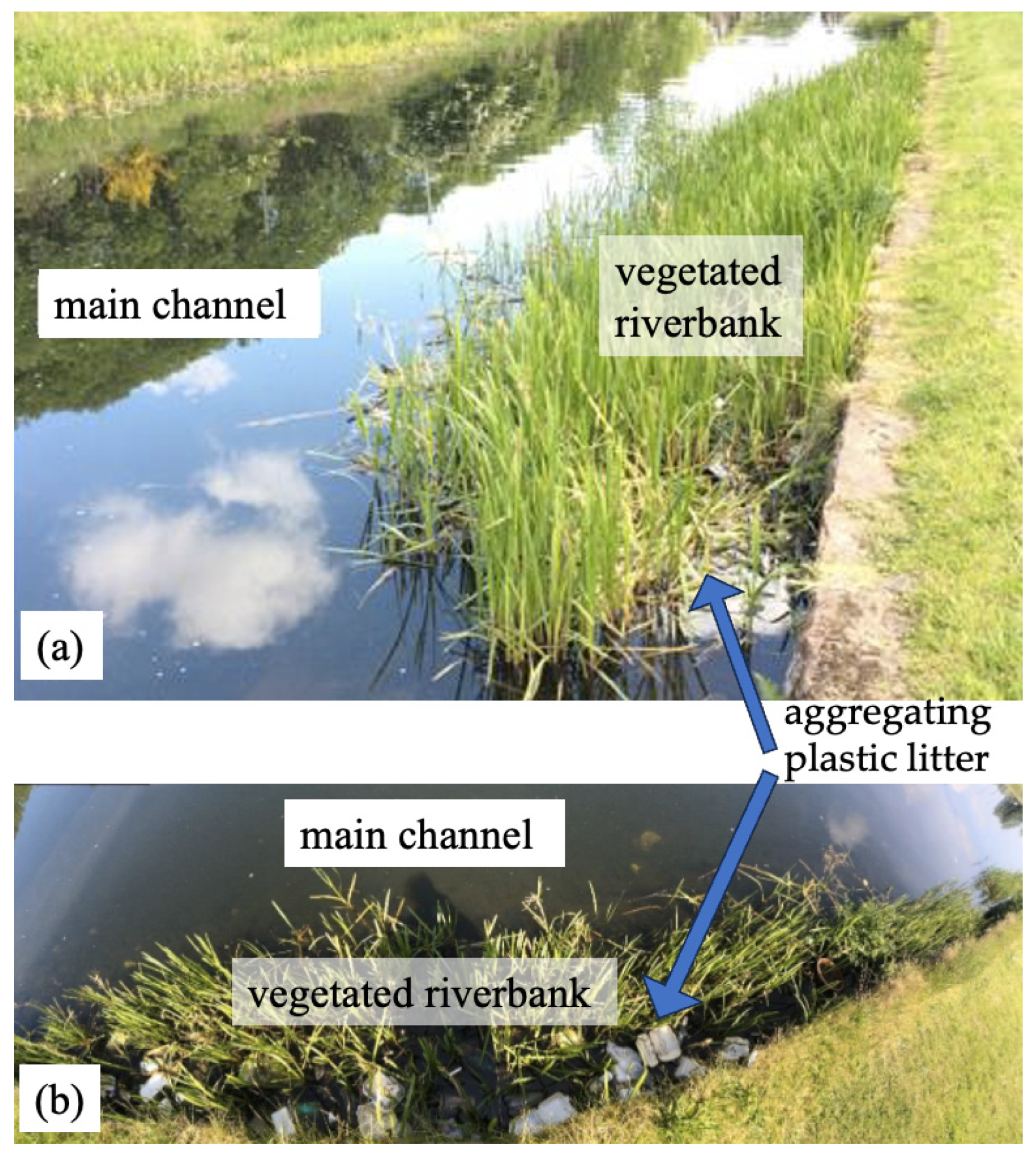
1. Introduction
2. Materials and Methods
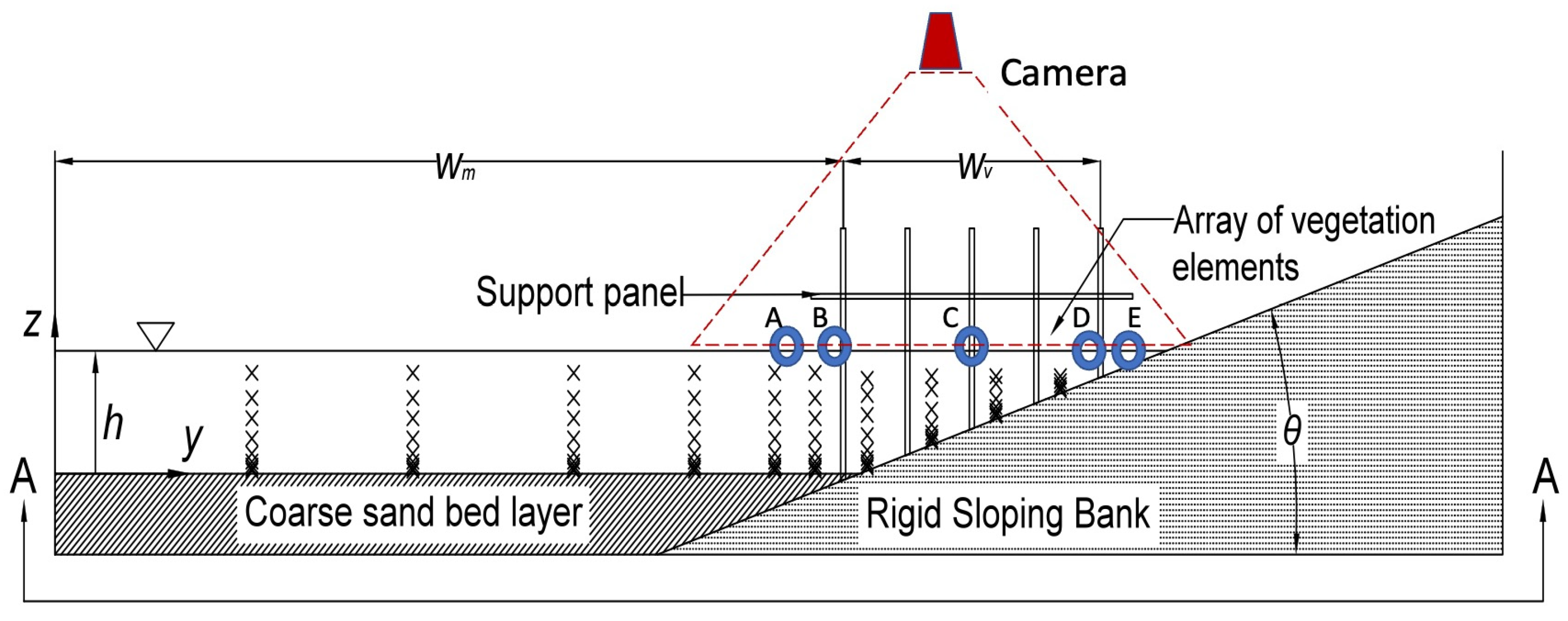
2.1. Experimental Design
2.2. Experimental Matrix
2.2.1. Trajectory Analysis of Floating Macro-Plastics
2.2.2. Acoustic Doppler Velocimetry
2.3. Field Campaigns
3. Results and Discussion
3.1. Qualitative Description of Transport Mechanics
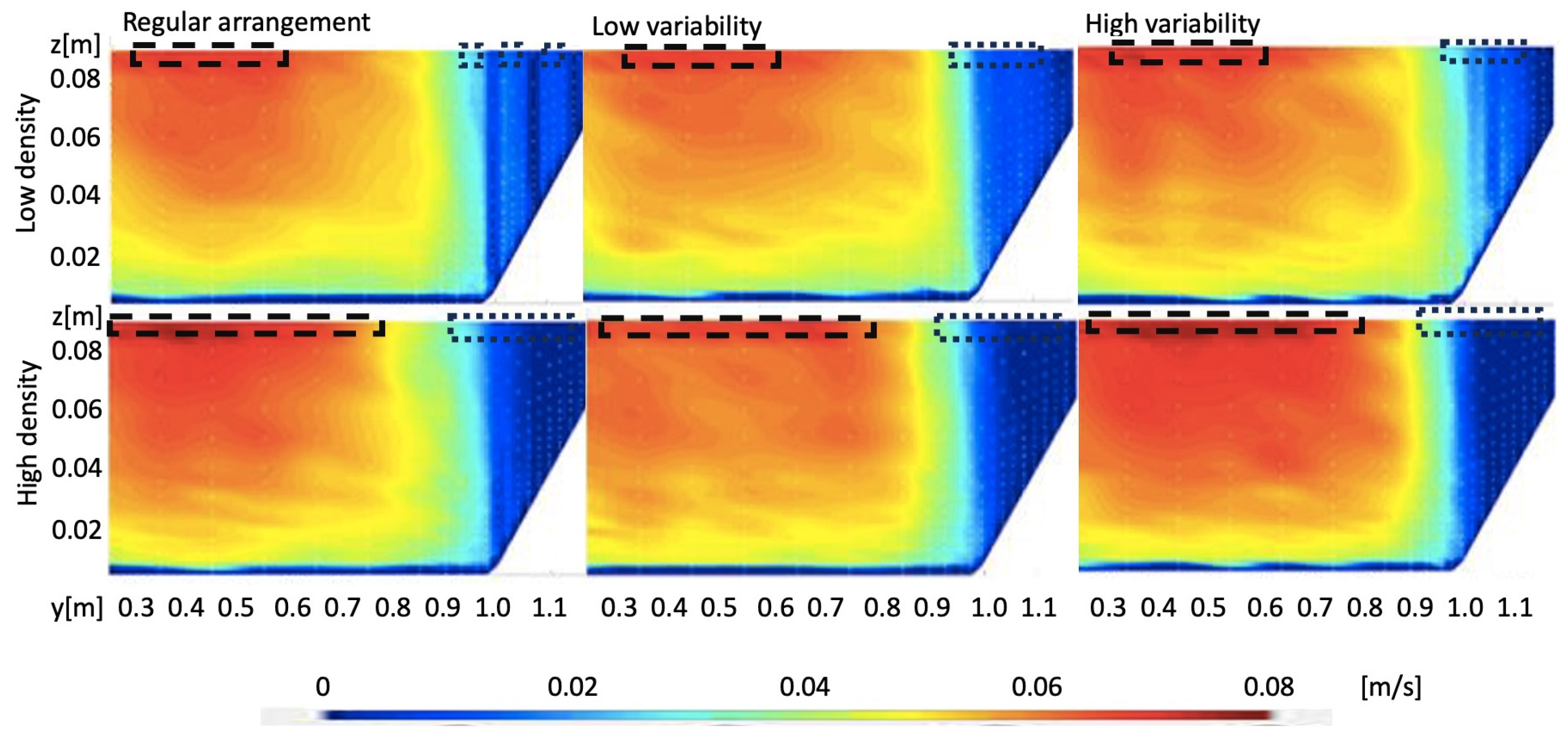
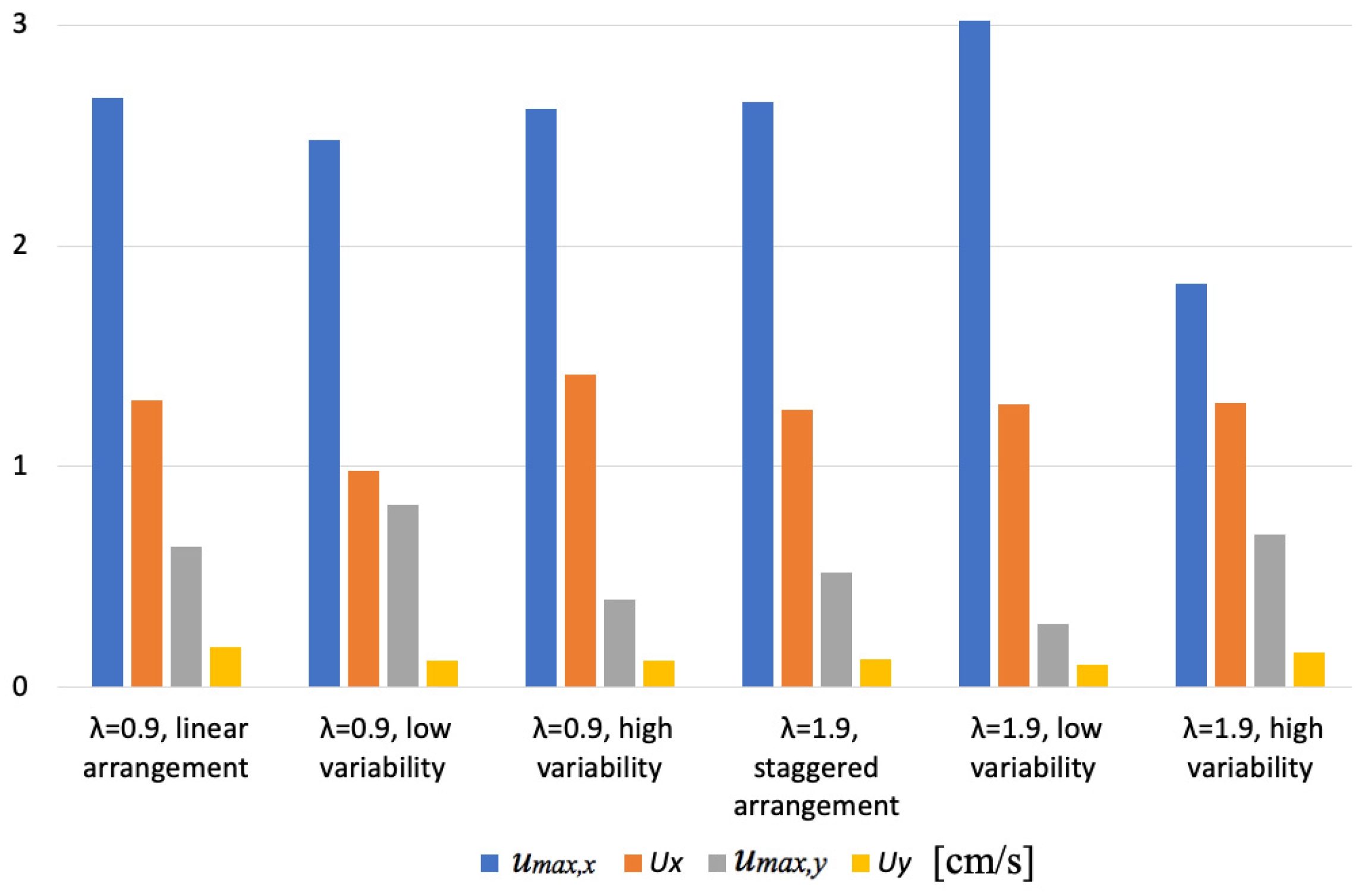
3.2. Transport Velocity
3.2.1. Effect of Riparian Vegetation Density and Arrangement
3.2.2. Effect of Size and Shape
3.2.3. Effect of Release Location
3.3. Rate of Rotation
3.4. Implications for Fluvial Storage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zulkernain, N.H.; Gani, P.; Chuck Chuan, N.; Uvarajan, T. Utilisation of plastic waste as aggregate in construction materials: A review. Constr. Build. Mater. 2021, 296, 123669. [Google Scholar] [CrossRef]
- Nelms, S.E.; Galloway, T.S.; Godley, B.J.; Jarvis, D.S.; Lindeque, P.K. Investigating microplastic trophic transfer in marine top predators. Environ. Pollut. 2018, 238, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.K.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Carney Almroth, B.M.; Åström, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N.-K. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. 2018, 25, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.M.; Waldron, S.; Phoenix, V.; Gauchotte-Lindsay, C. Micro- and Nanoplastic Pollution of Freshwater and Wastewater Treatment Systems. Springer Sci. Rev. 2017, 5, 19–30. [Google Scholar] [CrossRef]
- Schmaltz, E.; Melvin, E.C.; Diana, Z.; Gunady, E.F.; Rittschof, D.; Somarelli, J.A.; Virdin, J.; Dunphy-Daly, M.M. Plastic pollution solutions: Emerging technologies to prevent and collect marine plastic pollution. Environ. Int. 2020, 144, 106067. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, P.; Xu, R.; Wang, X.; Lei, L.; Schartup, A.T.; Peng, Y.; Pang, Q.; Wang, X.; Mai, L.; et al. Plastic waste discharge to the global ocean constrained by seawater observations. Nat. Commun. 2023, 14, 1372. [Google Scholar] [CrossRef] [PubMed]
- Talbot, R.; Chang, H. Microplastics in freshwater: A global review of factors affecting spatial and temporal variations. Environ. Pollut. 2022, 292, 118393. [Google Scholar] [CrossRef]
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean Plastic Soup: Synthetic polymers in Mediterranean surface waters. Sci. Rep. 2016, 6, 37551. [Google Scholar] [CrossRef]
- Lebreton, L.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef]
- Range, D.; Scherer, C.; Stock, F.; Ternes, T.A.; Hoffmann, T.O. Hydro-geomorphic perspectives on microplastic distribution in freshwater river systems: A critical review. Water Res. 2023, 245, 120567. [Google Scholar] [CrossRef] [PubMed]
- Gündoğdu, S.; Çevik, C.; Ayat, B.; Aydoğan, B.; Karaca, S. How microplastics quantities increase with flood events? An example from Mersin Bay NE Levantine coast of Turkey. Environ. Pollut. 2018, 239, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Meijer, L.J.J.; van Emmerik, T.; van der Ent, R.; Schmidt, C.; Lebreton, L. More than 1000 rivers account for 80% of global riverine plastic emissions into the ocean. Sci. Adv. 2021, 7, eaaz5803. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Krauth‚, T.; Wagner, S. Export of Plastic Debris by Rivers into the Sea. Environ. Sci. Technol. 2017, 51, 12246–12253. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Belay, B.S.; Moreno-Rodenas, A.; Kramer, M.; Franca, M.J. The key role of surface tension in the transport and quantification of plastic pollution in rivers. Water Res. 2022, 226, 119078. [Google Scholar] [CrossRef] [PubMed]
- Lofty, J.; Valero, D.; Wilson, C.A.M.E.; Franca, M.J.; Ouro, P. Microplastic and natural sediment in bed load saltation: Material does not dictate the fate. Water Res. 2023, 243, 120329. [Google Scholar] [CrossRef] [PubMed]
- van Emmerik, T.; Strady, E.; Kieu-Le, T.-C.; Nguyen, L.; Gratiot, N. Seasonality of riverine macroplastic transport. Sci. Rep. 2019, 9, 13549. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Sutherland, B.R.; DiBenedetto, M.; Kaminski, A.; van den Bremer, T. Fluid dynamics challenges in predicting plastic pollution transport in the ocean: A perspective. Phys. Rev. Fluids 2023, 8, 070701. [Google Scholar] [CrossRef]
- Morritt, D.; Stefanoudis, P.V.; Pearce, D.; Crimmen, O.A.; Clark, P.F. Plastic in the Thames: A river runs through it. Mar. Pollut. Bull. 2014, 78, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Nelms, S.E.; Coombes, C.; Foster, L.C.; Galloway, T.S.; Godley, B.J.; Lindeque, P.K.; Witt, M.J. Marine anthropogenic litter on British beaches: A 10-year nationwide assessment using citizen science data. Sci. Total Environ. 2017, 579, 1399–1409. [Google Scholar] [CrossRef]
- Horton, A.A.; Svendsen, C.; Williams, R.J.; Spurgeon, D.J.; Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK—Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017, 114, 218–226. [Google Scholar] [CrossRef]
- Gasperi, J.; Dris, R.; Bonin, T.; Rocher, V.; Tassin, B. Assessment of floating plastic debris in surface water along the Seine River. Environ. Pollut. 2014, 195, 163–166. [Google Scholar] [CrossRef] [PubMed]
- van Emmerik, T.; Roebroek, C.; De Winter, W.; Vriend, P.; Boonstra, M.; Hougee, M. Riverbank macrolitter in the Dutch Rhine–Meuse delta. Environ. Res. Lett. 2020, 15, 104087. [Google Scholar] [CrossRef]
- van Emmerik, T.; Vriend, P.; Roebroek, J. An Evaluation of the River-OSPAR Method for Quantifying Macrolitter on Dutch Riverbanks; Wageningen University: Wageningen, The Netherlands, 2008; p. 86. [Google Scholar] [CrossRef]
- Graca, B.; Bełdowska, M.; Wrzesień, P.; Zgrundo, A. Styrofoam debris as a potential carrier of mercury within ecosystems. Environ. Sci. Pollut. Res. 2014, 21, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Heo, N.W.; Hong, S.H.; Han, G.M.; Hong, S.; Lee, J.; Song, Y.K.; Jang, M.; Shim, W.J. Distribution of small plastic debris in cross-section and high strandline on Heungnam beach, South Korea. Ocean Sci. J. 2013, 48, 225–233. [Google Scholar] [CrossRef]
- Maes, T.; Van der Meulen, M.D.; Devriese, L.I.; Leslie, H.A.; Huvet, A.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastics Baseline Surveys at the Water Surface and in Sediments of the North-East Atlantic. Front. Mar. Sci. 2017, 4, 135. [Google Scholar] [CrossRef]
- Liu, D.; Valyrakis, M.; Williams, R.J. Flow Hydrodynamics across Open Channel Flows with Riparian Zones: Implications for Riverbank Stability. Water 2017, 9, 720. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Yagci, O.; Celik, F.; Kitsikoudis, V.; Ozgur Kirca, V.S.; Hodoglu, C.; Valyrakis, M.; Duran, Z.; Kaya, S. Scour patterns around individual vegetation elements. Adv. Water Resour. 2016, 97, 251–265. [Google Scholar] [CrossRef]
- Valyrakis, M.; Liu, D.; Turker, U.; Yagci, O. The role of increasing riverbank vegetation density on flow dynamics across an asymmetrical channel. Environ. Fluid Mech. 2021, 21, 643–666. [Google Scholar] [CrossRef]
- Xu, Y.; Valyrakis, M.; Gilja, G.; Michalis, P.; Yagci, O.; Przyborowski, L. Assessing riverbed surface destabilization risk downstream isolated vegetation elements. Water 2022, 14, 2880. [Google Scholar] [CrossRef]
- Valyrakis, M.; Al Hinai, J.K.; Liu, D. Transport of floating plastics along a channel with a vegetated riverbank. In Proceedings of the 12th International Symposium on Ecohydraulics ISE 2018, Tokyo, Japan, 19–24 August 2018. [Google Scholar]
- Nepf, H.M. Drag, turbulence, and diffusion in flow through emergent vegetation. Water Resour. Res. 1999, 35, 479–489. [Google Scholar] [CrossRef]
- Abeynayaka, A.; Kojima, F.; Miwa, Y.; Ito, N.; Nihei, Y.; Fukunaga, Y.; Yashima, Y.; Itsubo, N. Rapid Sampling of Suspended and Floating Microplastics in Challenging Riverine and Coastal Water Environments in Japan. Water 2020, 12, 1903. [Google Scholar] [CrossRef]
- Pasquier, G.; Doyen, P.; Carlesi, N.; Amara, R. An innovative approach for microplastic sampling in all surface water bodies using an aquatic drone. Heliyon 2022, 8, e11662. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Savino, I.; Pojar, I.; Massarelli, C.; Uricchio, V.F. A Practical Overview of Methodologies for Sampling and Analysis of Microplastics in Riverine Environments. Sustainability 2020, 12, 6755. [Google Scholar] [CrossRef]
- van Emmerik, T.; Schwarz, A. Plastic debris in rivers. WIREs Water 2020, 7, e1398. [Google Scholar] [CrossRef]
- de Lange, S.I.; Mellink, Y.; Vriend, P.; Tasseron, P.F.; Begemann, F.; Hauk, R.; Aalderink, H.; Hamers, E.; Jansson, P.; Joosse, N.; et al. Sample size requirements for riverbank macrolitter characterization. Front. Water 2023, 4, 1085285. [Google Scholar] [CrossRef]
- Chowdhury, Z.; Yokota, K.; Nguyen, M.N.; Inoue, T. Bank Accumulation of Macroplastic Samples in a Farmland River in Japan. Water Air Soil Pollut. 2023, 12, 234. [Google Scholar] [CrossRef]
- Cowger, W.; Markley, L.A.T.; Moore, S.; Gray, A.B.; Upadhyay, K.; Koelmans, A.A. How many microplastics do you need to (sub)sample? Ecotoxicol. Environ. Saf. 2024, 275, 116243. [Google Scholar] [CrossRef]
- Valyrakis, M.; Diplas, P.; Dancey, C.L. Entrainment of coarse grains in turbulent flows: An extreme value theory approach. Water Resour. Res. 2011, 47, W09512. [Google Scholar] [CrossRef]
- Valyrakis, M.; Diplas, P.; Dancey, C.L. Entrainment of coarse particles in turbulent flows: An energy approach. J. Geophys. Res. Earth Surf. 2013, 118, 42–53. [Google Scholar] [CrossRef]
- Courtene-Jones, W.; Quinn, B.; Gary, S.F.; Mogg, A.O.M.; Narayanaswamy, B.E. Microplastic pollution identified in deep-sea water and ingested by benthic invertebrates in the Rockall Trough, North Atlantic Ocean. Environ. Pollut. 2017, 231, 271–280. [Google Scholar] [CrossRef]
- Jodeau, M.; Hauet, A.; Paquier, A.; Le Coz, J.; Dramais, G. Application and evaluation of LS-PIV technique for the monitoring of river surface velocities in high flow conditions. Flow Meas. Instrum. 2008, 19, 117–127. [Google Scholar] [CrossRef]
- Gunawan, B.; Sun, X.; Sterling, M.; Shiono, K.; Tsubaki, R.; Rameshwaran, P.; Knight, D.W.; Chandler, J.H.; Tang, X.; Fujita, I. The application of LS-PIV to a small irregular river for inbank and overbank flows. Flow Meas. Instrum. 2012, 24, 1–12. [Google Scholar] [CrossRef]
- Lewis, Q.W.; Rhoads, B.L. Resolving two-dimensional flow structure in rivers using large-scale particle image velocimetry: An example from a stream confluence. Water Resour. Res. 2015, 51, 7977–7994. [Google Scholar] [CrossRef]
- Laxague, N.J.M.; Özgökmen, T.M.; Haus, B.K.; Novelli, G.; Shcherbina, A.; Sutherland, P.; Guigand, C.M.; Lund, B.; Mehta, S.; Alday, M.; et al. Observations of Near-Surface Current Shear Help Describe Oceanic Oil and Plastic Transport. Geophys. Res. Lett. 2018, 45, 245–249. [Google Scholar] [CrossRef]
- Fuchs, Y.; Scherbaum, S.; Huber, R.; Rüther, N.; Hartlieb, A. Removing Plastic Waste from Rivers: A Prototype-Scale Experimental Study on a Novel River-Cleaning Concept. Water 2024, 16, 248. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valyrakis, M.; Gilja, G.; Liu, D.; Latessa, G. Transport of Floating Plastics through the Fluvial Vector: The Impact of Riparian Zones. Water 2024, 16, 1098. https://doi.org/10.3390/w16081098
Valyrakis M, Gilja G, Liu D, Latessa G. Transport of Floating Plastics through the Fluvial Vector: The Impact of Riparian Zones. Water. 2024; 16(8):1098. https://doi.org/10.3390/w16081098
Chicago/Turabian StyleValyrakis, Manousos, Gordon Gilja, Da Liu, and Gaston Latessa. 2024. "Transport of Floating Plastics through the Fluvial Vector: The Impact of Riparian Zones" Water 16, no. 8: 1098. https://doi.org/10.3390/w16081098
APA StyleValyrakis, M., Gilja, G., Liu, D., & Latessa, G. (2024). Transport of Floating Plastics through the Fluvial Vector: The Impact of Riparian Zones. Water, 16(8), 1098. https://doi.org/10.3390/w16081098








