Detection of Autumnal Concentration of Coscinodiscus granii in the Southern Baltic—A Method for In Situ Measurement of Marine Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levassseur, M.; Therriault, J.C.; Legendre, L. Hierarchical control of phytoplankton succession by physical factors. Mar. Ecol. Prog. Ser. 1984, 19, 211–222. [Google Scholar] [CrossRef]
- Sommer, U. Nutrient status and nutrient competition of phytoplankton in a shallow, hypertrophic lake. Limnol. Oceanogr. 1989, 34, 1162–1173. [Google Scholar] [CrossRef]
- Gilabert, J. Seasonal plankton dynamics in a Mediterianian hypersaline coastal lagoon: The Mar Menor. J. Plankton Res. 2001, 23, 207–217. [Google Scholar] [CrossRef]
- Lau, S.S.S.; Lane, S.N. Biological and chemical factors influencing shallow lake eutrophication: A long term study. Sci. Total Environ. 2002, 288, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Kownacka, J.; Całkiewicz, J.; Kornijów, R. A turning point in the development of phytoplankton in the Vistula Lagoon (southern Baltic Sea) at the beginning of the 21st century. Oceanologia 2020, 62, 538–555. [Google Scholar] [CrossRef]
- Wielgat-Rychert, M.; Jarosiewicz, A.; Ficek, D.; Pawlik, M.; Rychert, K. Nutrient Fluxes and Their Impact on the Phytoplankton in a Shallow Coastal Lake. Pol. J. Environ. Stud. 2015, 24, 751–759. [Google Scholar] [CrossRef]
- Witek, B. Short-Term Fluctuations of Phytoplankton in the Coastal Zone of the Gulf of Gdansk; University of Gdańsk Publishing House: Gdańsk, Poland, 2010. (In Polish) [Google Scholar]
- Wasmund, N.; Pollehne, F.; Postel, L.; Siegel, H.; Zettler, M.L. Biologische Zustandseinschätzung der Ostsee im Jahre 2010; Meereswissenschaftliche Berichte No. 85; Institut für Ostseeforschung: Roctock, Germany, 2011; pp. 3–87. [Google Scholar]
- Konik, M.; Bradtke, K.; Stoń-Egiert, J.; Soja-Woźniak, M.; Sliwińska-Wilczewska, S.; Darecki, M. Cyanobacteria Index as a Tool for the Satellite Detection of Cyanobacteria Blooms in the Baltic Sea. Remote Sens. 2023, 15, 1601. [Google Scholar] [CrossRef]
- Witek, B.; Pliński, M. The first recorded bloom of Prorocentrum minimum (Pavillard) Schiller in the coastal zone of the Gulf of Gdańsk. Oceanologia 2000, 42, 433–446. [Google Scholar]
- Lund-Hansen, L.C. Development and dynamics of a coastal sub-surface phytoplankton bloom in the southwest Kattegat, Baltic Sea. Oceanologia 2006, 48, 29–36. [Google Scholar]
- Pliński, M.; Simm, A. Seasonal fluctuations in the composition, distribution and quantity of phytoplankton in the Vistula Lagoon in 1974 and 1975. Stud. Mater. Oceanol. Biol. 1978, 4, 53–80. (In Polish) [Google Scholar]
- Wasmund, N.; Dutz, J.; Pollehne, F.; Siegel, H.; Zettler, M.L. Biological Assessment of the Baltic Sea 2015; Meereswissenschaftliche Berichte No. 102; Leibniz-Institut für Ostseeforschung Warnemünde: Roctock, Germany, 2016. [Google Scholar] [CrossRef]
- Wasmund, N.; Dutz, J.; Pollehne, F.; Siegel, H.; Zettler, M.L. Biological Assessment of the Baltic Sea 2016; Meereswissenschaftliche Berichte No. 105; Leibniz-Institut für Ostseeforschung Warnemünde: Roctock, Germany, 2017. [Google Scholar] [CrossRef]
- Wasmund, N.; Dutz, J.; Pollehne, F.; Siegel, H.; Zettler, M.L. Biological Assessment of the Baltic Sea 2017; Meereswissenschaftliche Berichte No. 108; Leibniz-Institut für Ostseeforschung Warnemünde: Roctock, Germany, 2018. [Google Scholar] [CrossRef]
- Wasmund, N.; Dutz, J.; Kremp, A.; Zettler, M.L. Biological Assessment of the Baltic Sea 2018; Meereswissenschaftliche Berichte No. 112; Leibniz-Institut für Ostseeforschung Warnemünde: Roctock, Germany, 2019. [Google Scholar] [CrossRef]
- Kaczmarek, S.; Woźniak, B. The application of the optical classification of waters in the Baltic Sea (Case 2 Waters). Oceanologia 1995, 37, 285–297. [Google Scholar]
- Kowalczuk, P. Seasonal variability of yellow substance absorption in the surface layer of the Baltic Sea. J. Geophys. Res. 1999, 104, 30047–30058. [Google Scholar] [CrossRef]
- Woźniak, S.B.; Meler, J.; Lednicka, B.; Zdun, A.; Stoń-Egiert, J. Inherent optical properties of suspended particulate matter in the southern Baltic Sea. Oceanologia 2011, 53, 691–729. [Google Scholar]
- Jonasz, M. Nonsphericity of suspended marine particles and its influence on light scattering. Limnol. Oceanogr. 1987, 32, 1059–1065. [Google Scholar] [CrossRef]
- Jennings, B.R.; Parslow, K. Particle size measurement: The equivalent spherical diameter. Proc. R. Soc. Lond. 1988, 419, 137–149. [Google Scholar]
- Lee Karp-Boss, L.; Azevedo, L.; Boss, E. LISST-100 measurements of phytoplankton size distribution: Evaluation of the effects of cell shape. Limnol. Oceanogr. Methods 2007, 5, 396–406. [Google Scholar] [CrossRef]
- Anglès, A.; Jordi, A.; Garcés, E.; Masó, M.; Basterretxea, G. High-resolution spatio-temporal distribution of a coastal phytoplankton bloom using laser in situ scattering and transmissometry (LISST). Harmful Algae 2008, 7, 808–816. [Google Scholar] [CrossRef]
- Serra, T.; Colomer, J.; Cristina, X.P.; Vila, X.; Arellano, J.B.; Casamitjana, X. Evaluation of laser in-situ scattering instrument for measuring the concentration of phytoplankton, purpule sulfur bacteria, and suspended inorganic sediments in lakes. J. Environ. Eng. 2001, 127, 1023–1030. [Google Scholar] [CrossRef]
- Serra, T.; Casamitjana, X.; Colomer, J.; Granata, T.C. Observations of the particle size distribution and concentration in a coastal system using an in situ laser analyzer. Mar. Technol. Soc. J. 2002, 36, 59–69. [Google Scholar] [CrossRef]
- Pawlik, M.; Ficek, D. Pine pollen grains in coastal waters of the Baltic Sea. Int. J. Oceanol. Hydrobiol. Stud. 2016, 45, 35–41. [Google Scholar] [CrossRef]
- Pawlik, M.M.; Ficek, D. Spatial Distribution of Pine Pollen Grains Concentrations as a Source of Biologically Active Substances in Surface Waters of the Southern Baltic Sea. Water 2023, 15, 978. [Google Scholar] [CrossRef]
- Agrawal, Y.C.; Pottsmith, H.C. Instruments for particle size and settling velocity observations in sediment transport. Mar. Geol. 2000, 168, 89–114. [Google Scholar] [CrossRef]
- Edler, L. Phytoplankton and Chlorophyll: Recommendations on Methods for Marine Biological Studies in the Baltic Sea. Balt. Mar. Biol. Publ. 1979, 5, 38. [Google Scholar]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt Int. Verein. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- HELCOM. Manual for Marine Monitoring in the COMBINE Programme of HELCOM, Part C. Programme for Monitoring of Eutrophication and Its Effects, Annex C-6: Phytoplankton Species Composition, Abundance and Biomass; Baltic Marine Environment Protection Commission: Helsinki, Finland, 2001. [Google Scholar]
- Hällfors, G. Checklist of Baltic Sea Phytoplankton Species (Including Some Heterotrophic Protistan Groups)—Balt; Baltic Sea Environment Proceedings No. 95; Baltic Marine Environment Protection Commission: Helsinki, Finland, 2004; p. 208. [Google Scholar]
- Olenina, I.; Hajdu, S.; Edler, L.; Andersson, A.; Wasmund, N.; Busch, S.; Göbel, J.; Gromisz, S.; Huseby, S.; Huttunen, M.; et al. Biovolumes and size-classes of phytoplankton in the Baltic Sea. In HELCOM Baltic Sea Environment Proceedings; Baltic Marine Environment Protection Commission: Helsinki, Finland, 2006; No. 106; p. 144. [Google Scholar]
- Dutz, J.; Kremp, A.; Zettler, M.L. Biological assessment of the Baltic Sea 2020. In Meereswissenschaftliche Berichte; Leibniz-Institut für Ostseeforschung Warnemünde: Roctock, Germany, 2022; Volume 120. [Google Scholar] [CrossRef]
- Gromisz, S.; Witek, Z. Main phytoplankton assemblages in the Gulf of Gdańsk and the Pomeranian Bay from 1994 to 1997. Bull. Sea Fish. Inst. 2011, 2, 31–51. [Google Scholar]
- Sournia, A. Phytoplankton Manual. Monographs on Oceanographic Methodology 6; UNESCO: Paris, France, 1978. [Google Scholar]
- Chisholm, S.W. Phytoplankton size. In Primary Productivity and Biogeochemical Cycles in the Sea; Falkowski, P.G., Woodhead, A.D., Vivirito, K., Eds.; Springer Science & Business Media: Boston, MA, USA, 1992; pp. 213–237. [Google Scholar]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Sieburth, J.M.; Smetacek, V.; Lenz, J. Pelagic ecosystem structure: Heterotrophic copartments of the plankton and their relationship to plankton size fractions. Limnol. Oceanogr. 1978, 23, 1256–1263. [Google Scholar] [CrossRef]
- Löptien, U.; Dietze, H. Retracing cyanobacteria blooms in the Baltic Sea. Sci. Rep. 2022, 12, 10873. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.J.; Schoeman, D.S. Climate impact on plankton ecosystems in the northeast Atlantic. Science 2004, 305, 1609–1612. [Google Scholar] [CrossRef]
- Rousseaux, C.S.; Gregg, W.W. Recent decadal trends in global phytoplankton composition. Glob. Biogeochem. Cycles 2015, 29, 1674–1688. [Google Scholar] [CrossRef]
- Martin-Garcia, G.M. Oceanic impact on European climate changes during the quaternary. Geosciences 2019, 9, 119. [Google Scholar] [CrossRef]
- Kahru, M.; Elmgren, R.; Savchuk, O.P. Changing seasonality of the Baltic Sea. Biogeosciences 2016, 13, 1009–1018. [Google Scholar] [CrossRef]
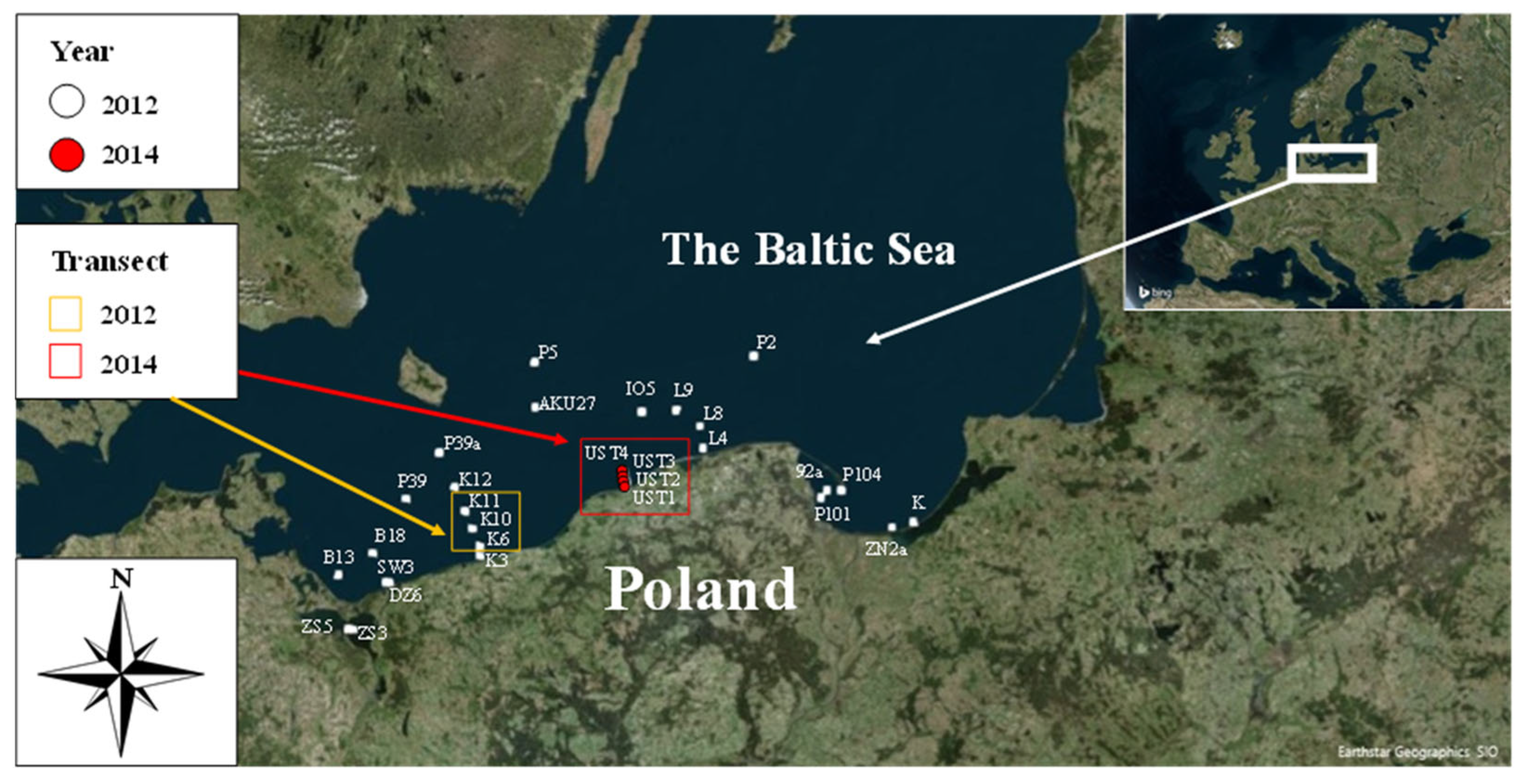
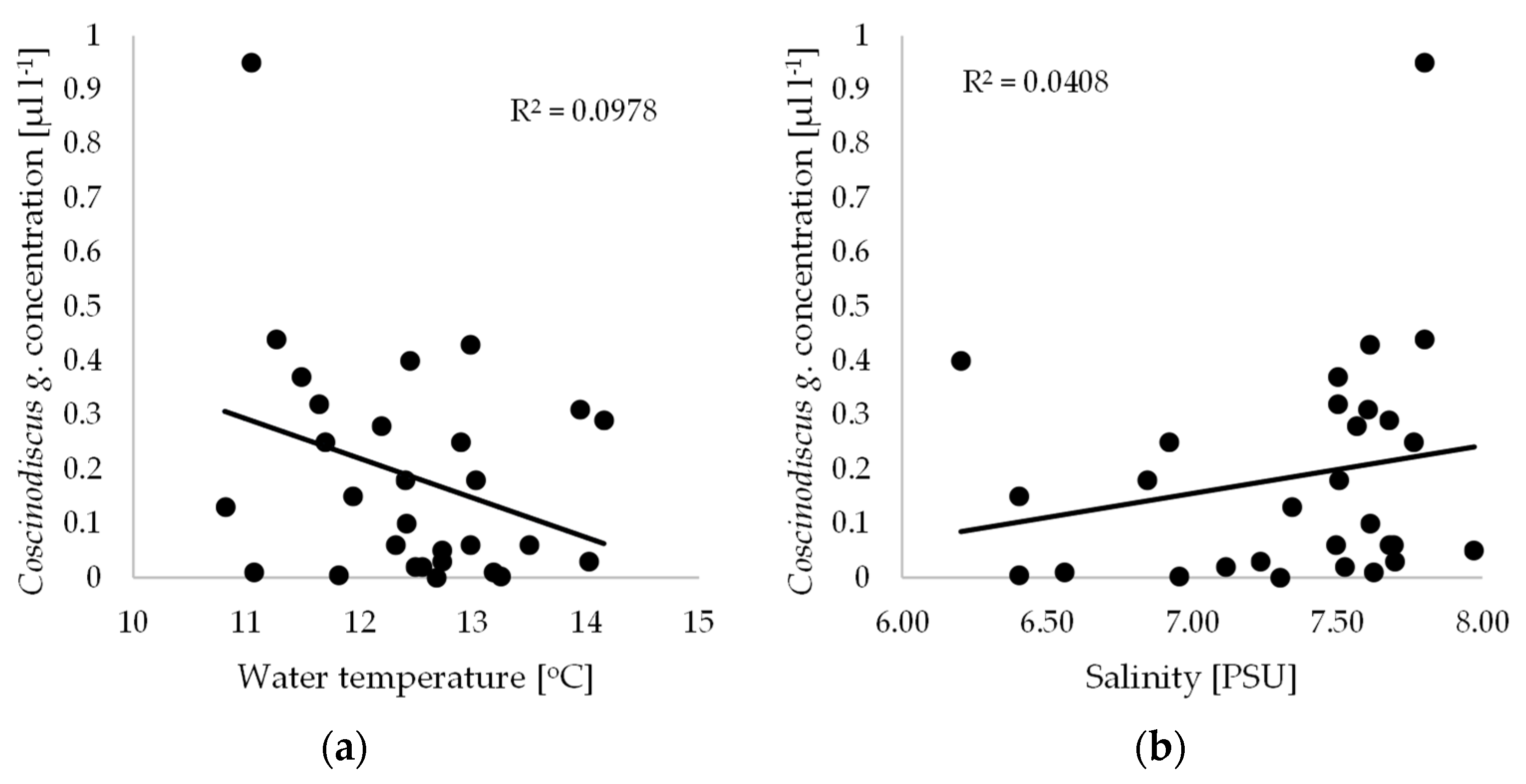
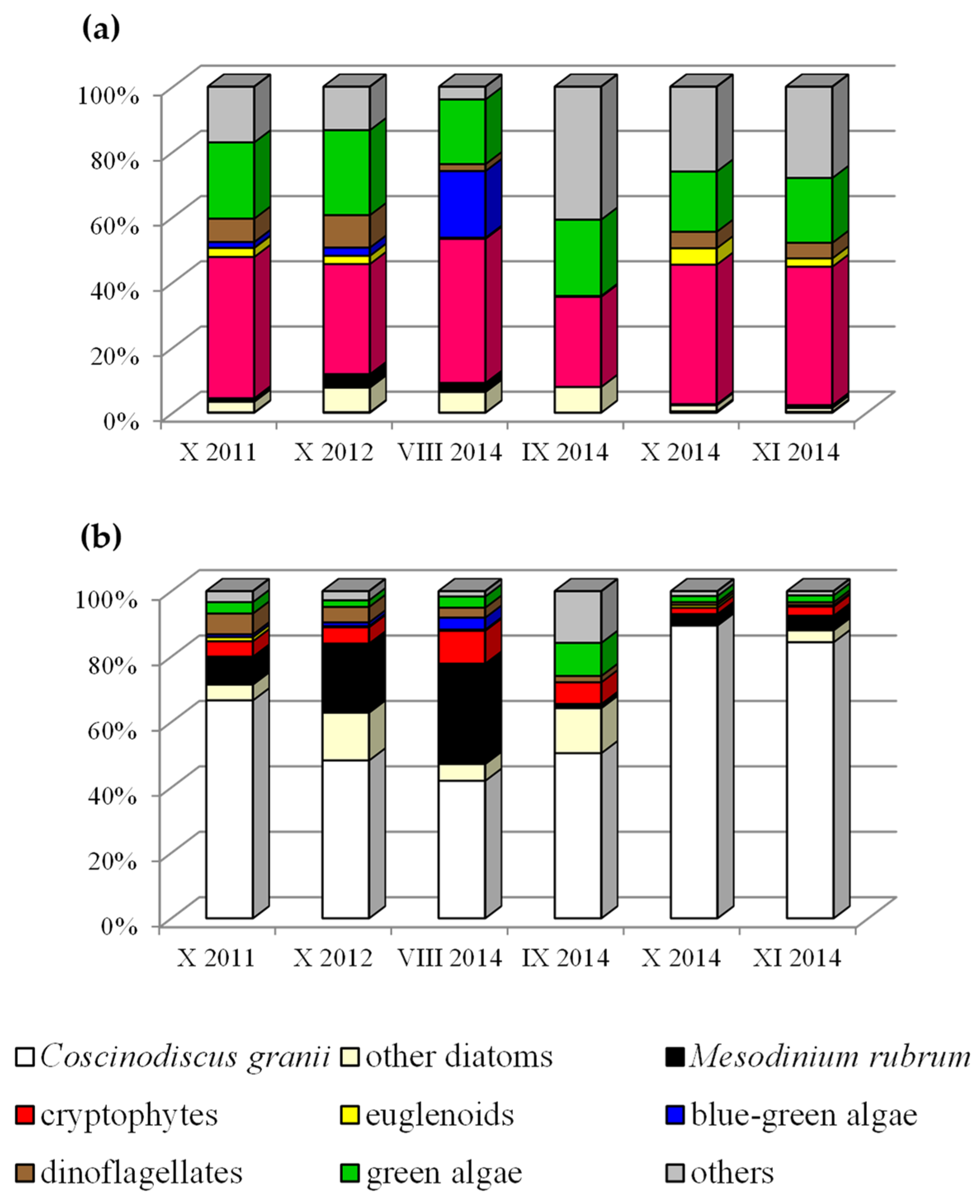
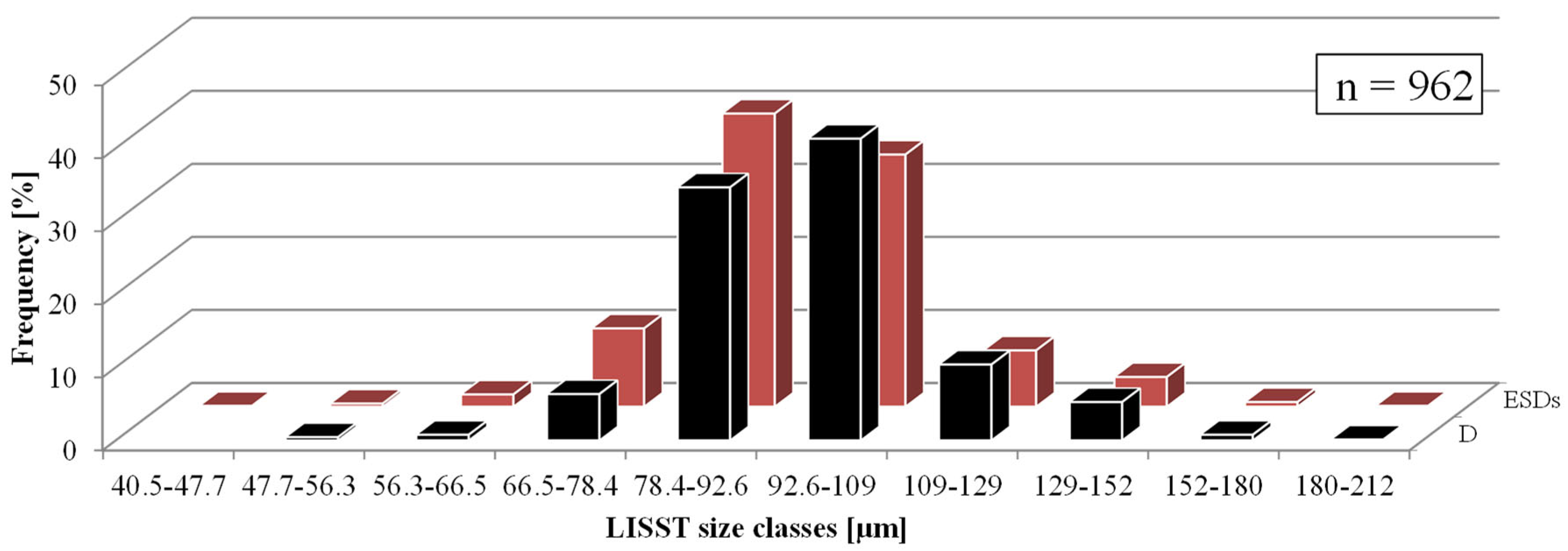
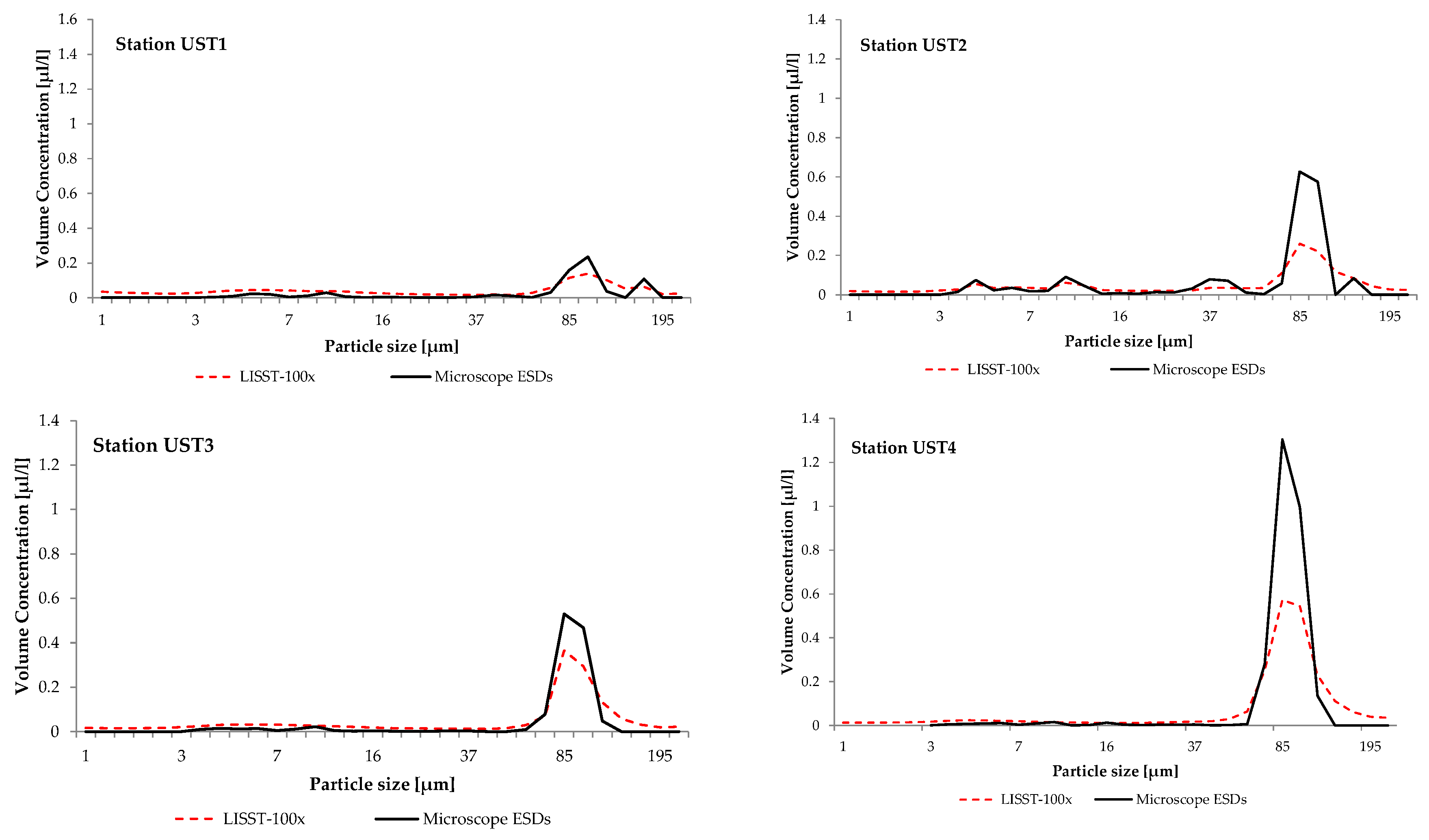
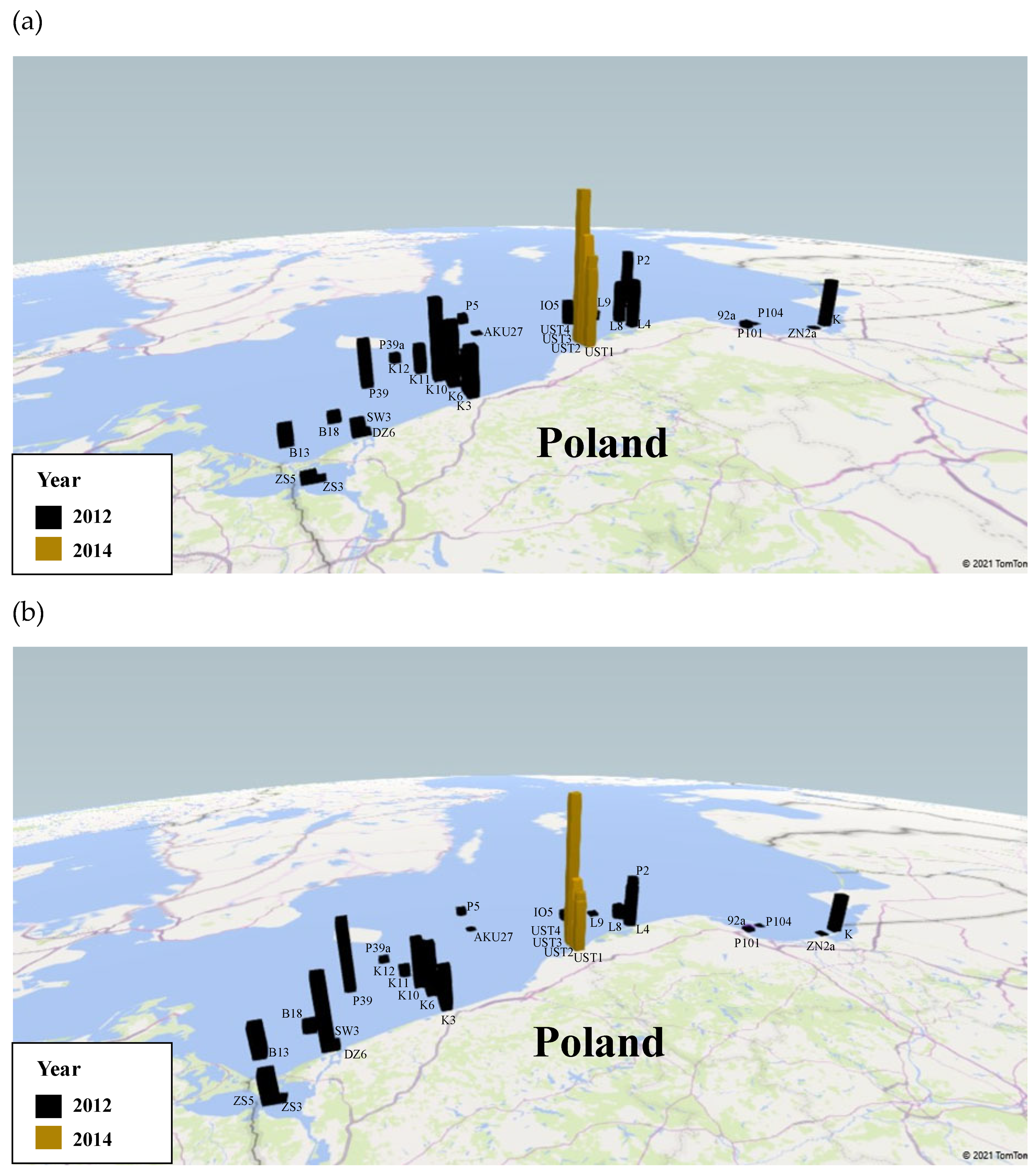
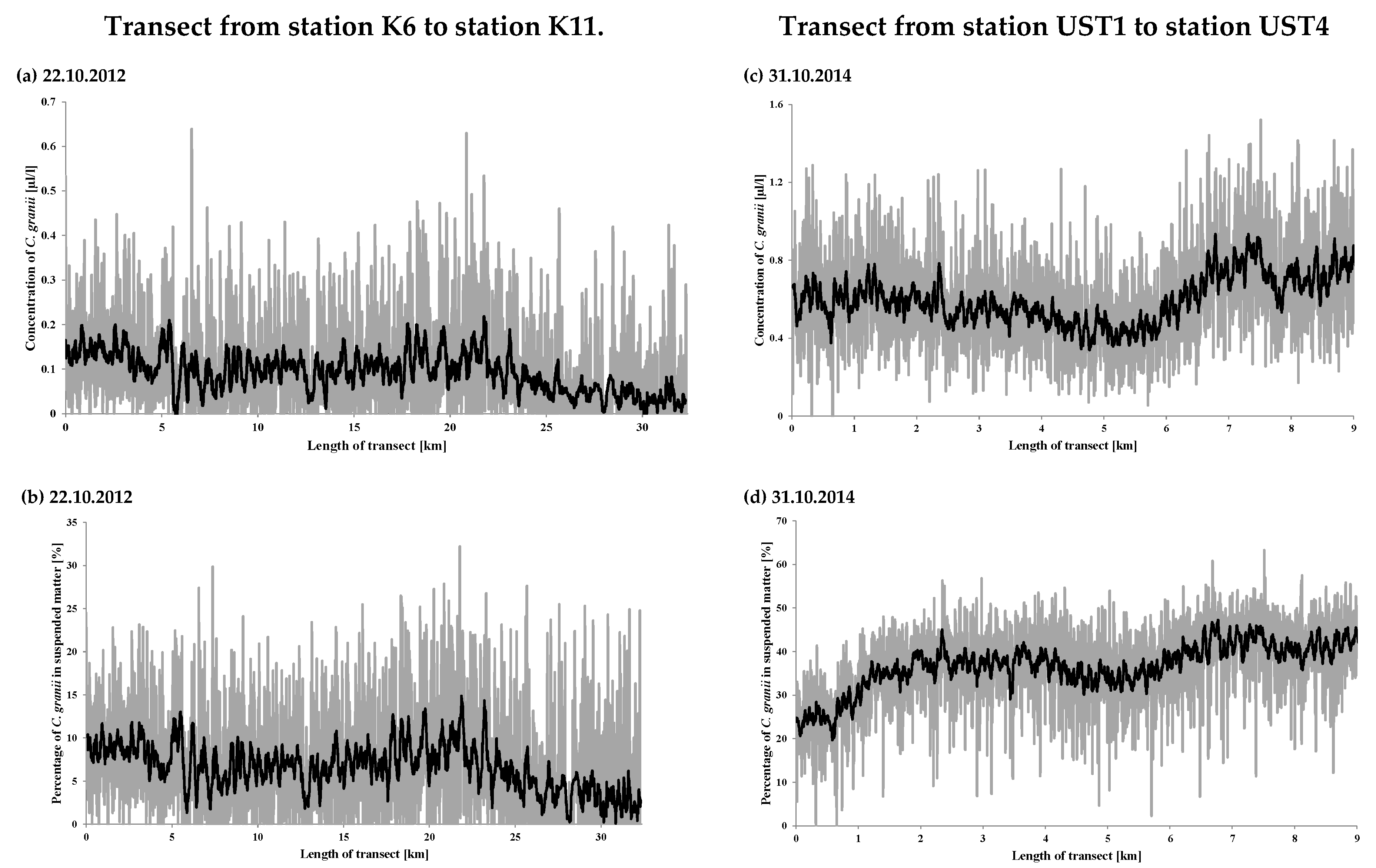
| Ship | Sampling Month | Number of Samples Collected for Species Indentification | Total | |
|---|---|---|---|---|
| s/y Oceania | October | 2011 | 9 | 49 |
| October | 2012 | 25 | ||
| Fishing boat “Sea Angel” | August | 2014 | 3 | |
| September | 2014 | 4 | ||
| October | 2014 | 4 | ||
| November | 2014 | 4 | ||
| Month | Year | Central Arkona Basin | Eastern Arkona Basin | Bornholm Basin | Southern Gotland Basin | Eastern Gotland Basin | References |
|---|---|---|---|---|---|---|---|
| XI | 2010 | 78.4 | 84.9 | 96.5 | 93.5 | 91.7 | [8] |
| XI | 2015 | 93.5 | 95.5 | 67.9 | no date | 50.6 | [13] |
| XI | 2016 | 8.4 | 7.4 | 55.8 | 79.8 | 81.2 | [14] |
| XI | 2017 | 5.7 | 9.7 | 50.5 | 12.3 | no date | [15] |
| XI | 2018 | No date | 64.0 | 77.1 | 94.8 | 79.7 | [16] |
| XI | 2020 | No date | No date | 19.5 | The Gotland basin > 70.0 | [34] | |
| Gulf of Gdańsk and Pomeranian Bay | |||||||
| X/XI | 1994 1997 | 73.2 | [35] | ||||
| Southern Baltic Sea | |||||||
| X | 2011 | 58.0 | Own research | ||||
| X | 2012 | 48.0 | Own research | ||||
| VIII/IX | 2014 | 49.0 | Own research | ||||
| X/XI | 2014 | 86.5 | Own research | ||||
| Diametr [µm] | Equivalent Spherical Diameter [µm] | |
|---|---|---|
| Minimum value | 48.0 | 47.4 |
| Maximum value | 188.0 | 188.0 |
| Average value | 97.3 | 89.7 |
| Median | 94.4 | 89.4 |
| Standard deviation | 17.2 | 14.9 |
| Station Symbol | Latitiude | Longitude | Date of Sampling | Absolute Concentration of Coscinodiscus spp. [µL L−1] | Relative Concentration of Coscinodiscus spp. [%] |
|---|---|---|---|---|---|
| AKU27 | 54°99.995 N | 15°99.975 E | 21 October 2012 | 0.01 | 0.85 |
| P5 | 55°14.395 N | 15°59.107 E | 21 October 2012 | 0.05 | 3.49 |
| K3 | 54°12.466 N | 15°31.972 E | 21 October 2012 | 0.25 | 14.76 |
| K6 | 54°15.380 N | 15°31.910 E | 22 October 2012 | 0.18 | 12.28 |
| K10 | 54°34.028 N | 15°17.027 E | 22 October 2012 | 0.31 | 19.43 |
| K11 | 54°26.499 N | 15°22.969 E | 22 October 2012 | 0.29 | 24.77 |
| K12 | 54°34.026 N | 15°17.019 E | 22 October 2012 | 0.06 | 8.73 |
| P39 | 54°74.255 N | 15°13.175 E | 22 October 2012 | 0.03 | 2.71 |
| P39a | 54°29.485 N | 14°50.460 E | 22 October 2012 | 0.43 | 14.01 |
| B13 | 54°03.985 N | 14°14.983 E | 24 October 2012 | 0.18 | 5.01 |
| B18 | 54°11.976 N | 14°33.277 E | 24 October 2012 | 0.06 | 2.30 |
| DZ6 | 54°02.512 N | 14°43.050 E | 24 October 2012 | 0.03 | 0.89 |
| SW3 | 53°57.073 N | 14°15.770 E | 24 October 2012 | 0.40 | 3.90 |
| ZS3 | 53°46.822 N | 14°21.685 E | 25 October 2012 | 0.15 | 1.66 |
| ZS5 | 53°46.798 N | 14°24.538 E | 25 October 2012 | 0.005 | 0.07 |
| IO5 | 54°59.470 N | 16°58.588 E | 27 October 2012 | 0.06 | 8.80 |
| L4 | 54°48.150 N | 17°32.489 E | 27 October 2012 | 0.28 | 17.38 |
| L9 | 55°00.343 N | 17°29.085 E | 27 October 2012 | 0.02 | 3.68 |
| L8 | 54°55.209 N | 17°30.620 E | 27 October 2012 | 0.10 | 15.11 |
| P101 | 54°32.543 N | 18°36.553 E | 28 October 2012 | 0.02 | 2.30 |
| 92a | 54°35.057 N | 18°40.001 E | 28 October 2012 | 0.002 | 0.35 |
| P104 | 54°34.895 N | 18°47.440 E | 28 October 2012 | 0.0002 | 0.03 |
| P2 | 55°17.501 N | 18°00.200 E | 29 October 2012 | 0.13 | 21.51 |
| ZN2a | 54°23.029 N | 19°15.036 E | 30 October 2012 | 0.01 | 0.54 |
| K | 54°24.522 N | 19°26.573 E | 30 October 2012 | 0.25 | 17.68 |
| UST4 | 54°40.422 N | 16°49.433 E | 31 October 2014 | 0.95 | 51.32 |
| UST3 | 54°38.085 N | 16°50.014 E | 31 October 2014 | 0.44 | 36.93 |
| UST2 | 54°36.701 N | 16°51.430 E | 31 October 2014 | 0.37 | 30.50 |
| UST1 | 54°35.453 N | 16°50.685 E | 31 October 2014 | 0.32 | 26.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik, M.M.; Ficek, D. Detection of Autumnal Concentration of Coscinodiscus granii in the Southern Baltic—A Method for In Situ Measurement of Marine Particles. Water 2024, 16, 1091. https://doi.org/10.3390/w16081091
Pawlik MM, Ficek D. Detection of Autumnal Concentration of Coscinodiscus granii in the Southern Baltic—A Method for In Situ Measurement of Marine Particles. Water. 2024; 16(8):1091. https://doi.org/10.3390/w16081091
Chicago/Turabian StylePawlik, Magdalena M., and Dariusz Ficek. 2024. "Detection of Autumnal Concentration of Coscinodiscus granii in the Southern Baltic—A Method for In Situ Measurement of Marine Particles" Water 16, no. 8: 1091. https://doi.org/10.3390/w16081091
APA StylePawlik, M. M., & Ficek, D. (2024). Detection of Autumnal Concentration of Coscinodiscus granii in the Southern Baltic—A Method for In Situ Measurement of Marine Particles. Water, 16(8), 1091. https://doi.org/10.3390/w16081091





