COVID-19 Pandemic Modulates the Environmental Contamination Level of Enteric Bacteria from WWTPs
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Microbiological Analysis
2.3. Statistically Analysis
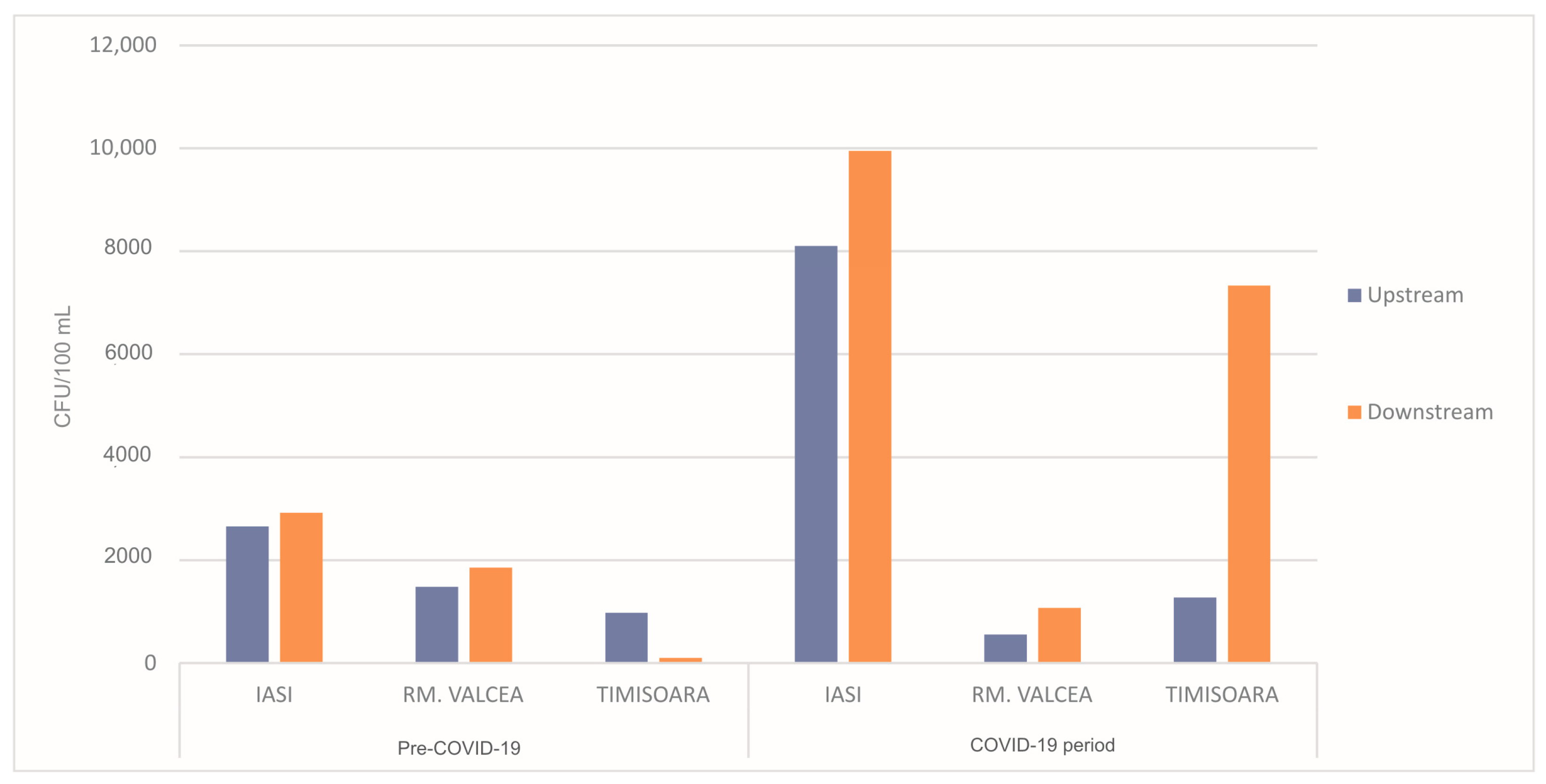
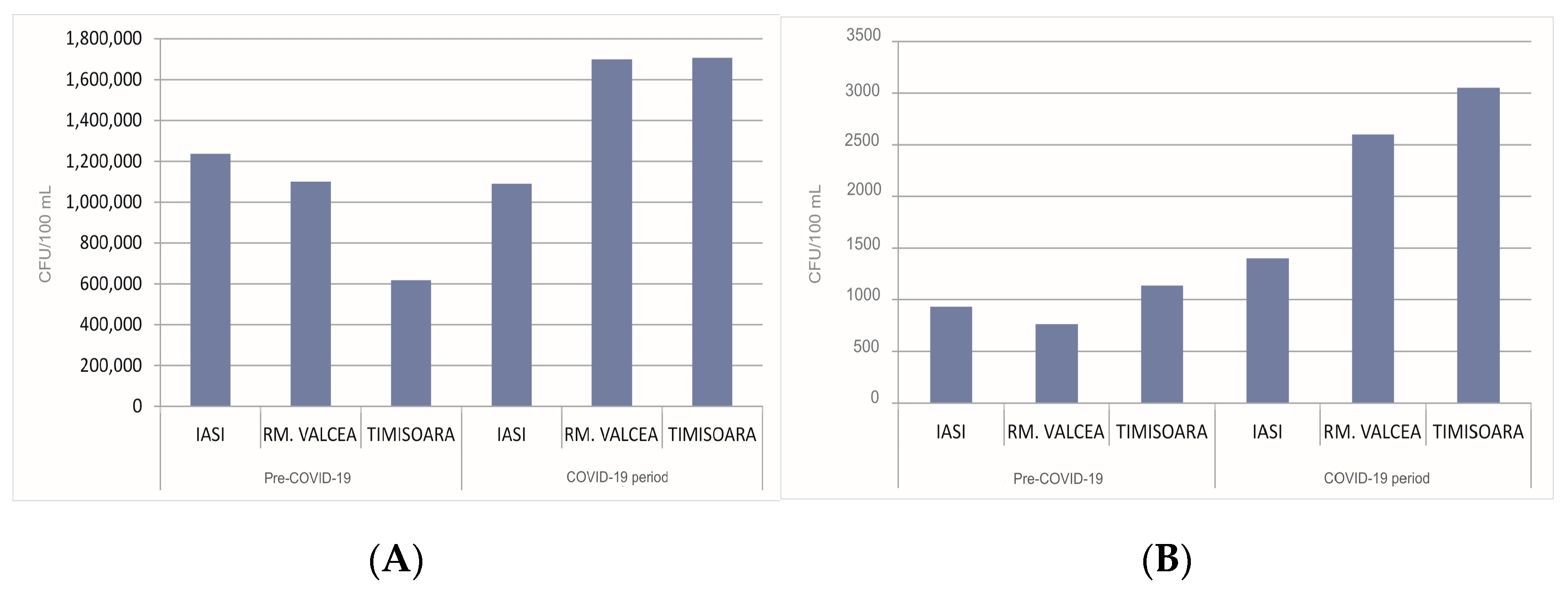
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cappelli, F.; Longoni, O.; Rigato, J.; Rusconi, M.; Sala, A.; Fochi, I.; Palumbo, M.T.; Polesello, S.; Roscioli, C.; Salerno, F.; et al. Suspect screening of wastewaters to trace anti-COVID-19 drugs: Potential adverse effects on aquatic environment. Sci. Total Environ. 2022, 824, 153756. [Google Scholar] [CrossRef] [PubMed]
- Boeras, I.; Burcea, A.; Banaduc, D.; Florea, D.I.; Curtean-Banaduc, A. Lotic ecosystem sediment microbial communities’ resilience to the impact of wastewater effluents in a polluted European Hotspot Mures Basin (Transylvania, Romania). Water 2024, 16, 402. [Google Scholar] [CrossRef]
- Osuolale, O.; Okoh, A. Human enteric bacteria and viruses in five wastewater treatment plants in the Eastern Cape, South Africa. J. Infect. Public Health 2017, 10, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Mare, R.; Mare, C.; Hadarean, A.; Hotupan, A.; Rus, T. COVID-19 and water variables: Review and scientometric analysis. Int. J. Environ. Res. Public Health 2023, 20, 957. [Google Scholar] [CrossRef] [PubMed]
- Zaneti, R.N.; Girardi, V.; Spilki, F.R.; Mena, K.; Campos-Westphalen, A.P.; da Costa-Colares, E.R.; Pozzebon, A.G.; Etchepare, R.G. Quantitative microbial risk assessment of SARS-CoV-2 for workers in wastewater treatment plants. Sci. Total Environ. 2021, 754, 142163. [Google Scholar] [CrossRef] [PubMed]
- Banciu, A.R.; Pascu, L.F.; Radulescu, D.M.; Stoica, C.; Gheorghe, S.; Lucaciu, I.; Ciobotaru, F.V.; Novac, L.; Manea, C. The COVID-19 pandemic impact of hospital wastewater on aquatic systems in Bucharest. Water 2024, 16, 245. [Google Scholar] [CrossRef]
- Johar, A.A.; Salih, M.A.; Abdelrahman, H.A.; Al Mana, H.; Hadi, H.A.; Eltai, N.O. Watewater-based epidemiology for tracking bacterial diversity and antibiotic resistance in COVID-19 isolation hospitals in Qatar. J. Hosp. Infect. 2023, 141, 209–220. [Google Scholar] [CrossRef]
- Yazdian, H.; Jamshidi, S. Performance evaluation of wastewater treatment plants under the sewage variations imposed by COVID-19 spread prevention action. J. Environ. Health Sci. Eng. 2021, 19, 1613–1621. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Z.; Fang, W.; Gao, G. Composition of bacterial communities in municipal wastewater treatment plant. Sci. Total Environ. 2019, 689, 1181–1191. [Google Scholar] [CrossRef]
- Makuwa, S.; Tiou, M.; Fosso-Kankeu, E.; Green, E. Evaluation of fecal coliform prevalence and physicochemical indicators in the effluent from a wastewater treatment plant in the North-West Province, South Africa. Int. J. Environ. Res. Public Health 2020, 17, 6381. [Google Scholar] [CrossRef]
- Aguilar-Salazar, A.; Martinez-Vazquez, A.V.; Aguilera-Arreola, G.; de Luna-Santillana, E.J.; Cruz-Hernandez, M.A.; Escobedo-Bonilla, C.M.; Lara-Ramirez, E.; Sanchez-Sanchez, M.; Guerrero, A.; Rivera, G.; et al. Prevalence of ESKAPE bacteria in surface water and wastewater sources: Multidrug resistance and molecular characterization, an updated review. Water 2023, 15, 3200. [Google Scholar] [CrossRef]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE pathogens in the environment: Antibiotic resistance status, community-acquired infection and risk to human health. Int. J. Hyg. Environ. 2022, 244, 114006. [Google Scholar] [CrossRef]
- Loyola-Cruz, M.A.; Gonzales-Avila, L.U.; Martinez-Trejo, A.; Saldana-Padilla, A.; Hernandez-Cortez, C.; Bello-Lopez, J.M.; Castro-Escarpulli, G. ESKAPE and beyond: The burden of coinfections in the COVID-19 pandemic. Pathogens 2023, 12, 743. [Google Scholar] [CrossRef]
- Gheorghe-Barbu, I.; Czobor-Barbu, I.; Popa, L.I.; Gradisteanu-Pircalaboiu, G.; Popa, M.; Marutescu, L.; Nita-Lazar, M.; Banciu, A.; Stoica, C.; Gheorghe, S.; et al. Temporo-spatial variations in resistance determinants and clonality of Acinetobacter baumannii and Pseudomonas aeruginosa strains from Romanian hospitals and wastewaters. Antimicrob. Resist. Infect. Control. 2022, 11, 115. [Google Scholar] [CrossRef]
- Popa, L.P.; Gheorghe, I.; Czobor Barbu, I.; Surleac, M.; Paraschiv, S.; Marutescu, L.; Popa, M.; Gradisteanu-Pircalaboiu, G.; Talapan, D.; Nita-Lazar, M.; et al. Multidrug resistant Klebsiella pneumoniae ST101 Clone survival chain from inpatients to hospital effluent after chlorine treatment. Front. Microbiol. 2021, 11, 610296. [Google Scholar] [CrossRef]
- Rodriguez-Molina, D.; Mang, P.; Schmit, H.; Chifiriuc, M.C.; Radon, K.; Wengenroth, L. Do wastewater treatment plants increase antibiotic resistant bacteria or genes in the environment? Protocol for a systematic review. Syst. Rev. 2019, 8, 304. [Google Scholar] [CrossRef]
- Wengenroth, L.; Berglund, F.; Blaak, H.; Chifiriuc, M.C.; Flach, C.-F.; Pircalabioru, G.G.; Larsson, D.G.J.; Marutescu, L.; van Passel, M.W.J.; Popa, M. Antibiotic Resistance in Wastewater Treatment Plants and Transmission Risks for Employees and Residents: The Concept of the AWARE Study. Antibiotics 2021, 10, 478. [Google Scholar] [CrossRef]
- Osunmakinde, C.O.; Selvarajan, R.; Mamba, B.B.; Msagati, T.A.M. Profiling bacterial diversity and potential pathogens in wastewater treatment plants using high-throughput sequencing analysis. Microorganisms 2019, 7, 506. [Google Scholar] [CrossRef]
- Kokkinos, P.; Mandilara, G.; Nikolaidou, A.; Velegraki, A.; Theodoratos, P.; Kampa, D.; Blougoura, A.; Christopoulou, A.; Smeti, E.; Kamizoulis, G.; et al. Performance of three small-scale wastewater treatment plants. A challenge for possible reuse. Environ. Sci. Pollut. Res. 2015, 22, 17744–17752. [Google Scholar] [CrossRef]
- ISO 19458; Water Quality—Sampling for Microbiological Analysis. International Organisation for Standardization: Geneva, Switzerland, 2007.
- ISO 9308-2; Water Quality—Detection and Enumeration of Coliform Organisms Thermotolerant Coliform Organisms and Presumptive Escherichia coli—Part 2: Multiple Tube (Most Probable Number) Method. International organization for Standardization: Geneva, Switzerland, 2014.
- ISO 7899-2; Water Quality—Detection and Enumeration of Intestinal Enterococci Part 2:—Membrane Filtration Method. International Organization for Standardization: Geneva, Switzerland, 2008.
- Durairajan, S.S.K.; Singh, A.K.; Saravanan, U.B.; Namachivayam, M.; Radhakrishnan, M.; Huang, J.D.; Dhodapkar, R.; Zhang, H. Gastrointestinal Manifestations of SARS-CoV-2: Transmission, pathogenesis, immunomodulation, microflora dysbiosis, and clinical implications. Viruses 2023, 15, 1231. [Google Scholar] [CrossRef]
- Barbu, I.C.; Barbu-Gheorghe, I.; Grigore, G.A.; Vrancianu, C.O.; Chifiriuc, M.C. Antimicrobial resistance in Romania: Updates 454 on Gram-Negative ESCAPE pathogens in the clinical, veterinary, and aqautic sectors. Int. J. Mol. Sci. 2023, 24, 7892. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Haque, R.; Rahman, M.Z.; Rahman, M.Z.; Mahmud, Z.H.; Hasan, R.; Islam, M.T.; Sarker, P.; Sarker, S.; Adnan, S.D.; et al. Dependency of sanitation infrastructure on the discharge of fecal coliform and SARS-CoV-2 viral RNA in wastewater from COVID and non-COVID hospitals in Dhaka, Bangladesh. Sci. Total. Environ. 2023, 867, 161424. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Ziquan, L.; Liangqiang, L.; Xiaowei, L.; Jian, X.; Suli, H.; Yuhua, C.; Yulin, F.; Changfeng, P.; Tingting, C.; et al. Impact of COVID-19 pandemic on profiles of antibiotic-resistant genes and bacteria in hospital wastewater. Environ. Pollut. 2023, 334, 122133. [Google Scholar] [CrossRef]
- Mugerwa, I.; Nabadda, S.N.; Midega, J.; Guma, C.; Kalyesubula, S.; Muwonge, A. Antimicrobial resistance situational analysis 2019-2020: Design and performance for human health surveillance in Uganda. Med. Infect. Dis. 2021, 6, 178. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Hays, J.P.; Kemp, A.; Okechukwu, R.; Murugaiyan, J.; Ekwanzala, M.D.; Alvarez, M.J.; Paul-Satyaseela, M.; Iwu, C.D.; Balleste-Delpiere, C.; et al. The potential impact of the COVID-19 pandemic on global antimicrobial and biocide resistance: An AMR insights global perspective. JAC Antimicrob. Resist. 2021, 3, dlab038. [Google Scholar] [CrossRef]
- Golli, A.L.; Zlatian, O.; Cara, M.L.; Olteanu, M. Pre- and post-COVID-19 antimicrobial resistance pattern of pathogens in an intensive care unit. Pharmaceuticals 2024, 17, 407. [Google Scholar] [CrossRef]
- Boccabella, L.; Palma, G.E.; Abenavoli, L.; Scarlata, G.G.M.; Boni, M.; Ianiro, G.; Santori, P.; Tack, J.F.; Scarpellini, E. Post-Coronavirus disease 2019 pandemic antimicrobial resistance. Antibiotics 2024, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Cao, W.; Wu, Q.; Cheng, S.; Jin, H.; Pang, H.; Zhou, A.; Feng, L.; Cao, J.; Luo, J. Dynamic microbiome disassembly and evolution induced by antimicrobial methylisothiazolinone in sludge anaerobic fermentation for volatile fatty acids generation. Water Res. 2024, 251, 121139. [Google Scholar] [CrossRef]
- Banciu, A.R.; Gheorghe, S.; Stoica, C.; Lucaciu, I.; Nita-Lazar, M. Post-pandemic effects on faecal pollution of aquatic systems. E-SIMI 2021, 1, 72–73. [Google Scholar] [CrossRef]
- Bumbac, C.; Manea, E.; Banciu, A.; Stoica, C.; Ionescu, I.; Badescu, V.; Nita-Lazar, M. Identification of physical, morphological and chemical particularities of mixed microalgae—Bacteria granules. Rev. Chim. 2019, 70, 275–277. [Google Scholar] [CrossRef]
- Bumbac, C.; Manea, E.; Banciu, A.; Stoica, C.; Ionescu, I.; Badescu, V.; Nita-Lazar, M. The intertrophic relationship between algae and bacteria from the activated microalgae granules. Rev. Chim. 2019, 70, 319–323. [Google Scholar] [CrossRef]
- Bumbac, C.; Manea, E.; Nita-Lazar, M.; Tiron, O. Microbial diversity of aerobic granular sludge under different operational Conditions. Rev. Chim. 2019, 70, 293–296. [Google Scholar] [CrossRef]
- Chiriac, L.F.; Stoica, C.; Iftode, C.; Pirvu, F.; Petre, V.A.; Paun, I.; Pascu, L.F.; Vasile, G.G.; Nita-Lazar, M. Bacterial Biodegradation of Perfluorooctanoic Acid (PFOA) and Perfluorosulfonic Acid (PFOS) Using Pure Pseudomonas Strains. Sustainability 2023, 15, 14000. [Google Scholar] [CrossRef]
- Chiriac, L.F.; Stoica, C.; Paun, I.; Pirvu, F.; Galaon, T.; Nita-Lazar, M. Biodegradation of two organic ultraviolet-Filters by single bacterial strains. Int. J. Environ. Sci. Technol. 2023, 884, 163810. [Google Scholar] [CrossRef]
- Zhang, T.; Li, P.; Fang, C.; Jiang, R. Phosphate recovery from animal manure wastewater by struvite crystallization and CO2 degasification reactor. Ecol. Chem. Eng. S 2014, 21, 89–99. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banciu, A.R.; Pascu, L.F.; Stoica, C.; Gheorghe, S.; Lucaciu, I.; Feodorov, L.; Nita-Lazar, M. COVID-19 Pandemic Modulates the Environmental Contamination Level of Enteric Bacteria from WWTPs. Water 2024, 16, 1092. https://doi.org/10.3390/w16081092
Banciu AR, Pascu LF, Stoica C, Gheorghe S, Lucaciu I, Feodorov L, Nita-Lazar M. COVID-19 Pandemic Modulates the Environmental Contamination Level of Enteric Bacteria from WWTPs. Water. 2024; 16(8):1092. https://doi.org/10.3390/w16081092
Chicago/Turabian StyleBanciu, Alina Roxana, Luoana Florentina Pascu, Catalina Stoica, Stefania Gheorghe, Irina Lucaciu, Laura Feodorov, and Mihai Nita-Lazar. 2024. "COVID-19 Pandemic Modulates the Environmental Contamination Level of Enteric Bacteria from WWTPs" Water 16, no. 8: 1092. https://doi.org/10.3390/w16081092
APA StyleBanciu, A. R., Pascu, L. F., Stoica, C., Gheorghe, S., Lucaciu, I., Feodorov, L., & Nita-Lazar, M. (2024). COVID-19 Pandemic Modulates the Environmental Contamination Level of Enteric Bacteria from WWTPs. Water, 16(8), 1092. https://doi.org/10.3390/w16081092







