Investigation of Used Water Sediments from Ceramic Tile Fabrication
Abstract
1. Introduction
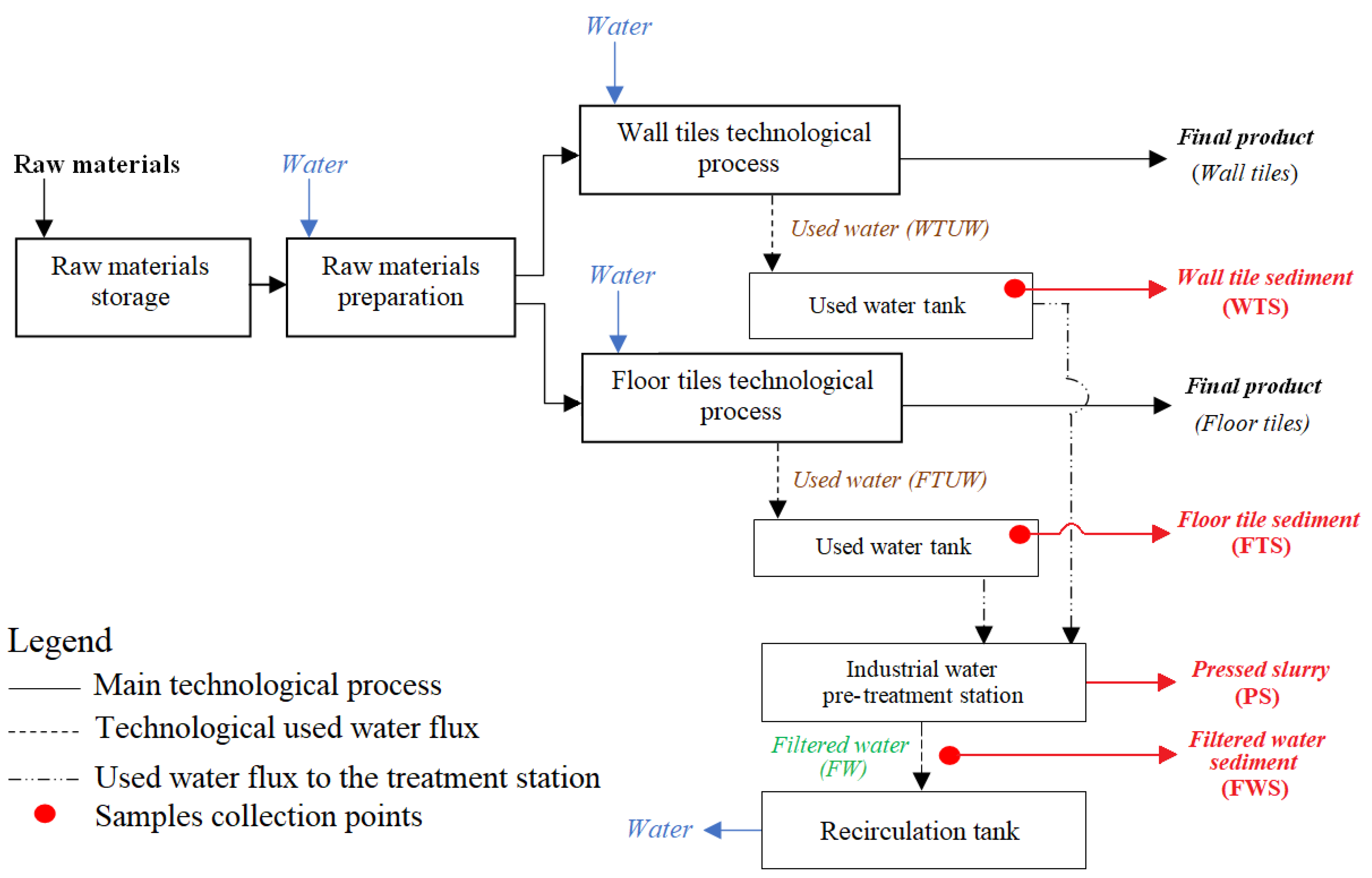
2. Materials and Methods
2.1. Samples Collection and Storage
2.2. Experimental Methods
2.2.1. Water Properties Measurement Method
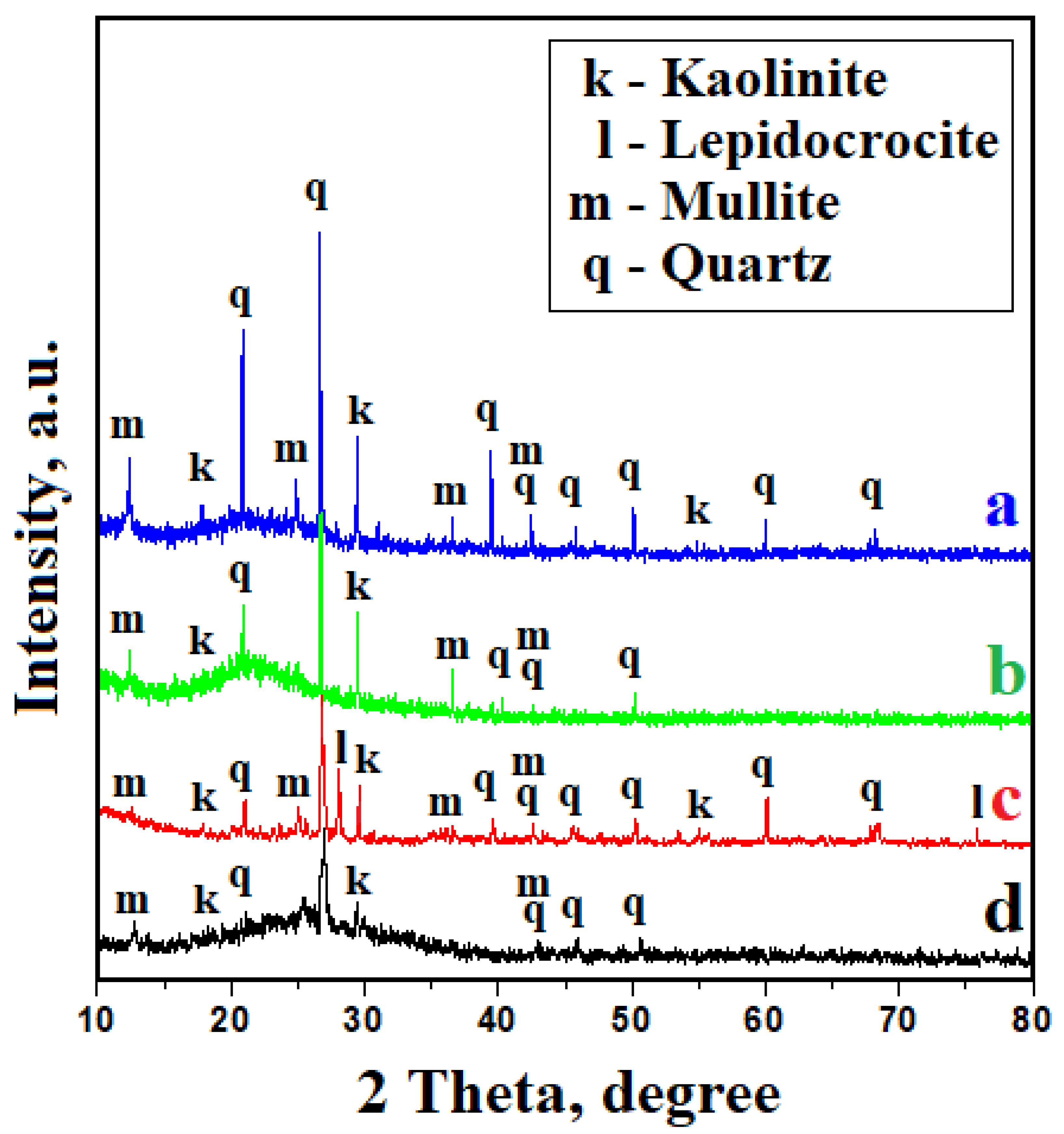
2.2.2. Mineralogical Investigations
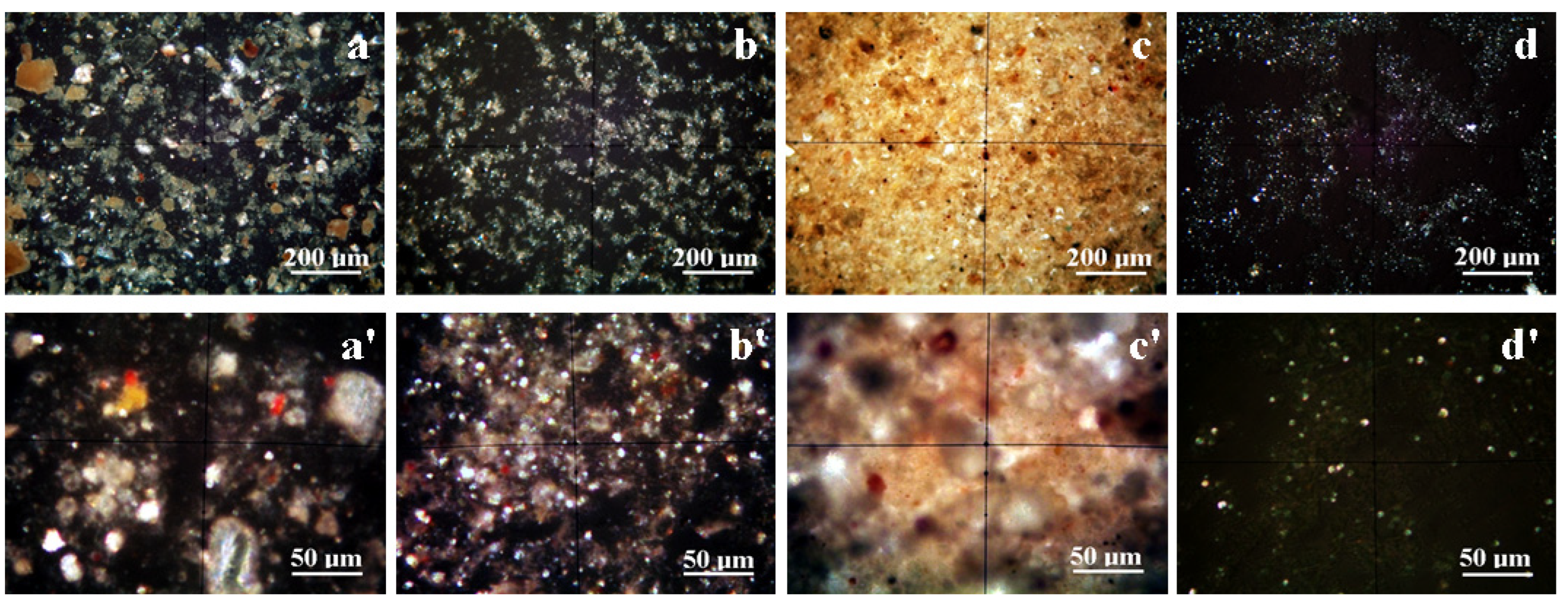
2.2.3. Microstructural Investigations
3. Results
3.1. Water Samples Properties
3.2. Mineral Constituents Assessment
3.3. Microstructural Observation and Elemental Spectroscopy
3.4. Ultra-Structure Observation
4. Discussion
5. Conclusions
- Both FTS and WTS sediments contain quartz, mullite, and kaolinite; FTS is slightly rich in mullite and WTS is rich in kaolinite. Traces of Fe were found in the FTS and WTS sediments, but there was no proof of crystallization.
- The efficacy of the filtering system was proven on the basis of the targeted particulate matters; these ensure the recirculation of water into technological processes. The sludge that is obtained after the filtration process presents with a dense grainy structure of sediment particles. These contain quartz, mullite, and kaolinite, along with traces of iron hydroxide crystallized as lepidocrocite.
- The pressed slurry cannot be reused in the technological flux because of the presence of iron hydroxide, which causes glaze staining; however, the observed microstructure, along with the mineralogical composition, indicates that it could be used for other applications, like ecological bricks or plasters. These potential applications will be investigated further.
- The liquid magnetic selective removal of the observed lepidocrocite clusters could offer a significant improvement in the process of used water pretreatment; this will allow the direct recirculation of pressed slurry into the main technological process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vieira, A.V.; Rosso, L.S.; Demarch, A.; Pasini, P.; Ruzza, S.P.; Arcaro, S.; Ribeiro, M.J.; Angioletto, E. Life cycle assessment in the ceramic tile industry: A review. J. Mater. Res. Technol. 2023, 23, 3904–3915. [Google Scholar] [CrossRef]
- Manikandan, K.P.; Nanthakumar, P.; Balachandar, M.; Gowri Shankar, D.; Vijayakumari, G. Partial replacement of aggregate with ceramic tile in concrete. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Zanelli, C.; Conte, S.; Molinari, C.; Soldati, R.; Dondi, M. Waste recycling in ceramic tiles: A technological outlook. Resour. Conserv. Recycl. 2012, 168, 105289. [Google Scholar] [CrossRef]
- Ziman, N. Contributions on Wall Tiles Technology with Implications in the Quality and Prices. Ph.D. Thesis, Romanian, University “Politehnica” of Timisoara, Timisoara, Romania, 2006. [Google Scholar]
- Baricza, A.; Bajnóczi, B.; Máté, S.; Tóth, M.; Bendő, Z.; Szabo, C. Deterioration of glazed architectural ceramics due to environmental factors: A comparative study of two buildings in Budapest. Carpathian J. Earth Environ. Sci. 2016, 11, 449–462. [Google Scholar]
- Balint, R.; Calotescu, L.G.; Boero, V.; Ajmone-Marsan, F. Clay minerals-clinoptilolite assemblages in two romanian tuffs. Carpathian J. Earth Environ. Sci. 2015, 10, 27–39. [Google Scholar]
- Ilyina, V.; Klimovskaya, E.; Bubnova, T. Ceramic Materials Based on Clay and Soapstone Waste: Thermo-Mechanical Properties and Application. Minerals 2023, 13, 1376. [Google Scholar] [CrossRef]
- Hosu Prack, G.A.; Petean, I.; Arghir, G.; Bobos, L.D.; Tomoaia-Cotisel, M. Nano-scale particulate matters found in urban street dust in Cluj–Napoca, Romania. Carpathian J. Earth Environ. Sci. 2016, 11, 539–546. [Google Scholar]
- Avram, S.E.; Filip, M.R.; Barbu Tudoran, L.; Borodi, G.; Petean, I. Investigation of ferrous conglomerate particles found in carwashslurry and their environmental implications. Stud. UBB Chem. 2023, 68, 57–70. [Google Scholar] [CrossRef]
- Rus, L.; Avram, S.E.; Micle, V. Determination and assessments of physicochemical parameters of the water from anthropo-saline lakes located in the protected area “Salina Turda”, Romania. Stud. UBB Chem. 2020, 65, 257–268. [Google Scholar] [CrossRef]
- Avram, S.E.; Rus, L.; Micle, V.; Hola, S.S. Evaluation and Evolution of the Physico-Chemical Parameters of Ocnei and Rotund Lakes Located near the “Salina Turda” Mine, Romania. Water 2022, 14, 2366. [Google Scholar] [CrossRef]
- Mohd, H.I.; Mohd, I.M.; Anis, N.I.; Syarifah, A.I.; Nabihah, O. Development of porous glass-ceramic using silica sand for wall tiles application. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Mourou, C.; Zamorano, M.; Ruiz, D.P.; Martín-Morales, M. Characterization of ceramic tiles coated with recycled waste glass particles to be used for cool roof applications. Constr. Build. Mater. 2023, 398, 132489. [Google Scholar] [CrossRef]
- Alves, C.L.; Skorych, V.; De Noni, A.; Hotza, D.; Gómez González, S.Y.; Heinrich, S.; Dosta, M. Improving the sustainability of porcelain tile manufacture by flowsheet simulation. Ceram. Int. 2023, 49, 24581–24597. [Google Scholar] [CrossRef]
- Contini, G.; Peruzzini, M.; Bulgarelli, S.; Bosi, G. Developing key performance indicators for monitoring sustainability in the ceramic industry: The role of digitalization and industry 4.0 technologies. J. Clean. Prod. 2023, 414, 137664. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, S.; Chu, J.; Chen, D.; Chen, J. Study on the copper and iron coexisted coloring glaze and the mechanism of the fambe. J. Eur. Ceram. Soc. 2018, 38, 3681–3688. [Google Scholar] [CrossRef]
- Gol, F.; Saritas, Z.G.; Cıbuk, S.; Ture, C.; Kacar, E.; Yilmaz, Y.; Arslan, M.; Sen, F. Coloring effect of iron oxide content on ceramic glazes and their comparison with the similar waste containing materials. Ceram. Int. 2022, 48, 2241–2249. [Google Scholar] [CrossRef]
- Muntean, D.F.; Ristoiu, D.; Arghir, G.; Campean, R.F.; Petean, I. Iron hydroxides occurrence in winter air particulate matters suspensions in Cluj–Napoca, Romania. Carpathian J. Earth Environ. Sci. 2012, 7, 175–182. [Google Scholar]
- Muntean, D.F.; Ivan, I.; Muresan, L. Environmental Implications Concerning the Chemical Composition and Particle Distribution of Anti–Skid Material. Stud. UBB Chem. 2015, 60, 207–218. [Google Scholar]
- Caulfield, J.T.; Tomlinson, E.L.; Chew, D.M.; Marks, M.A.V.; McKenna, C.A.; Ubide, T.; Smith, V.C. Microanalysis of Cl, Br and I in apatite, scapolite and silicate glass by LA-ICP-MS. Chem. Geol. 2020, 557, 119854. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, H.S. Glass-ceramic produced from a municipal waste incinerator fly ash with high Cl content. J. Eur. Ceram. Soc. 2004, 24, 2373–2382. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Raghavan, P. Significance of Impurity Mineral Identification in the Value Addition of Kaolin—A Case Study with Reference to an Acidic Kaolin from India. J. Miner. Mater. Charact. Eng. 2011, 10, 1007–1025. [Google Scholar] [CrossRef]
- Segura, J.C.F.; Reyes Cruz, V.E.; Jesús Pérez Bueno, J.; Lozada Ascencio, E.M.; García, F.L. Characterization and electrochemical treatment of a kaolin. Appl. Clay Sci. 2017, 146, 264–269. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Xu, X.; Zhu, Y. A Study on the Efficiency of Green Technology Innovation in Listed Chinese Water Environment Treatment Companies. Water 2024, 16, 510. [Google Scholar] [CrossRef]
- Santos, A.B.d.; Giacobbo, A.; Rodrigues, M.A.S.; Bernardes, A.M. Electrodeionization for Wastewater Reuse in Petrochemical Plants. Water 2024, 16, 401. [Google Scholar] [CrossRef]
- Boschi, G.; Bonvicini, G.; Masi, G.; Bignozzi, M.C. Recycling insight into the ceramic tile manufacturing industry. Open Ceram. 2023, 16, 100471. [Google Scholar] [CrossRef]
- Ding, K.; Li, A.; Lv, J.; Gu, F. Decarbonizing ceramic industry: Technological routes and cost assessment. J. Clean. Prod. 2023, 419, 138278. [Google Scholar] [CrossRef]
- Rao, F.; Ramirez-Acosta, F.J.; Sanchez-Leija, R.J.; Song, S.; Lopez-Valdivieso, A. Stability of kaolinite dispersions in the presence of sodium and aluminum ions. Appl. Clay Sci. 2011, 51, 38–42. [Google Scholar] [CrossRef]
- Dwari, R.K.; Mishra, B.K. Evaluation of flocculation characteristics of kaolinite dispersion system using guar gum: A green flocculant. Int. J. Min. Sci. Technol. 2019, 29, 745–755. [Google Scholar] [CrossRef]
- Markowski, Ł.; Kotliński, K.; Ostrowska, A. Sustainable Consumption and Production in the European Union—An Attempt to Assess Changes and Convergence from the Perspective of Central and Eastern European Countries. Sustainability 2023, 15, 16485. [Google Scholar] [CrossRef]
- Alresheedi, M.T.; Haider, H.; Albuaymi, A.M.; AlSaleem, S.S.; Shafiquzzaman, M.; Alharbi, A.; Ahsan, A. Sustainability of a Low-Cost Decentralized Treatment System for Wastewater Reuse: Resident Perception-Based Evaluation for Arid Regions. Water 2023, 15, 3458. [Google Scholar] [CrossRef]
- Chen, D.; Yen, M.; Lin, P.; Groff, S.; Lampo, R.; McInerney, M.; Ryan, J. A Corrosion Sensor for Monitoring the Early-Stage Environmental Corrosion of A36 Carbon Steel. Materials 2014, 7, 5746–5760. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Song, J.; Li, Y.; Wu, J.; Liu, X.; Zhang, C. The microstructure and properties of ceramic tiles from solid wastes of Bayer red muds. Constr. Build. Mater. 2019, 212, 266–274. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Corpas-Iglesias, F.A.; Martínez-Martínez, S.; Artiaga, R.; Pascual-Cosp, J. Manufacturing new ceramic materials from clay and red mud derived from the aluminium industry. Constr. Build. Mater. 2012, 35, 656–665. [Google Scholar] [CrossRef]
- Kuma, K.; Suzuki, Y.; Matsunaga, K. Solubility and dissolution rate of colloidal γ-FeOOH in seawater. Water Res. 1993, 27, 651–657. [Google Scholar] [CrossRef]
- Arghir, G.; Petean, I.; Munteanu, D.F.; Muresan, L.; Suciu, C. Raw material for powder metallurgy obtained from environmental iron hydroxides. Powder Metall. Prog. 2011, 3–4, 340–346. [Google Scholar]
- Avram, S.E.; Barbu Tudoran, L.; Cuc, S.; Borodi, G.; Birle, B.V.; Petean, I. Microstructural Investigations Regarding Sustainable Recycling of Ceramic Slurry Collected from Industrial Waste Waters. Sustainability 2024, 16, 1123. [Google Scholar] [CrossRef]
- Romero Quidel, G.; Soto Acuña, M.J.; Rojas Herrera, C.J.; Rodríguez Neira, K.; Cárdenas-Ramírez, J.P. Assessment of Modular Construction System Made with Low Environmental Impact Construction Materials for Achieving Sustainable Housing Projects. Sustainability 2023, 15, 8386. [Google Scholar] [CrossRef]
- Horabik, J.; Jozefaciuk, G. Structure and strength of kaolinite–soil silt aggregates: Measurements and modeling. Geoderma 2021, 382, 114687. [Google Scholar] [CrossRef]
- Kaminskas, R.; Savickaite, B. Expanded Clay Production Waste as Supplementary Cementitious Material. Sustainability 2023, 15, 11850. [Google Scholar] [CrossRef]



| Component | Kaolinite | Mullite | Quartz | Lepidocrocite | Glass |
|---|---|---|---|---|---|
| Formula | Al2Si2O5(OH)4 | Al6Si2O13 | SiO2 | γFeO(OH) | 6SiO2‧CaO‧Na2O |
| Color nuances | White-blue | Red-orange | Green-gray | Redish-brown | Black |
| FTS | |||||
| Amount, wt. % | 14 | 32 | 54 | 0 | * |
| Particle size, μm | 1–100 | 10–180 | 5–70 | - | 10–30 |
| WTS | |||||
| Amount, wt. % | 37 | 21 | 43 | 0 | * |
| Particle size, μm | 1–30 | 3–35 | 1–35 | - | 20–55 |
| FS | |||||
| Amount, wt. % | 41 | 32 | 19 | 8 | * |
| Particle size, μm | 1–100 | 1–180 | 1–70 | 3–20 | 10–60 |
| FWS | |||||
| Amount, wt. % | 68 | 6 | 26 | 0 | * |
| Particle size, μm | 1–2.5 | 1–10 | 1–15 | - | - |
| Element | Elemental Composition, At. % | ||
|---|---|---|---|
| FTS | WTS | PS | |
| O | 70.1 | 70.0 | 70.3 |
| Si | 14.7 | 17.5 | 17.6 |
| Al | 6.1 | 6.1 | 6.8 |
| Ca | 2.8 | 2.6 | 1.8 |
| Na | 1.6 | 1.7 | 1.6 |
| K | 0.8 | 0.8 | 0.8 |
| Fe | 0.7 | 0.7 | 0.4 |
| Cl | 0.3 | 0.3 | - |
| Mg | - | - | 0.4 |
| Ti | 0.2 | 0.2 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avram, S.E.; Birle, B.V.; Tudoran, L.B.; Borodi, G.; Petean, I. Investigation of Used Water Sediments from Ceramic Tile Fabrication. Water 2024, 16, 1027. https://doi.org/10.3390/w16071027
Avram SE, Birle BV, Tudoran LB, Borodi G, Petean I. Investigation of Used Water Sediments from Ceramic Tile Fabrication. Water. 2024; 16(7):1027. https://doi.org/10.3390/w16071027
Chicago/Turabian StyleAvram, Simona Elena, Bianca Violeta Birle, Lucian Barbu Tudoran, Gheorghe Borodi, and Ioan Petean. 2024. "Investigation of Used Water Sediments from Ceramic Tile Fabrication" Water 16, no. 7: 1027. https://doi.org/10.3390/w16071027
APA StyleAvram, S. E., Birle, B. V., Tudoran, L. B., Borodi, G., & Petean, I. (2024). Investigation of Used Water Sediments from Ceramic Tile Fabrication. Water, 16(7), 1027. https://doi.org/10.3390/w16071027







