Evaluating Groundwater Metal and Arsenic Content in Piatra, North-West of Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Sampling
2.2. Methods of Analysis
2.3. Statistical Analysis
2.4. Heavy Metal Evaluation Index (HEI)
2.5. Water Quality Index (WQI)
2.6. Human Health Risk Assessment
3. Results and Discussion
3.1. Physico-Chemical Characteristics of Groundwater Samples
3.2. Metal and as Content in Groundwater Samples
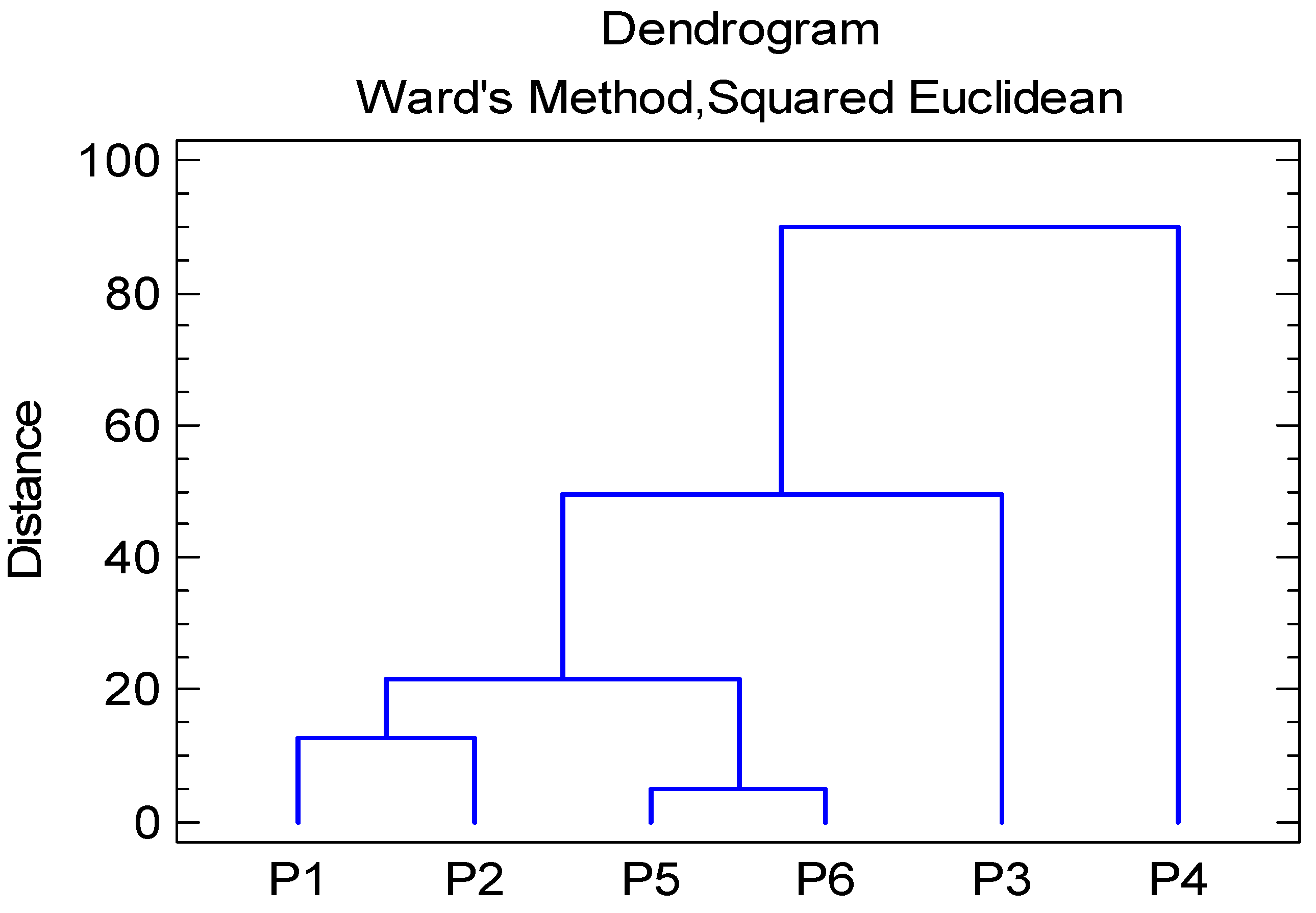
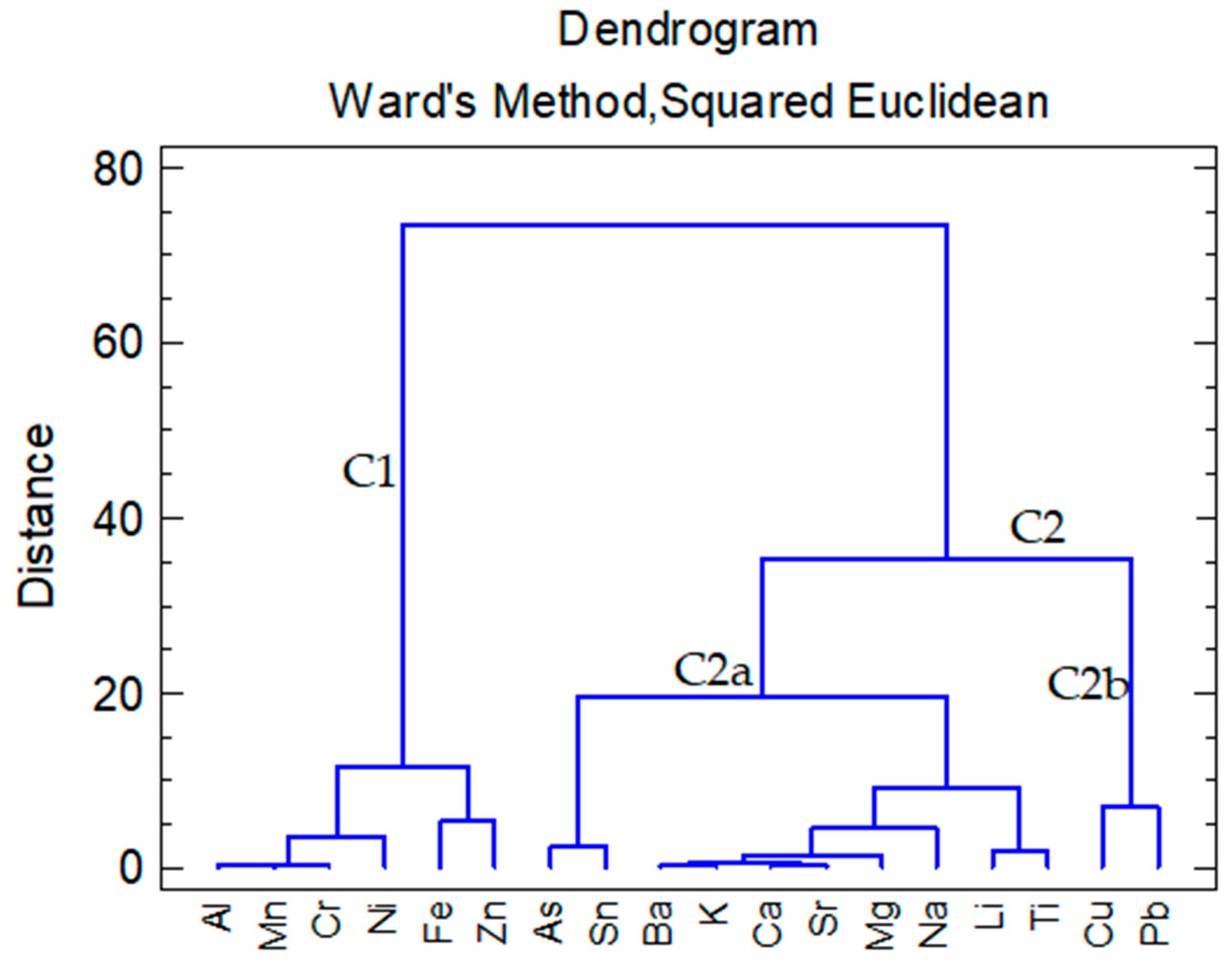
3.3. Cluster Analysis of Water Samples
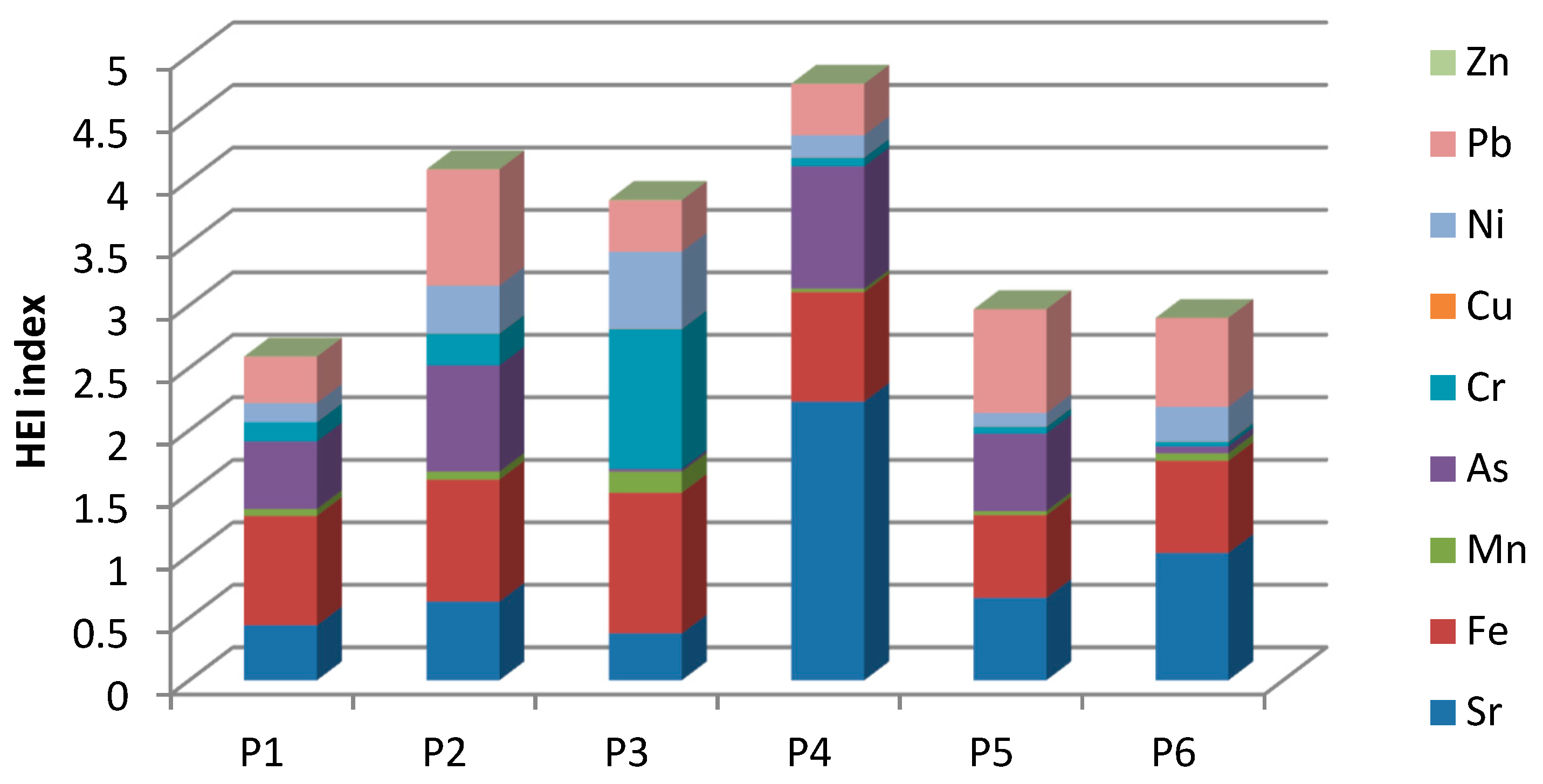
3.4. Heavy Metals Evaluation Index (HEI)
3.5. Water Quality Assessment by WQI
3.6. Human Health Risk Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abanyie, S.K.; Apea, O.B.; Abagale, S.A.; Amuah, E.E.Y.; Sunkari, E.D. Sources and factors influencing groundwater quality and associated health implication: A review. Emerg. Contam. 2023, 9, 100207. [Google Scholar] [CrossRef]
- Grönwall, J.; Danert, K. Regarding Groundwater and DrinkingWater Access through A Human Rights Lens: Self-Supply as A Norm. Water 2020, 12, 419. [Google Scholar] [CrossRef]
- UNESCO World Water Assessment Programme. The United Nations World Water Development Report. 2023. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000384659 (accessed on 3 December 2023).
- Burek, P.; Satoh, Y.; Fischer, G.; Kahil, M.T.; Scherzer, A.; Tramberend, S.; Nava, L.F.; Wada, Y.; Eisner, S.; Flörke, M.; et al. Water Futures and Solution: Fast Track Initiative (Final Report); IIASA Working Paper; International Institute for Applied Systems Analysis (IIASA): Laxenburg, Austria, 2016; Available online: https://pure.iiasa.ac.at/id/eprint/13008/ (accessed on 3 December 2023).
- Romania ClimThe World Bank Europe and Central Asia region. Romania Climate Change and Low Carbon Green Growth Program, Component B Sector Report, Integrated Water Resources Rapid Assessment, January 2014. Available online: https://documents1.worldbank.org/curated/en/675851468094456585/pdf/842640WP0P14660Box0382136B00PUBLIC0.pdf (accessed on 3 December 2023).
- Hussain, F.; Hussain, R.; Wu, R.-S.; Abbas, T. Rainwater Harvesting Potential and Utilization for Artificial Recharge of Groundwater Using Recharge Wells. Processes 2019, 7, 623. [Google Scholar] [CrossRef]
- Firoiu, D.; Ionescu, G.H.; Bandoi, A.; Florea, N.M.; Jianu, E. Achieving Sustainable Development Goals (SDG): Implementation of the 2030 Agenda in Romania. Sustainability 2019, 11, 2156. [Google Scholar] [CrossRef]
- Council Directive. 98/83/EC—On the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31998L0083 (accessed on 20 December 2023).
- Schuteima, G.; Hooks, T.; McDermott, F. Water quality perceptions and private well management: The role of perceived risks, worry and control. J. Environ. Manag. 2020, 267, 110654. [Google Scholar] [CrossRef]
- Sandulescu, M. Geotectonics of Romania; Editura Tehnică: București, Romania, 1984. (In Romanian) [Google Scholar]
- Cioruta, B.-V.; Coman, M. Definition, Role, and Functions of Soil Related to the Knowledge Society and the Somes,-Tisa Hydrographic Area (Romania). Sustainability 2022, 14, 8688. [Google Scholar] [CrossRef]
- Florea, N.; Munteanu, I. Sistemul Român de Taxonomie a Solurilor (SRTS); Institutul Naţional de Cercetare-Dezvoltare Pentru Pedologie, Agrochimie şi Protecţia Mediului–ICPA: Bucureşti, Romania, 2012. (In Romanian) [Google Scholar]
- Toth, G.; Montanarella, L.; Stolbovoy, V.; Mate, F.; Bodis, K.; Jones, A.; Panagos, P.; Liedekerke, M.V. Soils of the European Union. JCR–Scientific and Technical Reports, European Commision Joint Research Centre Institute for Environment and Sustainability. 2012. Available online: https://data.europa.eu/doi/10.2788/87029 (accessed on 2 December 2023).
- Stanila, A.-L.; Dumitru, M. Soil zones in Romania and pedogenetic processes. Agric. Agric. Sci. Procedia 2016, 10, 135–139. [Google Scholar] [CrossRef][Green Version]
- Oprea, R. Compendiu de Pedologie, 2nd ed.; Editura Universitara: Bucharest, Romania, 2013; ISBN 978-606-591-832-0. (In Romanian) [Google Scholar]
- Todd, D.K.; Mays, L.W. A Review Study on “Quality Assessment of Groundwater Resource in Centre India Region”. Int. J. Innov. Res. Technol. 2004, 4, 968–972. [Google Scholar]
- Arulnangai, R.; Sihabudeen, M.M.; Vivekanand, P.A.; Kamaraj, P. Influence of physico chemical parameters on potability of groundwater water in ariyalur of Tamil Nadu, India. Mater. Today Proc. 2021, 36, 923–928. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.L.; Akhtar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.S.; Faisal, A.A.H. Nanoparticles of (calcium/aluminium/CTAB) layered double hydroxide immobilization onto iron slag for removing of cadmium ions from aqueous environment. Arab. J. Chem. 2023, 16, 105031. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ahmed, K.; Ahmed, T.; Zafar, A.; Alrefaei, A.F.; Ashfaq, A.; Akhtar, S.; Mahpara, S.; Mehmood, N.; Ugulu, I. Evaluation of nickel tixicity and potential health implications of agriculturally diversely irrigated wheat crop varieties. Arab. J. Chem. 2023, 16, 104934. [Google Scholar] [CrossRef]
- Senze, M.; Kowalska-Goralska, M.; Czyz, K. Availability of aluminum in river water supplying dam reservoirs in Lower Silesia considering the hydrochemical conditions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100535. [Google Scholar] [CrossRef]
- Mohanty, S.; Benya, A.; Hota, S.; Kumar, M.S.; Singh, S. Eco-toxicity of hexavalent chromium and its adverse impact on environment and human health in Sukinda Valley of India: A review on pollution and prevention strategies. Environ. Chem. Ecotox. 2023, 5, 46–54. [Google Scholar] [CrossRef]
- Siraj, K.; Kitte, S.A. Analysis of Copper, Zinc and Lead using Atomic Absorption Spectrophotometer in ground water of Jimma town of Southwestern Ethiopia. Int. J. Chem. Anal. Sci. 2013, 4, 201–204. [Google Scholar] [CrossRef]
- Hossain, M.; Bhattacharya, P.; Frape, S.K.; Ahmed, K.M.; Jacks, G.; Hasan, M.A.; Bromssen, M.; Shahiruzzaman, M.; Morth, C.M. A potential source of low-manganese, arsenic-safe drinking water from Intermediate Deep Aquifers (IDA), Bangladesh. Groundw. Sustain. Dev. 2023, 21, 100906. [Google Scholar] [CrossRef]
- Markeb, A.A.; Moral-Vico, J.; Sanchez, A.; Font, X. Optimization of lead (II) removal from water and wastewater using a novel magnetic nanocomposite of aminopropyl triethoxysilane coated with carboxymethyl cellulose cross-linked with shitosan nanoparticles. Arab. J. Chem. 2023, 16, 105022. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, H.; Le, Q.V.; Peng, W.; Lam, S.S.; Yang, Y.; Li, C.; Sonne, C. Perspectives on phytoremediation of zinc pollution in air, water and soil. Sustain. Chem. Pharm. 2021, 24, 100550. [Google Scholar] [CrossRef]
- Beinabaj, S.M.H.; Heydariyan, H.; Aleii, H.M.; Hosseinzadeh, A. Concentration of heavy metals in leachate, soil, and plants in Teheran`s landfill: Investigation of the effect of landfill age on the intesity of polluation. Heliyon 2023, 9, e13017. [Google Scholar] [CrossRef]
- Chorol, L.; Gupta, S.K. Evalution of groudwater metal pollution index through analytical hierarchy process and its health risk assement via Monte Carlo simulation. Process Saf. Environ. Prot. 2023, 170, 855–864. [Google Scholar] [CrossRef]
- Rupias, O.J.B.; Pereira, S.Y.; Silva de Abreu, A.E. Hydrogeochemistry and groundwater quality assessment using the water quality index and heavy-metal pollution index in the alluvial plain of Atibaia river—Campinas/SP, Brazil. Groundw. Sustain. Dev. 2020, 15, 100661. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Han, Z.; Peng, J.; Chen, Y.; Zhan, L.; Li, J. Human health risk assessment for contaminated sites: A retrospective review. Environ. Int. 2023, 171, 107700. [Google Scholar] [CrossRef] [PubMed]
- Badeenezhad, A.; Soleimani, H.; Shahsavani, S.; Parseh, I.; Mohammadpour, A.; Azadbakht, O.; Javanmardi, P.; Faraji, H.; Nalosi, K.B. Comprehensive health risk analysis of heavy metal pollution using water quality indices and Monte Carlo simulation in R software. Sci. Rep. 2023, 13, 15817. [Google Scholar] [CrossRef] [PubMed]
- Munene, E.N.; Hashim, N.O.; Ambusso, W.N. Human health risk assessment of heavy metal concentration in surface water of Sosian river, Eldoret town, Uasin-Gishu County Kenya. MothodsX 2023, 11, 102298. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K. Sources and consequences of groundwater contamination. Arch. Environ. Contam. Toxicol. 2021, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Hou, Q.; Han, D.; Liu, R.; Song, J. Large scale occurrence of aluminium-rich shallow groundwater in the Pearl River Delta after the rapid urbanization: Co-effects of anthropogenic and geogenic factors. J. Contam. Hydrol. 2023, 254, 104130. [Google Scholar] [CrossRef] [PubMed]
- SR EN ISO 5667-3:2018; Calitatea apei. Prelevare. Partea 3: Conservarea ¸si Manevrarea Probelor de Apa. ISO: Geneva, Switzerland, 2018. (In Romanian)
- Dippong, T.; Mihali, C.; Cical, E. Metode de Determinare a Proprietatilor Fizico-Chimice ale Alimentelor; Risoprint: Cluj-Napoca, Romania, 2016; ISBN 978-973-53-1773-7. (In Romanian) [Google Scholar]
- ISO 7888:1985; Water Quality—Determination of Electrical Conductivity. ISO: Geneva, Switzerland, 1985.
- SR EN ISO 10523:2012; Calitatea apei. Determinarea pH-ului. ISO: Geneva, Switzerland, 2012. (In Romanian)
- Dippong, T.; Mihali, C.; Hoaghia, A.M.; Cical, E.; Cosma, A. Chemical modeling of groundwater quality in the aquifer of Seini town-Somes Plain, Northwestern Romania. Ecotoxicol. Environ. Saf. 2019, 168, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.A.H.; Islam, M.A.; Dampare, S.B.; Parvez, L.; Suzuki, S. Evaluation of hazardous metal pollution in irrigation and drinking water systems in the vicinity of a coal mine area of northwestern Bangladesh. J. Hazard. Mater. 2010, 179, 1065–1077. [Google Scholar] [CrossRef]
- Varol, M.; Gökot, B.; Bekleyem, A. Dissolved heavy metals in the Tigris River (Turkey): Spatial and temporal variations. Environ. Sci. Pollut. Res. 2013, 20, 6096–6108. [Google Scholar] [CrossRef]
- González, S.O.; Almeida, C.; Calderón, M.; Mallea, M.A.; González, P. Assessment of the water self-purification capacity on a river affected by organic pollution: Application of chemometrics in spatial and temporal variations. Environ. Sci. Pollut. Res. 2014, 21, 10583–10593. [Google Scholar] [CrossRef]
- Uddin, M.G.; Nash, S.; Agnieszka, I.; Olbert, A.I. A review of water quality index models and their use for assessing surface water quality. Ecol. Ind. 2021, 122, 107218. [Google Scholar] [CrossRef]
- Mare Roșca, O.; Dippong, T.; Marian, M.; Mihali, C.; Mihalescu, L.; Hoaghia, M.A.; Jelea, M. Impact of anthropogenic activities on water quality parameters of glacial lakes from Rodnei mountains, Romania. Environ. Res. 2020, 182, 109136. [Google Scholar] [CrossRef] [PubMed]
- Dippong, T.; Mihali, C.; Avram, A. Water Physico-Chemical Indicators and Metal Assessment of Teceu Lake and the Adjacent Groundwater Located in a Natura 2000 Protected Area, NW of Romania. Water 2023, 15, 3996. [Google Scholar] [CrossRef]
- Manoj, K.; Ghosh, S.; Padhy, P.K. Characterization and classification of hydrochemistry using multivariate graphical and hydrostatistical techniques. Res. Chem. Sci. 2013, 3, 32–42. [Google Scholar]
- Harter, T.; Davis, H.; Mathews, M.C.; Meyer, R.D. Shallow groundwater quality on dairy farms with irrigated forage crops. J. Contam. Hydrol. 2022, 55, 287–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Al-Maktoumi, A.; Al-Mamari, H.; Izady, A.; Nikoo, M.R.; Al-Busaidi, H. Oxygenation of aquifers with fluctuating water table: A laboratory and modeling study. J. Hydrol. 2020, 590, 125261. [Google Scholar] [CrossRef]
- Dimri, D.; Daverey, A.; Kuman, A.; Sharma, A. Monitoring water quality of River Ganga using multivariate techniques and WQI (Water Quality Index) in Western Himalayan region of Uttarakhand, India. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100375. [Google Scholar] [CrossRef]
- Lu, B.T.; Luo, J.L.; Norton, P.R. Environmentally assisted cracking mechanism of pipeline steel in near-neutral pH groundwater. Corros. Sci. 2010, 52, 1787–1795. [Google Scholar] [CrossRef]
- Wilkin, R.T.; DiGiulio, D.C. Geochemical impacts to groundwater from geologic carbon sequestration: Controls on pH and inorganic carbon concentrations from reaction path and kinetic modelling. Environ. Sci. Technol. 2010, 44, 4821–4827. [Google Scholar] [CrossRef]
- Mohammed, I.; Al-Khalaf, S.K.H.; Alwan, H.H.; Naje, A.S. Environmental assessment of Karbala water treatment plant using water quality index (WQI). Mater. Today Proc. 2022, 60, 1554–1560. [Google Scholar] [CrossRef]
- Nawaz, R.; Nasim, I.; Irfan, A.; Islam, A.; Naeem, A.; Ghani, N.; Irshad, M.A.; Latif, M.; Un Nisa, B.; Ullah, R. Water Quality Index and Human Health Risk Assessment of Drinking Water in Selected Urban Areas of a Mega City. Toxics 2023, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, U.C.; Chukwudi, M.I.; Monday, S.S.; Anthony, A.I. Human health risk assessment of heavy metals in drinking water sources in three senatorial districts of Anambra State, Nigeria. Toxicol. Rep. 2022, 9, 869–875. [Google Scholar] [CrossRef]
- Napo, G.; Akpataku, K.V.; Seyf-Laye, A.S.M.; Gnazou, M.D.T.; Bawa, L.M.; Djaneye-Boundjou, G. Assessment of Shallow Groundwater Quality Using Water Quality Index and Human Risk Assessment in the Vogan-Attitogon Plateau, Southeastern (Togo). J. Environ. Poll. Hum. Health 2021, 9, 50–63. [Google Scholar] [CrossRef]
- Average Height and Weight by Country. Available online: https://www.worlddata.info/average-bodyheight.php (accessed on 2 December 2023).
- United States Environmental Protection Agency. IRIS (Integrated Risk Information System). A–Z List of Substances; United States Environmental Protection Agency: Washington, DC, USA, 1987. Available online: https://www.epa.gov/iris (accessed on 3 December 2023).
- Qaiyum, M.S.; Shaharudin, M.S.; Syazwan, A.I.; Muhaimin, A. Health Risk Assessment after Exposure to Aluminium in Drinking Water between Two Different Villages. J. Water Resour. Prot. 2011, 3, 268–274. [Google Scholar] [CrossRef]
- Tsuji, S.J.; Perez, V.; Garry, M.R.; Alexander, D.D. Association of low-level arsenic exposure in drinking water with cardiovas-cular disease: A systematic review and risk assessment. Toxicology 2014, 323, 78–94. [Google Scholar] [CrossRef]
- Mawari, G.; Kumar, N.; Sarkar, S.; Frank, A.L.; Daga, M.K.; Singh, M.M.; Joshi, T.K.; Singh, I. Human Health Risk Assessment due to Heavy Metals in Ground and Surface Water and Association of Diseases With Drinking Water Sources: A Study from Maharashtra, India. Environ. Health Insights 2022, 16, 1–11. [Google Scholar] [CrossRef]
- Rivett, M.O.; Buss, S.R.; Morgan, P.; Smith, J.W.; Bemment, C.D. Nitrate attenuation in groundwater: A review of biogeochemical controlling processes. Water Res. 2008, 42, 4215–4232. [Google Scholar] [CrossRef]
- Laha, F.; Gashi, F.; Cadraku, H. Variations in the physico-chemical parameters of Grinaja catchment groundwater. IFAC-PapersOnLine 2019, 49, 200–205. [Google Scholar]
- Adesakin, T.A.; Oyewale, A.T.; Bayero, U.; Mohammed, A.N.; Aduwo, I.A.; Ahmed, P.Z.; Abubakar, N.D.; Barje, I.B. Assessment of bacteriological quality and physico-chemical parameters of domestic water sources in Samaru community, Zaria, Northwest Nigeria. Heliyon 2020, 6, e04773. [Google Scholar] [CrossRef]
- Dippong, T.; Mihali, C.; Mare Rosca, O.; Marian, M.; Payer, M.M.; Tîbîrnac, M.; Bud, S.; Coman, G.; Kovacs, E.; Hoaghia, M.-A. Physico-chemical characterization of water wells from Remeti, Maramures. Stud. Univ. Babes-Bolyai Chem. 2021, 66, 197–211. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011; pp. 1–541. ISBN 978-92-4-154815-1. [Google Scholar]
- Weisner, M.L.; Harris, M.S.; Mitsova, D.; Liu, W. Drinking water disparities and aluminum concentrations: Assessing socio-spatial dimensions across an urban landscape. Soc. Sci. Humanit. Open 2023, 8, 100536. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Mulligan, G.N. Study on the influence of water composition on iron nail corrosion and arsenic removal performace of the Kanchan arsenic filter (KAF). J. Hazard. Mater. Adv. 2023, 10, 100285. [Google Scholar] [CrossRef]
- Khan, M.; Din, I.; Aziz, F.; Qureshi, I.U.; Zahid, M.; Mustafa, G.; Sher, A.; Hakim, S. Chromium adsorption from water using mesoporous magnetic iron oxide-aluminum silicate absorbent: An investigation of adsorbtion isotherms and kinetics. Curr. Res. Green Sustain. Chem. 2023, 7, 100368. [Google Scholar] [CrossRef]
- Qu, Y.; Ji, S.; Sun, Q.; Zhao, F.; Li, Z.; Zhang, M.; Li, Y.; Zheng, L.; Song, H.; Zhang, W.; et al. Association of urinary nickel levels with diabetes and fasting blood glucose levels: A nationwide Chine population-based study. Ecotox. Environ. Saf. 2023, 252, 114601. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, F.; Liu, Q.; Zhao, X.; Song, K. Development of regional water quality criteria of lead for protecting aquatic organism in Taihu Lake, China. Ecotox. Environ. Saf. 2021, 222, 112479. [Google Scholar] [CrossRef] [PubMed]
- Abbaszade, G.; Tserendorj, D.; Salazar-Yanez, N.; Zachary, D.; Volgyesi, P.; Toth, E. Lead and stable lead isotopes as tracers of soil pollution and human health risk assessment in former industrail citisen of Hungary. Appl. Geochem. 2022, 145, 105397. [Google Scholar] [CrossRef]
- Obasi, P.N.; Akudinobi, B.B. Potential health risk and levels of heavy metals in water resources of lead–zinc mining communities of Abakaliki, southeast Nigeria. Appl. Water Sci. 2020, 10, 184. [Google Scholar] [CrossRef]
- Krok, B.; Mohammadian, S.; Noll, H.M.; Surau, C.; Markwort, S.; Fritzsche, A.; Nachev, M.; Sures, B.; Meckenstock, R.U. Remediation of zinc-contaminated groundwater by iron oxide in situ adsorption barriers—From lab to the field. Sci. Total Environ. 2022, 897, 151066. [Google Scholar] [CrossRef]
- Peng, H.; Yao, F.; Xiong, S.; Wu, Z.; Niu, G.; Lu, T. Strontium in public drinking water and associated public health risks in Chinese cities. Environ. Sci. Pollut. Res. 2021, 28, 23048–23059. [Google Scholar] [CrossRef]
- Manea, A.; Dumitru, M.; Vrinceanu, N.; Eftene, A.; Anghel, A.; Vrinceanu, A.; Ignat, P.; Dumitru, S.; Mocanu, V. Soil heavy metal status from Maramures county, Romania. In Proceedings of the GLOREP 2108 Conference, Timisoara, Romania, 15–17 November 2018. [Google Scholar]
- La Maestra, S.; D’Agostini, F.; Sanguineti, E.; González, A.Y.; Annis, S.; Militello, G.M.; Giovanni Parisi, G.; Scuderi, A.; Gaggero, L. Dispersion of Natural Airborne TiO2 Fibres in Excavation Activity as a Potential Environmental and Human Health Risk. Int. J. Environ. Res. Public Health 2021, 18, 6587. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef]
- Lorestani, B.; Merrikhpour, H.; Cheraghi, M. Assessment of heavy metals concentration in groundwater and their associated health risks near an industrial area. Environ. Health Eng. Manag. 2020, 7, 67–77. [Google Scholar] [CrossRef]
- Law 311 from 6rd June 2004 That Improves and Complements Law 458 from 29 July 2002 Regarding the Quality of Drinking Water. Official Gazette. Part I, no. 582/30.06.2004. (In Romanian). Available online: https://www.lege-online.ro/lr-LEGE-311%20-2004-(53106)-(1).html (accessed on 3 December 2023).
- Mishra, M.; Singhal, A.; Sriniva, R. Effect of urbanization on the urban lake water quality by using water quality index (WQI). Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Billong, P.T.N.; Feumba, R.; Ndjigui, P.-D. Hydrogeochemical appraisal of groundwater quality in Ngoua watershed (Doua-la-Cameroon): Implication for domestic purposes. Sci. Afr. 2023, 22, e01910. [Google Scholar] [CrossRef]
- Chen, F.; Yao, L.; Mei, G.; Shang, Y.; Xiong, F.; Ding, Z. Groundwater Quality and Potential Human Health Risk Assessment for Drinking and Irrigation Purposes: A Case Study in the Semiarid Region of North China. Water 2021, 13, 783. [Google Scholar] [CrossRef]
- Hopenhayn, C.; Ferreccio, C.; Browning, S.R.; Huang, B.; Peralta, C.; Gibb, H.; Hertz-Picciotto, I. Arsenic Exposure from Drinking Water and Birth Weight. Epidemiology 2003, 14, 593–602. [Google Scholar] [CrossRef] [PubMed]


| Element Content in Water, μg/L | P1 | P2 | P3 | P4 | P5 | P6 | Mean | SD | CV, % | Stnd. Skewness | Stnd. Kurtosis | MAC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC (μS/cm) | 763 | 398 | 172 | 1205 | 192 | 252 | 497 | 410 | 82.49 | 1.29 | 0.34 | 2500 |
| pH | 7.08 | 7.12 | 7.55 | 7.18 | 6.98 | 7.61 | 7.25 | 0.26 | 3.61 | 0.72 | −0.84 | 6.5–9.5 |
| DO (mg/L) | 6.79 | 5.65 | 9.06 | 5.33 | 9.77 | 9.55 | 7.69 | 2.01 | 26.13 | −0.16 | −1.34 | |
| T (NTU) | 1.31 | 3.92 | 8.75 | 1.14 | 2.56 | 5.48 | 3.86 | 2.90 | 75.20 | 1.01 | 0.22 | 5 |
| NH4+ (mg/L) | 0.88 | 0.48 | 0.28 | 1.09 | 0.04 | 0.77 | 0.59 | 0.39 | 66.82 | −0.21 | −0.64 | 0.5 |
| NO3− (mg/L) | 4.21 | 2.38 | 1.28 | 2.23 | 0.15 | 5.32 | 2.60 | 1.89 | 72.95 | 0.33 | −0.38 | 50 |
| ht (og) | 24.7 | 10.2 | 2.75 | 25.6 | 4.42 | 2.16 | 11.64 | 10.85 | 93.22 | 0.71 | −0.99 | min 5 |
| Cl− (mg/L) | 41.5 | 66.5 | 39.5 | 565.2 | 62.8 | 29.2 | 134.12 | 211.67 | 157.83 | 2.42 | 2.95 | 250 |
| PO43− (mg/L) | 0.86 | 0.13 | 0.15 | 0.19 | 0.17 | 0.14 | 0.27 | 0.29 | 105.44 | 2.42 | 2.94 | 0.4 |
| Element Content in Water, μg/L | P1 | P2 | P3 | P4 | P5 | P6 | Mean | SD | CV, % | Stnd. Skewness | Stnd. Kurtosis | MAC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li | 2.3 | 3.1 | 2.5 | 6.1 | 2.4 | 4.7 | 3.52 | 1.55 | 44.07 | 1.17 | −0.01 | 50 |
| Na | 18,345 | 16,889 | 25,455 | 59,745 | 24,332 | 11,045 | 25,969 | 17,361 | 66.85 | 1.95 | 2.12 | 200,000 |
| K | 2077 | 2045 | 2105 | 7245 | 2726 | 3044 | 3207 | 2020 | 62.99 | 2.23 | 2.57 | 10,000 |
| Ca | 1890 | 3558 | 1345 | 8921 | 2432 | 3779 | 3654 | 2746 | 75.15 | 1.83 | 1.86 | 100,000 |
| Mg | 10,124 | 20,144 | 8142 | 45,224 | 6885 | 12,784 | 17,217 | 14,505 | 84.25 | 1.93 | 1.90 | 50,000 |
| Ba | 17.2 | 20.1 | 15.5 | 80.2 | 31.6 | 27.3 | 31.93 | 24.41 | 76.31 | 2.12 | 2.35 | 700 |
| Sr | 88 | 126 | 75 | 446 | 132 | 204 | 178.5 | 138.6 | 77.64 | 1.92 | 1.92 | 200 |
| Al | 27.3 | 18.3 | 88.3 | 4.3 | 19.1 | 17.5 | 29.13 | 29.91 | 102.68 | 2.11 | 2.42 | 200 |
| Fe | 175 | 195 | 225 | 175 | 132 | 147 | 174.83 | 33.27 | 19.03 | 0.28 | −0.13 | 200 |
| Mn | 2.8 | 3.2 | 8.5 | 1.4 | 1.7 | 3.1 | 3.45 | 2.58 | 74.89 | 1.99 | 2.20 | 50 |
| Sn | 22.1 | 94.5 | 18.3 | 68.9 | 57.4 | 11.3 | 45.42 | 33.31 | 73.34 | 0.49 | −0.76 | - |
| Ti | 14.3 | 19.4 | 10.3 | 48.6 | 31.0 | 41.2 | 27.47 | 15.37 | 55.95 | 0.35 | −0.89 | - |
| As | 5.4 | 8.5 | 0.22 | 9.8 | 6.2 | 0.55 | 5.11 | 3.99 | 77.99 | −0.33 | −0.88 | 10 |
| Cr | 7.6 | 12.6 | 55.8 | 3.4 | 2.6 | 1.7 | 13.95 | 20.90 | 149.81 | 2.25 | 2.60 | 50 |
| Cu | 2.5 | 4.1 | 3.3 | 2.5 | 5.1 | 6.4 | 3.98 | 1.55 | 38.84 | 0.70 | −0.34 | 100 |
| Ni | 3.1 | 7.7 | 12.4 | 3.6 | 2.2 | 5.6 | 5.77 | 3.80 | 65.94 | 1.22 | 0.53 | 20 |
| Pb | 3.7 | 9.3 | 4.1 | 4.1 | 8.3 | 7.1 | 6.10 | 2.44 | 40.05 | 0.30 | −1.21 | 10 |
| Zn | 4.2 | 5.3 | 21.3 | 15.3 | 1.2 | 1.8 | 8.18 | 8.20 | 100.23 | 1.04 | −0.30 | 5000 |
| Physico-Chemical Parameter, Measure Units | Si * Value (pvi) | Weight, wi | Relative Weight, Wi | Variation of Q |
|---|---|---|---|---|
| EC (μS/cm) | 2500 | 4 × 10−4 | 0.17 × 10−6 | 6.88–48.2 |
| pH (pH units) | 9.5 | 0.11 | 0.000322 | 0.8–24.4 |
| Turbidity, NTU | 5 | 0.2 | 0.000585 | 22.8–175 |
| NH4+ mg/L | 0.5 | 2 | 0.00585 | 8–218 |
| NO3−, mg/L | 50 | 0.02 | 5.85 × 10−5 | 0.3–10.64 |
| Cl−, mg/L | 250 | 0.04 | 1.17 × 10−5 | 11.68–226.08 |
| PO43−, mg/L | 0.4 | 2.5 | 0.007319 | 32.5–215 |
| Li, mg/L | 0.05 | 20 | 0.058548 | 4.6–12.2 |
| Na, mg/L | 200 | 0.005 | 1.46 × 10−5 | 5.52–29.87 |
| K, mg/L | 10 | 0.1 | 0.000293 | 20.45–72.45 |
| Ca, mg/L | 100 | 0.01 | 2.93 × 10−5 | 1.35–8.92 |
| Mg, mg/L | 50 | 0.02 | 5.85 × 10−5 | 13.77–90.45 |
| Ba, mg/L | 0.7 | 1.429 | 0.004182 | 2.21–11.46 |
| Sr, mg/L | 0.2 | 5 | 0.014637 | 37.5–223.0 |
| Al, mg/L | 0.2 | 5 | 0.014637 | 2.15–44.15 |
| Fe, mg/L | 0.2 | 5 | 0.014637 | 66.0–112.5 |
| Mn, mg/L | 0.05 | 20 | 0.058548 | 2.8–17.0 |
| As, mg/L | 0.01 | 100 | 0.292742 | 2.2–98 |
| Cr, mg/L | 0.05 | 20 | 0.058548 | 3.4–111.6 |
| Cu, mg/L | 0.1 | 10 | 0.029274 | 2.5–6.4 |
| Ni, mg/L | 0.02 | 50 | 0.146371 | 11–62 |
| Pb, mg/L | 0.01 | 100 | 0.292742 | 37–93 |
| Zn, mg/L | 5 | 0.2 | 0.000585 | 0.024–0.426 |
| Groundwater Sample/Pollutant | P1 | P2 | P3 | P4 | P5 | P6 |
|---|---|---|---|---|---|---|
| NH4+ | ||||||
| Male | 0.0204 | 0.01116 | 0.0065 | 0.0254 | 0.0009 | 0.0179 |
| Female | 0.0206 | 0.0112 | 0.0065 | 0.0255 | 0.0009 | 0.0180 |
| Children | 0.0870 | 0.0475 | 0.0277 | 0.1078 | 0.0040 | 0.0761 |
| NO3− | ||||||
| Male | 0.0594 | 0.0336 | 0.0180 | 0.0314 | 0.0021 | 0.0750 |
| Female | 0.0596 | 0.0337 | 0.0181 | 0.0316 | 0.0021 | 0.0754 |
| Children | 0.2523 | 0.1426 | 0.0767 | 0.1336 | 0.0090 | 0.3188 |
| Sr | ||||||
| Male | 0.0033 | 0.0047 | 0.0028 | 0.0168 | 0.0050 | 0.0077 |
| Female | 0.0033 | 0.0048 | 0.0028 | 0.0168 | 0.0050 | 0.0077 |
| Children | 0.0055 | 0.0079 | 0.0047 | 0.0279 | 0.0083 | 0.0128 |
| Fe | ||||||
| Male | 0.0056 | 0.0063 | 0.0073 | 0.0056 | 0.0043 | 0.0047 |
| Female | 0.0057 | 0.0063 | 0.0073 | 0.0057 | 0.0043 | 0.0048 |
| Children | 0.0240 | 0.0267 | 0.0308 | 0.0240 | 0.0181 | 0.0201 |
| As | ||||||
| Male | 0.4061 | 0.6392 | 0.0165 | 0.7370 | 0.4662 | 0.0414 |
| Female | 0.4080 | 0.6421 | 0.0166 | 0.7404 | 0.4684 | 0.0416 |
| Children | 1.7260 | 2.7169 | 0.0703 | 3.1324 | 1.9817 | 0.1758 |
| Cr | ||||||
| Male | 0.5715 | 0.9475 | 4.1962 | 0.2557 | 0.1955 | 0.1278 |
| Female | 0.5742 | 0.9519 | 4.2155 | 0.2569 | 0.1964 | 0.1284 |
| Children | 2.4292 | 4.0274 | 17.8355 | 1.0868 | 0.8310 | 0.5434 |
| Cu | ||||||
| Male | 0.1128 | 0.1850 | 0.1489 | 0.1128 | 0.2301 | 0.2888 |
| Female | 0.1133 | 0.1858 | 0.1496 | 0.1133 | 0.2312 | 0.2901 |
| Children | 0.4795 | 0.7863 | 0.6329 | 0.4795 | 0.9781 | 1.2274 |
| Ni | ||||||
| Male | 0.0130 | 0.0322 | 0.0518 | 0.0150 | 0.0092 | 0.0234 |
| Female | 0.0130 | 0.0323 | 0.0520 | 0.0151 | 0.0092 | 0.0235 |
| Children | 0.0550 | 0.1367 | 0.2202 | 0.0639 | 0.0391 | 0.0994 |
| Pb | ||||||
| Male | 0.2782 | 0.6994 | 0.3083 | 0.3083 | 0.6242 | 0.5339 |
| Female | 0.2795 | 0.7026 | 0.3097 | 0.3097 | 0.6270 | 0.5364 |
| Children | 1.1826 | 2.9726 | 1.3105 | 1.3105 | 2.6530 | 2.2694 |
| HI | ||||||
| Male | 1.38 | 2.50 | 4.72 | 1.43 | 1.53 | 1.02 |
| Female | 1.39 | 2.51 | 4.74 | 1.44 | 1.53 | 1.02 |
| Children | 6.24 | 10.86 | 20.21 | 6.37 | 6.52 | 4.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dippong, T.; Mihali, C.; Avram, A. Evaluating Groundwater Metal and Arsenic Content in Piatra, North-West of Romania. Water 2024, 16, 539. https://doi.org/10.3390/w16040539
Dippong T, Mihali C, Avram A. Evaluating Groundwater Metal and Arsenic Content in Piatra, North-West of Romania. Water. 2024; 16(4):539. https://doi.org/10.3390/w16040539
Chicago/Turabian StyleDippong, Thomas, Cristina Mihali, and Alexandra Avram. 2024. "Evaluating Groundwater Metal and Arsenic Content in Piatra, North-West of Romania" Water 16, no. 4: 539. https://doi.org/10.3390/w16040539
APA StyleDippong, T., Mihali, C., & Avram, A. (2024). Evaluating Groundwater Metal and Arsenic Content in Piatra, North-West of Romania. Water, 16(4), 539. https://doi.org/10.3390/w16040539







