Abstract
Although mangrove forests occupy only 0.5% of the global coastal area, they account for 10–15% of coastal organic carbon (OC) storage, and 49–98% of OC is stored in sediments. The biogeochemistry of iron minerals and OC in marine sediments is closely related. To better reveal the role of iron minerals in OC preservation in mangrove sediments, an established dithionite–citrate–bicarbonate (DCB) extraction method was used to extract iron-bound OC (Fe-OC), and then the parameters of OC, Fe-OC, iron content, carbon isotopes, infrared spectroscopy, and XRD diffractions of sediments at a 1 m depth in four typical mangrove communities in the Gaoqiao Mangrove Reserve, Guangdong, China, were systematically measured. XRD diffractograms showed that the iron minerals in mangrove sediments may mainly exist in the form of goethite, which is consistent with the predominant types of iron minerals in marine sediments. About 10% of OC is directly bound to iron, and it is further estimated that about 2.4 × 1012–3.8 × 1012 g OC is preserved in global mangrove forests each year based on the high burial rate of OC in mangrove sediments. Lower Fe-OC/OC molar ratios indicated that iron mainly binds to OC via adsorption mechanisms. More depleted δ13CFe-OC relative to δ13Cbulk indicated that iron minerals are mainly associated with terrigenous OM, and the infrared spectra also revealed that iron minerals preferentially bind to terrigenous aromatic carbon. This work supports the “giant rusty sponge” view, elucidating that iron plays an important role in the preservation of OC in mangrove sediments.
1. Introduction
Protecting organic carbon stored in forests is considered important for mitigating climate change. Like terrestrial ecosystems, coastal ecosystems also store large amounts of carbon known as blue carbon. As typical coastal blue carbon ecosystems, mangrove forests play an important role in the global carbon cycle and are a place of material exchange between marine and terrestrial ecosystems [1,2]. Although mangrove forests occupy only 0.5% of the global coastal area, they account for 10–15% of coastal carbon storage [3], and 49–98% of organic carbon (OC) is stored in sediments [4]. However, despite the increasing recognition of the urgent need for climate change mitigation, stability assessments of sediment carbon pools for mangrove ecosystems have rarely been conducted.
The protective effect minerals have on OC is the product of a highly important stabilizing mechanism. More than 90% of organic matter (OM) in marine sediments cannot be separated from clay minerals and iron minerals, and this holds especially true for iron minerals that are closely associated with the long-term preservation of OC [5]. A previous study found that iron-bound OC (Fe-OC) in Arctic marine sediments persists below the uppermost oxygenated sediment layer over thousands of years [6]. In addition, the binding of iron and carbon in sediments can reduce the rate of OC decomposition through adsorption and co-precipitation due to the characteristics of iron minerals, such as their large specific surface area, strong adsorption capacity, and high reactivity [7].
As for the quantitative assessment of Fe-OC, the protective effect of iron minerals on OC in sediments of forests [8], grasslands [9], farmlands [10], plateau frozen soils [11], lakes [12], and other terrestrial ecosystems has been studied, with the results showing that about 40% of OC is preserved by iron. As for marine ecosystems, a recent study found that about 21.5% of the OC in global marine sediments is directly bound to iron minerals [13]. More recent research has investigated the percentage of iron-bound carbon (fFe-OC) in continental margins (20.71–26.59%) [6,14,15], continental shelves (4.50–36.88%) [16,17,18], estuaries (7.40–22.96%) [19,20,21,22,23], deltas (8.1–22.2%) [24], and volcanic ash (79%) [16]. In one study, the fFe-OC was up to 79%, which strongly supports the view that iron serves as a “rust sink” for OC. Longman et al. (2022) further estimated that the marine Fe-OC sinks were between 31 and 70 Mt C/yr−1 (average 52 Mt C/yr−1) [25].
The carbon sequestration rate of mangrove sediments is high, being twice that of coastal salt marsh, 10 times that of lake sediments, and 22 times that of dryland [26]. However, the importance of iron minerals’ contribution to the mangrove sediment carbon pool and the characterization of Fe-OC in mangrove sediments have not been systematically studied and reported, respectively; therefore, sediment core samples from four typical mangrove communities in the Gaoqiao Mangrove Reserve in the Guangdong province were collected to assess the characteristics of Fe-OC and analyze the factors influencing these characteristics. The main aims of this study were as follows: (1) to carry out a quantitative evaluation of iron minerals’ contribution to carbon sequestration and the burial rate of Fe-OC in mangrove sediments; (2) to analyze the factors influencing the binding of iron and OC; and (3) to analyze the binding mechanism and molecular composition of Fe-OC.
2. Sample and Methods
2.1. Sample Sites
The Gaoqiao Mangrove Reserve is located at the core of the Zhanjiang Mangrove National Nature Reserve in the Guangdong province, China, within the Yingluo Bay at the entrance of the Ximi River in the Beibu Bay. Their mangrove communities are mainly composed of Aegiceras corniculatum, Bruguiera gymnorrhiza, Kandelia obvolata, Rhizophora stylosa, and Avicennia marina communities, among others [27]. The natural zonation of the mangrove species is obviously influenced by various ecological and environmental factors, including geographical location, tidal conditions, soil composition, etc. [28]. The Kandelia obvolata plus Bruguiera gymnorrhiza communities, Aegiceras corniculatum community, Rhizophora stylosa community, and Avicennia marina community were selected for sediment collection in this study. The locations of the sampling sites are shown in Figure 1. The community structures and elevations of the different sampling sites are shown in Table 1. The Avicennia marina community had the lowest elevation (1.22 m) compared with the other three sites (Kandelia obvolata plus Bruguiera gymnorrhiza communities: 1.98 m; Aegiceras corniculatum community: 2.2 m; Rhizophora stylosa community: 1.84 m), indicating that this site is at low tide.
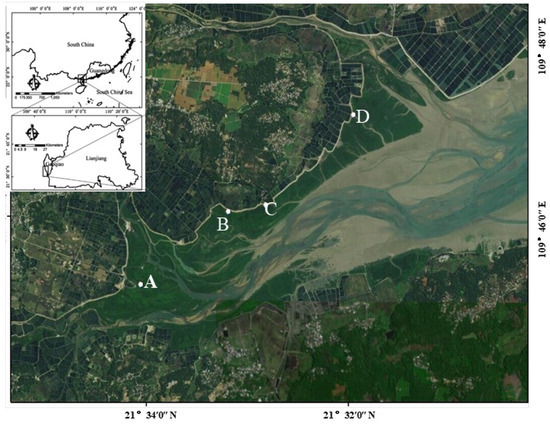
Figure 1.
Locations of the sampling sites in this study.

Table 1.
Community compositions and mudflat elevations of the sampling sites.
2.2. Sediment Collection
Sediment samples were collected from quadrats using a PVC pipe that was 7.5 cm in diameter and 1.2 m in length. Within each plant quadrat, three sediment columnar samples were collected before being divided into seven layers (0–5 cm, 5–10 cm, 10–20 cm, 20–30 cm, 30–40 cm, 40–60 cm, and 60–100 cm) and mixed evenly. All segmented samples were then sealed and stored in a plastic bag and transported to the laboratory for freezing at −4 °C. Dead roots, stems, stones, and large visible particles were removed from the sediment samples; then, each sample was ground using a mortar and sifted through a 60-mesh sieve.
2.3. Mineral Composition
The mineral compositions of the subsamples were analyzed using an X-ray diffractometer equipped with a Ni filter and Cu Kα radiation (40 kV and 40 mA). The diffraction patterns were collected from 2° to 55° 2θ at a scanning rate of 3° 2θ min−1.
2.4. OC and Stable Carbon Isotope Analyses
The OC content and stable carbon isotope values of the bulk mangrove sediments were determined in the Guangzhou Institute of Geochemistry, Chinese Academy of Sciences. The carbonate minerals were removed from the sediment samples with 10% hydrochloric acid, rinsed with deionized water, and dried at 40 °C. The OC concentrations were determined using a Sunset laboratory elemental analyzer, and the stable carbon isotope values were determined using an isotope mass spectrometer (IRMS). The error was no more than 1‰. The standard consisted of a homogenous batch of protein (casein) standard and was used to determine the carbon isotope values. The mean carbon isotope value was −26.98 ± 0.13‰.
2.5. Iron Extraction and Fe-OC Analyses
After analyzing the OC and carbon isotope values, iron minerals in the mangrove sediments were further extracted. A modified dithionite–citrate–bicarbonate method that involved the release of Fe-OC while being gentle with clay minerals was used for iron extraction [13,29]. The procedure involved the following: 0.5 g freeze-dried sediments were added into a 30 mL solution containing 0.27 mol/L trisodium citrate and 0.11 mol/L NaHCO3-neutral buffer in a 50 mL centrifuge tube and heated to 80 °C in a water bath. Then, 0.5 g sodium dithionite was added to the solution, which had been maintained at 80 °C for 15 min. Then, the solution was centrifuged at 3000 rpm for 10 min, the supernatant was separated, and the solid residue was cleaned thrice with pure deionized water. The rinse water was mixed with the supernatant, acidified, filtered, and diluted 10 times. Finally, iron concentration was measured using an atomic absorption spectrometer. The solid residue was dried and ground to analyze the OC and stable carbon isotope values.
Control experiments were carried out under the same conditions as the reduction treatment to prevent overestimates of the amount of iron-bound carbon (CFe-OC), but we replaced trisodium citrate and sodium dithionite with sodium chloride with an equivalent ionic strength. Inorganic minerals in the mangrove sediments were removed by using a 10% hydrochloric acid solution, and the OC (OCcontrol) and stable carbon isotope (δ13Ccontrol) values were subsequently determined. The content, proportion, and carbon isotope values of Fe-OC were determined using Equations (1)–(3), respectively.
CFe-OC = OCcontrol − OCexperiment
fFe-OC = CFe-OC/OCcontrol × 100%
δ13CFe-OC = δ13Ccontrol − δ13Cexperiment × (1 − fFe-OC)/fFe-OC
2.6. The Molecular Composition of Fe-OC
Fourier-transform infrared spectroscopy was carried out using a Bruker VERTEX 70V (origin: Germany) equipped with dual detectors based on DTGS (room temperature; origin: Germany) and InGaAs (room temperature; origin: Germany) to characterize the molecular composition of Fe-OC. The following simple steps were carried out: the freeze-dried sediment was mixed with potassium bromide (mass ratio 1:100), ground to a paste under an infrared lamp, and then pressed. Infrared spectrograms were obtained by using the aforementioned infrared spectrophotometer at room temperature.
2.7. Statistical Analyses
Statistical analyses were conducted using SPSS 27 software. A Pearson correlation analysis with a two-tailed test was carried out to identify correlations between all measured parameters. Based on the 95% confidence intervals, significant statistical differences were determined using a one-way analysis of variance and a t test. Regression analysis was performed to determine the relationship between the iron and OC concentrations.
3. Experimental Results
3.1. Mineral Composition
The mineral composition of the mangrove sediments in the four typical mangrove communities mainly consists of quartz and kaolinite minerals, accompanied by a small amount of calcite (Figure 2). It is worth noting that goethite was found in all subsamples, which is consistent with the conclusion that goethite is the main iron mineral in marine sediments (Figure 2) [13,21].
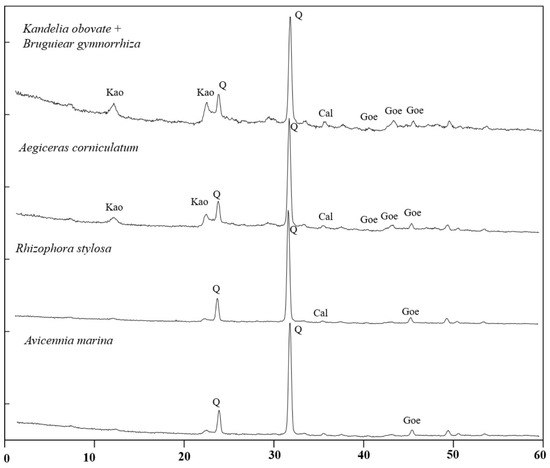
Figure 2.
Mineral composition of sediments of four typical mangrove communities in Gaoqiao Mangrove Reserve. Kao: kaolinite; Q: quartz; Cal: calcite; Goe: Goethite.
3.2. Bulk OC Concentration and Carbon Isotope Signatures
The OC concentrations in the subsamples of the four mangrove communities gradually decreased with increasing depth, as shown by the vertical axis in Figure 3a. OC gradually decreased after reaching the maximum value of 1.23% at 20 cm depth in the Avicennia marina sediments, while a maximum value of 2.59% was achieved at 30 cm depth in the in Rhizophora stylosa sediments; a maximum value of 2.82% was achieved at 40 cm depth in the Kandelia obvolata plus Bruguiera gymnorrhiza sediments, and a maximum value of 3.14% was achieved at 60 cm depth in the Aegiceras corniculatum sediments. On average, the OC concentrations were lowest in the Avicennia marina sediments, with an average value of 0.78 ± 0.39%, but the highest OC concentrations were found in the Kandelia obvolata plus Bruguiera gymnorrhiza sediments, with an average value of 2.24 ± 0.35%. The average OC concentrations in the Aegiceras corniculatum and Rhizophora stylosa sediments were 1.65 ± 0.77% and 1.63 ± 0.62%, respectively. In addition, the OC concentrations in the Avicennia marina sediments were significantly different compared with those of the other three sites (p < 0.001).
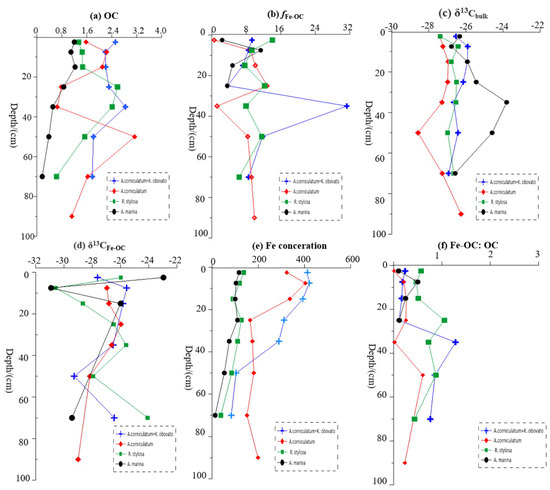
Figure 3.
The characterization of iron-bound OC (Fe-OC) in mangrove sediments at four sampling sites in Gaoqiao Mangrove Reserve; (a) OC concentration (wt/%); (b) percentage of iron-bound OC (%); (c) carbon isotope values for bulk sediment (‰); (d) carbon isotope value of Fe-OC (‰); (e) iron concentration (μmol/g); (f) molar ratio of Fe-OC/OC.
3.3. Characterization of Fe-OC and Carbon Isotope Values
The content and percentage of iron-bound carbon (CFe-OC, fFe-OC) were evaluated based on the differences in OC concentration in the extraction and control experiments (Figure 3b and Figure 4). The values of CFe-OC and fFe-OC were the highest (0.27 ± 0.25%, 11.48 ± 8.4%) in the Kandelia obvolata plus Bruguiera gymnorrhiza sediments and the lowest (0.06 ± 0.04%, 5.54 ± 3.45%) in the Avicennia marina sediments. The values of fFe-OC in the Aegiceras corniculatum and Rhizophora stylosa sediments were 7.32 ± 4.28% and 9.88 ± 2.62%, respectively. On average, it was estimated that about 10% of the OC in the subsamples from the four typical mangrove communities was directly bound to iron.
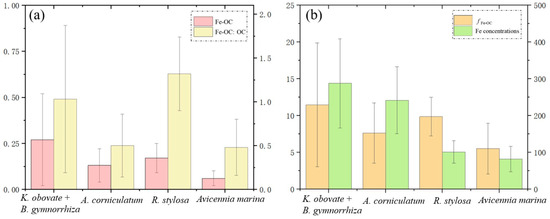
Figure 4.
Average content of Fe-OC and molar ratio of Fe-OC/OC. (a) Average percentage of Fe-OC (fFe-OC) and iron concentration at four sampling sites (b) in the Gaoqiao Mangrove Reserve.
Although no significant differences between the δ13C values for the iron-bound carbon (δ13CFe-OC) and bulk sediments (δ13Cbulk) were found (p > 0.05), the average values of δ13CFe-OC were visibly more depleted 13C than that of the bulk sediments (Figure 3c,d and Figure 5) (difference: up to 2‰).
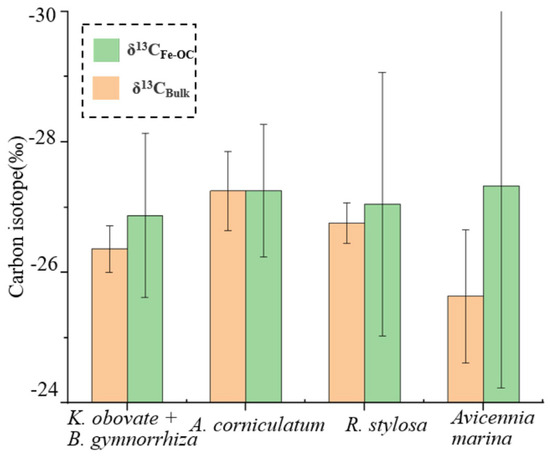
Figure 5.
Average carbon isotope values of bulk sediments (δ13Cbulk) and Fe-OC (δ13CFe-OC) at four sampling sites in the Gaoqiao Mangrove Reserve.
3.4. Iron Concentration
The iron concentrations gradually decreased with the increase in sampling depth, but the iron concentrations in the Aegiceras corniculatum sediments reached the lowest value at 30 cm (164.74 μmol/g) and then remained basically unchanged with increasing depth, ranging from 151.35 to 404.08 μmol/g (241.77 ± 90.93 μmol/g). The iron concentrations ranged from 13.61 to 116.84 μmol/g (average: 81.82 ± 34.59 μmol/g), 38.24 to 137.63 μmol/g (average: 101.33 ± 30.84 μmol/g), and 151.35 to 404.08 μmol/g (average: 287.92 ± 121.04 μmol/g) in the Avicennia marina, Rhizophora stylosa, and Kandelia obvolata plus Bruguiera gymnorrhiza sediments, respectively, while the Avicennia marina sediments had the lowest iron concentration (Figure 3e). Additionally, there were significant differences between the iron concentrations in the Avicennia marina sediments and those of the other three sites (p < 0.05).
3.5. Relationship between Iron Content and OC Concentration
A significant linear positive correlation between the iron content and OC concentration was observed in the four sample sites (R2 = 0.367, p < 0.001) (Figure 6). In addition, the average molar ratio of Fe-OC/Fe in the four sample sites was 0.8 ± 0.02, which is lower than 1.0 (Figure 3f). At low tide, the Avicennia marina sediments had the lowest molar ratio (0.48 ± 0.32), but the highest ratio (1.32 ± 0.42) was found in the Rhizophora stylosa sediments. The molar ratios of Fe-OC/Fe in the Aegiceras corniculatum and Kandelia obvolata plus Bruguiera gymnorrhiza sediments were 0.50 ± 0.36 and 1.03 ± 0.84, respectively.
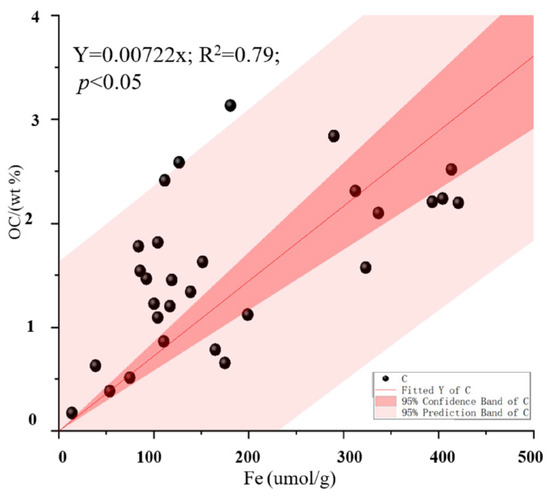
Figure 6.
Scatter graph of iron concentration (μmol/g) vs. OC content (wt %) in all collected subsamples.
3.6. The Molecular Composition of Fe-OC
An analysis of the mangrove sediments before and after iron extraction, carried out via attenuated total reflectance–Fourier-transform infrared spectroscopy (ATR−FTIR), was performed to qualitatively analyze the molecular composition of Fe-OC. The experimental results showed that obvious mineral peaks, including kaolinite and montmorillonite, appeared at 800–1200 cm−1 whether iron minerals were extracted or not. Aliphatic carbon was enriched at 2800–3000 cm−1 after extracting iron in the Kandelia obvolata plus Bruguiera gymnorrhiza sediments and Aegiceras corniculatum sediments. However, there was no obvious peaks at 2800–3000 cm−1 in the Avicennia marina sediments before and after iron extraction, which may be due to the lower OC content.
4. Discussion
4.1. Percentage of Iron-Bound Carbon and Influencing Factors
In this study, the average OC concentration was lowest in the Avicennia marina sediments at low tide but highest in the Kandelia obvolata plus Bruguiera gymnorrhiza sediments at middle/high tide. The amount and dynamics of OC vary greatly among different mangrove forests, which are largely affected by tidal gradient, vegetation biomass, and primary productivity, as well as species composition and suspended matter deposition [30]. Higher tidal levels may increase the OC concentration for two possible reasons. Firstly, the increased productivity along tidal gradients leads to more plant litter and root exudates entering into the anaerobic soils [31,32]. Secondly, the extensive root system of mangrove forests at middle or high tide promotes the retention of organic matter (OM) and the deposition of fine sediment components with high OC concentrations [33].
More importantly, we observed that a significant amount of OC was bound to iron in the mangrove sediments (Figure 3b and Figure 4). Our results show that the average value of fFe-OC in the four sampling sites is about 10%, which is slightly lower than that of classical marine sediments (21.5 ± 8.6%) [13] but higher than that of estuarine sediments (7.4 ± 3.5%) [19]. The binding of iron and OC is influenced by multiple factors, such as the deposition rate, the redox state, and hydrodynamic strength [21,25]. Previous studies have found that fFe-OC values are significantly different in continental margins, continental shelves, estuarine sediments, delta sediments, coastal sediments, tephra depositions, and other marine sedimentary environments [13], indicating that the sedimentary environment significantly affects the binding of iron and OC.
In mangrove sediments, the concentrations of OC and Fe-OC are affected by multiple factors, such as tree species, the tidal range, and mangrove productivity [31,32]. A previous study revealed that the OC concentration in Avicennia marina sediments is much lower than that of Kandelia obvolata plus Bruguiera gymnorrhiza sediments and Rhizophora stylosa sediments in the Gaoqiao Mangrove Reserve, which may indicate that community composition and tidal level significantly affect the OC concentration [33]. In addition, the accumulation of OC and the binding of iron to OC are also significantly influenced by tidal level [19,21]. Frequent leaching at low tide may prevent the binding of OC and iron minerals, but the less frequent leaching and relatively stable sedimentary environment at middle and high tide is beneficial for the binding of iron and OC [24,34]. In this study, the average value of fFe-OC in the Avicennia marina sediments at low tide was also the lowest compared with the other three sites, which indicates that the tidal level may influence the binding of iron and OC.
4.2. Binding Mechanism of Iron and OC
The binding of iron and OC mainly occurs through adsorption and co-precipitation mechanisms, which can be determined based on the molar ratio of Fe-OC/Fe [35]. Adsorption refers to the ligand exchange between the carbonyl/hydroxyl functional groups on the surface of OM and the functional groups of iron [36], which have the characteristics of a high adsorption heat, low desorption, and high bond stability [37]. In addition, the amount of OC adsorbed by iron is relatively smaller, and adsorption occurs on the surface of minerals, so the molar ratio of Fe-OC/Fe is often lower than 1.0 [38,39]. Co-precipitation refers to the combination of Fe3+ in solution with acidic functional groups of dissolved OM (DOM) to form insoluble an Fe3+–organic ligand complex and the subsequent transfer of OM to deeper anoxic sedimentary layers through deposition, physical modification, and bioturbation [40]. More OC is adsorbed by co-precipitation than by adsorption, and the molar ratio of Fe-OC/Fe is much higher than 1.0 [13,30].
In our experimental results, the average molar ratios of Fe-OC/Fe at the four sampling sites (A, B, C and D) were all close to 1.0 (1.03 ± 0.84, 0.50 ± 0.36, 1.32 ± 0.42, and 0.48 ± 0.32, respectively), indicating that adsorption was the main iron and OC binding mechanism. The adsorption mechanisms mainly included hydrogen bonding, hydrophobic interactions, ligand exchange, and cation bridging [41]. It has been found that the average molar ratios of Fe-OC/Fe in most mangrove sediments from coastal estuaries in China are lower than 1.0, which is consistent with our results [42]. However, other studies have also found that the average molar ratios of Fe-OC/Fe for mangrove sediments in six coastal areas of the Philippines and marine sediments are much greater than 1.0, suggesting that iron and OC are mainly bound by co-precipitation [30]. Differences in molar ratios in different regions may be influenced by sediment type, mineral compositions, and iron concentration [25]. The sediments featured in this study are mainly composed of clay minerals, goethite, and quartz, and they only contain a small amount of calcite; however, the sediments in the Philippines mangrove forests are mainly composed of silicate minerals, and their iron contents are relatively low [30]. Therefore, differences in mineral composition may affect the binding of iron to OC [13,21].
4.3. Carbon Isotope and Molecular Characteristics of Fe-OC
The OC in the mangrove sediments mainly included two sources, namely allochthonnus (tidally induced marine input and fluvially transported upstream sediments) and autochthonous (mangrove biomass) sources [43]. Additionally, the material circulation of mangrove ecosystems was promoted through the exchange of terrestrial and marine OM in mangrove ecosystems [44]. Stable carbon isotopes can be used to distinguish whether OC was sourced from terrestrial or marine sources, and it is generally believed that the carbon isotope of marine OC is more rich in 13C than that in terrestrial OC (δ13Cmarine: −20.9‰; δ13Cterrigenous: −26.5‰) [45,46], so the source of OC in mangrove sediments can be roughly determined. In our study, the carbon isotope values of the mangrove sediments (δ13Cbulk) ranged from −28.53‰ to −23.81‰ (average: −26.55 ± 0.85‰), showing typical terrigenous input characteristics. However, the average value of δ13C in the Avicennia marina sediments (−25.64 ± 1.02‰) was significantly lower than that of the other three sites (p < 0.05) (Kandelia obvolata plus Bruguiera gymnorrhiza: −26.36 ± 0.36‰; Aegiceras corniculatum: −27.25 ± 0.6‰; Rhizophora stylosa: −26.76 ± 0.31‰), indicating that the Avicennia marina sediments were more affected by marine OM input, which may be because the Avicennia marina community was located at lower tides, leading to greater marine OM deposition [33]. Furthermore, the carbon isotope value of Fe-OC (δ13CFe-OC) in all subsamples was more depleted in 13C than in the bulk sediments (Figure 5), which indicated that more terrigenous OM preferentially combined with iron minerals.
To further analyze the molecular composition of Fe-OC, the molecular differences between the subsamples were compared via FTIR analysis. It has been found that infrared spectrum peaks at 1600–1750 cm−1 and 2800–3000 cm−1 can be used to study the molecular composition of OM, and spectra peaks in the range of 1600–1750 cm−1 usually contain fingerprint peaks of amide, carboxylic acid, and aromatic functional groups [47]. Peaks at 2895 cm−1–2930 cm−1 indicate aliphatic carbon [48], while peaks at 500–1200 cm−1 indicate the presence of clay minerals and other iron/aluminum minerals, such as kaolinite and montmorillonite, which peak at 850–1200 cm−1 [49,50]. In this study, obvious mineral peaks occurred at 400–1200 cm−1 before and after the removal of Fe-OC (Figure 7), which was consistent with the diffraction peak of clay minerals found in our XRD analysis. More importantly, the spectra at 2895–2930 cm−1 were more prominent after the removal of Fe-OC, indicating that the aliphatic carbon was enriched after extraction. Zhao et al. (2016) demonstrated that the Fe-OC in forest soils was less aliphatic, more carboxylic, and enriched in 13C by considering an FTIR analysis, NEXAFS analysis (near-edge X-ray absorption fine structure analysis), and 13C [48]; this finding is consistent with our experimental results.
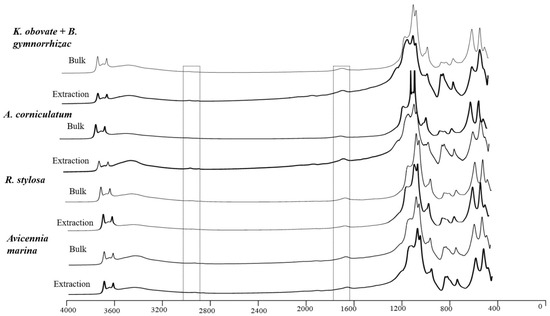
Figure 7.
Comparison of infrared spectra before and after Fe-OC extraction in sediments of four typical mangrove communities.
4.4. Estimation of the Burial Rate of Fe-OC
Mangroves are highly productive wetlands that photosynthetically sequester atmospheric CO2 as OC. A varying proportion of OC was buried in tidally inundated, hypoxic acid and anoxic sediments, and thus largely prevented from returning to the atmosphere. Based on the fFe-oc in this study and sediment OC burial rate in mangrove ecosystems on a global scale (24–38.3 Tg C a−1) [51], we believe that an average of 10% OC (2.4 × 1012–3.8 × 1012 g C a−1) was buried by binding with iron at the global mangrove ecosystem (4). A previous study has shown that about 21% of OC in marine sediments is preserved by binding with iron minerals [13], which may overestimate the role of iron minerals in marine sediments. In fact, the deposition of nutrients and plant litter carried by seawater tides into the sediment could lead to higher OC burial rates in mangrove ecosystems [51,52,53,54]. In this study, although the fFe-OC was relatively low, the higher OC burial rate in mangrove ecosystems still lead to higher Fe-OC burial rates. Thus, our study suggests that iron plays an important role in the preservation of OC in mangrove sediments.
Fe-OCburial rate = OCburial rate × fFe-oc
5. Conclusions
More than 90% of OM in marine sediments cannot be separated from clay minerals and iron minerals (with this holding especially true for the latter), but the role of iron minerals in mangrove sediments has been rarely revealed. In this study, four one-meter soil cores were collected from Aegiceras corniculatum plus Kandelia obovate, Aegiceras corniculatum, Rhizophora stylosa, and Avicennia marina sediments in the Gaoqiao Mangrove Reserve, Guangdong province, China, respectively. A dithionite–citrate–bicarbonate reduction procedure was employed to extract iron from these sediments. The OC concentrations, carbon isotope values, mineral compositions, and iron contents were measured before and after sediment extraction. About 10% of OC in mangrove sediments is bound by iron through an adsorption mechanism, and the binding between iron and OC was mainly influenced by community composition and tidal level. Carbon isotope signatures of Fe-OC (δ13CFe-OC) indicate that iron preferentially preserved terrigenous organic matter, implying that iron aids the incorporation of terrigenous OC. The differences in the infrared spectra before and after iron extraction also indicated that the iron-bound OC was less than aliphatic carbon. Based on the global OC burial rate in mangrove sediments, it is estimated that a global mass of 2.1 × 1012–3.8 × 1012 g OC a−1 is preserved by iron. Thus, we conclude that iron plays an important role in stabilizing terrestrial OC in mangrove sediments. Furthermore, iron serves as an effective rust sink for the preservation of OC in sediments and contributes to the global carbon cycle.
Author Contributions
K.L.: data collection, formal analysis, visualization, and writing—original draft. H.H.: conceptualization, validation, formal analysis, writing—review and editing, supervision, and funding acquisition. D.D.: data collection and analysis. S.Z.: formal analysis. R.Y.: formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Marine Economy Special Project of the Guangdong Province in 2024 for the “Identification and Model Prediction of Key Restoration Areas of Typical Blue Carbon Ecosystem”. Science and Technology Project of Guangdong Forestry Administration (2024): Monitoring and Ecological Value Assessment of Coastal Wetland Resources in the Guangdong Province. The Science and Technology Project of Guangdong Forestry Administration (2023): Verification of Human Activities in Marine Protected Areas and Monitoring and Assessment of Typical Ecosystems. The Science and Technology Project of Guangdong Forestry Administration (2023): Research on Carbon Storage Verification, Potential Assessment and Car-bon Sink Trading Mechanism of Typical Coastal Wetlands. Marine Economy Special Project of Guangdong Province in 2023 for “Marine Carbon Source, Carbon Sink, Carbon Flux Investigation and Assessment of Carbon Sequestration Technology and Application Research”. The APC was funded by the Science and Technology Project of Guangdong Forestry Administration (2023): Verification of Human Activities in Marine Protected Areas and Monitoring and Assessment of Typical Eco-systems.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Thanks to the two reviewers and editors for their valuable suggestions on this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Atwood, T.B.; Connolly, R.M.; Almahasheer, H. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Chang. 2017, 7, 523–528. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon Sequestration in Mangrove Forests. Carbon Manag. 2012, 3, 313–322. [Google Scholar] [CrossRef]
- Duarte, C.M.; Middelburg, J.J.; Caraco, N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2005, 2, 1–8. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Hedges, J.I.; Keil, R.G. Sedimentary organic matter preservation: An assessment and speculative synthesis. Mar. Chem. 1995, 49, 81–115. [Google Scholar] [CrossRef]
- Faust, J.C.; Tessin, A.; Fisher, B.J.; Zindorf, M.; Papadaki, S.; Hendry, K.R.; Doyle, K.A.; März, C. Millennial scale persistence of organic carbon bound to iron in Arctic marine sediments. Nat. Commun. 2021, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Boudot, J.P.; Hadj, A.B.; Steiman, R.; Seigle-Murandi, F. Biodegradation of synthetic organo-metallic complexes of iron and aluminium with selected metal to carbon ratios. Soil Biol. Biochem. 1989, 21, 961–966. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Hall, S.J.; Hui, D.; Li, J.; Chen, G.; Sun, L.; Zhang, D.; Deng, Q. Increased interactions between iron oxides and organic carbon under acid deposition drive large increases in soil organic carbon in a tropical forest in southern China. Biogeochemistry 2022, 158, 287–301. [Google Scholar] [CrossRef]
- Che, M.; Gong, Y.; Xu, M.; Kang, C.; Lv, C.; He, S.; Zheng, J. Effects of elevation and slope aspect on the distribution of the soil organic carbon associated with Al and Fe mineral phases in alpine shrub–meadow soil. Sci. Total Environ. 2021, 753, 141933. [Google Scholar] [CrossRef]
- Hu, J.; Zeng, Q.; Chen, H.; Dong, H. Effect of bacterial cell addition on Fe (III) reduction and soil organic matter transformation in a farmland soil. Geochim. Cosmochim. Acta. 2022, 325, 25–38. [Google Scholar] [CrossRef]
- Mu, C.C.; Zhang, T.J.; Zhao, Q.; Gao, H.; Zhong, W.; Su, H.; Wu, Q.B. Soil organic carbon stabilization by iron in permafrost regions of the Qinghai-Tibet Plateau. Geophys. Res. Lett. 2016, 43, 286–294. [Google Scholar] [CrossRef]
- Peter, S.; Sobek, S. High variability in iron-bound organic carbon among five boreal lake sediments. Biogeochemistry 2018, 139, 19–29. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gelinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Zhu, M.X.; Yang, G.P.; Li, T. Iron geochemistry and organic carbon preservation by iron (oxyhydr) oxides in surface sediments of the East China Sea and the south Yellow Sea. J. Mar. Syst. 2018, 178, 62–74. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, M.X.; Yang, G.P.; Ma, W.W. Reactive iron and iron-bound organic carbon in surface sediments of the river-dominated Bohai Sea (China) Versus the Southern Yellow Sea. J. Geophys. Res. Biogeosci. 2019, 124, 79–98. [Google Scholar] [CrossRef]
- Longman, J.; Gernon, T.M.; Palmer, M.R.; Manners, H.R. Tephra deposition and bonding with reactive oxides enhances burial of organic carbon in the Bering Sea. Global Biogeochem. Cycles 2021, 35, e2021GB007140. [Google Scholar] [CrossRef]
- Salvadó, J.A.; Tesi, T.; Andersson, A.; Ingri, J.; Dudarev, O.V.; Semiletov, I.P.; Gustafsson, O. Organic carbon remobilized from thawing permafrost is resequestered by reactive iron on the Eurasian Arctic Shelf. Geophys. Res. Lett. 2015, 42, 8122–8130. [Google Scholar] [CrossRef]
- Ghaisas, N.A.; Maiti, K.; Roy, A. Iron-Mediated Organic Matter Preservation in the Mississippi River-Influenced Shelf Sediments. J. Geophys. Res-Biogeo. 2021, 126, e2020JG006089. [Google Scholar] [CrossRef]
- Zhao, B.; Yao, P.; Bianchi, T.S.; Cui, X.Q.; Zhang, X.W.; Huang, X.Y.; Schroeder, C.; Zhao, J.; Yu, Z.G. The role of reactive iron in the preservation of terrestrial organic carbon in estuarine sediments. J. Geophys. Res-Biogeo. 2018, 123, 3556–3569. [Google Scholar] [CrossRef]
- Sirois, M.; Couturier, M.; Barber, A.; Gelinas, Y.; Chaillou, G. Interactions between iron and organic carbon in a sandy beach subterranean estuary. Mar. Chem. 2018, 202, 86–96. [Google Scholar] [CrossRef]
- Zhao, B.; Yao, P.; Bianchi, T.S.; Shields, M.R.; Cui, X.Q.; Zhang, X.W.; Huang, X.Y.; Schroeder, C.; Zhao, J.; Yu, Z.G. Preferential preservation of pre-aged terrestrial organic carbon by reactive iron in estuarine particles and coastal sediments of a large river-dominated estuary. Geochim. Cosmochim. Acta. 2023, 345, 34–49. [Google Scholar] [CrossRef]
- Sun, C.H.; Zhu, M.X.; Ma, W.W.; Sun, Z.L.; Zhang, X.R.; Ding, K.Y.; Liu, S.H. Examining bulk and iron-associated organic carbon through depth in margin sea sediments (China) under contrasting depositional settings: Chemical and NEXAFS spectral characterization. J Mar. Syst. 2020, 207, 103344. [Google Scholar] [CrossRef]
- Bai, J.; Luo, M.; Yang, Y.; Xiao, S.; Zhai, Z.; Huang, J. Iron-bound carbon increases along a freshwater−oligohaline gradient in a subtropical tidal wetland. Soil Biol. Biochem. 2021, 154, 108128. [Google Scholar] [CrossRef]
- Shields, M.R.; Bianchi, T.S.; Gélinas, Y.; Allison, M.A.; Twilley, R. Enhanced terrestrial carbon preservation promoted by reactive iron in deltaic sediments. Geophys. Res. Lett. 2016, 43, 1149–1157. [Google Scholar] [CrossRef]
- Longman, J.; Faust, J.C.; Bryce, C.; Homoky, W.B.; Marz, C. Organic carbon burial with reactive iron across global environments. Glob. Biogeochem. Cycles 2022, 36, e2022GB007447. [Google Scholar] [CrossRef]
- Bouillon, S.; Borges, A.V.; Castañeda-Moya, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.; et al. Mangrove production and carbon sinks: A revision of global budget estimates. Global Biogeochem. Cycles 2008, 22, 2. [Google Scholar] [CrossRef]
- Lin, P. Ecological notes on mangroves in southeast coast of China including Taiwan Province and Hainan Island. Acta Ecol Sin. 1981, 1, 283–290. [Google Scholar]
- Hong, P.N.; San, H.T. Mangroves of Vietnam; IUCN. The World Conservation Union: Bangkok, Thailand, 1993; p. 193. [Google Scholar]
- Mehra, O.P.; Jackson, M.L. Iron oxide removal from soils and clays by a dithionite–citrate system buffered with sodium bicarbonate. In Clays and Clay Minerals; Pergamon: Oxford, UK, 2013; pp. 317–327. [Google Scholar]
- Dicen, G.P.; Navarrete, I.A.; Rallos, R.V.; Salmo, S.G.; Garcia, M.C.A. The role of reactive iron in long-term carbon sequestration in mangrove sediments. J. Soils Sediments. 2019, 19, 501–510. [Google Scholar] [CrossRef]
- Gleason, S.M.; Ewel, K.C. Organic matter dynamics on the forest floor of a micronesian mangrove forest: An investigation of species composition Shifts1. Biotropica 2002, 34, 190–198. [Google Scholar] [CrossRef]
- Sherman, R.E.; Fahey, T.J.; Martinez, P. Spatial patterns of biomass and aboveground net primary productivity in a mangrove ecosystem in the Dominican Republic. Ecosystems 2003, 6, 384–398. [Google Scholar] [CrossRef]
- Wang, G.; Guan, D.; Peart, M.R.; Chen, Y.; Peng, Y. Ecosystem carbon stocks of mangrove forest in Yingluo Bay, Guangdong Province of South China. For. Ecol. Manag. 2013, 310, 539–546. [Google Scholar] [CrossRef]
- Fisher, B.J.; Moore, O.W.; Faust, J.C.; Peacock, C.L.; Marz, C. Experimental evaluation of the extractability of iron bound organic carbon in sediments as a function of carboxyl content. Chem. Geol. 2020, 556, 119853. [Google Scholar] [CrossRef]
- Johnson, D.W.; Todd, D.E. Relationships among iron, aluminum, carbon, and sulfate in a variety of forest soils. Soil Sci. Soc. Am. J. 1983, 47, 792–800. [Google Scholar] [CrossRef]
- Mikutta, R.; Mikutta, C.; Kalbitz, K.; Torn, M. Biodegradation of forest floor organic matter bound to minerals via different binding mechanisms. Geochim. Cosmochim. Acta. 2007, 71, 2569–2590. [Google Scholar] [CrossRef]
- Gu, B.; Schmitt, J.; Chen, Z.; Liang, L. Adsorption and desorption of natural organic matter on iron oxide: Mechanisms and models. Environ. Sci. Technol. 1994, 28, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Dynes, J.J.; Wang, J.; Sparks, D.L. Properties of Fe-organic matter associations via coprecipitation versus adsorption. Environ. Sci. Technol. 2014, 48, 13751–13759. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Keiluweit, M.; Yang, Y.; Jin, J.; Sun, H.; Wu, F.; Xing, B. Mobilization of ferrihydrite-associated organic carbon during Fe reduction: Adsorption versus coprecipitation. Chem. Geol. 2019, 503, 61–68. [Google Scholar] [CrossRef]
- Riedel, T.; Zak, D.; Biester, H.; Dittmar, T. Iron traps terrestrially derived dissolved organic matter at redox interfaces. Proc. Natl. Acad. Sci. USA 2013, 110, 10101–10105. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.; Brandes, J.; Leri, A.; Lalonde, K.; Balind, K.; Wirick, S.; Wang, J.; Gelinas, Y. Preservation of organic matter in marine sediments by inner-sphere interactions with reactive iron. Sci. Rep. 2017, 7, 366. [Google Scholar] [CrossRef]
- He, T.R. Preservation of Organic Carbon Promoted by Iron in Estuarine Wetland Sediments; East China Normal University: Shanghai, China, 2021. [Google Scholar]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Nieuwenhuize, J. Carbon and nitrogen stable isotopes in suspended matter and sediments from the Schelde Estuary. Mar. Chem. 1998, 60, 217–225. [Google Scholar] [CrossRef]
- Fontugne, M.R.; Jouanneau, J.M. Modulation of the particulate organic carbon flux to the ocean by a macrotidal estuary: Evidence from measurements of carbon isotopes in organic matter from the Gironde system. Estuar. Coast. Shelf Sci. 1987, 24, 377–387. [Google Scholar] [CrossRef]
- Kendall, C.; Silva, S.R.; Kelly, V.J. Carbon and nitrogen isotopic compositions of particulate organic matter in four large river systems across the United States. Hydrol. Process 2001, 15, 1301–1346. [Google Scholar] [CrossRef]
- Parikh, S.J.; Goyne, K.W.; Margenot, A.J. Soil chemical insights provided through vibrational spectroscopy. Adv. Agron. 2014, 126, 1–148. [Google Scholar]
- Zhao, Q.; Poulson, S.R.; Obrist, D.; Sumaila, S.; Dynes, J.J.; McBeth, J.M.; Yang, Y. Iron-bound organic carbon in forest soils: Quantification and characterization. Biogeosciences 2016, 13, 4777–4788. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Senesi, N.; D’Orazio, V.; Ricca, G. Humic acids in the first generation of EUROSOILS. Geoderma 2003, 116, 325–344. [Google Scholar] [CrossRef]
- Wang, F.; Sanders, C.J.; Santos, I.R.; Tang, J.; Schuerch, M.; Kirwan, M.L.; Kopp, R.E.; Zhu, K.; Li, X.; Yuan, J.; et al. Global blue carbon accumulation in tidal wetlands increases with climate change. Natl. Sci. Rev. 2021, 8, nwaa296. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Wang, G.; Guan, D.; Xiao, L.; Peart, M.R. Ecosystem carbon storage affected by intertidal locations and climatic factors in three estuarine mangrove forests of South China. Reg. Environ. Chang. 2019, 19, 1701–1712. [Google Scholar] [CrossRef]
- Yu, C.; Feng, J.; Liu, K.; Wang, G.; Zhu, Y.; Chen, H.; Guan, D. Changes of ecosystem carbon stock following the plantation of exotic mangrove Sonneratia apetala in Qi’ao Island, China. Sci. Total Environ. 2020, 717, 137142. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).