Stocks and Sources of Soil Carbon and Nitrogen in Non-Native Kandelia obovata Afforestation and Spartina alterniflora Invasion: A Case Study on Northern Margin Mangroves in the Subtropical Coastal Wetlands of China
Abstract
1. Introduction
2. Materials and Methods
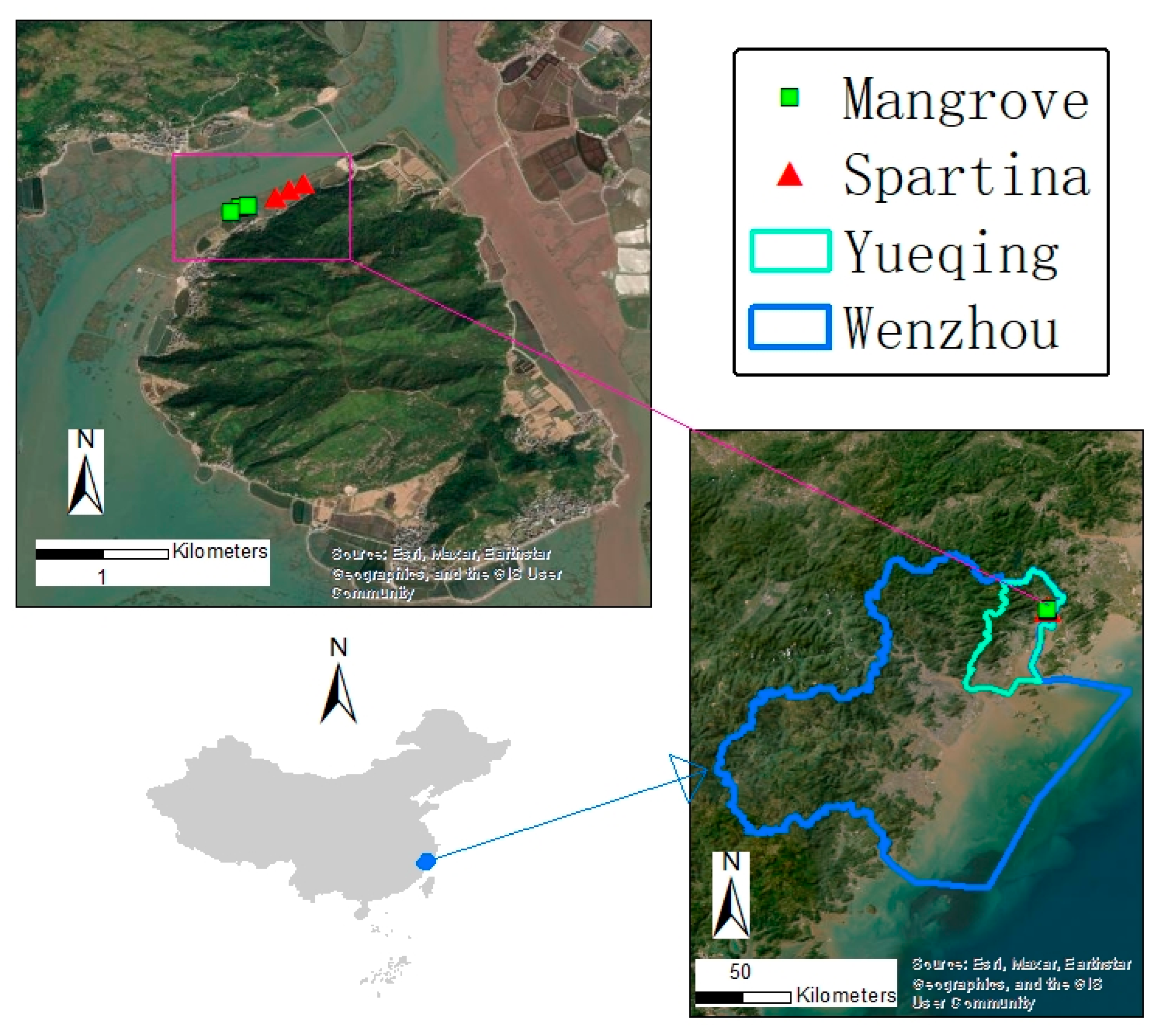
2.1. Study Area
2.2. Soil Sampling and Analysis
2.3. Data Analysis
3. Results
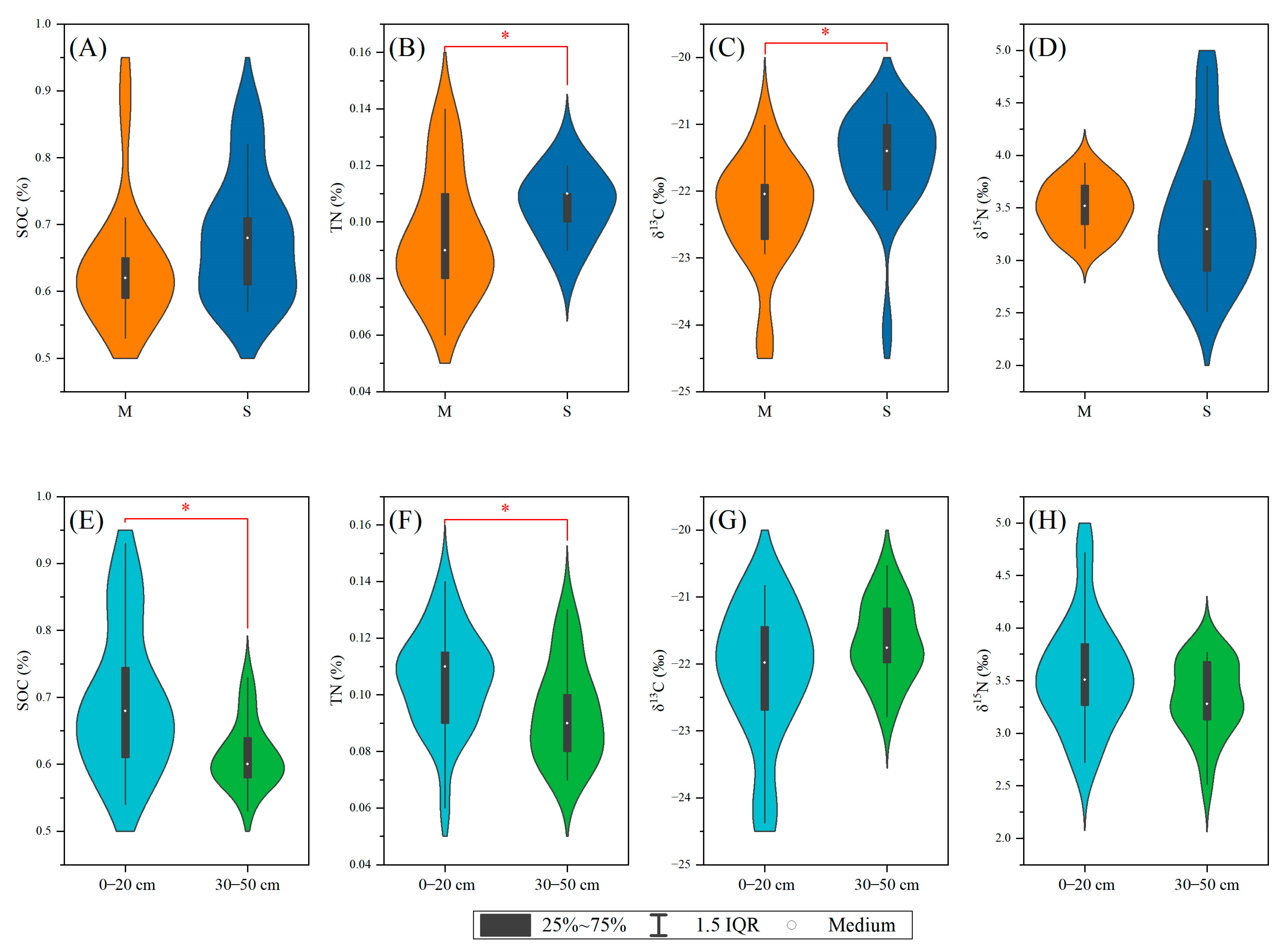
3.1. Vertical Distribution Characteristics of Soil TC, SOC, and TN
3.2. Vertical Distribution Characteristics of Soil δ13C, δ15N, and C/N Ratio
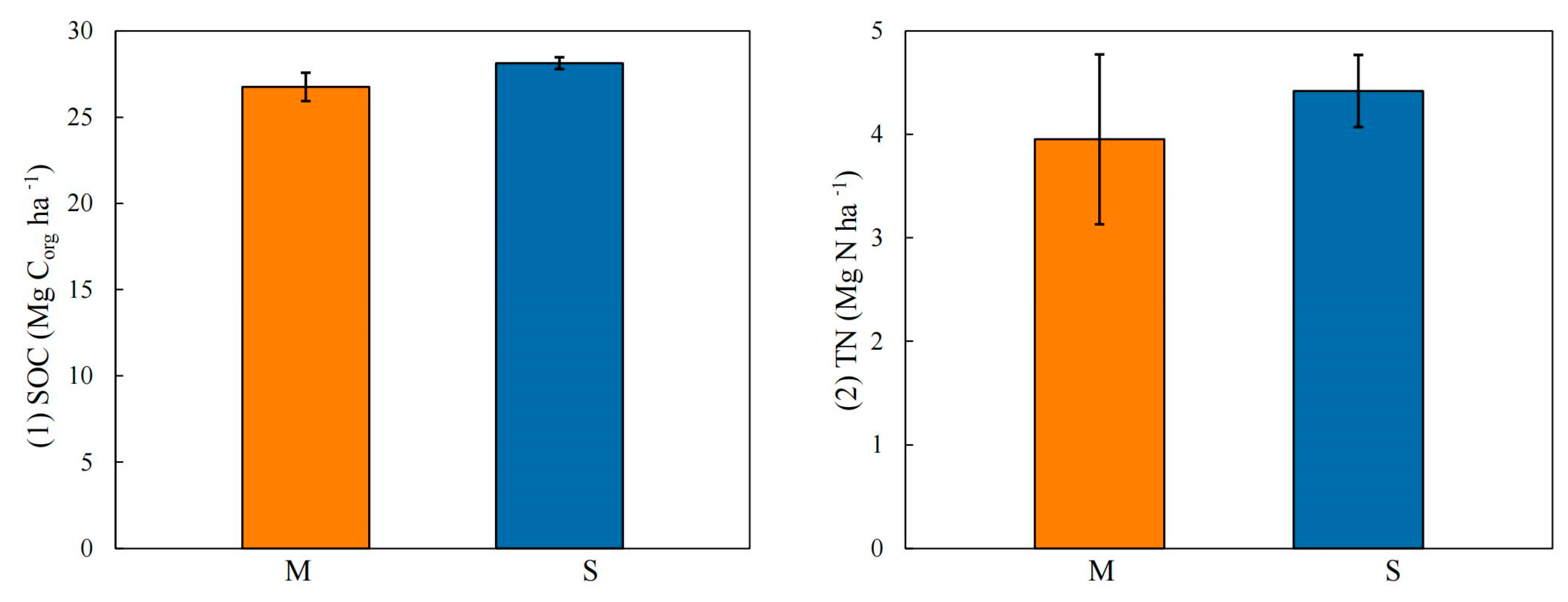
3.3. Differences between Ecotypes and Soil Layers
4. Discussion
4.1. Stocks and Sources of Soil Carbon in Non-Native K. obovata Afforestation and S. alterniflora Invasion
4.2. Stocks and Sources of Soil Nitrogen in Non-Native K. obovata Afforestation and S. alterniflora Invasion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macreadie, P.I.; Costa, M.D.; Atwood, T.B.; Friess, D.A.; Kelleway, J.J.; Kennedy, H.; Lovelock, C.E.; Serrano, O.; Duarte, C.M. Blue carbon as a natural climate solution. Nat. Rev. Earth Environ. 2021, 2, 826–839. [Google Scholar] [CrossRef]
- Wilson, A.M.W.; Forsyth, C. Restoring near-shore marine ecosystems to enhance climate security for island ocean states: Aligning international processes and local practices. Mar. Policy 2018, 93, 284–294. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. Wetlands; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Blaber, S.; Bouillon, S.; Green, P.; Haywood, M.; Kirton, L.; Meynecke, J.-O.; Pawlik, J.; Penrose, H.; Sasekumar, A. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquat. Bot. 2008, 89, 155–185. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Anton, A.; Raven, J.A.; Beaumont, N.; Connolly, R.M.; Friess, D.A.; Kelleway, J.J.; Kennedy, H.; Kuwae, T.; Lavery, P.S. The future of Blue Carbon science. Nat. Commun. 2019, 10, 3998. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Li, Q.Q.; Zhang, Y.; Yang, S.; Osland, M.J.; Huang, J.; Peng, C. Mangrove species’ responses to winter air temperature extremes in China. Ecosphere 2017, 8, e01865. [Google Scholar] [CrossRef]
- Lu, W.-X.; Zhang, B.-H.; Zhang, Y.-Y.; Yang, S.-C. Differentiation of cold tolerance in an artificial population of a mangrove species, Kandelia obovata, is associated with geographic origins. Front. Plant Sci. 2022, 12, 695746. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Zhang, Y.; Lin, G. Recent progresses in mangrove conservation, restoration and research in China. J. Plant Ecol. 2009, 2, 45–54. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Gu, J.-D. Ecological responses, adaptation and mechanisms of mangrove wetland ecosystem to global climate change and anthropogenic activities. Int. Biodeterior. Biodegrad. 2021, 162, 105248. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, S.; Feng, Z.; Liu, G.; Gan, Q.; Peng, S. Use of exotic plants to control Spartina alterniflora invasion and promote mangrove restoration. Sci. Rep. 2015, 5, 12980. [Google Scholar] [CrossRef]
- Atwood, T.B.; Connolly, R.M.; Almahasheer, H.; Carnell, P.E.; Duarte, C.M.; Ewers Lewis, C.J.; Irigoien, X.; Kelleway, J.J.; Lavery, P.S.; Macreadie, P.I. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Chang. 2017, 7, 523–528. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Kuzyakov, Y.; Lubis, A.A.; Murdiyarso, D.; Hutley, L.B.; Bachri, S.; Friess, D.A.; Martius, C.; Borchard, N. Organic carbon burial and sources in soils of coastal mudflat and mangrove ecosystems. Catena 2020, 187, 104414. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon sequestration in mangrove forests. Carbon Manag. 2012, 3, 313–322. [Google Scholar] [CrossRef]
- Sarker, S.; Masud-Ul-Alam, M.; Hossain, M.S.; Rahman Chowdhury, S.; Sharifuzzaman, S. A review of bioturbation and sediment organic geochemistry in mangroves. Geol. J. 2021, 56, 2439–2450. [Google Scholar] [CrossRef]
- Alongi, D.M. Cycling and global fluxes of nitrogen in mangroves. Glob. Environ. Res. 2013, 17, 173–182. [Google Scholar]
- Sanders, C.J.; Eyre, B.D.; Santos, I.R.; Machado, W.; Luiz-Silva, W.; Smoak, J.M.; Breithaupt, J.L.; Ketterer, M.E.; Sanders, L.; Marotta, H. Elevated rates of organic carbon, nitrogen, and phosphorus accumulation in a highly impacted mangrove wetland. Geophys. Res. Lett. 2014, 41, 2475–2480. [Google Scholar] [CrossRef]
- Xia, S.; Song, Z.; Singh, B.P.; Guo, L.; Bolan, N.; Wang, W.; Lin, G.; Fang, Y.; Wen, X.; Wang, J. Contrasting patterns and controls of soil carbon and nitrogen isotope compositions in coastal wetlands of China. Plant Soil 2023, 489, 483–505. [Google Scholar] [CrossRef]
- Ray, R.; Majumder, N.; Das, S.; Chowdhury, C.; Jana, T.K. Biogeochemical cycle of nitrogen in a tropical mangrove ecosystem, east coast of India. Mar. Chem. 2014, 167, 33–43. [Google Scholar] [CrossRef]
- Ellison, A.M. Mangrove restoration: Do we know enough? Restor. Ecol. 2000, 8, 219–229. [Google Scholar] [CrossRef]
- Junk, W.J.; An, S.; Finlayson, C.; Gopal, B.; Květ, J.; Mitchell, S.A.; Mitsch, W.J.; Robarts, R.D. Current state of knowledge regarding the world’s wetlands and their future under global climate change: A synthesis. Aquat. Sci. 2013, 75, 151–167. [Google Scholar] [CrossRef]
- Wang, C.; Cui, L.; Wang, D. Distribution and pollution assessment of heavy metals in surface sediments of aquaculture area in Yueqing bay. Trans. Chin. Soc. Agric. Eng. 2015, 31, 204–210. [Google Scholar]
- Thura, K.; Serrano, O.; Gu, J.L.; Fang, Y.Y.; Htwe, H.Z.; Zhu, Y.J.; Huang, R.Q.; Agusti, S.; Duarte, C.M.; Wang, H.L.; et al. Mangrove restoration built soil organic carbon stocks over six decades: A chronosequence study. J. Soils Sediments 2023, 23, 1193–1203. [Google Scholar] [CrossRef]
- Wang, A.; Chen, J.; Jing, C.; Ye, G.; Wu, J.; Huang, Z.; Zhou, C. Monitoring the invasion of Spartina alterniflora from 1993 to 2014 with Landsat TM and SPOT 6 satellite data in Yueqing Bay, China. PLoS ONE 2015, 10, e0135538. [Google Scholar] [CrossRef]
- Hu, J.; Loh, P.S.; Pradit, S.; Le, T.P.Q.; Oeurng, C.; Mohamed, C.A.R.; Lee, C.W.; Lu, X.; Anshari, G.Z.; Kandasamy, S. Assessing the effect of age and geomorphic setting on organic carbon accumulation in high-latitude human-planted mangroves. Forests 2022, 13, 105. [Google Scholar] [CrossRef]
- Chen, S.; Chen, B.; Chen, G.; Ji, J.; Yu, W.; Liao, J.; Chen, G. Higher soil organic carbon sequestration potential at a rehabilitated mangrove comprised of Aegiceras corniculatum compared to Kandelia obovata. Sci. Total Environ. 2021, 752, 142279. [Google Scholar] [CrossRef]
- Feng, J.X.; Zhou, J.; Wang, L.M.; Cui, X.W.; Ning, C.X.; Wu, H.; Zhu, X.S.; Lin, G.H. Effects of short-term invasion of Spartina alterniflora and the subsequent restoration of native mangroves on the soil organic carbon, nitrogen, and phosphorus stock. Chemosphere 2017, 184, 774–783. [Google Scholar] [CrossRef]
- Gu, J.; Wu, J. Blue carbon effects of mangrove restoration in subtropics where Spartina alterniflora invaded. Ecol. Eng. 2023, 186, 106822. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, N.; Yang, S.; Li, Y.; Yang, L.; Cao, W. Source and stability of soil organic carbon jointly regulate soil carbon pool, but source alteration is more effective in mangrove ecosystem following Spartina alterniflora invasion. Catena 2024, 235, 107681. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, R.-H.; Ouyang, Z.-T.; Shao, C.-L.; Chen, J.-Q.; Zhang, T.-T.; Guo, H.-Q.; Tang, J.-W.; Zhao, F.; Zhuang, P.; et al. Enhanced Lateral Exchange of Carbon and Nitrogen in a Coastal Wetland with Invasive Spartina alterniflora. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005459. [Google Scholar] [CrossRef]
- Yang, W.; An, S.; Zhao, H.; Xu, L.; Qiao, Y.; Cheng, X. Impacts of Spartina alterniflora invasion on soil organic carbon and nitrogen pools sizes, stability, and turnover in a coastal salt marsh of eastern China. Ecol. Eng. 2016, 86, 174–182. [Google Scholar] [CrossRef]
- Wang, Q.; Kang, Q.; Zhao, B.; Li, H.; Lu, H.; Liu, J.; Yan, C. Effect of land-use and land-cover change on mangrove soil carbon fraction and metal pollution risk in Zhangjiang Estuary, China. Sci. Total Environ. 2022, 807, 150973. [Google Scholar] [CrossRef]
- Yu, X.; Yang, J.; Liu, L.; Tian, Y.; Yu, Z. Effects of Spartina alterniflora invasion on biogenic elements in a subtropical coastal mangrove wetland. Environ. Sci. Pollut. Res. Int. 2015, 22, 3107–3115. [Google Scholar] [CrossRef]
- Feng, J.; Wang, S.; Wang, S.; Ying, R.; Yin, F.; Jiang, L.; Li, Z. Effects of invasive Spartina alterniflora loisel. and subsequent ecological replacement by Sonneratia apetala Buch. -Ham. on soil organic carbon fractions and stock. Forests 2019, 10, 171. [Google Scholar] [CrossRef]
- Meng, W.; Feagin, R.A.; Hu, B.; He, M.; Li, H. The spatial distribution of blue carbon in the coastal wetlands of China. Estuar. Coast. Shelf Sci. 2019, 222, 13–20. [Google Scholar] [CrossRef]
- Meyers, P.A. Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org. Geochem. 1997, 27, 213–250. [Google Scholar] [CrossRef]
- Bouillon, S.; Connolly, R.; Lee, S. Organic matter exchange and cycling in mangrove ecosystems: Recent insights from stable isotope studies. J. Sea Res. 2008, 59, 44–58. [Google Scholar] [CrossRef]
- Rani, V.; Nandan, S.B.; Schwing, P.T. Carbon source characterisation and historical carbon burial in three mangrove ecosystems on the South West coast of India. Catena 2021, 197, 12. [Google Scholar] [CrossRef]
- Chmura, G.; Aharon, P. Stable carbon isotope signatures of sedimentary carbon in coastal wetlands as indicators of salinity regime. J. Coast. Res. 1995, 11, 124–135. [Google Scholar]
- Lamb, A.L.; Wilson, G.P.; Leng, M.J. A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and C/N ratios in organic material. Earth-Sci. Rev. 2006, 75, 29–57. [Google Scholar] [CrossRef]
- Martiny, A.; Vrugt, J.A.; Primeau, F.W.; Lomas, M.W. Regional variation in the particulate organic carbon to nitrogen ratio in the surface ocean. Glob. Biogeochem. Cycles 2013, 27, 723–731. [Google Scholar] [CrossRef]
- Tue, N.T.; Nguyen, P.T.; Quan, D.M.; Dung, L.V.; Quy, T.D.; Nhuan, M.T.; Thai, N.D. Sedimentary composition and organic carbon sources in mangrove forests along the coast of northeast Vietnam. Reg. Stud. Mar. Sci. 2018, 17, 87–94. [Google Scholar] [CrossRef]
- Xu, G.; Liu, J.; Hu, G.; Jonell, T.N.; Chen, L.L. Distribution and source of organic matter in surface sediment from the muddy deposit along the Zhejiang coast, East China Sea. Mar. Pollut. Bull. 2017, 123, 395–399. [Google Scholar] [CrossRef]
- Wang, D.B.; Huang, W.; Liang, R.W.; Li, F.S. Effects of Spartina alterniflora Invasion on Soil Quality in Coastal Wetland of Beibu Gulf of South China. PLoS ONE 2016, 11, 17. [Google Scholar] [CrossRef]
- Li, B.; Liao, C.-H.; Zhang, X.-D.; Chen, H.-L.; Wang, Q.; Chen, Z.-Y.; Gan, X.-J.; Wu, J.-H.; Zhao, B.; Ma, Z.-J.; et al. Spartina alterniflora invasions in the Yangtze River estuary, China: An overview of current status and ecosystem effects. Ecol. Eng. 2009, 35, 511–520. [Google Scholar] [CrossRef]
- Huang, J.X.; Xu, X.; Wang, M.; Nie, M.; Qiu, S.Y.; Wang, Q.; Quan, Z.X.; Xiao, M.; Li, B. Responses of soil nitrogen fixation to Spartina alterniflora invasion and nitrogen addition in a Chinese salt marsh. Sci. Rep. 2016, 6, 8. [Google Scholar] [CrossRef]
- Morris, J.T. The nitrogen uptake kinetics of Spartina alterniflora in culture. Ecology 1980, 61, 1114–1121. [Google Scholar] [CrossRef]
- Joesting, H.M.; Blaylock, R.; Biber, P.; Ray, A. The use of marine aquaculture solid waste for nursery production of the salt marsh plants Spartina alterniflora and Juncus roemerianus. Aquac. Rep. 2016, 3, 108–114. [Google Scholar] [CrossRef]
- Craft, C.; Reader, J.; Sacco, J.N.; Broome, S.W. Twenty-five years of ecosystem development of constructed Spartina alterniflora (Loisel) marshes. Ecol. Appl. 1999, 9, 1405–1419. [Google Scholar] [CrossRef]



| Variable | Mean | Standard Error | n | Skewness | Kurtosis | Shapiro–Wilk Test of Normality | |
|---|---|---|---|---|---|---|---|
| Statistic W Value | p | ||||||
| Total Carbon (%) | 1.33 | 0.04 | 42 | 1.04 | 2.20 | 0.931 | 0.014 * |
| Soil Organic Carbon (%) | 0.66 | 0.01 | 42 | 1.21 | 0.98 | 0.884 | 0.000 ** |
| Total Nitrogen (%) | 0.10 | 0.00 | 42 | 0.00 | −0.47 | 0.972 | 0.377 |
| C/N | 6.75 | 0.21 | 42 | 0.97 | 1.59 | 0.934 | 0.018 * |
| δ13C (‰) | −21.93 | 0.14 | 42 | −1.05 | 1.36 | 0.917 | 0.005 ** |
| δ15N (‰) | 3.47 | 0.07 | 42 | 0.73 | 1.53 | 0.954 | 0.092 |
| Subtropic Coastal Wetlands | Latitude | Dominate Species | Soil Depth | Soil Organic Carbon | Soil Total Nitrogen | C/N | δ13C | δ15N | References | |
|---|---|---|---|---|---|---|---|---|---|---|
| cm | Mg C ha−1 | Mg N ha−1 | ‰ | ‰ | ||||||
| Native + Invasive species | Mangrove | 23°53′ N | Kandelia obovate, Avicennia marina, and Aegiceras corniculatum | 0–30 | 65.6 | 5.6 | 5.62~20.04 | −28.42~−21.74 | 3.95~7.59 | [31] |
| 24°24′ N | K. obovata | 0–60 | 39.7 | 4.5 | [29] | |||||
| 23°53′ N | S. alterniflora | 0–30 | 52.1 | 5.4 | 6.82~14.62 | −23.02~−18.15 | 4.51~7.54 | [31] | ||
| 24°24′ N | S. alterniflora | 0–60 | 39.5 | 4.9 | [29] | |||||
| Mudflat | 23°53′ N | 0–30 | 33.3 | 4.1 | 7.17~26.25 | −25.70~−23.21 | 3.53~5.72 | [31] | ||
| Mudflat | 24°24′ N | 0–60 | 41 | 4.45 | [29] | |||||
| Non-native + Invasive species | Northern Margin Mangrove | 28°20′ N | K. obovata | 0–100 | 65.53 | 6.7 | −22.30 | [27] | ||
| 28°20’ N | K. obovata | 0–50 | 26.76 | 3.95 | 4.29~10.83 | −24.38~−21.02 | 3.11~3.93 | This study | ||
| 28°21′ N | S. alterniflora | 0–100 | 71.72 | [27] | ||||||
| 28°20’ N | S. alterniflora | 0–50 | 28.14 | 4.42 | 5.27~7.91 | −24.09~−20.53 | 2.52~4.85 | This study | ||
| Mudflat | 28°20′ N | 0–100 | 69.52 | −21.6~−22.5 | [27] | |||||
| Invasive species | Salt marsh | 31°30′ N | Phragmites australis | 0–20 | 1.6 | 19.7 | [32] | |||
| 32°48′ N | Phragmites australis | 0–30 | 16.84 | −24.97 | 2.37 | [33] | ||||
| 31°30′ N | S. alterniflora | 0–20 | 1.78 | 18.6 | [32] | |||||
| 32°48′ N | S.alterniflora | 0–30 | 37.83 | −18.41 | 3.93 | [33] | ||||
| Mudflat | 32°48′ N | 0–30 | 4.49 | −21.11 | 0.48 | [33] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Q.; Hou, C.; Wang, Q.; Gao, C.; Stefanik, K.; Li, F.; Jiang, B. Stocks and Sources of Soil Carbon and Nitrogen in Non-Native Kandelia obovata Afforestation and Spartina alterniflora Invasion: A Case Study on Northern Margin Mangroves in the Subtropical Coastal Wetlands of China. Water 2024, 16, 866. https://doi.org/10.3390/w16060866
Ye Q, Hou C, Wang Q, Gao C, Stefanik K, Li F, Jiang B. Stocks and Sources of Soil Carbon and Nitrogen in Non-Native Kandelia obovata Afforestation and Spartina alterniflora Invasion: A Case Study on Northern Margin Mangroves in the Subtropical Coastal Wetlands of China. Water. 2024; 16(6):866. https://doi.org/10.3390/w16060866
Chicago/Turabian StyleYe, Qianwen, Cuicui Hou, Qiang Wang, Changjun Gao, Kay Stefanik, Feng Li, and Bingbing Jiang. 2024. "Stocks and Sources of Soil Carbon and Nitrogen in Non-Native Kandelia obovata Afforestation and Spartina alterniflora Invasion: A Case Study on Northern Margin Mangroves in the Subtropical Coastal Wetlands of China" Water 16, no. 6: 866. https://doi.org/10.3390/w16060866
APA StyleYe, Q., Hou, C., Wang, Q., Gao, C., Stefanik, K., Li, F., & Jiang, B. (2024). Stocks and Sources of Soil Carbon and Nitrogen in Non-Native Kandelia obovata Afforestation and Spartina alterniflora Invasion: A Case Study on Northern Margin Mangroves in the Subtropical Coastal Wetlands of China. Water, 16(6), 866. https://doi.org/10.3390/w16060866





