Identification of Microplastics Using µ-Raman Spectroscopy in Surface and Groundwater Bodies of SE Attica, Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
- A karstic aquifer with a high capacity (discharge rate over 100 m3/h) as it is also fractured. Along the coasts of Porto Rafti and Artemis, scattered underwater sources of this karst water have been identified, confirming the hydraulic connection with seawater.
- The alternation of permeable and impermeable layers favors the development of unconfined and confined aquifers in the Neogene deposits. The capacity of these systems is low (discharge rates up to 5 m3/h). The shallow aquifer is overexploited by extensive pumping to meet irrigation and industrial needs in the region.
- A phreatic aquifer has developed in Quaternary deposits with low capacity (discharge rates of 15 up to 35 m3/h). The phreatic aquifer is exploited by many wells, resulting in seawater intrusion in the coastal parts of the aquifer system. This fact is also reflected in the groundwater quality, as it is characterized as brackish.
- Fractured aquifers are mainly developed in the Rafina area, due to secondary porosity, created by metamorphic and tectonic processes and the weathering of impermeable formations.
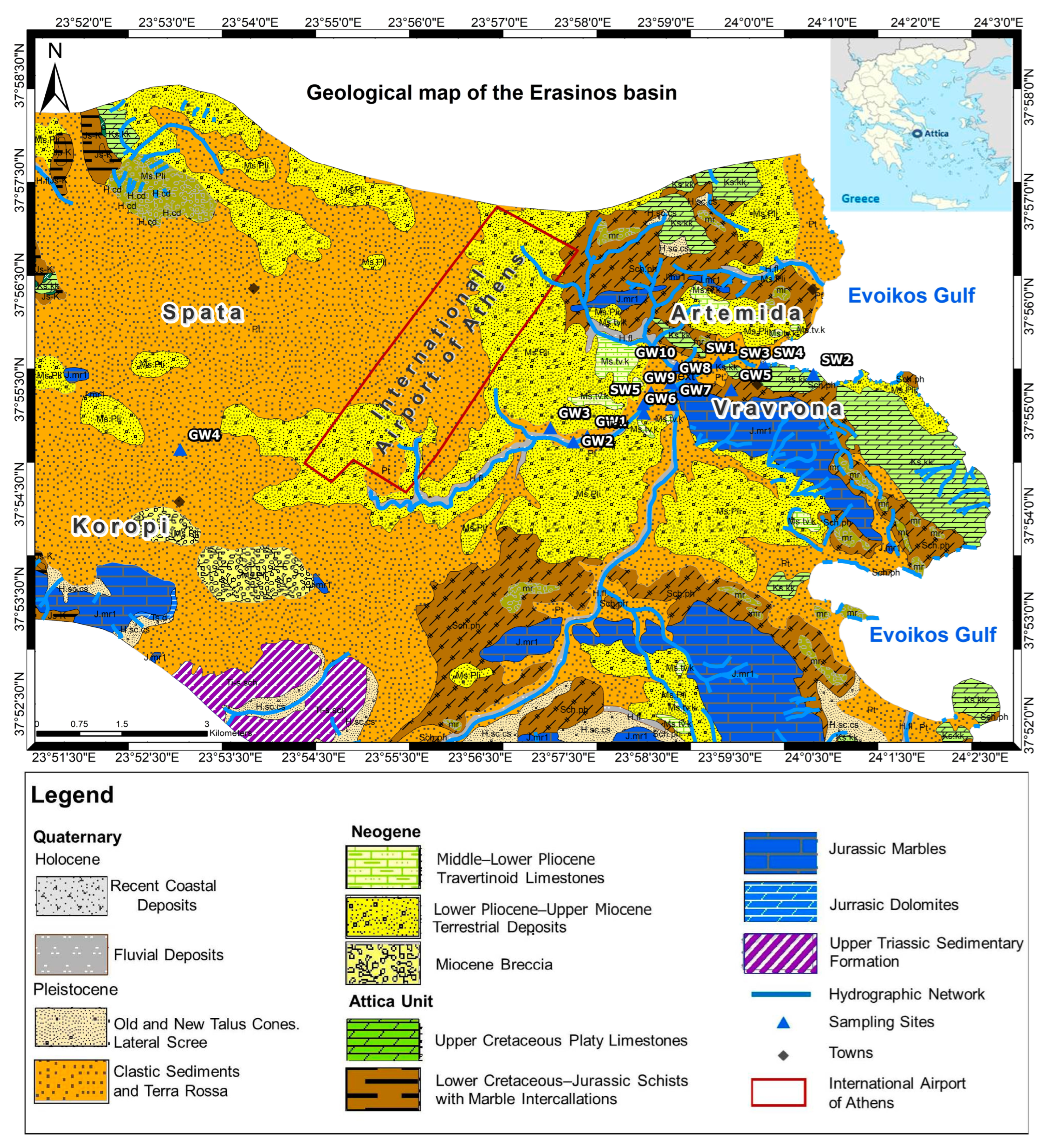
2.2. Sampling
2.3. Geochemical Modeling
2.4. Sample Extraction
3. Results
3.1. Visual Presorting
3.2. Raman Spectroscopy
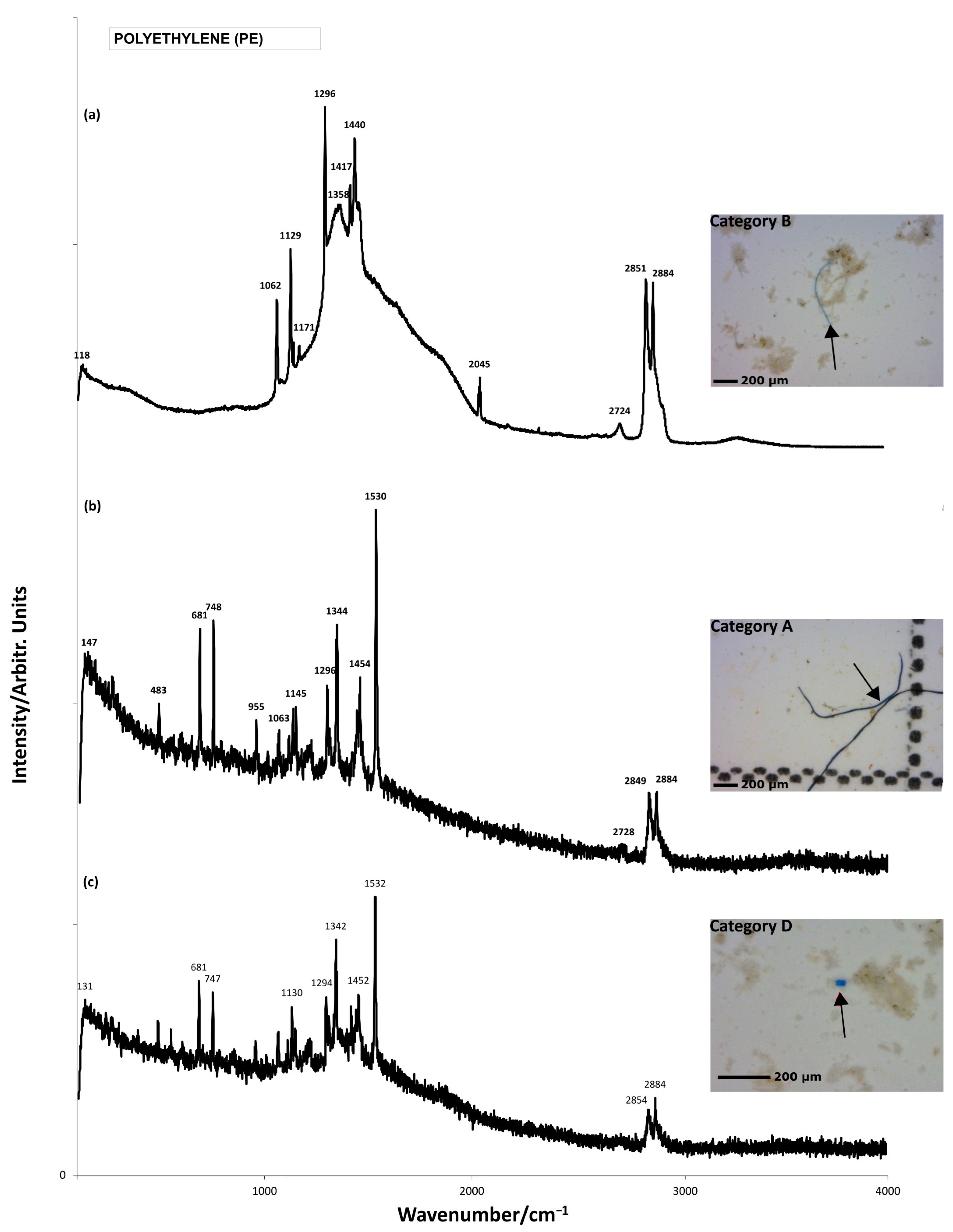

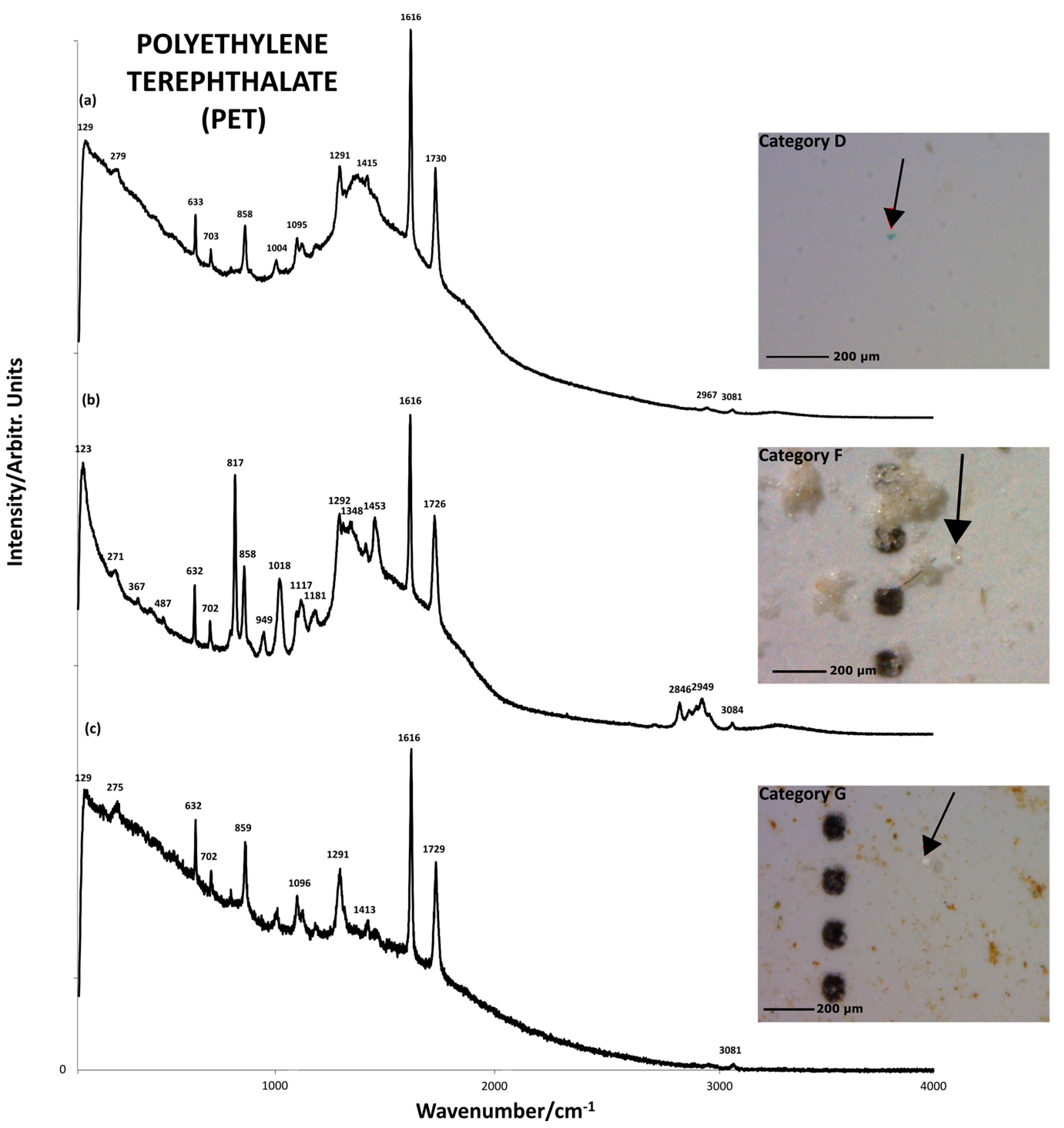
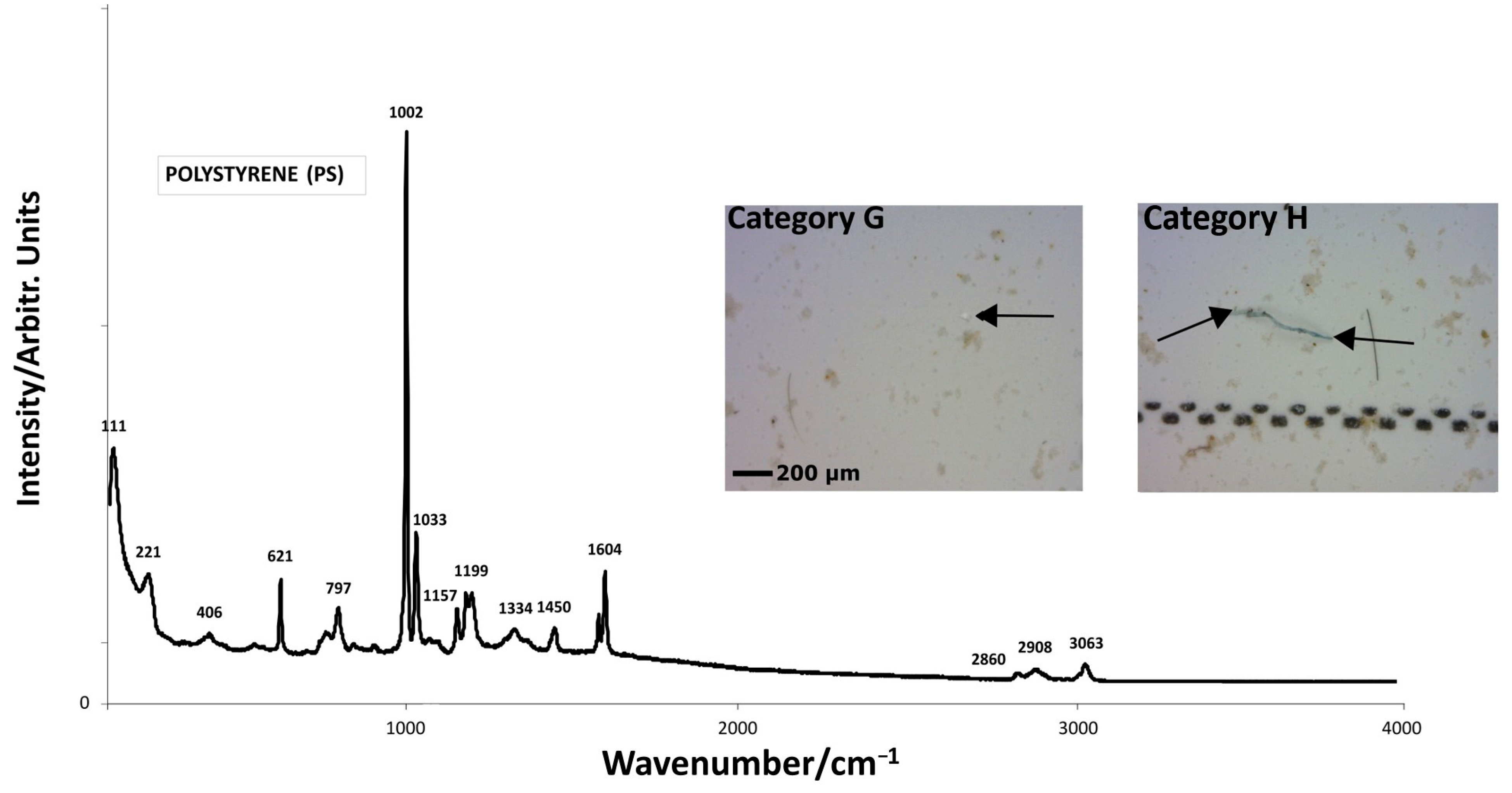
| Polymer Types | Polymer Formula | Raman Peaks (cm−1) | Sample | Visual Categories |
|---|---|---|---|---|
| PE | 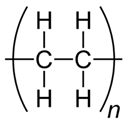 (C2H4)n | 1062s (νasC–C) [40,41,42] ~1129s (νsC–C) [40,41,42] 1296s (τCH2) [40,41,42] ~1417m (ωCH2) [42] ~1440s (δCH2) [40,41] 1512w (δCH2) [40,41] ~2850 (νsCH2) [43,44] ~2884s (νasCH2) [43,44] | GW1, GW2, GW4 | A,B,D,E |
| PP | 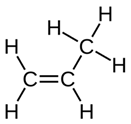 (C3H6)n | 396s (ωCH2,δCH) [45,46] 809vs (ρCH2, vCC, vC–CH3 [42,45,46] 841s (pCH2, vCCb, vC–CH3, pCH3) [45,46] 973m (ρCH3 + vC–C chain) [45,46] 1153s (vCCb, vC–CH3, δCH, pCH3) [45,46] ~1330s (δCH) [45,46] ~1460s (δCH3) [45,46] ~2842m(νCH2) [43,44] 2860m(νCH2) [43,44] ~2885s (νsCH3) [43,44] 2954-2963m (νaCH3) [43,44] | GW1, GW2, GW3 | A,C,D,F |
| PS | 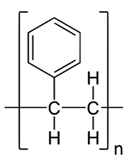 (C8H8)n | 621s (νC–C) [37,38] 797m (νsC–C) [37,38] 1002s (νC–C) [45] 1033 (δC–H) [37] 1157w (ν15 mode) [37] 1450w [ν19b or δ(CH2)] [37] 1604m (νsC–C) [37] 2860w (νsCH2) [42] 2908w (νasCH2) [42] 3063w (νC–H) [42] | GW1 | G,H |
| PET | 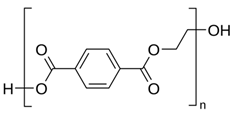 (C10H8O4)n | 860s (ρCH2) 1097m (νC–O, νC–C, δC–O–C) [47] 1612-1617s (C–C ring of phenyl) [43,47] 1726-1730s (νC=O) [47] 2850w,m (νCH2) [43,47] 2883w,m (νCH2) [43,47] 3081w (C–H of phenyl) [43,47] | GW1, GW2, GW3, GW4 | D,F,G |
4. Discussion
5. Conclusions
- ▪
- The characterization of MPs by Raman microspectroscopy revealed the presence of the following:
- a.
- PE and PP in the form of fibers and fragments;
- b.
- PET as angular irregular particles;
- c.
- PS as reflective irregular angular particles and blue-colored fibers.
- ▪
- Microplastics are more prevalent in shallow aquifers, as expected, reaching up to 513 MPs/L. In the deeper aquifers (water table ~70 m), the abundance of MPs is lower (up to 16 MPs/L); however, this is no less significant, as it may indicate that MPs are able to migrate to greater depths through water infiltration.
- ▪
- The types of MPs appear to vary due to different land use and the vulnerability of groundwater systems. A high amount of microplastics (3399 particles/ per 16 Liters) was found where intensive anthropogenic activities (agricultural, industrial, and urban) take place.
- ▪
- The mechanism of MPs admission into the deep (water table ~70 m) aquifer needs further investigation. Seawater intrusion, which is reflected in the qualitative characteristics of groundwater, could potentially be a source of microplastics. The extent at which seawater MP contamination interacts with other potential sources such as agricultural activities requires further in-depth geochemical investigations, to document the correlation between MPs and key chemical parameters.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cowger, W.; Gray, A.; Christiansen, S.H.; DeFrond, H.; Deshpande, A.D.; Hemabessiere, L.; Lee, E.; Mill, L.; Munno, K.; Ossmann, B.E.; et al. Critical Review of Processing and Classification Techniques for Images and Spectra in Microplastic Research. Appl. Spectrosc. 2020, 74, 989–1010. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics in the Environment: Challenges in Analytical Chemistry—A Review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.; Baker, J.E.; Bamford, H.A. NOAA Technical Memorandum NOS-OR&R-30. In Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, University of Washington Tacoma, Tacoma, WA, USA, 9–11 September 2008. [Google Scholar]
- Ziani, K.; Ioniță-Mîndrican, C.-B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.-T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef]
- An, L.; Liu, Q.; Deng, Y.; Wu, W.; Gao, Y.; Ling, W. Sources of Microplastic in the Environment. In Microplastics in Terrestrial Environments: Emerging Contaminants and Major Challenges; He, D., Luo, Y., Eds.; The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2020; pp. 143–159. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Yee Leung, K.M.; Wu, F. Color: An Important but Overlooked Factor for Plastic Photoaging and Microplastic Formation. Environ. Sci. Technol. 2022, 56, 9161–9163. [Google Scholar] [CrossRef]
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean Plastic Soup: Synthetic Polymers in Mediterranean Surface Waters. Sci. Rep. 2016, 6, 37551. [Google Scholar] [CrossRef]
- Isobe, A.; Iwasaki, S.; Uchida, K.; Tokai, T. Abundance of Non-Conservative Microplastics in the Upper Ocean from 1957 to 2066. Nat. Commun. 2019, 10, 417. [Google Scholar] [CrossRef]
- Peeken, I.; Primpke, S.; Beyer, B.; Gütermann, J.; Katlein, C.; Krumpen, T.; Bergmann, M.; Hehemann, L.; Gerdts, G. Arctic Sea Ice Is an Important Temporal Sink and Means of Transport for Microplastic. Nat. Commun. 2018, 9, 1505. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Velázquez, D.; Edo, C.; Carretero, O.; Gago, J.; Baron-Sola, Á.; Hernández, L.E.; Yousef, I.; Quesada, A.; Leganés, F.; et al. Fibers Spreading Worldwide: Microplastics and Other Anthropogenic Litter in an Arctic Freshwater Lake. Sci. Total Environ. 2020, 722, 137904. [Google Scholar] [CrossRef]
- Nguyen, N.B.; Kim, M.-K.; Le, Q.T.; Ngo, D.N.; Zoh, K.-D.; Joo, S.-W. Spectroscopic Analysis of Microplastic Contaminants in an Urban Wastewater Treatment Plant from Seoul, South Korea. Chemosphere 2021, 263, 127812. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Zhao, J.; Liu, L.; Zhang, Y.; Wang, X.; Wu, F. A Novel Way to Rapidly Monitor Microplastics in Soil by Hyperspectral Imaging Technology and Chemometrics. Environ. Pollut. 2018, 238, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Min, J.; Jiang, W.; Li, Y.; Zhang, W. Separation, Characterization and Identification of Microplastics and Nanoplastics in the Environment. Sci. Total Environ. 2020, 721, 137561. [Google Scholar] [CrossRef] [PubMed]
- Käppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Analysis of Environmental Microplastics by Vibrational Microspectroscopy: FTIR, Raman or Both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef]
- Käppler, A.; Windrich, F.; Löder, M.G.J.; Malanin, M.; Fischer, D.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Identification of Microplastics by FTIR and Raman Microscopy: A Novel Silicon Filter Substrate Opens the Important Spectral Range below 1300 Cm−1 for FTIR Transmission Measurements. Anal. Bioanal. Chem. 2015, 407, 6791–6801. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Cha, J.; Ha, K.; Viaroli, S. Microplastic Pollution in Groundwater: A Systematic Review. Environ. Pollut. Bioavailab. 2024, 36, 2299545. [Google Scholar] [CrossRef]
- Ma, H.; Chao, L.; Wan, H.; Zhu, Q. Microplastic Pollution in Water Systems: Characteristics and Control Methods. Diversity 2024, 16, 70. [Google Scholar] [CrossRef]
- Li, S.; Yang, M.; Wang, H.; Jiang, Y. Adsorption of Microplastics on Aquifer Media: Effects of the Action Time, Initial Concentration, Ionic Strength, Ionic Types and Dissolved Organic Matter. Environ. Pollut. 2022, 308, 119482. [Google Scholar] [CrossRef] [PubMed]
- Ioakeimidis, C.; Fotopoulou, K.N.; Karapanagioti, H.K.; Geraga, M.; Zeri, C.; Papathanassiou, E.; Galgani, F.; Papatheodorou, G. The Degradation Potential of PET Bottles in the Marine Environment: An ATR-FTIR Based Approach. Sci. Rep. 2016, 6, 23501. [Google Scholar] [CrossRef]
- Tziourrou, P.; Megalovasilis, P.; Tsounia, M.; Karapanagioti, H.K. Characteristics of Microplastics on Two Beaches Affected by Different Land Uses in Salamina Island in Saronikos Gulf, East Mediterranean. Mar. Pollut. Bull. 2019, 149, 110531. [Google Scholar] [CrossRef]
- Tsangaris, C.; Digka, N.; Valente, T.; Aguilar, A.; Borrell, A.; de Lucia, G.A.; Gambaiani, D.; Garcia-Garin, O.; Kaberi, H.; Martin, J.; et al. Using Boops Boops (Osteichthyes) to Assess Microplastic Ingestion in the Mediterranean Sea. Mar. Pollut. Bull. 2020, 158, 111397. [Google Scholar] [CrossRef]
- Karkanorachaki, K.; Kiparissis, S.; Kalogerakis, G.C.; Yiantzi, E.; Psillakis, E.; Kalogerakis, N. Plastic Pellets, Meso- and Microplastics on the Coastline of Northern Crete: Distribution and Organic Pollution. Mar. Pollut. Bull. 2018, 133, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Jacobshagen, V. Geologie von Griechenland. Available online: https://www.schweizerbart.de/publications/detail/isbn/9783443110192/Geologie_von_Griechenland (accessed on 27 January 2024).
- Katsikatsos, G. Geology of Greece; University of Patras: Patras, Greece, 1992. [Google Scholar]
- Champidi, P.; Stamatis, G.; Zagana, E. Groundwater Quality Assessment and Geogenic and Anthropogenic Effect Estimation in Erasinos Basin (E. Attica). Eur. Water 2011, 33, 11–27. [Google Scholar]
- Stamatis, G.; Lambrakis, N.; Alexakis, D.; Zagana, E. Groundwater Quality in Mesogea Basin in Eastern Attica (Greece). Hydrol. Process. 2006, 20, 2803–2818. [Google Scholar] [CrossRef]
- Latsoudas, X. Geological Map “Koropi-Plaka”, Scale 1:50.000; Institute of Geology and Mineral Exploration: Athens, Greece, 2003. [Google Scholar]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in Freshwaters and Drinking Water: Critical Review and Assessment of Data Quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D.; Elizalde-Martínez, I. Common Laboratory Reagents: Are They a Double-Edged Sword in Microplastics Research? Sci. Total Environ. 2023, 875, 162610. [Google Scholar] [CrossRef] [PubMed]
- Merkel, B.; Friedrich, B. Groundwater Geochemistry: A Practical Guide to Modeling of Natural and Contaminated Aquatic Systems; Nordstorm, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Shruti, V.C.; Kutralam-Muniasamy, G. Blanks and Bias in Microplastic Research: Implications for Future Quality Assurance. Trends Environ. Anal. Chem. 2023, 38, e00203. [Google Scholar] [CrossRef]
- Jin, N.; Song, Y.; Ma, R.; Li, J.; Li, G.; Zhang, D. Characterization and Identification of Microplastics Using Raman Spectroscopy Coupled with Multivariate Analysis. Anal. Chim. Acta 2022, 1197, 339519. [Google Scholar] [CrossRef]
- Scherrer, N.C.; Stefan, Z.; Francoise, D.; Annette, F.; Renate, K. Synthetic Organic Pigments of the 20th and 21st Century Relevant to Artist’s Paints: Raman Spectra Reference Collection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 505–524. [Google Scholar] [CrossRef]
- Vedad, J.; Reilly, L.; Desamero, R.Z.B.; Mojica, E.-R.E. Quantitative Analysis of Xylene Mixtures Using a Handheld Raman Spectrometer. In Raman Spectroscopy in the Undergraduate Curriculum; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2018; Volume 1305, pp. 129–152. [Google Scholar] [CrossRef]
- Lapa, H.M.; Martins, L.M.D.R.S. P-Xylene Oxidation to Terephthalic Acid: New Trends. Molecules 2023, 28, 1922. [Google Scholar] [CrossRef]
- Zhou, X.-X.; Liu, R.; Hao, L.-T.; Liu, J.-F. Identification of Polystyrene Nanoplastics Using Surface Enhanced Raman Spectroscopy. Talanta 2021, 221, 121552. [Google Scholar] [CrossRef]
- Kellar, E.J.C.; Galiotis, C.; Andrews, E.H. Raman Vibrational Studies of Syndiotactic Polystyrene. 1. Assignments in a Conformational/Crystallinity Sensitive Spectral Region. Macromolecules 1996, 29, 3515–3520. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Cosentino, A.; Mangone, A. Pigments Checker Version 3.0, a Handy Set for Conservation Scientists: A Free Online Raman Spectra Database. Microchem. J. 2016, 129, 123–132. [Google Scholar] [CrossRef]
- Gall, M.J.; Hendra, P.J.; Peacock, C.J.; Cudby, M.E.A.; Willis, H.A. Laser-Raman Spectrum of Polyethylene: Part 1. Structure and Analysis of the Polymer. Polymer 1972, 13, 104–108. [Google Scholar] [CrossRef]
- Fischer, J.; Wallner, G.M.; Pieber, A. Spectroscopical Investigation of Ski Base Materials. Macromol. Symp. 2008, 265, 28–36. [Google Scholar] [CrossRef]
- Nava, V.; Frezzotti, M.L.; Leoni, B. Raman Spectroscopy for the Analysis of Microplastics in Aquatic Systems. Appl. Spectrosc. 2021, 75, 1341–1357. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhang, Q.; Xing, X.; Chen, W.; She, Z.; Luo, Z. Raman Spectra and Surface Changes of Microplastics Weathered under Natural Environments. Sci. Total Environ. 2020, 739, 139990. [Google Scholar] [CrossRef] [PubMed]
- Kniggendorf, A.-K.; Wetzel, C.; Roth, B. Microplastics Detection in Streaming Tap Water with Raman Spectroscopy. Sensors 2019, 19, 1839. [Google Scholar] [CrossRef] [PubMed]
- De Baez, M.A.; Hendra, P.J.; Judkins, M. The Raman spectra of oriented isotactic polypropylene. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 2117–2124. [Google Scholar] [CrossRef]
- Andreassen, E. Infrared and Raman Spectroscopy of Polypropylene. In Polypropylene; Karger-Kocsis, J., Ed.; Polymer Science and Technology Series; Springer: Dordrecht, The Netherlands, 1999; Volume 2, pp. 320–328. [Google Scholar] [CrossRef]
- Ciera, L.; Beladjal, L.; Almeras, X.; Gheysens, T.; Van Landuyt, L.; Mertens, J.; Nierstrasz, V.; Van Langenhove, L. Morphological and Material Properties of Polyethyleneterephthalate (PET) Fibres with Spores Incorporated. Fibres Text. East. Eur. 2014, 22, 29–36. [Google Scholar]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for Sampling and Detection of Microplastics in Water and Sediment: A Critical Review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Martí, E.; Martin, C.; Galli, M.; Echevarría, F.; Duarte, C.M.; Cózar, A. The Colors of the Ocean Plastics. Environ. Sci. Technol. 2020, 54, 6594–6601. [Google Scholar] [CrossRef]
- Gregory, P. Industrial Applications of Phthalocyanines. J. Porphyr. Phthalocyanines 2000, 4, 432–437. [Google Scholar] [CrossRef]
- Leiser, R.; Jongsma, R.; Bakenhus, I.; Möckel, R.; Philipp, B.; Neu, T.R.; Wendt-Potthoff, K. Interaction of Cyanobacteria with Calcium Facilitates the Sedimentation of Microplastics in a Eutrophic Reservoir. Water Res. 2021, 189, 116582. [Google Scholar] [CrossRef] [PubMed]
- Dodhia, M.S.; Rogers, K.L.; Fernández-Juárez, V.; Carreres-Calabuig, J.A.; Löscher, C.R.; Tisserand, A.A.; Keulen, N.; Riemann, L.; Shashoua, Y.; Posth, N.R. Microbe-Mineral Interactions in the Plastisphere: Coastal Biogeochemistry and Consequences for Degradation of Plastics. Front. Mar. Sci. 2023, 10, 1134815. [Google Scholar] [CrossRef]
- Panno, S.V.; Kelly, W.R.; Scott, J.; Zheng, W.; McNeish, R.E.; Holm, N.; Hoellein, T.J.; Baranski, E.L. Microplastic Contamination in Karst Groundwater Systems. Groundwater 2019, 57, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Severini, E.; Ducci, L.; Sutti, A.; Robottom, S.; Sutti, S.; Celico, F. River–Groundwater Interaction and Recharge Effects on Microplastics Contamination of Groundwater in Confined Alluvial Aquifers. Water 2022, 14, 1913. [Google Scholar] [CrossRef]
- Gong, X.; Tian, L.; Wang, P.; Wang, Z.; Zeng, L.; Hu, J. Microplastic Pollution in the Groundwater under a Bedrock Island in the South China Sea. Environ. Res. 2023, 239, 117277. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Pei, D. Relationships between Microplastic Pollution and Land Use in the Chongqing Section of the Yangtze River. Front. Ecol. Evol. 2023, 11, 1202562. [Google Scholar] [CrossRef]
- Wang, F.; Shih, K.M.; Li, X.Y. The Partition Behavior of Perfluorooctanesulfonate (PFOS) and Perfluorooctanesulfonamide (FOSA) on Microplastics. Chemosphere 2015, 119, 841–847. [Google Scholar] [CrossRef]
- Shockley, D.J.; Lapworth, D.J. Microplastics in Groundwater: A Literature Review. Available online: https://nora.nerc.ac.uk/id/eprint/532669/ (accessed on 27 January 2024).
- Alvarado-Zambrano, D.; Rivera-Hernández, J.R.; Green-Ruiz, C. First Insight into Microplastic Groundwater Pollution in Latin America: The Case of a Coastal Aquifer in Northwest Mexico. Environ. Sci. Pollut. Res. 2023, 30, 73600–73611. [Google Scholar] [CrossRef]
- Sangkham, S.; Aminul Islam, M.d.; Adhikari, S.; Kumar, R.; Sharma, P.; Sakunkoo, P.; Bhattacharya, P.; Tiwari, A. Evidence of Microplastics in Groundwater: A Growing Risk for Human Health. Groundw. Sustain. Dev. 2023, 23, 100981. [Google Scholar] [CrossRef]
- Mu, H.; Wang, Y.; Zhang, H.; Guo, F.; Li, A.; Zhang, S.; Liu, S.; Liu, T. High Abundance of Microplastics in Groundwater in Jiaodong Peninsula, China. Sci. Total Environ. 2022, 839, 156318. [Google Scholar] [CrossRef] [PubMed]
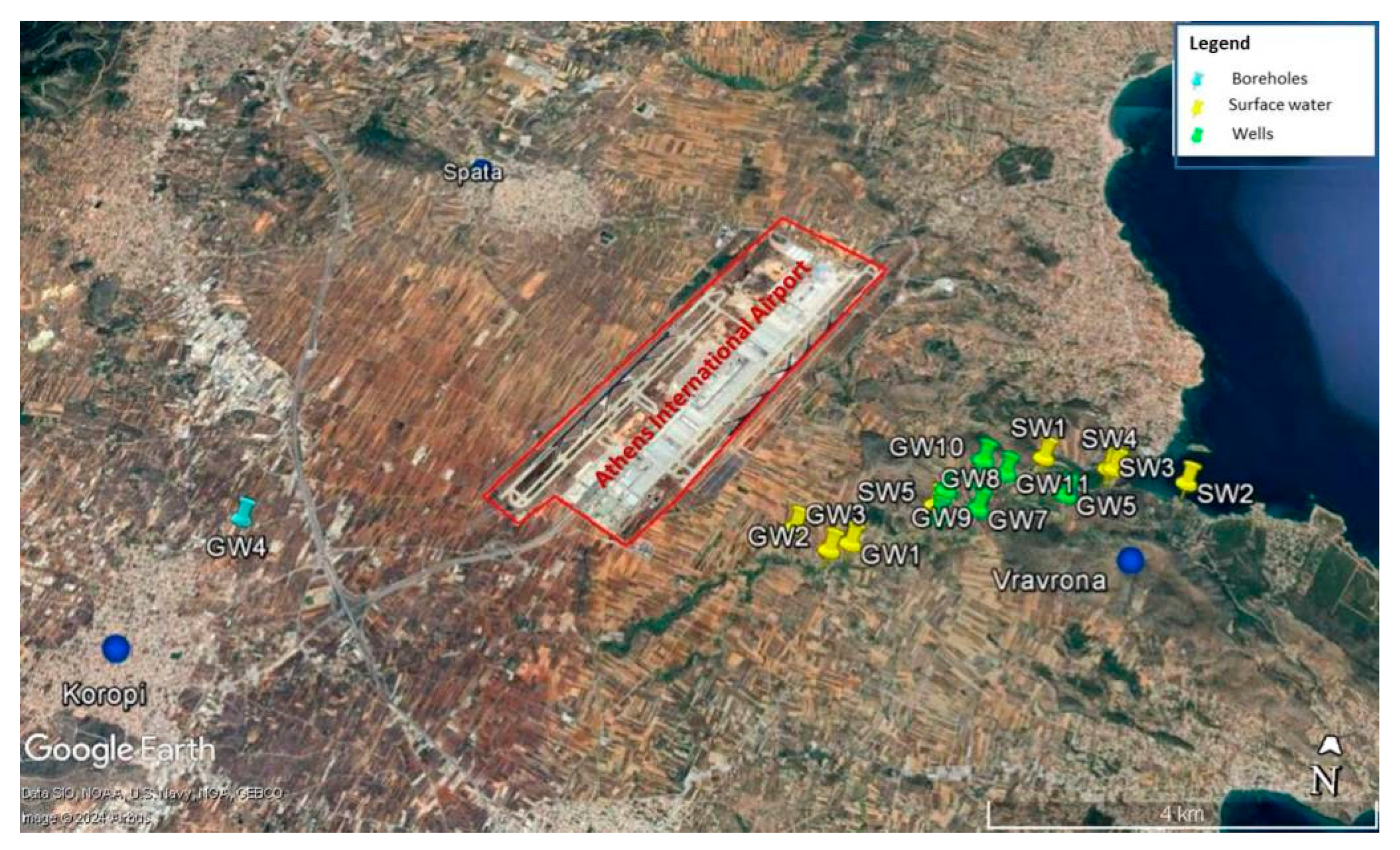

| Sample ID | X Longitude | Y Latitude | Elevation (m.a.s.l.) * | Notes |
|---|---|---|---|---|
| GW1 | 23°57′57.77″ E | 37°54′53.56″ N | 25 m | Well—phreatic water table |
| GW2 | 23°57′43.44″ E | 37°54′50.45″ N | 33 m | Well—phreatic water table |
| GW3 | 23°57′27.13″ E | 37°54′58.95″ N | 41 m | Well—phreatic water table |
| GW4 | 23°52′59.76″ E | 37°54′53.20″ N | 103 m | Borehole |
| SW1 | 23°59′29.80″ E | 37°55′33.56″ N | 10 m | Surface water |
| GW5 | 23°59′38.50″ E | 37°55′17.50″ N | 9.5 m | Well—phreatic water table |
| SW2 | 24°0′37.90″ E | 37°55′24.90″ N | 0 m | Surface water—river mouth to the sea |
| SW3 | 24°0′0.85″ E | 37°55′29.64″ N | 8.5 m | Surface water |
| SW4 | 24°0′3.50″ E | 37°55′29.85″ N | 0 m | Surface water |
| SW5 | 23°58′33.4″ E | 37°55′7.40″ N | 19.5 m | Surface water |
| GW6 | 23°58′32.55″ E | 37°55′5.16″ N | 19.8 m | Well—phreatic water table |
| GW7 | 23°58′55.15″ E | 37°55′10.10″ N | 15.2 m | Well—phreatic water table |
| GW8 | 23°58′55.11″ E | 37°55′22.59″ N | 12 m | Well—phreatic water table |
| GW9 | 23°58′40.60″ E | 37°55′17.65″ N | 14 m | Well—phreatic water table |
| GW10 | 23°58′59.85″ E | 37°55′31.39″ N | 13.5 m | Well—phreatic water table |
| GW11 | 23°59′10.14″ E | 37°55′25.90″ N | 5.2 m | Well—phreatic water table |
| Sample ID | pH | TDS | E.C. | Temp | Na+ | K+ | Ca2+ | Mg2+ | Cl− | HCO3− | NO3− | Si | SO42− | PO43− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | µS/cm | °C | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | ||
| SW1 | 7.4 | 1126 | 1732 | 21 | 99.3 | 4 | 136 | 39.4 | 212.8 | 366 | 31.7 | 10.7 | 120 | 0.034 |
| GW5 | 7.4 | 1807 | 2780 | 22.6 | 346 | 3.4 | 208.8 | 74.2 | 531.9 | 317.2 | 72.2 | 6 | 470 | 0.14 |
| SW2 | 8.1 | 35,750 | 55,000 | 20 | 10,300 | 420 | 435.2 | 1393.6 | 12,198.6 | 170.8 | 11 | 0.8 | 2640 | 0.055 |
| SW3 | 7.8 | 1251 | 1925 | 22 | 206.6 | 5 | 147.2 | 81.4 | 425.5 | 396.5 | 31.2 | 10.3 | 162.5 | 0.145 |
| SW4 | 7.8 | 11,440 | 17,600 | 21 | 208 | 70 | 227.2 | 506.6 | 6241.1 | 549 | 22 | 8.1 | 195.5 | 0.13 |
| SW5 | 7.7 | 965 | 1485 | 24.8 | 93.3 | 3.2 | 116.8 | 43.7 | 248.2 | 329.4 | 36.1 | 9.8 | 43.8 | 0.069 |
| GW6 | 7.4 | 1043 | 1605 | 22.5 | 95.1 | 4 | 142.4 | 47.7 | 283.7 | 347.7 | 40.5 | 10.5 | 115 | 0.11 |
| GW7 | 7.3 | 1040 | 1600 | 22 | 101 | 3.8 | 123.2 | 69.1 | 319.2 | 427 | 16.7 | 8.9 | 72.5 | 0.07 |
| GW8 | 7.9 | 917 | 1410 | 21.5 | 86.6 | 6.4 | 120 | 39.3 | 248.2 | 384.3 | 12.3 | 9.5 | 17.5 | 0.935 |
| GW9 | 7.1 | 1339 | 2060 | 21.2 | 114 | 3.6 | 174.4 | 57.5 | 372.3 | 366 | 61.2 | 13.1 | 125 | 0.14 |
| GW10 | 6.9 | 1411 | 2170 | 24 | 122 | 2.2 | 209.6 | 53.2 | 407.8 | 396.5 | 48 | 9.9 | 87.5 | 0.07 |
| GW11 | 7.5 | 897 | 1380 | 25 | 91.6 | 4.2 | 123.2 | 66.7 | 319.2 | 372.1 | 36.5 | 8 | 50 | 0.155 |
| Sample ID | SI (Calcite) | SI (Dolomite) | SI (Quartz) |
|---|---|---|---|
| SW1 | 0.45 | 0.66 | 0.62 |
| GW5 | 0.49 | 0.84 | 0.35 |
| SW2 | 0.71 | 2.25 | −0.42 |
| SW3 | 0.87 | 1.8 | 0.59 |
| SW4 | 0.94 | 2.55 | 0.52 |
| SW5 | 0.7 | 1.32 | 0.52 |
| GW6 | 0.46 | 0.77 | 0.59 |
| GW7 | 0.39 | 0.85 | 0.53 |
| GW8 | 0.93 | 1.68 | 0.56 |
| GW9 | 0.19 | 0.2 | 0.71 |
| GW10 | 0.13 | 0.01 | 0.55 |
| GW11 | 0.6 | 1.28 | 0.44 |
| Category/Samples No of MPs Particles/L | A | B | C | D | E | F | G | H | Total |
|---|---|---|---|---|---|---|---|---|---|
| SW1 (MPs particles/L) | 26 | 7 | 2 | 4 | 2 | 89 | 0 | 40 | 170 |
| GW5 (MPs particles/L) | 11 | 4 | 3 | 10 | 1 | 458 | 0 | 26 | 513 |
| SW2 (MPs particles/L) | 16 | 6 | 7 | 7 | 3 | 347 | 1 | 16 | 403 |
| SW3 (MPs particles/L) | 14 | 2 | 2 | 7 | 0 | 166 | 1 | 15 | 207 |
| SW4 (MPs particles/L) | 27 | 7 | 7 | 44 | 12 | 303 | 13 | 72 | 485 |
| SW5 (MPs particles/L) | 3 | 0 | 3 | 8 | 1 | 95 | 0 | 16 | 126 |
| GW6 (MPs particles/L) | 17 | 5 | 4 | 16 | 0 | 220 | 0 | 18 | 280 |
| GW7 (MPs particles/L) | 8 | 2 | 2 | 7 | 0 | 29 | 1 | 8 | 57 |
| GW8 (MPs particles/L) | 12 | 1 | 1 | 5 | 3 | 229 | 0 | 13 | 264 |
| GW9 (MPs particles/L) | 13 | 0 | 5 | 8 | 20 | 362 | 2 | 21 | 431 |
| GW10 (MPs particles/L) | 9 | 2 | 2 | 8 | 3 | 77 | 0 | 11 | 112 |
| GW11 (MPs particles/L) | 11 | 1 | 1 | 5 | 2 | 128 | 0 | 9 | 157 |
| GW1 (MPs particles/L) | 17 | 1 | 0 | 2 | 3 | 31 | 4 | 9 | 67 |
| GW2 (MPs particles/L) | 18 | 0 | 3 | 6 | 2 | 36 | 0 | 5 | 70 |
| GW3 (MPs particles/L) | 1 | 0 | 0 | 2 | 0 | 37 | 0 | 1 | 41 |
| GW4 (MPs particles/L) | 4 | 0 | 0 | 0 | 0 | 5 | 6 | 1 | 16 |
| Total per category | 207 | 38 | 42 | 139 | 52 | 2612 | 28 | 281 | 3399 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perraki, M.; Skliros, V.; Mecaj, P.; Vasileiou, E.; Salmas, C.; Papanikolaou, I.; Stamatis, G. Identification of Microplastics Using µ-Raman Spectroscopy in Surface and Groundwater Bodies of SE Attica, Greece. Water 2024, 16, 843. https://doi.org/10.3390/w16060843
Perraki M, Skliros V, Mecaj P, Vasileiou E, Salmas C, Papanikolaou I, Stamatis G. Identification of Microplastics Using µ-Raman Spectroscopy in Surface and Groundwater Bodies of SE Attica, Greece. Water. 2024; 16(6):843. https://doi.org/10.3390/w16060843
Chicago/Turabian StylePerraki, Maria, Vasilios Skliros, Petros Mecaj, Eleni Vasileiou, Christos Salmas, Ioannis Papanikolaou, and Georgios Stamatis. 2024. "Identification of Microplastics Using µ-Raman Spectroscopy in Surface and Groundwater Bodies of SE Attica, Greece" Water 16, no. 6: 843. https://doi.org/10.3390/w16060843
APA StylePerraki, M., Skliros, V., Mecaj, P., Vasileiou, E., Salmas, C., Papanikolaou, I., & Stamatis, G. (2024). Identification of Microplastics Using µ-Raman Spectroscopy in Surface and Groundwater Bodies of SE Attica, Greece. Water, 16(6), 843. https://doi.org/10.3390/w16060843








