Effect of Aeration and External Carbon Source on Nitrogen Removal and Distribution Patterns of Related-Microorganisms in Horizontal Subsurface Flow Constructed Wetlands
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Setup Location
2.2. Experimental Design
2.3. Water Samples Analysis
2.4. DNA Extraction, Bacterial Sequencing, and qPCR
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Aeration and External Carbon Source on the Overall Performance of HSSFCWs
3.2. Effect of Aeration and External Carbon Sources on Bacterial Community Structure
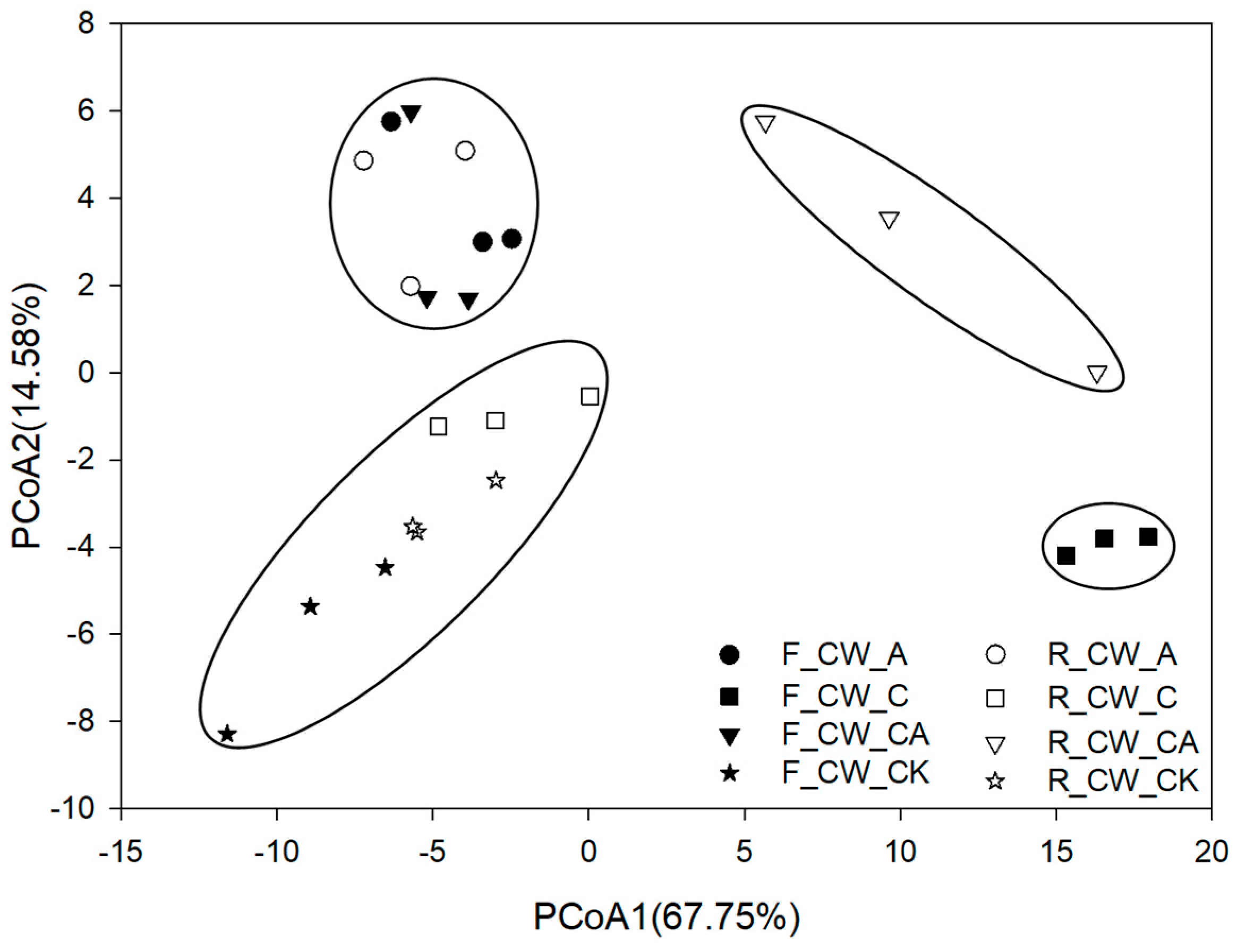
3.2.1. α-Diversity
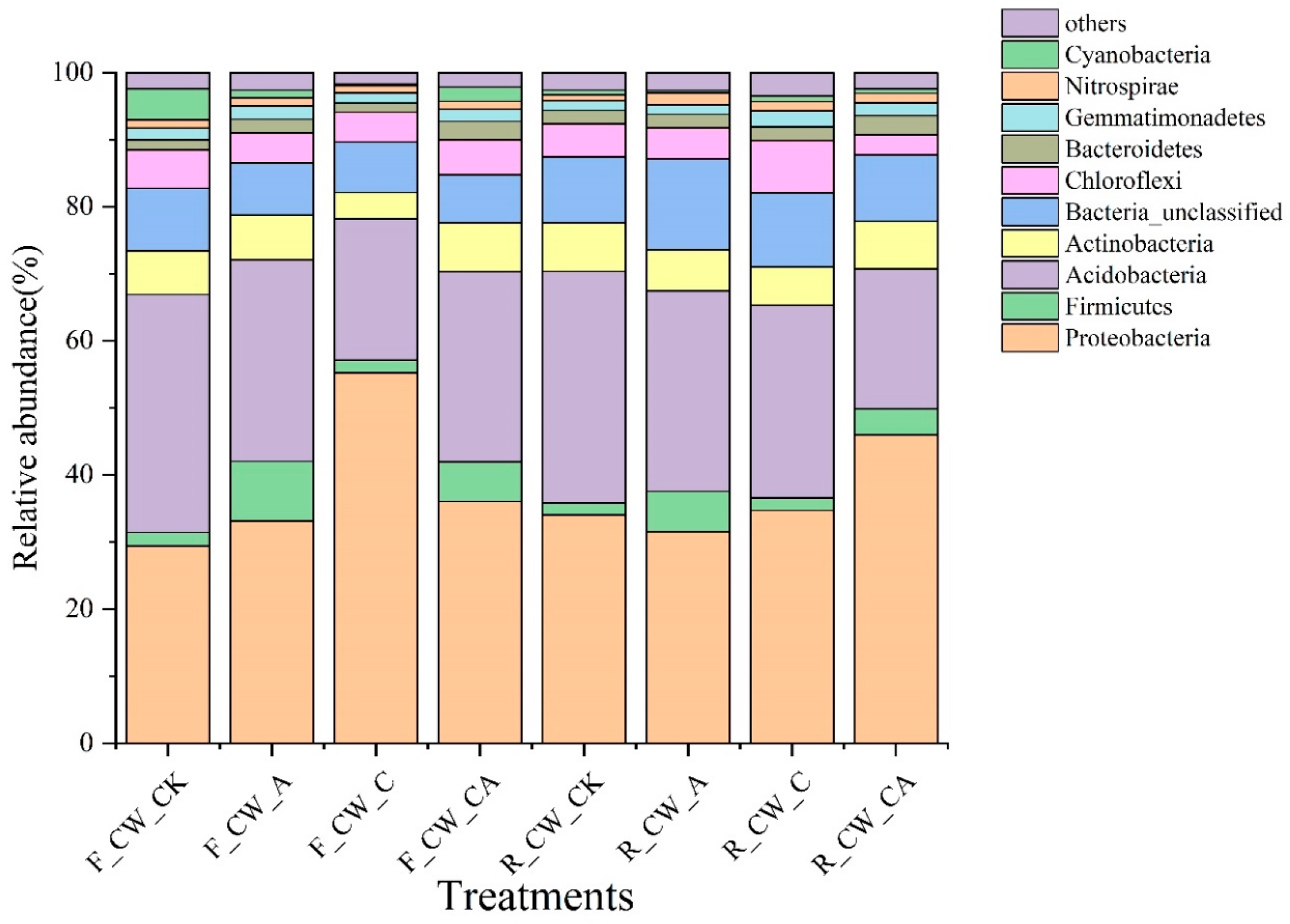
3.2.2. Bacterial Community Composition
3.3. Microbial Mechanism of Aeration and External Carbon Source on Nitrogen Transformation
3.3.1. Genera Related to Nitrogen Transformation
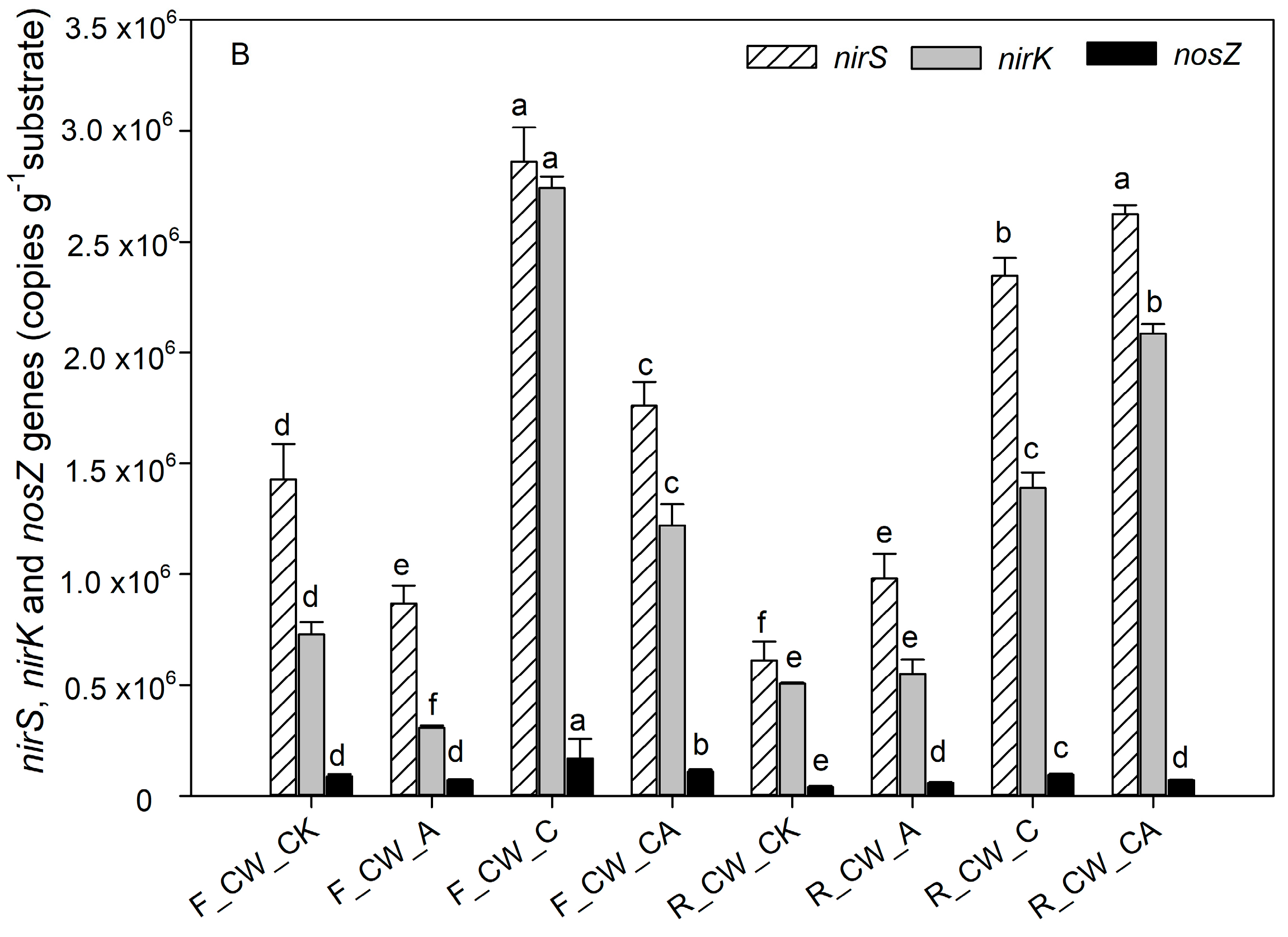
3.3.2. Functional Genes Related to Nitrogen Transformation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Price, J.R.; Ledford, S.H.; Ryan, M.O.; Toran, L.; Sales, C.M. Wastewater treatment plant effluent introduces recoverable shifts in microbial community composition in receiving streams. Sci. Total Environ. 2018, 613–614, 1104–1116. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment: Five Decades of Experience. Environ. Sci. Technol. 2011, 45, 61–69. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, W.; Song, X.; Wang, Y. Spatial distribution characteristics of environmental parameters and nitrogenous compounds in horizontal subsurface flow constructed wetland treating high nitrogen-content wastewater. Ecol. Eng. 2014, 70, 446–449. [Google Scholar] [CrossRef]
- Liu, G.; He, T.; Liu, Y.; Chen, Z.; Li, L.; Huang, Q.; Xie, Z.; Xie, Y.; Wu, L.; Liu, J. Study on the purification effect of aeration-enhanced horizontal subsurface-flow constructed wetland on polluted urban river water. Environ. Sci. Pollut. Res. 2019, 26, 12867–12880. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, C.; Huang, X.; Liu, J.; Lu, L.; Peng, K.; Li, S. Research progress in solid carbon source-based denitrification technologies for different target water bodies. Sci. Total Environ. 2021, 782, 146669. [Google Scholar] [CrossRef]
- Wu, S.; Kuschk, P.; Brix, H.; Vymazal, J.; Dong, R. Development of constructed wetlands in performance intensifications for wastewater treatment: A nitrogen and organic matter targeted review. Water Res. 2014, 57, 40–55. [Google Scholar] [CrossRef]
- Ong, S.A.; Uchiyama, K.; Inadama, D.; Ishida, Y.; Yamagiwa, K. Performance evaluation of laboratory scale up-flow constructed wetlands with different designs and emergent plants. Bioresour. Technol. 2010, 101, 7239–7244. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, X.; Liu, H.; Wu, H. Elucidating the impact of influent pollutant loadings on pollutants removal in agricultural waste-based constructed wetlands treating low C/N wastewater. Bioresour. Technol. 2019, 273, 529–537. [Google Scholar] [CrossRef]
- Wang, J.; Hou, J.; Xia, L.; Jia, Z.; He, X.; Li, D.; Zhou, Y. The combined effect of dissolved oxygen and COD/N on nitrogen removal and the corresponding mechanisms in intermittent aeration constructed wetlands. Biochem. Eng. J. 2020, 153, 107400. [Google Scholar] [CrossRef]
- Wu, H.; Fan, J.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Lv, J. Optimization of organics and nitrogen removal in intermittently aerated vertical flow constructed wetlands: Effects of aeration time and aeration rate. Int. Biodeterior. Biodegrad. 2016, 113, 139–145. [Google Scholar] [CrossRef]
- Zhu, H.; Yan, B.; Xu, Y.; Guan, J.; Liu, S. Removal of nitrogen and COD in horizontal subsurface flow constructed wetlands under different influent C/N ratios. Ecol. Eng. 2014, 63, 58–63. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Yan, B.; Shutes, B.; Tian, L.; Wen, H. Optimal influent COD/N ratio for obtaining low GHG emissions and high pollutant removal efficiency in constructed wetlands. J. Clean. Prod. 2020, 267, 122003. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, H.B.; Xu, X.Y.; Wang, S.Q.; Pan, J. Does carbon-nitrogen ratio affect nitrous oxide emission and spatial distribution in subsurface wastewater infiltration system? Bioresour. Technol. 2018, 250, 846–852. [Google Scholar] [CrossRef]
- Fu, G.; Yu, T.; Ning, K.; Guo, Z.; Wong, M.-H. Effects of nitrogen removal microbes and partial nitrification-denitrification in the integrated vertical-flow constructed wetland. Ecol. Eng. 2016, 95, 83–89. [Google Scholar] [CrossRef]
- Vymazal, J. The use constructed wetlands with horizontal sub-surface flow for various types of wastewater. Ecol. Eng. 2009, 35, 1–17. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, J.; Guo, W.; Liang, S.; Wu, H. Enhanced long-term organics and nitrogen removal and associated microbial community in intermittently aerated subsurface flow constructed wetlands. Bioresour. Technol. 2016, 214, 871–875. [Google Scholar] [CrossRef]
- Wu, H.; Ma, W.; Kong, Q.; Liu, H. Spatial-temporal dynamics of organics and nitrogen removal in surface flow constructed wetlands for secondary effluent treatment under cold temperature. Chem. Eng. J. 2018, 350, 445–452. [Google Scholar] [CrossRef]
- Sun, H.; Yang, Z.; Wei, C.; Wu, W. Nitrogen removal performance and functional genes distribution patterns in solid-phase denitrification sub-surface constructed wetland with micro aeration. Bioresour. Technol. 2018, 263, 223–231. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Technical Guide of Constructed Wetland Water Purification System; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2021. [Google Scholar]
- GB 18918-2002; Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2002.
- Wang, W.; Gao, J.; Guo, X.; Li, W.; Tian, X.; Zhang, R. Long-term effects and performance of two-stage baffled surface flow constructed wetland treating polluted river. Ecol. Eng. 2012, 49, 93–103. [Google Scholar] [CrossRef]
- Fu, G.; Huangshen, L.; Guo, Z.; Zhou, Q.; Wu, Z. Effect of plant-based carbon sources on denitrifying microorganisms in a vertical flow constructed wetland. Bioresour. Technol. 2017, 224, 214–221. [Google Scholar] [CrossRef]
- Jia, L.; Li, C.; Zhang, Y.; Chen, Y.; Li, M.; Wu, S.; Wu, H. Microbial community responses to agricultural biomass addition in aerated constructed wetlands treating low carbon wastewater. J. Environ. Manag. 2020, 270, 110912. [Google Scholar] [CrossRef]
- Lai, X.; Zhao, Y.; Pan, F.; Yang, B.; Wang, H.; Wang, S.; He, F. Enhanced optimal removal of nitrogen and organics from intermittently aerated vertical flow constructed wetlands: Relative COD/N ratios and microbial responses. Chemosphere 2020, 244, 125556. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhou, Y.; Wang, J. Comparison of denitrification performance and microbial diversity using starch/polylactic acid blends and ethanol as electron donor for nitrate removal. Bioresour. Technol. 2013, 131, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, Z.; Zhang, Y.; Zhang, J.; Xie, H.; Liang, S. Microbial nitrogen removal of ammonia wastewater in poly (butylenes succinate)-based constructed wetland: Effect of dissolved oxygen. Appl. Microbiol. Biotechnol. 2018, 102, 9389–9398. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Luo, P.; Chen, X.; Chen, J.; Huang, Z.; Peng, J.; Xiao, R.; Wu, J. Stimulation of optimized influent C:N ratios on nitrogen removal in surface flow constructed wetlands: Performance and microbial mechanisms. Sci. Total Environ. 2019, 694, 133575. [Google Scholar] [CrossRef]
- Song, S.; Pan, J.; Wu, S.; Guo, Y.; Yu, J.; Shan, Q. Effects of chemical oxygen demand (COD)/N ratios on pollutants removal in the subsurface wastewater infiltration systems with/without intermittent aeration. Water Sci. Technol. 2016, 73, 2662–2669. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, W.; Liu, X.; Song, X.; Wang, Y.; Ullman, J.L. Intensified nitrogen removal of constructed wetland by novel integration of high rate algal pond biotechnology. Bioresour. Technol. 2016, 219, 757–761. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, M.; Wen, C.; Liu, D. Nitrogen release and its influence on anammox bacteria during the decay of Potamogeton crispus with different values of initial debris biomass. Sci. Total Environ. 2019, 650, 604–615. [Google Scholar] [CrossRef]
- Wang, W.; Song, X.; Li, F.; Ji, X.; Hou, M. Intensified nitrogen removal in constructed wetlands by novel spray aeration system and different influent COD/N ratios. Bioresour. Technol. 2020, 306, 123008. [Google Scholar] [CrossRef] [PubMed]
- Hocaoglu, S.M.; Insel, G.; Cokgor, E.U.; Orhon, D. Effect of low dissolved oxygen on simultaneous nitrification and denitrification in a membrane bioreactor treating black water. Bioresour. Technol. 2011, 102, 4333–4340. [Google Scholar] [CrossRef]
- Ye, F.; Li, Y. Enhancement of nitrogen removal in towery hybrid constructed wetland to treat domestic wastewater for small rural communities. Ecol. Eng. 2009, 35, 1043–1050. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, R.; Liu, H.; Wu, S.; Wu, H. Nitrogen removal responses to biochar addition in intermittent-aerated subsurface flow constructed wetland microcosms: Enhancing role and mechanism. Ecol. Eng. 2019, 128, 57–65. [Google Scholar] [CrossRef]
- Liu, F.F.; Fan, J.; Du, J.; Shi, X.; Zhang, J.; Shen, Y. Intensified nitrogen transformation in intermittently aerated constructed wetlands: Removal pathways and microbial response mechanism. Sci. Total Environ. 2019, 650, 2880–2887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; You, J.; An, N.; Zhao, J.; Wang, L.; Cheng, Z.; Ye, J.; Chen, D.; Chen, J. Gaseous toluene powered microbial fuel cell: Performance, microbial community, and electron transfer pathway. Chem. Eng. J. 2018, 351, 515–522. [Google Scholar] [CrossRef]
- Shu, D.; He, Y.; Yue, H.; Wang, Q. Metagenomic and quantitative insights into microbial communities and functional genes of nitrogen and iron cycling in twelve wastewater treatment systems. Chem. Eng. J. 2016, 290, 21–30. [Google Scholar] [CrossRef]
- Chen, D.; Gu, X.; Zhu, W.; He, S.; Huang, J.; Zhou, W. Electrons transfer determined greenhouse gas emissions in enhanced nitrogen-removal constructed wetlands with different carbon sources and carbon-to-nitrogen ratios. Bioresour. Technol. 2019, 285, 121313. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, L.; Wei, C.; Wu, W.; Zhao, X.; Lu, T. Enhanced nitrogen removal using solid carbon source in constructed wetland with limited aeration. Bioresour. Technol. 2018, 248, 98–103. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, W.; Huang, J.; He, S.; Yan, Y.; Zhu, W.; Wu, S.; Zhang, X. Nitrogen removal by the enhanced floating treatment wetlands from the secondary effluent. Bioresour. Technol. 2017, 234, 243–252. [Google Scholar] [CrossRef]
- Button, M.; Nivala, J.; Weber, K.P.; Aubron, T.; Muller, R.A. Microbial community metabolic function in subsurface flow constructed wetlands of different designs. Ecol. Eng. 2015, 80, 162–171. [Google Scholar] [CrossRef]
- Liu, L.; Li, N.; Tao, C.; Zhao, Y.; Gao, J.; Huang, Z.; Zhang, J.; Gao, J.; Zhang, J.; Cai, M. Nitrogen removal performance and bacterial communities in zeolite trickling filter under different influent C/N ratios. Environ. Sci. Pollut. Res. Int. 2020, 28, 15909–15922. [Google Scholar] [CrossRef]
- Zhu, T.; Gao, J.; Huang, Z.; Shang, N.; Gao, J.; Zhang, J.; Cai, M. Comparison of performance of two large-scale vertical-flow constructed wetlands treating wastewater treatment plant tail-water: Contaminants removal and associated microbial community. J. Environ. Manag. 2021, 278, 111564. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Liu, G.; She, Z.; Gao, M.; Zhao, Y.; Guo, L.; Jin, C. Performance and bacterial communities in unsaturated and saturated zones of a vertical-flow constructed wetland with continuous-feed. Bioresour. Technol. 2020, 315, 123859. [Google Scholar] [CrossRef] [PubMed]
- Coban, O.; Kuschk, P.; Kappelmeyer, U.; Spott, O.; Martienssen, M.; Jetten, M.S.; Knoeller, K. Nitrogen transforming community in a horizontal subsurface-flow constructed wetland. Water Res. 2015, 74, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; He, F.; Ma, L.; Zhang, Y.; Wu, Z. Microbial nitrogen removal pathways in integrated vertical-flow constructed wetland systems. Bioresour. Technol. 2016, 207, 339–345. [Google Scholar] [CrossRef]
- Pelissari, C.; Avila, C.; Trein, C.M.; Garcia, J.; de Armas, R.D.; Sezerino, P.H. Nitrogen transforming bacteria within a full-scale partially saturated vertical subsurface flow constructed wetland treating urban wastewater. Sci. Total Environ. 2017, 574, 390–399. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, B.; Zhang, J.; Ngo, H.H.; Guo, W.; Liu, F.; Guo, Y.; Wu, H. Intermittent aeration strategy to enhance organics and nitrogen removal in subsurface flow constructed wetlands. Bioresour. Technol. 2013, 141, 117–122. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.T.; Hu, H.W.; Cai, Z.J.; Lei, Y.R.; Li, W.; Zhang, M.Y.; Li, Z.M.; Zhu, Y.N.; Cui, L.J. Changes of the denitrifying communities in a multi-stage free water surface constructed wetland. Sci. Total Environ. 2019, 650, 1419–1425. [Google Scholar] [CrossRef]
- Hou, J.; Wang, X.; Wang, J.; Xia, L.; Zhang, Y.; Li, D.; Ma, X. Pathway governing nitrogen removal in artificially aerated constructed wetlands: Impact of aeration mode and influent chemical oxygen demand to nitrogen ratios. Bioresour. Technol. 2018, 257, 137–146. [Google Scholar] [CrossRef]
- Harter, J.; Krause, H.M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J. 2014, 8, 660–674. [Google Scholar] [CrossRef] [PubMed]


| Item | Effluent Concentration | Removal Rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| CW_CK | CW_A | CW_C | CW_CA | CW_CK | CW_A | CW_C | CW_CA | |
| COD (mg·L−1) | 20.79 ± 0.76b | 16.86 ± 1.04c | 30.01 ± 1.13a | 21.74 ± 0.91b | 48.02 ± 1.61b | 57.84 ± 1.52a | 53.83 ± 1.27b | 66.56 ± 1.32a |
| NH4+-N (mg·L−1) | 0.70 ± 0.03a | 0.47 ± 0.01b | 0.78 ± 0.03a | 0.53 ± 0.02b | 64.78 ± 1.14b | 76.27 ± 2.06a | 61.21 ± 1.52b | 73.51 ± 1.65a |
| NO3−-N (mg·L−1) | 4.02 ± 0.13b | 4.76 ± 0.24a | 2.52 ± 0.32c | 2.38 ± 0.24c | 65.01 ± 1.97b | 58.59 ± 2.58c | 78.07 ± 2.28a | 79.31 ± 2.82a |
| TN (mg·L−1) | 4.77 ± 0.38a | 3.66 ± 0.13c | 4.22 ± 0.41b | 3.10 ± 0.21d | 63.28 ± 1.38d | 71.81 ± 1.29b | 67.52 ± 2.77c | 76.19 ± 2.19a |
| DO (mg·L−1) | 0.50 ± 0.01b | 1.81 ± 0.05a | 0.10 ± 0.01c | 1.69 ± 0.03a | - | - | - | - |
| pH | 7.57 ± 0.01a | 7.48 ± 0.01a | 7.46 ± 0.01a | 7.56 ± 0.01a | - | - | - | - |
| Treatments | Observed_Species | Chao1 | Shannon | Simpson |
|---|---|---|---|---|
| F_CW_CK | 2373 ± 146a | 3778 ± 252a | 9.76 ± 0.199a | 0.996 ± 0.001a |
| F_CW_A | 2336 ± 231a | 3695 ± 347a | 9.66 ± 0.388a | 0.994 ± 0.003a |
| F_CW_C | 2064 ± 55b | 3482 ± 251b | 8.50 ± 0.089b | 0.973 ± 0.003b |
| F_CW_CA | 2495 ± 111a | 3919 ± 230a | 9.94 ± 0.015a | 0.997 ± 0.001a |
| R_CW_CK | 2187 ± 115b | 3616 ± 309b | 9.739 ± 0.098a | 0.997 ± 0.002a |
| R_CW_A | 2353 ± 94a | 4104 ± 201a | 9.740 ± 0.141a | 0.995 ± 0.001a |
| R_CW_C | 2450 ± 44a | 4134 ± 288a | 10.099 ± 0.034a | 0.998 ± 0.001a |
| R_CW_CA | 2478 ± 199a | 4326 ± 104a | 9.163 ± 1.144b | 0.964 ± 0.052b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, R.; Shi, Y.; Pan, F. Effect of Aeration and External Carbon Source on Nitrogen Removal and Distribution Patterns of Related-Microorganisms in Horizontal Subsurface Flow Constructed Wetlands. Water 2024, 16, 632. https://doi.org/10.3390/w16050632
Zhang H, Li R, Shi Y, Pan F. Effect of Aeration and External Carbon Source on Nitrogen Removal and Distribution Patterns of Related-Microorganisms in Horizontal Subsurface Flow Constructed Wetlands. Water. 2024; 16(5):632. https://doi.org/10.3390/w16050632
Chicago/Turabian StyleZhang, Hao, Rongxin Li, Yue Shi, and Fuxia Pan. 2024. "Effect of Aeration and External Carbon Source on Nitrogen Removal and Distribution Patterns of Related-Microorganisms in Horizontal Subsurface Flow Constructed Wetlands" Water 16, no. 5: 632. https://doi.org/10.3390/w16050632
APA StyleZhang, H., Li, R., Shi, Y., & Pan, F. (2024). Effect of Aeration and External Carbon Source on Nitrogen Removal and Distribution Patterns of Related-Microorganisms in Horizontal Subsurface Flow Constructed Wetlands. Water, 16(5), 632. https://doi.org/10.3390/w16050632





