Non-Lethal Assessment of Land Use Change Effects in Water and Soil of Algerian Riparian Areas along the Medjerda River through the Biosentinel Bufo spinosus Daudin
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Soil Physico-Chemical Characterization
2.3. Water Evaluation
2.4. Toad Collection and Skin Analysis
2.5. HM Bioaccumulation
2.6. Statistical Approach
3. Results
3.1. Soil and General Water Characteristics
3.2. HM Concentration in Water, Soil and Toad Skin
3.3. HM Contamination Indices
3.4. HM Bioaccumulation in Toad Skin
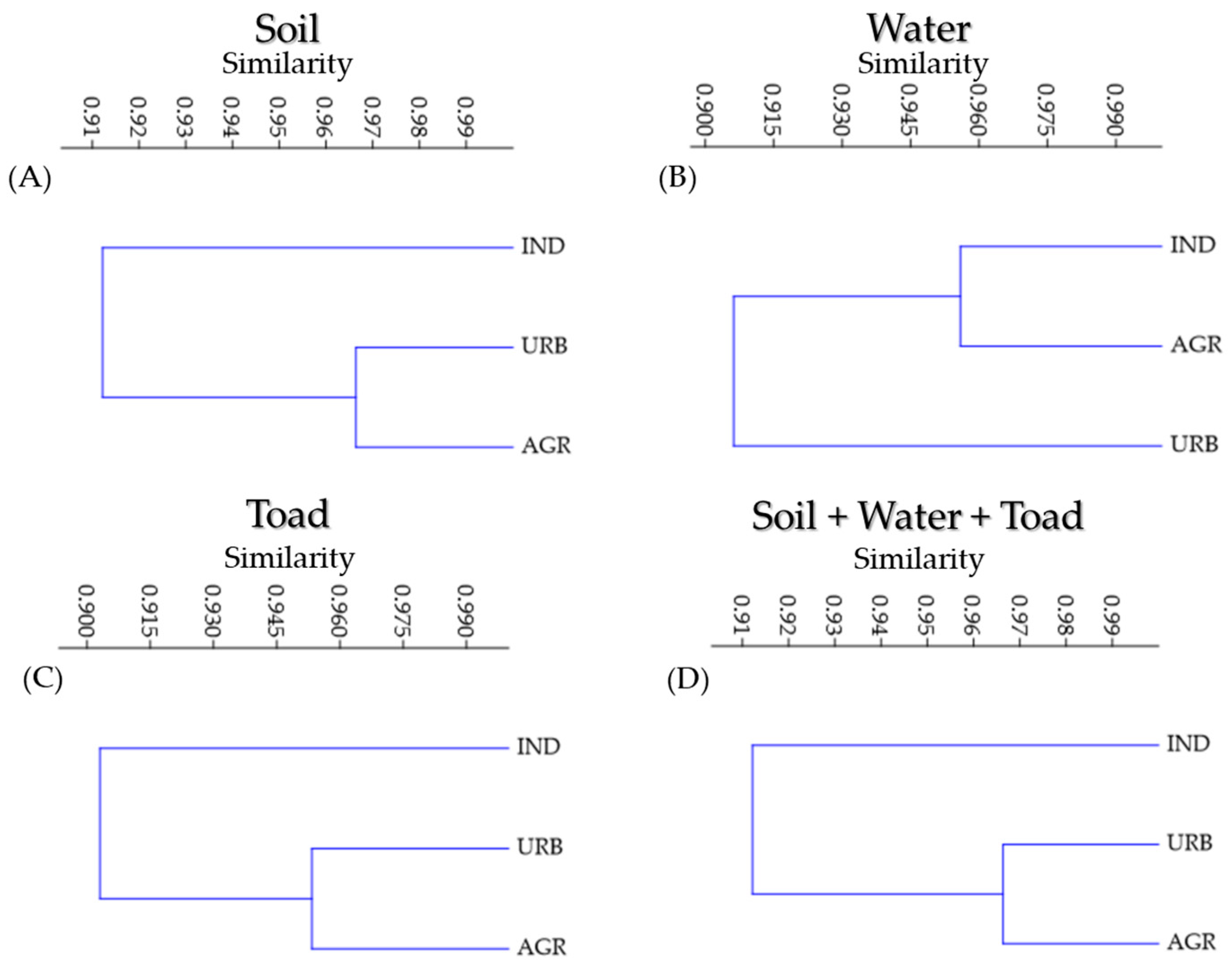
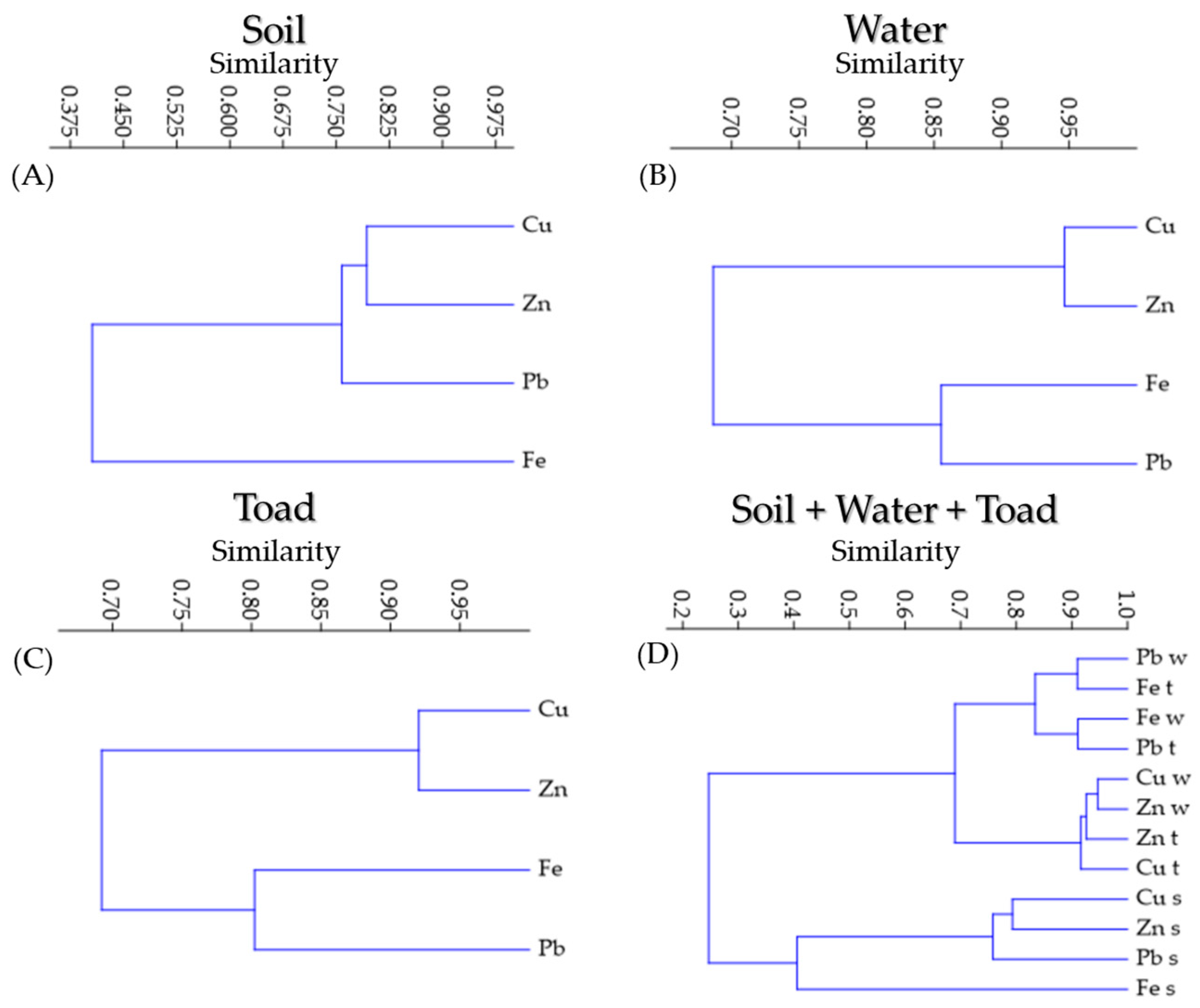
3.5. Hierarchical Clustering
4. Discussion
4.1. Impact of Land Uses on Soil and Water
4.2. Responses to Different Land Uses of Bufo spinosus and Relationships with Soil and Water Matrices
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderon, M.R.; González, S.P.; Pérez-Iglesias, J.M.; Jofré, M.B. Anthropogenic impacts on rivers: Use of multiple indicators to assess environmental quality status. Hydrobiologia 2023, 850, 469–487. [Google Scholar] [CrossRef]
- Rakshit, D.; Sahu, G.; Mohanty, A.K.; Satpathy, K.K.; Jonathan, M.P.; Murugan, K.; Sarkar, S.K. Bioindicator role of tintinnid (Protozoa: Ciliophora) for water quality monitoring in Kalpakkam, Tamil Nadu, south east coast of India. Mar. Pollut. Bull. 2017, 114, 134–143. [Google Scholar] [CrossRef]
- Abas, A. A systematic review on biomonitoring using lichen as the biological indicator: A decade of practices, progress and challenges. Ecol. Indic. 2021, 121, 107197. [Google Scholar] [CrossRef]
- Itziou, A.; Dimitriadis, V.K. Introduction of the land snail Eobania vermiculata as a bioindicator organism of terrestrial pollution using a battery of biomarkers. Sci. Total Environ. 2011, 409, 1181–1192. [Google Scholar] [CrossRef]
- Deeb, M.; Desjardins, T.; Podwojewski, P.; Pando, A.; Blouin, M.; Lerch, T.Z. Interactive effects of compost, plants and earthworms on the aggregations of constructed Technosols. Geoderma 2017, 305, 305–313. [Google Scholar] [CrossRef]
- Zina, V.; Ordeix, M.; Franco, J.C.; Ferreira, M.T.; Fernandes, M.R. Ants as bioindicators of riparian ecological health in catalonian rivers. Forests 2021, 12, 625. [Google Scholar] [CrossRef]
- Coller, E.; Longa, C.M.O.; Morelli, R.; Zanoni, S.; Ippolito, M.C.C.; Pindo, M.; Cappelletti, C.; Ciutti, F.; Menta, C.; Zanzotti, R.; et al. Soil communities: Who responds and how quickly to a change in agricultural system? Sustainability 2022, 14, 383. [Google Scholar] [CrossRef]
- Testi, A.; Fanelli, G.; Crosti, R.; Castigliani, V.; D’Angeli, D. Characterizing river habitat quality using plant and animal bioindicators: A case study of Tirino River (Abruzzo Region, Central Italy). Ecol. Indic. 2012, 20, 24–33. [Google Scholar] [CrossRef]
- Panda, B.P.; Mohanta, Y.K.; Parida, S.P.; Pradhan, A.; Mohanta, T.K.; Patowary, K.; Wan Mahari, W.A.; Lam, S.S.; Ghfar, A.A.; Guerriero, G.; et al. Metal pollution in freshwater fish: A key indicator of contamination and carcinogenic risk to public health. Environ. Pollut. 2023, 330, 121796. [Google Scholar] [CrossRef] [PubMed]
- De Agostini, A.; Cortis, P.; Cogoni, A. Monitoring of air pollution by moss bags around an oil refinery: A critical evaluation over 16 years. Atmosphere 2020, 11, 272. [Google Scholar] [CrossRef]
- Özkök, E.A.; Çobanoğlu, G. Spatial evaluation of air quality by biomonitoring of toxic element accumulation in lichens in urban green areas and nature parks on the Anatolian side of Istanbul. Environ. Monit. Assess. 2023, 195, 908. [Google Scholar] [CrossRef]
- Addesso, R.; Gonzalez-Pimentel, J.L.; D’Angeli, I.M.; De Waele, J.; Saiz-Jimenez, C.; Jurado, V.; Miller, A.Z.; Cubero, B.; Vigliotta, G.; Baldantoni, D. Microbial Community Characterizing Vermiculations from Karst Caves and Its Role in Their Formation. Microb. Ecol. 2021, 81, 884–896. [Google Scholar] [CrossRef]
- Celletti, S.; Fedeli, R.; Ghorbani, M.; Loppi, S. Impact of starch-based bioplastic on growth and biochemical parameters of basil plants. Sci. Total Environ. 2023, 856, 159163. [Google Scholar] [CrossRef]
- Monaci, F.; Ancora, S.; Paoli, L.; Loppi, S.; Franzaring, J. Air quality in post-mining towns: Tracking potentially toxic elements using tree leaves. Environ. Geochem. Health 2023, 45, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Piano, E.; Biagioli, F.; Nicolosi, G.; Coleine, C.; Poli, A.; Prigione, V.; Zanellati, A.; Addesso, R.; Varese, G.C.; Selbmann, L.; et al. Tourism affects microbial assemblages in show caves. Sci. Total Environ. 2023, 871, 162106. [Google Scholar] [CrossRef] [PubMed]
- Rascio, I.; Gattullo, C.E.; Porfido, C.; Allegretta, I.; Spagnuolo, M.; Tiziani, R.; Celletti, S.; Cesco, S.; Mimmo, T.; Terzano, R. Fire-induced effects on the bioavailability of potentially toxic elements in a polluted agricultural soil: Implications for Cr uptake by durum wheat plants. Environ. Sci. Pollut. Res. 2023, 30, 6358–6372. [Google Scholar] [CrossRef] [PubMed]
- Salo, J.V.B.; Solania, C.L. Article 2 Part of the Biodiversity Commons, Terrestrial and Aquatic Ecology Commons, and the Zoology Commons Recommended Citation Recommended Citation Salo. J. Bioresour. Manag. 2022, 9, 9–22. [Google Scholar]
- Çömden, E.A.; Yenmiş, M.; Çakır, B. The Complex Bridge between Aquatic and Terrestrial Life: Skin Changes during Development of Amphibians. J. Dev. Biol. 2023, 11, 6. [Google Scholar] [CrossRef]
- Gauthier, L.; Tardy, E.; Mouchet, F.; Marty, J. Biomonitoring of the genotoxic potential (micronucleus assay) and detoxifying activity (EROD induction) in the River Dadou (France), using the amphibian Xenopus laevis. Sci. Total Environ. 2004, 323, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal. Chim. Acta 2008, 606, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.G.; Wojtaszek, B.F.; Staznik, B.; Chartrand, D.T.; Stephenson, G.R. Chemical and biomonitoring to assess potential acute effects of Vision® herbicide on native amphibian larvae in forest wetlands. Environ. Toxicol. Chem. 2004, 23, 843–849. [Google Scholar] [CrossRef]
- Guezgouz, N.; Parisi, C.; Boubsil, S.; Grieco, G.; Hana, S.A.; Guerriero, G. Heavy Metals Assessment in the Medjerda River Basin (Northeastern Algeria): A Preliminary Water Analysis and Toad Skin Biopsy. Proc. Zool. Soc. 2021, 74, 104–113. [Google Scholar] [CrossRef]
- Napoletano, P.; Guezgouz, N.; Di Iorio, E.; Colombo, C.; Guerriero, G.; De Marco, A. Anthropic impact on soil heavy metal contamination in riparian ecosystems of northern Algeria. Chemosphere 2023, 313, 137522. [Google Scholar] [CrossRef]
- Meek, R. Long-Term Changes in Four Populations of the Spiny Toad, Bufo spinosus, in Western France; Data from Road Mortalities. Conservation 2022, 2, 248–261. [Google Scholar] [CrossRef]
- Zaimes, G.N.; Iakovoglou, V. Assessing riparian areas of Greece-an overview. Sustainability 2021, 13, 309. [Google Scholar] [CrossRef]
- Groffman, P.M.; Bain, D.J.; Band, L.E.; Belt, K.T.; Brush, G.S.; Grove, J.M.; Pouyat, R.V.; Yesilonis, I.C.; Zipperer, W.C. Down by the riverside: Urban riparian ecology. Front. Ecol. Environ. 2003, 1, 315–321. [Google Scholar] [CrossRef]
- Santolini, R.; Morri, E.; Pasini, G.; Giovagnoli, G.; Morolli, C.; Salmoiraghi, G. Assessing the quality of riparian areas: The case of River Ecosystem Quality Index applied to the Marecchia river (Italy). Int. J. River Basin Manag. 2015, 13, 1–16. [Google Scholar] [CrossRef]
- Kooch, Y.; Tavakoli, M.; Akbarinia, M. Microbial/biochemical indicators showing perceptible deterioration in the topsoil due to deforestation. Ecol. Indic. 2018, 91, 84–91. [Google Scholar] [CrossRef]
- Ndalilo, L.A.; Maranga, E.K.; Kirui, B.K. Land Use and Land Cover Change along River Lumi Riparian Ecosystem in Kenya: Implications on Local Livelihoods. Open J. For. 2021, 11, 206–221. [Google Scholar] [CrossRef]
- Kindu, M.; Schneider, T.; Teketay, D.; Knoke, T. Drivers of land use/land cover changes in Munessa-Shashemene landscape of the south-central highlands of Ethiopia. Environ. Monit. Assess. 2015, 187, 452. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Rahman, S.; Islam, M.S.; Rakib, M.R.J.; Hossen, S.; Rahman, M.Z.; Kormoker, T.; Idris, A.M.; Phoungthong, K. Distribution of heavy metals in water and sediment of an urban river in a developing country: A probabilistic risk assessment. Int. J. Sediment Res. 2022, 37, 173–187. [Google Scholar] [CrossRef]
- Abd Elnabi, M.K.; Elkaliny, N.E.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Abd Elaty, A.E.; et al. Toxicity of Heavy Metals and Recent Advances in Their Removal: A Review. Toxics 2023, 11, 580. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Santorufo, L.; Memoli, V.; Panico, S.C.; Esposito, F.; Vitale, L.; Di Natale, G.; Trifuoggi, M.; Barile, R.; De Marco, A.; Maisto, G. Impact of Anthropic Activities on Soil Quality under Different Land Uses. Int. J. Environ. Res. Public Health 2021, 18, 8423. [Google Scholar] [CrossRef]
- Joimel, S.; Cortet, J.; Jolivet, C.C.; Saby, N.P.A.; Chenot, E.D.; Branchu, P.; Consalès, J.N.; Lefort, C.; Morel, J.L.; Schwartz, C. Physico-chemical characteristics of topsoil for contrasted forest, agricultural, urban and industrial land uses in France. Sci. Total Environ. 2016, 545–546, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Belon, E.; Boisson, M.; Deportes, I.Z.; Eglin, T.K.; Feix, I.; Bispo, A.O.; Galsomies, L.; Leblond, S.; Guellier, C.R. An inventory of trace elements inputs to French agricultural soils. Sci. Total Environ. 2012, 439, 87–95. [Google Scholar] [CrossRef]
- El Khalil, H.; Schwartz, C.; Elhamiani, O.; Kubiniok, J.; Morel, J.; Boularbah, A. Contribution of technic materials to the mobile fraction of metals in urban soils in Marrakech (Morocco). J. Soils Sediments 2008, 8, 17–22. [Google Scholar] [CrossRef]
- Al-Kahtany, K.; Nour, Y.H.E.; El-Sorogy, A.S.; Alharbi, T. Ecological and health risk assessment of heavy metals contamination in mangrove sediments, Red Sea coast. Mar. Poll. Bull. 2023, 192, 115000. [Google Scholar] [CrossRef] [PubMed]
- Berhail, S.; Katipoğlu, O.M. Spatiotemporal detection of abrupt change in trends of rainfall and dry and wet periods at different time scales: The case of the Medjerda basin in northeast Algeria. Acta Geophys. 2023, 71, 2497–2516. [Google Scholar] [CrossRef]
- Dridi, A.; Tlili, A.; Fatnassi, S.; Hamrouni, H.; Gueddari, M. Effects of boron distribution on sugar beet crop yield in two Tunisian soils. Arab. J. Geosci. 2018, 11, 400. [Google Scholar] [CrossRef]
- IUSS. IUSS Working Group WRB: World Reference Base for Soil Resources 2014, Update 2015; World Soil Resources Report No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Colombo, C.; Miano, T. Metodi di Analisi Chimica del Suolo; Pubblicità & Stampa: Bari, Italy, 2015. [Google Scholar]
- De Marco, A.; Fioretto, A.; Giordano, M.; Innangi, M.; Menta, C.; Papa, S.; De Santo, A.V. C stocks in forest floor and mineral soil of two mediterranean beech forests. Forests 2016, 7, 181. [Google Scholar] [CrossRef]
- Pribyl, D.W. A critical review of the conventional SOC to SOM conversion factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- Lindsay, W.N.; Norwell, W.A. Development of a DTPA micronutrient soil test. Agron. Abstr. 1969, 84, 69–87. [Google Scholar]
- Srodek, D.; Rahmonov, O. The Properties of Black Locust Robinia pseudoacacia L. to Selectively Accumulate Chemical Elements from Soils of Ecologically Transformed Areas. Forests 2022, 13, 7. [Google Scholar] [CrossRef]
- Mueller, L.; Schindler, U.; Mirschel, W.; Graham Shepherd, T.; Ball, B.C.; Helming, K.; Rogasik, J.; Eulenstein, F.; Wiggering, H. As-sessing the productivity function of soils. A review. Agron. Sustain. Dev. 2010, 30, 601–614. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Okedeyi, O.O.; Dube, S.; Awofolu, O.R.; Nindi, M.M. Assessing the enrichment of heavy metals in surface soil and plant (Digitaria eriantha) around coal-fired power plants in South Africa. Environ. Sci. Pollut. Res. 2014, 21, 4686–4696. [Google Scholar] [CrossRef]
- Hakanson, L. Ecological risk index for aquatic pollution control, a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Rahmonov, O.; Sobala, M.; Srodek, D.; Karkosz, D.; Pytel, S.; Rahmonov, M. The spatial distribution of potentially toxic elements in the mountain forest topsoils (the Silesian Beskids, southern Poland). Sci. Rep. 2024, 14, 338. [Google Scholar] [CrossRef]
- Ahamad, M.I.; Song, J.; Sun, H.; Wang, X.; Mehmood, M.S.; Sajid, M.; Su, P.; Khan, A.J. Contamination level, ecological risk, and source identification of heavy metals in the hyporheic zone of the Weihe river, China. Int. J. Environ. Res. Public Health 2020, 17, 1070. [Google Scholar] [CrossRef]
- Heyer, R.; Donnelly, M.A.; Foster, M.; Mcdiarmid, R. Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians; Smithsonian Institution: Washington, DC, USA, 2014. [Google Scholar]
- D’Errico, G.; Vitiello, G.; De Tommaso, G.; Abdel-Gawad, F.K.; Brundo, M.V.; Ferrante, M.; De Maio, A.; Trocchia, S.; Bianchi, A.R.; Ciarcia, G.; et al. Electron Spin Resonance (ESR) for the study of Reactive Oxygen Species (ROS) on the isolated frog skin (Pelophylax bergeri): A non-invasive method for environmental monitoring. Environ. Res. 2018, 165, 11–18. [Google Scholar] [CrossRef]
- Kuziemska, B.; Wysokinski, A.; Klej, P. The Content, Uptake and Bioaccumulation Factor of Copper and Nickel in Grass Depending on Zinc Application and Organic Fertilization. Agriculture 2023, 13, 1676. [Google Scholar] [CrossRef]
- Ionescu, P.; Radu, V.M.; Deak, G.; Ciobotaru, I.E.; Marcu, E.; Diacu, E.; Pipirigeanu, M. Bioaccumulation of potentially toxic elements in fish species from aquatic environments located in crowded areas of southern Romania. Tech. Rom. J. Appl. Sci. Technol. 2019, 1, 53–58. [Google Scholar] [CrossRef]
- Lin, H. Three Principles of Soil Change and Pedogenesis in Time and Space. Soil Sci. Soc. Am. J. 2011, 75, 2049–2070. [Google Scholar] [CrossRef]
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various natural and anthropogenic factors responsible for water quality degradation: A review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Oyeyiola, A.O.; Davidson, C.M.; Olayinka, K.O.; Alo, B.I. Fractionation and ecotoxicological implication of potentially toxic metals in sediments of three urban rivers and the Lagos Lagoon, Nigeria, West Africa. Environ. Monit. Assess. 2014, 186, 7321–7333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guasmi, I.; Kherici-Bousnoubra, H.; Kherici, N.; Hadji, F. Assessing the organic pollution of surface water of Medjerda watershed (NE Algeria). Environ. Earth Sci. 2010, 60, 985–992. [Google Scholar] [CrossRef]
- Ben Ayed, L.; Horry, M.; Sabbahi, S.; Nouiri, I.; Karanis, P. Physico-chemical quality of the Medjerda River in Tunisia and suitability for irrigation during the moist and the dry seasons. Bull. Soc. R. Sci. Liege 2022, 91, 23–43. [Google Scholar] [CrossRef]
- Fayiga, A.O.; Ipinmoroti, M.O.; Chirenje, T. Environmental Pollution in Africa; Springer: Dordrecht, The Netherlands, 2018; Volume 20, ISBN 1066801698944. [Google Scholar]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water and food crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Shifaw, E. Review of heavy metals pollution in China in agricultural and urban soils. J. Health Pollut. 2018, 8, 180607. [Google Scholar] [CrossRef] [PubMed]
- Jdid, E.A.; Blazy, P.; Kamoun, S.; Guedria, A.; Marouf, B.; Kitane, S. Environmental impact of mining activity on the pollution of the Medjerda River, north-west Tunisia. Bull. Eng. Geol. Environ. 1999, 57, 273–280. [Google Scholar] [CrossRef]
- AFNOR. Qualité des sols. In Recueil de Normes Françaises; AFNOR: Paris, France, 1996. [Google Scholar]
- Bartoli, G.; Papa, S.; Sagnella, E.; Fioretto, A. Heavy metal content in sediments along the Calore river: Relationships with physical-chemical characteristics. J. Environ. Manag. 2012, 95, S9–S14. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Evaluating Household Water Treatment Options: Health-Based Targets and Microbiological Performance Specifications; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Prokof’eva, T.V.; Kiryushin, A.V.; Shishkov, V.A.; Ivannikov, F.A. The importance of dust material in urban soil formation: The experience on study of two young Technosols on dust depositions. J. Soils Sediments 2017, 17, 515–524. [Google Scholar] [CrossRef]
- Boubehziz, S.; Khanchoul, K.; Benslama, M.; Benslama, A.; Marchetti, A.; Francaviglia, R.; Piccini, C. Predictive mapping of soil organic carbon in Northeast Algeria. Catena 2020, 190, 104539. [Google Scholar] [CrossRef]
- Adegbidi, H.G.; Briggs, R.D.; Volk, T.A.; White, E.H.; Abrahamson, L.P. Effect of organic amendments and slow-release nitrogen fertilizer on willow biomass production and soil chemical characteristics. Biomass Bioenergy 2003, 25, 389–398. [Google Scholar] [CrossRef]
- Panico, S.C.; Esposito, F.; Memoli, V.; Vitale, L.; Polimeno, F.; Magliulo, V.; Maisto, G.; De Marco, A. Variations of agricultural soil quality during the growth stages of sorghum and sunflower. Appl. Soil Ecol. 2020, 152, 103569. [Google Scholar] [CrossRef]
- Tao, H.; Bobaker, A.M.; Ramal, M.M.; Yaseen, Z.M.; Hossain, M.S.; Shahid, S. Determination of biochemical oxygen demand and dissolved oxygen for semi-arid river environment: Application of soft computing models. Environ. Sci. Pollut. Res. 2019, 26, 923–937. [Google Scholar] [CrossRef]
- Yadav, R.K.; Goyal, B.; Sharma, R.K.; Dubey, S.K.; Minhas, P.S. Post-irrigation impact of domestic sewage effluent on composition of soils, crops and ground water—A case study. Environ. Int. 2002, 28, 481–486. [Google Scholar] [CrossRef]
- Kolář, L.; Klimeš, F.; Ledvina, R.; Kužel, S. A method to determine mineralization kinetics of a decomposable part of soil organic matter in the soil. Plant Soil Environ. 2003, 49, 8–11. [Google Scholar] [CrossRef]
- Guerriero, G.; Brundo, M.V.; Labar, S.; Bianchi, A.R.; Trocchia, S.; Rabbito, D.; Palumbo, G.; Abdel-Gawad, F.K.; De Maio, A. Frog (Pelophylax bergeri, Günther 1986) endocrine disruption assessment: Characterization and role of skin poly(ADP-ribose) polymerases. Environ. Sci. Pollut. Res. 2018, 25, 18303–18313. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; D’Errico, G.; Di Giaimo, R.; Rabbito, D.; Olanrewaju, O.S.; Ciarcia, G. Reactive oxygen species and glutathione antioxidants in the testis of the soil biosentinel Podarcis sicula (Rafinesque 1810). Environ. Sci. Pollut. Res. 2018, 25, 18286–18296. [Google Scholar] [CrossRef]
- Kaufmann, K.; Dohmen, P. Adaption of a dermal in vitro method to investigate the uptake of chemicals across amphibian skin. Environ. Sci. Eur. 2016, 28, 10. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Verma, R.K.; Chopade, R.L.; Pandit, P.P.; Nagar, V.; Aseri, V.; Choudhary, S.K.; Awasthi, G.; Awasthi, K.K. Heavy Metal Contamination of Water and Their Toxic Effect on Living Organisms. In The Toxicity of Environmental Pollutants; IntechOpen: London, UK, 2022. [Google Scholar]
- DeForest, D.K.; Brix, K.V.; Adams, W.J. Assessing metal bioaccumulation in aquatic environments: The inverse relationship between bioaccumulation factors, trophic transfer factors and exposure concentration. Aquat. Toxicol. 2007, 84, 236–246. [Google Scholar] [CrossRef]
- Dobrovoljc, K.; Falnoga, I.; Žnidarič, M.T.; Mazej, D.; Ščančar, J.; Bulog, B. Cd, Cu, Zn, Se, and metallothioneins in two amphibians, necturus maculosus (Amphibia, Caudata) and Bufo bufo (Amphibia, Anura). Biol. Trace Elem. Res. 2012, 150, 178–194. [Google Scholar] [CrossRef]
- Da Silva Veronez, A.C.; Salla, R.V.; Baroni, V.D.; Barcarolli, I.F.; Bianchini, A.; dos Reis Martinez, C.B.; Chippari-Gomes, A.R. Genetic and biochemical effects induced by iron ore, Fe and Mn exposure in tadpoles of the bullfrog Lithobates catesbeianus. Aquat. Toxicol. 2016, 174, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Y.; Duan, R.Y.; Ji, X. Chronic effects of environmentally-relevant concentrations of lead in Pelophylax nigromaculata tadpoles: Threshold dose and adverse effects. Ecotoxicol. Environ. Saf. 2014, 104, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Herkovits, J.; Alejandra Helguero, L. Copper toxicity and copper-zinc interactions in amphibian embryos. Sci. Total Environ. 1998, 221, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Calfee, R.D.; Little, E.E. Toxicity of Cadmium, Copper, and Zinc to the Threatened Chiricahua Leopard Frog (Lithobates [Rana] chiricahuensis). Bull. Environ. Contam. Toxicol. 2017, 99, 679–683. [Google Scholar] [CrossRef] [PubMed]


| Particle Size (%) | ||||||
|---|---|---|---|---|---|---|
| Sand | Silt | Clay | WHC (%) | pH | OM (% d.w.) | |
| AGR | 66.8 ± 1.8 | 21.4 ± 1.6 ab | 11.8 ± 1.2 | 30.7 ± 1.5 | 8.49 ± 0.05 b | 9.21 ± 0.84 a |
| URB | 65.1 ± 1.6 | 23.5 ± 1.5 a | 11.4 ± 1.4 | 29.8 ± 0.9 | 8.66 ± 0.05 a | 7.15 ± 0.22 b |
| IND | 67.0 ± 1.7 | 20.6 ± 1.2 b | 12.4 ± 1.6 | 30.1 ± 1.8 | 8.31 ± 0.10 b | 6.92 ± 0.82 b |
| pH | Turbidity (NTU) | DO (mg L−1) | BOD (mg L−1) | |
|---|---|---|---|---|
| AGR | 6.4 ± 0.4 | 4.0 ± 0.3 | 9.8 ± 0.3 a | 6.9 ± 0.2 a |
| URB | 6.3 ± 0.5 | 3.7 ± 0.3 | 9.1 ± 0.2 b | 6.3 ± 0.3 b |
| IND | 6.0 ± 0.2 | 3.5 ± 0.5 | 9.0 ± 0.4 b | 6.2 ± 0.2 b |
| Cu | Fe | Pb | Zn | |
|---|---|---|---|---|
| % | ||||
| AGR | 54.4 ± 3.0 a | 0.14 ± 0.01 ab | 70.5 ±2.2 a | 13.6 ± 2.0 |
| URB | 52.1 ± 2.2 a | 0.12 ± 0.01 b | 65.5 ± 0.5 b | 11.7 ± 2.4 |
| IND | 37.4 ± 3.0 b | 0.16 ± 0.01 a | 64.7 ± 0.8 b | 15.3 ± 2.1 |
| Cu | Fe | Pb | Zn | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RI (≤150–≥600) | Igeo (0–≥5) | EF (≤2–≥40) | CF (≤1–≥6) | Igeo (0–≥5) | CF (≤1–≥6) | Igeo (0–≥5) | EF (≤2–≥40) | CF (≤1–≥6) | Igeo (0–≥5) | EF (≤2–≥40) | CF (≤1–≥6) | |
| AGR | 316 ± 60 b | 0.06 ± 0.009 b | 1.39 ± 0.23 c | 0.28 ± 0.05 c | 0.04 ± 0.002 ab | 0.20 ± 0.007 ab | 0.025 ± 0.003 b | 0.63 ± 0.07 b | 0.13 ± 0.01 b | 0.67 ± 0.12 ab | 17.06 ± 3.41 | 3.32 ± 0.59 a |
| URB | 537 ± 25 a | 0.08 ± 0.005 b | 2.14 ± 0.13 b | 0.40 ± 0.02 b | 0.04 ± 0.003 b | 0.19 ± 0.01 b | 0.03 ± 0.001 a | 0.94 ± 0.07 a | 0.17 ± 0.003 a | 0.45 ± 0.13 b | 11.28 ± 2.85 | 2.22 ± 0.65 b |
| IND | 390 ± 58 b | 0.16 ± 0.02 a | 3.46 ± 0.41 a | 0.79 ± 0.09 a | 0.05 ± 0.003 a | 0.23 ± 0.02 a | 0.03 ± 0.001 a | 0.77 ± 0.06 ab | 0.17 ± 0.004 a | 0.80 ± 0.10 a | 18.06 ± 3.0 | 3.99 ± 0.50 a |
| BFw | BFs | |||||||
|---|---|---|---|---|---|---|---|---|
| Cu | Fe | Pb | Zn | Cu | Fe | Pb | Zn | |
| AGR | ||||||||
| Fe > Zn > Cu > Pb | Pb > Zn > Cu > Fe | |||||||
| URB | ||||||||
| Fe = Zn > Pb > Cu | Zn > Pb > Cu > Fe | |||||||
| IND | ||||||||
| Fe > Zn > Cu > Pb | Pb > Zn > Fe > Cu | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napoletano, P.; Guezgouz, N.; Benradia, I.; Benredjem, S.; Parisi, C.; Guerriero, G.; De Marco, A. Non-Lethal Assessment of Land Use Change Effects in Water and Soil of Algerian Riparian Areas along the Medjerda River through the Biosentinel Bufo spinosus Daudin. Water 2024, 16, 538. https://doi.org/10.3390/w16040538
Napoletano P, Guezgouz N, Benradia I, Benredjem S, Parisi C, Guerriero G, De Marco A. Non-Lethal Assessment of Land Use Change Effects in Water and Soil of Algerian Riparian Areas along the Medjerda River through the Biosentinel Bufo spinosus Daudin. Water. 2024; 16(4):538. https://doi.org/10.3390/w16040538
Chicago/Turabian StyleNapoletano, Pasquale, Noureddine Guezgouz, Imen Benradia, Sarra Benredjem, Costantino Parisi, Giulia Guerriero, and Anna De Marco. 2024. "Non-Lethal Assessment of Land Use Change Effects in Water and Soil of Algerian Riparian Areas along the Medjerda River through the Biosentinel Bufo spinosus Daudin" Water 16, no. 4: 538. https://doi.org/10.3390/w16040538
APA StyleNapoletano, P., Guezgouz, N., Benradia, I., Benredjem, S., Parisi, C., Guerriero, G., & De Marco, A. (2024). Non-Lethal Assessment of Land Use Change Effects in Water and Soil of Algerian Riparian Areas along the Medjerda River through the Biosentinel Bufo spinosus Daudin. Water, 16(4), 538. https://doi.org/10.3390/w16040538













