Abstract
Climate change is expected to alter the timing and intensity of precipitation and river discharge patterns, leading to hydrological extremes. Compared to forested watersheds, highly urbanized and cultivated areas are prone to sediment and nutrient loads from agricultural fields, impacting river water quality. On the other hand, prolonged low discharge periods limit the rivers’ dilution capacity, and result in hyporheic water stagnation and the accumulation of metabolic end products. Hydrological extremes may, therefore, produce severe implications for river water quality and, consequently, for aquatic life; however, this important aspect is poorly explored in the literature. In this context, three boreal streams that represent spawning and juvenile rearing habitats for anadromous salmonids were analyzed comparatively with respect to land use, anthropization level, and seasonal variability in water chemistry, during low and high discharge events. A set of chemical parameters depicting the water quality are discussed in relation to different land cover features, high discharge events, and seasonality. Finally, potential negative implications for the incubation period of salmonid embryos and juvenile rearing are outlined.
1. Introduction
In the context of river ecology, hydrological extremes are intended as exceptional prolonged low-flow periods or intense flood events [1] and are regarded as one of the most crucial challenges of our century [2]. Hydrological extremes are triggered by the interplay of ongoing climate change and human pressures; they have the potential to compromise river functioning and the resilience of riverine ecosystems, leading to habitat loss and ecological community degradation [3,4].
On one hand, watercourses are experiencing low-flow events out of range, and water scarcity episodes are becoming more common in temperate climates [5]. This leads to the hydrological intermittency phenomenon, also called the Mediterraneization process [6,7] a pattern already common in watercourses of the Mediterranean regions. The absence of perennial flow determines multilayer consequences on the functioning of a running water environment [8]. Low-flow periods cause alteration of biogeochemical processes, dissolved and particulate matter transport, changes in surface–groundwater interactions, and shifts in community structure [9,10]. This is because longitudinal water transport is a pivotal element of such lotic environments [11,12], and during low discharge events, the limited dilution capacity results in nutrient accumulation, favoring primary producers’ growth [13]. In low flushed, stagnant areas, this can lead to organic matter accumulation and oxygen depletion [14,15], a scenario that is worsened by temperature rise [16]. Additionally, hydrological intermittency can also lead to a loss in sediment transport activity from the running water. This translates into an increased deposition and accumulation of the fine fraction over the riverbed [17]. Interstices’ clogging prevents water circulation beneath the streambed, leading to suffocation of the intragravel environment. Clogged areas are then unsuitable for the most sensitive macrofauna taxa and the majority of the lithophilic fish in intragravel stages [18,19]. Moreover, flow reduction inevitably leads to a nutrient concentration rise. This can become harmful to sensitive cold-water species like salmonids, if combined with the increased temperatures and oxygen drops [20,21].
On the other hand, climate change is also connected with the increasing intensity of rainfall and flood events. The latter, if associated with artificialized stream paths and with land use that favors highly erosive phenomena, may further increase siltation and riverbed scouring potential [22,23]. The mechanical clogging resulting from high and prolonged loads of suspended solids exerts negative impacts on the stream system. They span from gill functioning impairment in fish [24] to streambed suffocation [19,25,26,27]. Flood phases result in higher sediment and organic matter loads with the associated increment in nitrogen (N) and phosphorus (P) concentrations [28,29]. Additionally, higher infiltration rates in the streambed and intragravel waterflow decrement result in oxygen deficiency. This may lead to the production and accumulation of potentially toxic endogenous N-compound by-products [15,30,31].
Land use has the potential to affect the response of a drainage system during the hydrological extremes. Unvegetated soil surfaces without well-established root systems are more prone to soil erosion during rainfall events. This may lead to higher loads of suspended solids into the watercourses [19,25,27]. Anthropogenic pressures in the proximity of rivers (e.g., bank and riverbed alteration, road construction) or spread within the watershed (e.g., agricultural practices, tree-felling, and wastewater from industries and housing) enhance the chemical inputs (inorganic N and P fertilizers, pesticides, and heavy metals) [14,32] to freshwater bodies. They also increase the mechanical action of running water on the streambed, leading to increased scouring events and impacting spawning areas exploited by lithophilic fish communities [31,33,34]. It is widely accepted that the presence of forested areas and buffer zones near watercourses can intercept nutrients and solid inputs during rainfall events [19,25,35]. These interfaces have the potential to metabolize organic substances prior to entering the stream environment [36]. Additionally, the mechanical action of roots and branches buffers surface water runoff entering the system, contributing to a slower rise in water levels and a dampening of the scouring potential [37,38,39]. On the contrary, impermeable surfaces allow the water to massively enter the stream channel, favoring the bed scour action [25,40]. The presence of wastewater treatment plants or septic tanks directly connected to the watercourse significantly increases nutrient availability in the riverine ecosystem, triggering eutrophication phenomena [41,42].
Under the present climate change scenario [43], the latest predictions for Lithuanian rivers for the end of the 21st century are a significant decrement in spring floods, an increment in summer low-flow events, and an increment in water temperature of more than 5 °C [44,45]. Systems that already show altered patterns in water quality during hydrological extremes are likely to see further deterioration in their conditions. Events that currently represent a stressor for the cold-water stenoecian fish species in the future will seriously hamper their survival. Therefore, it is important to raise awareness of the watershed-related responses occurring during the hydrological extremes. Understanding how the system functions during low-flow and flood events will provide insights into the potential risks to the physicochemical suitability of the environment for stenoecian fish species. This study aims to address knowledge gaps in this topic. Its main general objective is to understand how water quality varies seasonally across three boreal river systems during extreme hydrological events, and to analyze if land use affects water quality along with changes in discharge. The specific objectives of this work are (1) to analyze seasonal variations in the water quality of three riverine systems, in particular during hydrological extremes; (2) to explore links between land use and riverine water quality (i.e., its ecological status with respect to the Water Framework Directive—WFD 2000/60/EC); and (3) to outline which physicochemical factors can hamper salmonid fish during early ontogenetic stages.
A synergistic interaction between the effects of catchments’ land use and extreme meteorological events on water quality is hypothesized. Such interaction can affect the early life stages of anadromous salmonid species.
2. Materials and Methods
2.1. Study Areas and Land Use
The three sampled watercourses are all located in western Lithuania, and are considered lowland streams, with slopes between 0.1% and 0.3%, and a pool-riffle structure [46] (Figure 1).
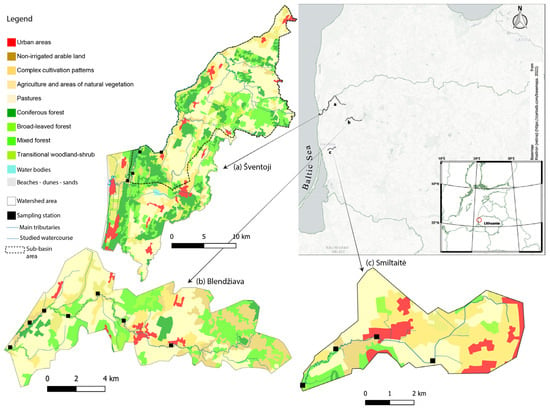
Figure 1.
Map of the study area reporting the three investigated watercourses with their respective watersheds, tributaries, sampling stations, and land use concerning the 3rd level of classification from the CORINE land cover project. Dotted line delimits the Šventoji sub-basin area.
The Blendžiava Stream (Bl) is in the upper part of the Minija River catchment, between the Samogitian Highland and the west Samogitian Plateau, in the Kretinga district. It is a third-order stream with a catchment area of 86 km2 and an average annual discharge of 1.06 m3 s−1 [46]. The investigated stretch falls under a protected area for salmonid spawning ground and juvenile rearing protection [44,47]. Its ecological status was classified as “Good” following the Water Framework Directive prescriptions by the Lithuanian Environmental Protection Agency (Aplinkos Apsaugos Agentūra https://vanduo.old.gamta.lt, accessed on 30 May 2024).
The Smiltaitė Stream (Sm) has a catchment area of 32 km2 and an average annual discharge of 0.14 m3 s−1. It flows into the Klaipeda district and is the main tributary of the Smeltalė River, which discharges its water into the Curonian Lagoon [47]. Sm has a self-sustaining sea trout population that registered a negative trend over the years [48]. This situation may be due to the poor ecological status of this watershed [49,50], mainly caused by point source pollution from stormwater runoff discharges and household pollution spreads [50].
The Šventoji River (Sv) is a transboundary river marking the Latvian border in the Skuodas district, and it flows directly into the Baltic Sea near Palanga Town. Sv has an overall catchment area of 472 km2 [47] and an average annual discharge of 5.3 m3 s−1 [51], supporting self-sustaining sea trout and Atlantic salmon populations [52]. However, only the draining basin upstream of the sampling stations (dotted contour in Figure 1) was considered when the land use percentages were calculated; therefore, we refer to an area of 268 km2. Indeed, the downstream section to the confluence with the Baltic Sea is not suited for spawning and juvenile rearing and, therefore, not judged pertinent for the scope of his work. Although Sv globally holds a “Good” ecological status, the spawning areas in the investigated section fall inside the stretch from the Latvian border up to the mouth, which failed to achieve a good chemical status [51]. The area is characterized by diffuse input pollution from agricultural runoff, delivering inorganic nutrients and organic matter to the river [51,52].
The water supply of Bl is the R-us type: 53% of the supply is from precipitation (R), 25% is from groundwater (u), and 22% is from snowmelt water (s). On the contrary, the water supply for Sm and Sv is classified as R-s, with 67% of water inputs from precipitation, 26% from snowmelt, and 7% from groundwater [53]. Sampling stations in these three watercourses are located within elective stretches for sea trout (Bl and Sm) and salmon (Sv) spawning [52,54].
Differences in land cover among the three basins were evaluated using the European project CORINE Land Cover (CLC) 2018 at the 3rd level of accuracy (Copernicus Land Monitoring Service—https://land.copernicus.eu, accessed on 10 November 2023). The decision to attain a high level of resolution for land use is driven by the fact that the same land use category can have significant differences in the specific management typology. For example, different land layouts can be grouped under the same heading of “agricultural areas”, like “pastures”, “not-irrigated arable land”, and “complex cultivation patterns”, although their surface management will be different [55,56]. Land use coverages were normalized for the respective basin areas and expressed as percentages in the contingency table (Table S7).
2.2. Sampling Strategy
Samplings were performed between October 2021 and August 2022 to capture physicochemical variation on a seasonal basis, following similar surveys aimed at understanding water quality in relation to the watershed land usage [36,57]. Within each season, we prioritized periods of hydrological extremes, such as high flow, i.e., flood, and low flow. This aligns with previous works that aimed to understand changes in nutrient concentrations in relation to different hydrological conditions [28,58,59]. In addition to temporal variation, spatial variation was accounted for as well; water samples were collected throughout the lengths of the investigated stretches. This allowed us to account for any upstream–downstream variations, as already proved elsewhere [28,57,58,59].
In boreal ecosystems, ice cover during the winter months and light limitations have the potential to decrease primary production [60]. This may translate into higher export rates of unprocessed nutrients through the watercourse [61]. Nevertheless, in the three investigated systems, the ice cover during the sampled period was moderate, and the sampling stations were always ice-free. This reflects a global trend in which the ice cover is becoming rapidly lost in running water systems [61]. Additionally, the three systems lie close to the Baltic Sea, and are, therefore, influenced by its climate [45]. Winter thaws systematically remove the forming ice from the watercourse surfaces, leaving them free for the entire sampling period. Because of this, the effect of the ice cover on the physicochemical water quality was regarded as a minor variable in this study.
Periods of flood and low flow were detected with respect to the water level changes in the real-time data from the Lithuanian Hydrometeorological Service (https://www.meteo.lt/) and validated with on-site surveys. For the flood moments, an overflow of water into the dryland was observed. Low-flow moments were assumed when macrophytes and epiphyton that were submersed under average water levels appeared dry and exposed to air. Such empirical observations were validated by the exceedance probability curves (Figure 2), where flood and low-flow events were connected to the probability of falling in the first (Q1) and the fourth (Q4) quartiles, respectively.
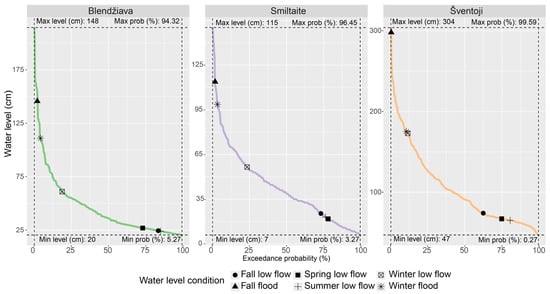
Figure 2.
Water level exceedance probability curves for the sampled period.
During the wettest conditions, all rivers were sampled at the Q1, and the fall flood sampling occurred during periods with a less than 5% probability of occurrence, capturing peak discharge events. The driest periods during the fall, spring, and summer appeared to be positioned at the Q4 of water levels. This was true except for the fall low-flow sample from Šventoji, which was taken at conditions below the third quartile (Q3). These driest conditions were characterized by a probability of over 80%, representing typical low-flow conditions.
To describe the physicochemical status of the water, eleven (11) environmental variables were chosen. A YSI multiparameter probe (Pro 1030, YSI, Yellow Springs, OH, USA) was employed to measure in situ dissolved oxygen (DO, both as mg L−1 and saturation %), pH, electrical conductivity (EC, μS cm−1), and temperature (T, °C). Filtered water aliquots (GF/F filters, Frisenette, 0.7 μm mesh size) were collected to detect concentrations (mg L−1) of ammonium (N-NH4+), nitrate (N-NO3-), nitrite (N-NO2-), and soluble reactive phosphorous (P-PO43-). Nutrient concentrations were analyzed via standard spectrophotometric methods. Alkalinity (mmol L−1) was calculated using the Gran titration method [62], while total suspended solids (TSS, mg L−1) were measured with the gravimetric method [24].
2.3. Statistical Analyses
Correspondence analysis (CA) was used to infer the degrees of similarity and difference between the three watersheds concerning the different land use configurations. Results are presented in the form of an asymmetric biplot [63] where rows, indicating the different land use features, are depicted on the principal coordinates, i.e., “rowprincipal”, [64], while the watershed contribution is expressed in terms of columns (Table S5) [64].
The 11 environmental variables were summarized and visualized via Principal Component Analysis (PCA). PCAs were performed for each season and event, and, although all 11 environmental variables were graphically displayed, in the result description, only those variables that contributed most to the definition of the first two principal components (PCs) are reported. The expected average contribution was used as a cut-off point to retain the statistically significant variables. This is the value if the contribution of the variables were uniform, and it was calculated as 1/number of variables × 100 [65]. The complete list of all the contributing variables for each PCA is reported in the Supplementary Materials (Table S1).
For each watershed, variations in water chemistry between flood and low-flow periods were evaluated during the fall and winter seasons, when hydrological extremes occurred. A paired samples t-test was employed after checking the normality and homoscedasticity assumptions. For data with unequal variances, the paired Welch t-test was employed. If data did not comply with the normality assumption, the non-parametric Wilcoxon signed-rank test for paired data was used (Table S2). Among the three watersheds, variations among seasons and events were evaluated. One-way ANOVA was employed (Table S3), followed by the Tukey HSD test for multiple pairwise comparisons. Prior to ANOVAs, the normality and homoscedasticity assumptions were checked. If data did not meet the assumptions, comparisons were made using the Kruskal–Wallis test, followed by Dunn’s Test for multiple comparisons (Table S4). All the statistical analyses were performed in the R language [66] and conducted with the packages rstatix and factomineR [67]. Charts were built using factoextra [68] and ggplot2 [69] packages. All the statistical tests were performed at an alpha level of 0.05, and p-values from the pairwise tests were corrected using the Bonferroni–Holm method to account for the familywise error.
3. Results
3.1. Land Cover
The three drainage basins are mainly composed of agricultural areas (Bl: 77%, Sm: 70%, and Sv: 58%). The Sv watershed has the highest percentage of forests (40%), while 13% of that of Sm consists of urban areas. Focusing on the third CLC level, the “not-irrigated arable land” is the primary feature of Sv and Sm, with 43 and 34%, respectively. At the same time, Bl exhibits the highest percentage of “complex cultivation patterns”. The statistical analysis confirmed that the three basins have different land uses (chi-square: 110, p < 0.001, df: 16), and the asymmetric biplot (Figure 3) visually depicts the relationships in terms of land use for the three watersheds outlined in the contingency table (Table S7).
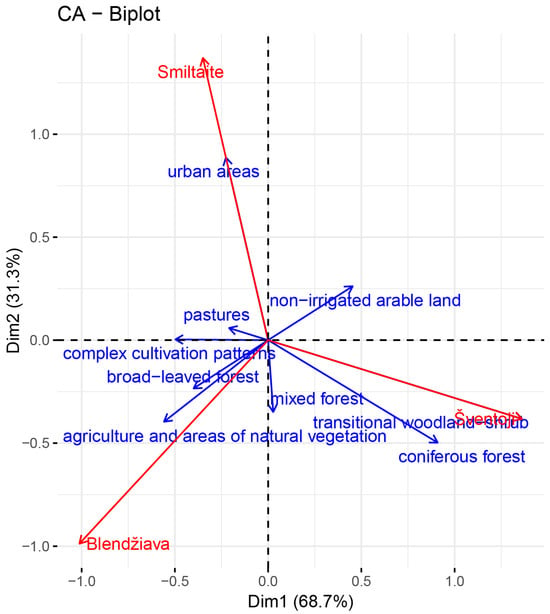
Figure 3.
Asymmetric biplot portraying the relationships between different land uses within the three investigated basins.
The first dimension of the asymmetric biplot (69% of the variability explained) is represented by “complex cultivation patterns” as opposed to “coniferous forest” and “transitional woodland-shrub” in terms of land use, and by “Šventoji” in terms of watershed (Table S5). Indeed, the Sv watershed holds the highest share in coverage of these land use features. Their correlation is further supported by the reciprocal proximity in the asymmetric biplot. “Complex cultivation patterns” is in opposition to these two land features and is typical of the Bl and Sm basins.
The second dimension (31% of the variability explained) is mainly constituted by “mixed forest” and “urban areas” in terms of land use, and by “Smiltaitė” in terms of watershed (Table S7). The overlapping between Sm and “urban areas” confirms the record for the Sm basin in terms of this feature (13%). In the opposite direction, the “mixed forests” are a more typical trait of the other two watersheds. “not-irrigated arable land”, “broad-leaved forest”, and “agriculture and areas of natural vegetation” equally contribute to the two dimensions, being the first feature shared by the Sv and Sm basins. On the other hand, the other two features characterize Bl (15% of “agriculture and areas of natural vegetation” and 6% of “broad-leaved forest”) more than Sm and Sv, where the percentages are much smaller. Moreover, Blendžiava holds, among the watersheds, the lowest percentages of “urban areas” (2%) and “not-irrigated arable land” (8%), as suggested by the strong contraposition of these vectors in the CA (Figure 3). Finally, “pastures” are similarly represented in all basins with small percentages, contributing minimally to both dimensions.
3.2. Seasonal Variations in Water Quality Among Rivers
3.2.1. Fall
During the fall low-flow period (Figure 4a), the three basins are well separated through the first PC. Bl stations have the highest pH values (p < 0.001, Figure 5e) among the three basins. DO and % sat. values at Bl stations are higher than in the Sm stations (DO: p < 0.01, % sat.: p < 0.05, Figure 5a,b). Sm stations dominate the right side of the PC, having the highest values in EC (p < 0.001, Figure 4a and Figure 5c) among the three watersheds. N-NO3−, N-NO2−, and alkalinity are significantly higher (p < 0.05, Figure 5g,h,j) only in comparison to Bl. Sv points are also related to the second dimension, showing a vertical pattern reflecting the decreasing trend in TSS from the upper station, 2.23 mg L−1, to the bottom valley one, <DL (0.5 mg L−1). This pattern is also recorded for Sm, with TSS values dropping from 4.0 mg L−1 in the upstream station to <DL in the downstream station.
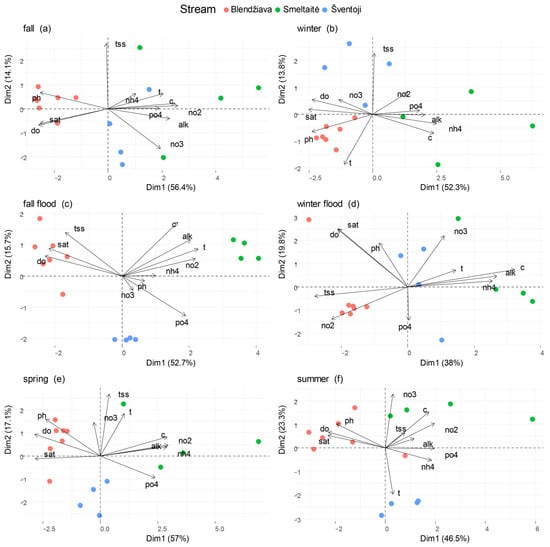
Figure 4.
PCA biplots portraying the associations among the stations (dots) of the three different basins and the environmental variables, across all the seasons and the two flood periods. Environmental variables labels refer to do: dissolved oxygen (DO, mg L−1), sat: percentage of oxygen saturation (% sat.), ph: pH, c: conductivity (EC, μS cm−1), t: temperature (T °C), nh4: ammonium N-NH4+, no3: nitrate N-NO3−, no2: nitrite N-NO2−, po4: soluble reactive phosphorous P-PO43−, all expressed in mg L−1, alk: alkalinity (mmol L−1), tss: total suspended solids (TSS, mg L−1).
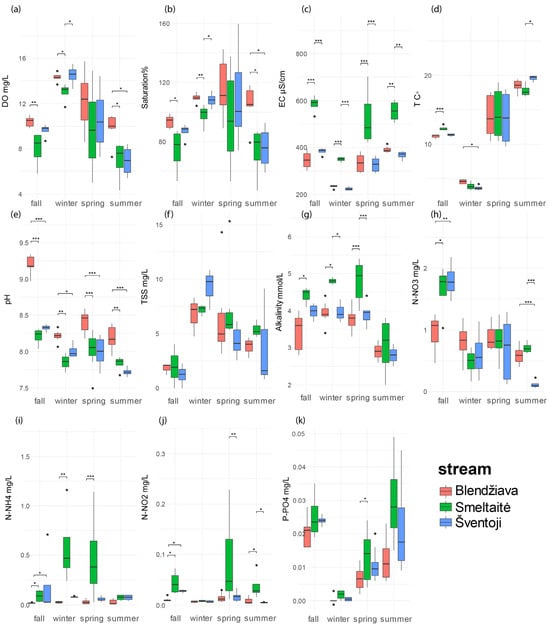
Figure 5.
Variations in the 11 environmental parameters across the three watersheds, considering each season only during the low-flow periods. p < 0.05 (*), p < 0.01 (**), p < 0.001 (***). (a) dissolved oxygen (DO, mg L−1), (b) percentage of oxygen saturation (% saturation), (c) conductivity (EC, μS cm−1), (d) temperature (T °C), (e) pH, (f) total suspended solids (TSS, mg L−1), (g) alkalinity (alkalinity mmol L−1), (h) nitrate (N-NO3−, mg L−1), (i) ammonium (N-NH4+, mg L−1), (j) nitrite (N-NO2−, mg L−1), (k) soluble reactive phosphorous (P-PO43−, mg L−1).
During the fall flood period (Figure 4c), the variables most contributing to the definition of the first PC are DO and % sat. on the left side. These are opposed to T, N-NO2−, P-PO43−, and alkalinity on the right side. The second PC is dominated by TSS, C, and alkalinity, pointing to the upper part of the chart, in contrast to P-PO43−. The partition among the three watercourses is still clear, with the Bl stations showing a strong correlation with % sat. (p < 0.001, Figure S1b), DO (p < 0.001, Figure S1a), and TSS (p > 0.05, Figure S1g). Bl is negatively related to the P-PO43− vector due to significantly lower concentrations compared to the other two watersheds (p < 0.001, Figure S1d).
During the flood period, EC, N-NO2−, and alkalinity remained related to Sm stations. Indeed, Sm presents the highest EC values, and they are significantly higher (p < 0.001, Figure S1c) compared to Sv. Additionally, Sm stations had higher N-NO2− (p < 0.001, Figure S1j) and alkalinity (p < 0.01, Figure S1h) concentrations between the two basins. Sm stations registered an average increment of 3 °C in temperature compared to the other two basins, showing a significant difference only with respect to Bl stations (p < 0.01, Figure S1f).
Sv stations are clustered on the vertical axis with homogeneity of the sampling points, and the environmental variables fall in between the two other watersheds (Figure S1c).
3.2.2. Winter
During the winter low-flow period (Figure 4b), the variables most contributing to the definition of the first PC were N-NH4+, EC (positively correlated), and, in opposition, pH, DO, and % sat., defining the other side of the first PC. The vertical axis is mostly composed of TSS, contrasting with T. Bl stations are in opposition to Sm ones. The separation is driven by significantly higher values in pH for Bl stations (p < 0.05, Figure 5e), while Sm holds the lowest values in % sat. and DO with respect to Bl (DO: p = 0.02, % sat.: p = 0.008, Figure 5a,b). Bl points are also positioned toward the T vector, being the highest among the basins and significantly different with respect to Sv (p < 0.02, Figure 5), which has the lowest T.
In agreement with the previous season, Sm presents markedly higher N-NH4+ concentrations (p < 0.01, Figure 5i), alkalinity (p < 0.05, Figure 5g), and EC (p < 0.001, Figure 5c).
Sv sampling stations had slightly higher TSS concentrations, although they were not significantly different from the other two basins (p > 0.05, Figure 5f). Sv held intermediate characteristics with respect to the other two watersheds, with no statistically significant differences.
During the winter flood period (Figure 4d), the variables most contributing to the definition of the first PC are EC, alkalinity, and N-NH4+, positioned on the right side of the chart, while TSS and N-NO2− dominate the left side. The second dimension is mainly composed of DO, % sat., and pH, which point to the upper left quadrant, while N-NO3− is oriented on the right side. In contrast with the previous periods, DO, % sat., N-NO3−, and pH do not show any significant difference among the three systems.
Bl stations mainly lie on the N-NO2− vector, with concentrations significantly higher than those in the other two basins (p < 0.01, Figure S1j), by up to two orders of magnitude. A similar trend can also be described for TSS (p < 0.05, Figure S1g), which increases up to six times in Bl with respect to the other two basins, where average values range from 9 to 18 mg L−1. EC values in Sm samples are the highest among the three watercourses (p < 0.001, Figure S1c). The same is true for N-NH4+ (p < 0.01, Figure S1k) and alkalinity (p < 0.05, Figure S1h). In line with the other PCAs, Sv stations occupy the middle part of the PCA and have intermediate characteristics in between the two other rivers. Sv samples have lower N-NO2− (p < 0.05, Figure S1j) and N-NH4+ concentrations (p < 0.01, Figure S1k), also significantly different compared to Sm (N-NO2−: Sv vs. Sm p < 0.05, N-NH4+: Sv vs. Sm p< 0.01, Figure S1j,k). Additionally, Sv presents intermediate values in the EC profile, but it is significantly higher with respect to Bl (p < 0.001, Figure S1c).
3.2.3. Spring
During the spring low-flow period (Figure 4e), the variables most contributing to the definition of the first PC are the same as for the previous seasons. Contrarily, the second dimension is formed by the TSS, N-NO3−, and T vectors going in the same direction. The left side of the biplot is dominated by Bl stations grouping around the DO, % sat., and pH vectors, although only pH registers significantly higher values for Bl samples (p < 0.001, Figure 5e).
On the right side of the PCA biplot, Sm stations present higher levels of EC (p < 0.01, Figure 5c) and alkalinity (p < 0.01, Figure 5g). Only in comparison with Bl samples, Sm samples have significantly higher N-NH4+ and P-PO43− concentrations (N-NH4+: p < 0.001, P-PO43−: p = 0.02, Figure 5i,k).
In Sv samples, all parameters are not different from the other two rivers (p > 0.05, Figure 5).
3.2.4. Summer
During the summer low-flow period (Figure 4f), pH, DO, and % sat. lie on the left side, and in opposition to the vectors of the N-NO2−, N-NH4+, P-PO43−, and EC. The second PC is mainly constituted by N-NO3− and EC in opposition to T. Watershed separation is still clear. Bl stations have significantly higher pH (p < 0.01, Figure 5e), DO (p < 0.05, Figure 5a), and % sat. (p < 0.05, Figure 5b) with respect to the other two basins.
Sm holds the highest N-NO2− concentrations (p < 0.02, Figure 5j) and EC values (p ≤ 0.01, Figure 5c) compared to the two other watersheds, whereas Sv stations hold an off-center position with respect to most of the vectors representing the environmental variables. Indeed, samples from Sv attain the lowest values in terms of N-NO3− (p < 0.001, Figure 5h) and the highest T (+2 °C on average), which is, however, not significantly different from the two other watersheds (p > 0.05, Figure 5d). Finally, in the summer, no significant differences were registered among the three watersheds in terms of N-NH4+ and P-PO43−.
3.3. Rivers’ Behavior Under Extreme Hydrological Events
Overall, the low-flow phases were characterized by a generalized increment in EC, T, alkalinity, and % sat., and to a lesser extent, by pH and DO. N-compounds and TSS generally show stable values between the two low-flow events. On the contrary, P-PO43− globally rose during the flood moments (Figure 6j).
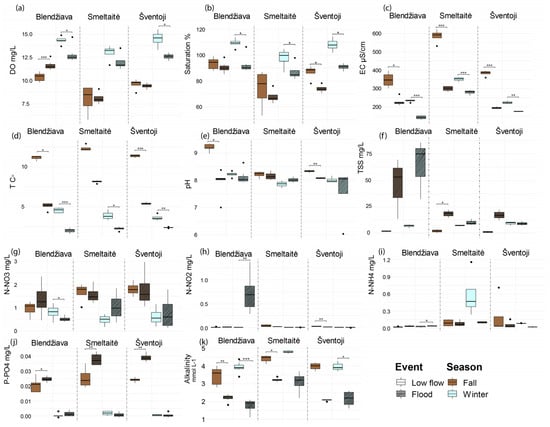
Figure 6.
Variations between the flooding and low-flow phases during the fall and winter seasons for the 11 environmental variables in the three watercourses. p ≤ 0.05 (*), p < 0.01 (**), p < 0.001 (***). (a) dissolved oxygen (DO, mg L−1), (b) percentage of oxygen saturation (% saturation), (c) conductivity (EC, μS cm−1), (d) temperature (T °C), (e) pH, (f) total suspended solids (TSS, mg L−1), (g) nitrate (N-NO3−, mg L−1), (h) nitrite (N-NO2−, mg L−1), (i) ammonium (N-NH4+, mg L−1), (j) soluble reactive phosphorous (P-PO43−, mg L−1), (k) alkalinity (alkalinity mmol L−1).
Particularly, the three systems always displayed significant increments in EC (Figure 6c) values during the low-flow periods registered in fall (p < 0.01) and winter (p < 0.01) when compared to the respective flood situations. Temperatures increased during the low-flow periods in all the rivers in winter (Bl p < 0.001, Sm p < 0.05, Sv p < 0.01, Figure 6d), but only partially in fall (Bl p < 0.05, Sv p < 0.001). pH shows a stable trend between the two hydrologic conditions, except for Bl (p < 0.05, Figure 6e) and Sv (p = 0.01), which had fall low-flow values that were significantly higher than during the flood period.
Globally, DO rose during low-flow periods, with significant increases observed in the Bl and Sv watersheds during winter (p < 0.05, Figure 6a). This is further confirmed by a similar significant increase in % sat. across all watersheds in winter (p < 0.05, Figure 6b).
TSS incremented only during the fall flood event (p < 0.05, Figure 6f) in Sm samples. Globally, N-compound concentrations were not influenced by the event type. Indeed, N-NO3− significantly rose only in Bl during the winter low-flow event (p < 0.05, Figure 6g), while N-NH4+ increased only during the winter flood (p < 0.05, Figure 6i) in the Bl stream. N-NO2− incremented significatively only during the winter flood in Bl samples (p < 0.01, Figure 6h) and the fall low flow in Sv (p < 0.01). P-PO43− consistently rose during the fall flood period in all three systems (Bl p < 0.05, Sm & Sv p < 0.01, Figure 6j). Alkalinity rose during the low-flow moments for Bl (fall p < 0.01, winter p < 0.001, Figure 6k) and partially for Sm in fall (p < 0.05) and Sv in winter (p < 0.05).
4. Discussion
4.1. Land Use as the Main Driver of Water Quality
From the PCA results, the three watersheds’ layouts are positioned in distinct areas of the charts and are usually related to the same array of vectors consistently across different seasons and hydrological conditions. This multivariate analysis led to a clear differentiation of samples from the different basins in a consistent fashion over time. This suggests that such differences are driven by consistent features, i.e., land use peculiarities for each watershed, rather than seasonal or faster phenomena.
Bl stations are grouped on the left sides of the PCA biplots and are coupled with DO, % sat., and pH vectors, which dominate these parts of the charts in most of the sampled seasons. Conversely, this watershed is distant from the nutrient (N and P forms) and alkalinity vectors. Those are considered indexes of a higher trophic status [26,70,71] and point toward the right PC areas where Sm stations lie. Such separations in the PCs’ space follow the differences in land use that these watersheds hold. Higher N and P concentrations in the other two watersheds can trigger eutrophication events [70,72], a situation already ascertained for the Sm basin [48,49]. A parallel situation is already found in lentic boreal ecosystems surrounded by urbanized areas. This anthropogenic disturbance, united with the rising temperatures, triggered unprecedented cyanobacteria blooms [73].
The pH vector usually lies on the left side of the first PC’s axis and, except during flood events, is highly correlated with Bl stations. Indeed, Bl exhibits significantly higher pH values during low-flow periods, ranging from 8.1 to 9.2 (Table S6), compared to the other two watersheds (means range, Sm: 7.8–8.2; Sv: 7.6–8.3; Table S6) throughout the year. This can be partially explained by the clay loams in the Bl streambed [74] and their buffer capacity due to the high presence of exchange sites [75,76]. N and P nutrients in the other two watersheds can trigger eutrophication events [70,72], a situation already ascertained for the Sm basin [48,49]. Thus, the accumulation of decomposing algal mats and dead macrophytes following these eutrophication events leads to the release of carbon dioxide, principally responsible for the lowering of the pH in the water [77,78]. If not contrasted, this phenomenon can severely compromise the ecosystem’s capacity to host stenoecian fish species, like the brown trout, as already pointed out in boreal rivers subjected to strong acidification conditions [79].
Although the Bl watershed has a consistent percentage of agricultural areas, the number of uncovered surfaces subjected to possible nutrient and organic matter leaching into the watercourse is low. The main cultivated layout is “complex cultivation patterns”, 42%, which, according to the definition from the CLC Copernicus project (Copernicus Land Monitoring Service—https://land.copernicus.eu, accessed on 10 November 2023), is a “mosaic of arable and permanent crops and grasslands”. This, combined with the consistent presence of “agriculture and areas of natural vegetation”, returns a complex mosaic of cultivated patches with little presence of uncovered soil. As a result, these sheltered surfaces help buffer potential nutrient and organic matter inputs into the watercourse [80,81]. Similar findings were already ascertained in a multidecade study on boreal soils cultivated with perennial crops. This study highlighted how these covers guarantee small N losses and minimize the need for external N fertilizers [82].
The Sm watershed holds a high share of “not-irrigated arable land”, 34%, connected with “urbanized areas”, 13%. These features lead to a higher share of uncovered soil subjected to possible runoff and leaching of nutrients and organic matter into the stream. EC and alkalinity vectors usually share the same direction of the aforementioned variables in all the PCA biplots. It was already ascertained how high values in these two parameters are regarded as indicators of weathering processes [26] marking eutrophic conditions [71,83]. Similar values were also recorded in a watercourse vocated to salmonid production in the same context of a highly exploited agricultural catchment [84]. The situation is further worsened by the higher presence of wastewater septic tanks connected to household leakages located in the Sm watershed [49]. Thus, it is not surprising if N and P vectors are generally correlated together through the first PC axis and point toward the Sm stations. This supports why Sm sampling stations are likely to have a poor water quality status, as additionally confirmed by previous ecological assessments [48,49] under the Water Framework Directive.
Sv stations hold an intermediate position on the PC maps, being closer to Bl, fall low flow, or closer to Sm, winter low flow, or equidistant from the two basins during the other events. Among the three basins, Sv shares the highest percentage of forested areas, 40%, a trait that might have contributed to keeping its station points off-center with respect to the vectors of N and P nutrients, EC, and alkalinity. Indeed, according to the CA, “coniferous forest” is a distinct trait of this basin that additionally shares the “mixed forest” vector with Bl. However, Sv shares the “not-irrigated arable land” vector with Sm (Sv: 43%, Sm: 34%), which turns out to be the primary agricultural layout in this watershed. As a result, Sv stations remain off-center with respect to Bl points and their related vectors. In fact, the investigated transect falls in the Sv stretch classified as a water body at risk, missing the “Good” ecological status due to agricultural pollution spreads [50,52] and livestock farming [51]. These two forms of point and non-point pollution can represent the main pool of nutrient input in lotic ecosystems holding a similar agricultural background [85,86]. Therefore, also in terms of N and P vectors, Sv samples hold, alternatively, an intermediate position between the other two basins (e.g., N-NH4+ and P- P-PO43− in spring, Table S6), or they share similar values with Sm samples (N-NOx-, N-NH4+ and P-PO43− in fall, Table S6).
Vector zonation according to the watershed land use is also reflected in the annual values of N and P nutrients as well as DO, marking the different pollution degrees of the three systems. In these terms, Sm holds the highest means (or the lowest, if DO is considered), followed by Sv and Bl. Nonetheless, P-PO43− concentrations fall in the range for “High” ecological status (0.05 mg L−1, Table 1), and the same can be stated for N-NO3− concentrations. N-NO3− values, except for one single station in Bl and Sv during the flooding events, fall within the “Good” ecological status range, with concentrations lower than 2.3 mg L−1 (Table 1).

Table 1.
Annual averages (±sd) and respective range (min-max) of the parameters for which ecological status classes have been established pursuant to the Water Framework Directive (WFD, 2000/60/EC) for Lithuania and summarized in the Nemunas River basin district [49]. Limits for salmonid protection are listed.
Therefore, P-PO43− and N-NO3− may not be considered as primary variables affecting these three watercourses. Conversely, N-NH4+ concentrations in Sm are remarkably higher during the winter and spring low-flow periods, being up to one order of magnitude above the other two basins (Table S6). N-NH4+ values fall in the “Moderate” and “Poor” ecological status classes (Table 1), with more than 0.6 mg N-NH4+ L−1. Concentrations of this compound peak above 1 mg N-NH4+ L−1 in the stations in proximity to the urbanized areas. This trend can be explained by the low efficiency of wastewater treatment plants and septic tanks with low temperatures during the winter months, which slows down the nitrification processes of this compound [16]. The situation is further worsened by the prolonged low-flow period, lasting also into spring, that shrinks the water column, weakening its dilution effects [95]. Additionally, early spring can still be a relatively cold season for these latitudes (water T in Sm: 14.4 ± 3.6 °C), therefore postponing and lowering primary producers’ growth and, thus, their nutrient sequestration capacity [96].
Bl and Sv samples presented DO values that correspond to the “Good” ecological status class for this parameter (Table 1), as confirmed by the vector arrangement in the PCAs. This is partially true for Sm stations, although alarming concentrations corresponding to the “Moderate” and “Poor” classes were recorded in late spring and summer. Here, the water peaks in temperature (18 ± 1 °C) and the prolonged dry moment surely contributed to macrophyte and epiphyton growth, leading to nocturnal DO depletion.
4.2. Accounting for the Seasonality and Hydrological Extremes Shifts
The strength of the relationships between environmental variables and sampled points varies in the PCAs according to the seasonality and the occurrence of hydrological extremes. Indeed, low-flow events amplify the effects of point source pollution (i.e., wastewater treatment plants), as was already underlined in similar studies that assessed the water quality based on land cover [36] and hydrological extremes [95]. Parallel findings suggest how the presence of urbanized areas plays a crucial role in the peak release of nutrients in the adjacent river stretch [97]. Furthermore, the reduction in river discharge necessarily leads to lowered dilution followed by mass concentration [98]. This triggers the increase in electrical conductivity and alkalinity [26] patterns, confirmed during the low-flow events inside each watershed (Figure 6). In this regard, the presence of higher groundwater input during the low-flow events could be an additional explanation for the augmented C and alkalinity values [26]. N and P nutrients present only partial differences in their concentrations when low-flow and flood events are compared inside each watershed (Figure 6). Indeed, only P concentrations partially rise during the fall flood period across all the watersheds. This was already ascertained in similar watersheds holding high agricultural shares in the same study region [99] and during different hydrologic conditions [100]. Agricultural areas usually undergo fertilization practices [99]; this can provoke the release of P forms during intense rainfall events as a result of soil leaching [100]. However, the weak changes in nutrient concentrations during the hydrological events may suggest limited control by non-point sources during the rainfall period or high baseflow concentrations delivered by point source activities (e.g., wastewater in Sm). A similar pattern is observed with the fluctuating DO patterns. We speculate that the cold season (4.2 ± 1 °C) during which we sampled the only two flood events favored DO dissolution. This reduced the physical changes in DO due to water level fluctuations.
Prolonged low-flow periods exacerbated differences among the three watersheds in terms of significantly higher N-NO2-, N-NH4+, EC, and alkalinity values for the Sm stream. This contrasts with the remarkably higher DO, % sat., and pH values observed for Bl (Figure 5). These differences could be attributed to the Sm watershed having the highest share of urbanized areas (13%). These findings are further supported by the poor ecological status assigned during the last nationwide inventory [49,50] under the Water Framework Directive and within the scope of sea trout spawning habitat improvement actions [48]. These recent studies demonstrate that surface water pollution from household leakage is the primary concern in this watershed [49,50]. The transport of reduced N forms in the watercourse occurs when oxidation treatment is absent [30,101] or inhibited by low temperatures [16,102]. This is confirmed by the significantly higher concentrations of N-NO2- and N-NH4+ observed in winter and spring compared to the other two systems (Figure 5). Even a small number of rural dwellings not connected to the modern wastewater drainage system can heavily impact small freshwater environments, given the untreated nature of their sewage waters [103]. This situation is already underlined for the Sm watershed, as its N-NH4+ and BOD7 values exceed the thresholds for a good ecological status [49]. Further evidence of these eutrophic conditions is supported by the EC and alkalinity values, which are consistently the highest for this watershed (Figure 5).
In contrast, the Bl basin has a low percentage of urbanized areas (2%) that are located far from the sampled stretches. In situ investigations within the scope of this study confirm the almost total absence of point sources entering the Bl sampled area. These are sewage water treatment plant outlets or rural dwellings not connected to the sewage drainage. Furthermore, during low-flow periods, the nutrient inputs coming from the agricultural runoff may be negligible [37,38,104]. This explains why, although the mean annual discharge is of a small quantity, 1.06 m3 s−1 [46], and, therefore, the concentration phenomena of polluting substances can be enhanced, low-flow periods are always characterized by low N-NO2− and N-NH4+. This is significant in comparison with the more urbanized Sm watershed. Furthermore, the well-expressed riparian belts along the river channel [74] contribute to the sequestration of pollutants leached from uncovered soils [39] as their degradation occurs [105]. A parallel study on boreal watercourses found how the presence of riparian belts enhances soil saturation with related reducing conditions associated with enhanced nitrate removal rates [105]. Therefore, nutrient scarcity resulting from the aforementioned factors likely contributed to maintaining low biochemical and chemical oxygen demand (BOD, COD). This, in turn, resulted in significantly higher levels in DO and % sat. in Bl during low-flow events compared to the two other watercourses.
On the other hand, flood events enhance non-point source pollution via field leaching during rainfall [32]. This is confirmed by the alternatively higher N-NO2− and N-NH4+ concentrations in the Sm and Bl basins (Figure S1), which have a greater portion of cultivated land (Bl: 70%, Sm: 77%) compared to Sv (58%). Indeed, it has been previously stated that the conversion of pasture to cropland is the main driver of increased N exports from the principal Lithuanian rivers [106]. Moreover, in addition to the exogenous contribution from cultivated area runoff [107,108], high-water periods are also characterized by endogenous inputs from metabolic processes. Endogenous inputs are driven by increased suspended solids and infiltration rates in the hyporheic zone [14,15]. Therefore, higher shares of uncovered areas may favor the mobility of these reduced N forms in the surface runoff. Nevertheless, it is unlikely that these compounds will be metabolized (e.g., oxidized to N-NO3−) by the system due to the slowing of biogeochemical processes caused by the low temperatures (4–5 °C, Table S6) typical of the cold season [16,96,109]. This may help explain why N-NO3− trends were similar across the three watersheds, as well as within each watershed, a similarity also registered between the flood and low-flow events in the late fall and winter, when temperatures were low. In addition to leakage from agricultural areas, flooding can severely hinder the functioning of wastewater treatment plants and septic tanks by overloading them with increased flow rates or inundating the operational units [41,42]. This may lead to the release of sludges directly into the watercourse. Consequently, when the three watersheds were contrasted during the flooding moments, the N-NH4+, alkalinity, and EC were still higher for Sm (Figure 6). During the flood events, differences in DO and % sat. were partially smoothed out among the three systems. This is due to the physical mixing in the water column, aided by the low temperatures registered in fall and winter across the three systems.
T vectors are mostly relegated to the second PC’s axes, which is regarded as a minor variable explaining watershed differences. Indeed, there is no clear contraposition in terms of this parameter among the watersheds. T values hold alternatively higher records across the sampling moments (Figure 5 and Figure S1) and globally increase as the warm period advances. Nevertheless, T significantly rises in each watershed during the low-flow moments, even though these periods occur in the cold season. Low-flow periods are characterized by the reduction in the wetted stream area, coupled with slower current velocity. These factors lead to increased retention times and, consequently, an increment in the water insulation, connected with a higher T [110]. Conversely, pH differences during flood events are less pronounced across the three watersheds. This is most probably due to the dilution effect of the high-water period and the acidifying impact of rainwater [111].
TSS accounts for the amount of inorganic and organic particles carried by the water [24,112]. In the indicative list of the main pollutants (annex VIII of the WFD 2000/60/EC), they are loosely defined as “materials in suspensions” without specific guidance on their monitoring and ecological thresholds [112]. To our knowledge, Lithuania has not yet established such assessment criteria for running waters. In our appraisal, TSS vectors laid on the second PC’s axes during the low-flow periods explain little variability in the dataset. This is not surprising, since the three systems hold average values from 4.4 to 5.9 mg L−1 for all low-flow events, classifying them as low-turbidity systems, <25 mg L−1 [74]. Consequently, there are no significant differences among the systems during low-flow events (Figure 5). On the contrary, TSS concentrations are more pronounced during flooding phases, with higher values observed at Bl stations. During the two high-water periods, a well-expressed dichotomy was registered, with Sm and Sv having comparable mean values of 14.2 and 12.3 mg L−1, respectively, from three to four times lower than Bl. This is further highlighted in the boxplots in which the winter flood registered significantly lower TSS values for Sm and Sv if compared to Bl (Figure S1). This is also reflected within each watershed, where TSS values for Sm and Sv are generally similar during both flood and low-flow events. However, for Bl, they greatly increase during the flood period, although this is associated with high variability (Figure 6). Indeed, high runoff generated during rainy events has the potential to erode uncovered and cultivated soils, bringing high amounts of sediment inside the watercourses [22,23]. Such phenomena are more typical during cold seasons, where high soil humidity associated with low temperatures increases the amount of washed-off sediments [36]. We speculate that higher TSS loads found in Bl could be related to its high share of cultivated features, 70%, that, although slightly lower than Sm, 77%, rely on a drainage basin that is more than twice as big with respect to Sm. This scenario is worsened, given the pastures’ conversion in Lithuania to cultivated land [106], as has happened for other basins vocated to salmonid production in central Europe [25,26,27]. Similar studies focusing on the effects of land cover on the water quality for sustaining salmonid populations pointed out how crop areas are land features vulnerable to soil erosion [25,40]. This weakness can deliver high amounts of fine sediments (<0.85 mm) to the spawning grounds, reducing their suitability [25,40]. Moreover, the steeper bed slope of Bl, 0.33% [47], compared to the gentler one of Sm, 0.007%, and Sv, 0.006% [49,51], surely contributes to increased velocity in surface runoff, leading to higher soil erosion and, thus, higher TSS delivery to the stream. On the other hand, the presence of forests dampens TSS loads in the watercourse, as is evident from Sv values (forest coverage: Sv: 40%, Bl: 28%, Sm: 10%). Sv holds the lowest TSS values among most of the sampling events (Table S6), and always during the flooding periods (Figure 6). Since the three systems respond differently in TSS loads during flooding events, we speculate how the origin of TSS could be more exogenous (i.e., brought through the runoff) rather than endogenous (i.e., resuspension during high flow), and, thus, connected to the land cover composition. Similar studies have already ascertained how the presence of forested areas can sequester the suspended solids entering the streams during intense runoff events [25,35,40].
4.3. Implication for Salmonid Incubation and Juvenile Rearing Phases
The Atlantic salmon and sea trout incubation season in these systems lasts from November/December to May [74]. During this period, DO values ranged from 9.7 to 14.4 mg L−1, falling inside the optimal ranges defined for the waters able to sustain successful survival rates for the intragravel stages [89,90]. Overall, during the flooding events, DO and % sat. profiles were high for the three watersheds due to the high-water mixing and low temperatures registered in these periods. These two factors contributed to the physical DO dissolution in the system [36,113]. However, minima of 5 mg L−1 were recorded in Sm toward the end of the incubation period in the stations in proximity to the urbanized areas. This situation indicates a poor ecological status, as previously highlighted [49,50], primarily due to point source pollution from urbanized areas [50]. Therefore, these DO values have the potential to impair the intragravel stages of salmonid larvae, as even short hypoxia events can be harmful [18,84]. Additionally, larvae in the last phases, prior to the emergence, require more oxygen than those in the earlier stages [114,115]. As summer approaches, the higher temperatures typical of this period necessarily limit DO concentration to a certain extent [36]. Despite this, the three systems show appropriate mean concentrations (7–10 mg L−1) to sustain salmonid juvenile rearing (Crisp 2000/8, WDOE 2002). Nevertheless, minima from 5.4 to 4.3 mg L−1 were recorded in Sv and Sm sampling stretches, respectively. Indeed, such DO concentrations in the Sv transect further support previous assessments of the poor ecological status [50,51] of the last stretch of this river, mainly due to non-point source pollution from cultivated lands [50,52]. The DO concentrations can trigger active avoidance behavior in juveniles [116]. Therefore, a DO shortage would not be as harmful as it would be for the intragravel stages, due to the higher mobility of the fingerlings and their ability to move to more suitable areas [117,118,119]. Moreover, although not directly lethal, prolonged periods of low intragravel DO concentrations can hamper larval fitness, with premature hatching related to a smaller body size, thus making such offspring more vulnerable to predators like other salmonids, bullheads, or stonefly nymphs [120,121,122]. In addition to this, environments presenting DO content deficiencies enhance the harmful effects of N-compounds even at concentrations considered safe for embryo development. They can synergistically contribute to possible low survival fitness [123,124] or even deplete more DO because of their oxidation processes [21].
Regarded as a cold-water species [125], salmonids are sensitive to temperature fluctuations. These regulate the length of the incubation period [34,126] in terms of the growth rate and swimming speed during the juvenile and adult stages [127,128]. Moreover, the temperature has a direct influence on the DO content by increasing its solubility in cold water, and on the salmonid metabolic rates, and, thus, on their oxygen demand [129,130]. Therefore, adequate temperature ranges in waters vocated to sustaining salmonid populations are pivotal for all the developmental stages [125].
Sm held the highest T values in fall, then Bl in winter, and, finally, Sv in summer (Figure 5). Although significant differences were found, gaps of 1–1.5 °C among the three investigated systems still do not, apparently, constitute a specific threat since the temperature profiles were falling inside the adequacy range for spawning [34] and the early incubation season [89]. Moreover, the significantly higher temperature regime found in Bl (Table S6), possibly derived from its groundwater supply [53,74], can be beneficial for the eggs in avoiding anchor ice formation [34] and fastening their development [131,132]. Nevertheless, during the last phase of the intragravel development (i.e., April–May), temperatures averaging 14 °C, with peaks up to 17–19 °C, exceeded the upper lethal limits defined for Atlantic salmon and brown trout intragravel stages [34,89,133]. If, on one side, this can represent a threat to the newly emerging alevins, on the other side, it should be recalled that late intragravel stages have the capacity to move and, thus, eschew unsuitable conditions [116,117,118]. Nevertheless, increasing temperatures during springtime can accelerate the breakdown of organic matter, which accumulates over winter due to low temperatures. If this organic matter is not mechanically removed by the spring thaws, it can suddenly be processed when temperatures start rising, potentially leading to episodes of DO depletion in the hyporheic zone [31]. Average values of T recorded during the summer period ranged from 17.8 to 19.6 °C, approaching the critical limits for juvenile rearing [89,92,127,128]. This alarming situation was already outlined in the last assessment on salmonid productivity in the Sv basin [52]. This scenario will likely worsen given the ongoing anthropogenic climate warming [125] and hydrologic intermittency [12,134]. Nevertheless, the presence of groundwater-fed patches inside these three systems [53,74] may constitute thermal refugia during low-flow and high-temperature conditions. This ecosystem service has been found for intermittent streams at the edge of salmonid distribution [12,135,136].
Among nutrients, multiple sources of evidence point out N-compounds as harmful molecules for aquatic animals [137,138,139], with specific regard to salmonids, considered one of the most sensitive families [21,27,140]. N-compounds are generated from point sources, like wastewater treatment plants, and non-point sources, like agricultural runoff [30,32]. They can be the by-product of decaying embryos [21] and organic matter [16] embedded in the intragravel environment. Above certain thresholds, N-compounds can have a direct effect on embryo development and performance in terms of hatching and size [30,87,124,140].
Although ionized ammonium is relatively harmless because of its inability to penetrate biological membranes, the unionized form is a real concern [87,140], whose concentration is primarily dictated by pH and temperature variations [21,30,141]. Within the pH and temperature ranges found in the three systems, we calculated N-NH3 concentrations ranging from 0.001 to 0.011 μL−1, similar to what was also reported by [141]. These concentrations are below the toxicity thresholds from 0.017 [87,142] to 0.021 mg L−1 [140] established to protect salmonid populations in the natural environment. These are also lower than the 0.05 mg L−1 threshold determined under laboratory conditions [124]. Although such values refer to adult stages, for the early ontogenetic phases, much higher thresholds are reported, from 16.8 [30] to 58 mg L−1 [124], given that embryos prior to hatching, thanks to the presence of the chorion, can be up two times more resistant than sack fries and juveniles [91,143]. However, it must be recalled that laboratory assays can be biased by the optimal conditions under which animals are tested, thus leading to underestimation of their real tolerance in natural environments [144]. Similar conclusions can be drawn for N-NOx− compounds, with overall means ranging from 0.8 to 1 mg L−1 for N-NO3− and from 0.01 to 0.04 mg L−1 for N-NO2−. These values are far below the threshold of 14 mg L−1 for N-NO2− [21,91], above which retardation in hatching and embryo growth has been observed in the early ontogenetic and fry stages of Atlantic salmon. Reported values are also below the thresholds of 20 and 34 mg L−1 of N-NO3−, which are known to trigger negative responses in developing fry of Chinook salmon and Cutthroat trout. However, N-compounds and the related organic matter can have an indirect effect on aquatic organisms, given their potential to contribute to eutrophication issues. N-compounds can fuel the trophic chain and, thus, subtract oxygen from the aquatic environment [34,87,123].
Concern can be pointed out during the intragravel stages of salmonids, from December to April. Due to the low temperatures typical of winter months, unprocessed organic matter deriving from biological treatments can accumulate in the spawning areas. Following spring temperature increments, organic matter may be oxidized, leading to a decline in DO concentrations [16,18,31]. In this regard, spawning grounds placed in the Sm basin can be highly vulnerable to episodes of low DO, considering the higher nutrient concentrations found during low-flow periods with respect to the other two watersheds. Also, organic matter and inorganic nutrients fuel biofilm growth, such as algal and bacterial mats, on spawning gravel. These mats can clog the interstices, leading to physical suffocation of the underneath intragravel environment [15,145,146] and, thus, impair embryo survival. This can lead to knock-on effects near the egg pockets, where N-compounds released as a by-product of embryo metabolism can locally accumulate, with negative consequences for the densely packed eggs [26,89]. Unfavorable physicochemical conditions, such as DO shortage and increased temperatures, can, in turn, augment the N-compounds’ effects [137], as already observed by Massa, Bagliniere [123], who reported increased post-hatch mortality with N-NO2− concentrations as low as 0.006 mg L−1 under low oxygen conditions.
High TSS concentrations decrease and worsen the available habitat for intragravel stages of stenoecian fish species [18,23,84] and macroinvertebrates [24]. Clogging of the interstices reduces intragravel flow velocity, leading to suffocation episodes within the intragravel matrix. This impairs the survival of intragravel stages that cannot actively move (i.e., eggs) [16,19,23,27].
In this regard, although Lithuania hosts many salmonid-vocated waters, the country does not have an established sampling protocol and assessment for the ecological TSS thresholds. This lack follows the common scenario of other European countries [112]. Parallelly, most US states indirectly monitor TSS using a critical value based on the exceedance (generally over 10%) of the seasonally established background value in the turbidity scale (NTU) for each watershed [24]. Despite this, only reliable monitoring and quantification of TSS loads can effectively depict their negative effects on aquatic biota [24,94,112].
The three investigated systems fall within the clear waters classification, <25 mg L−1 [74], and the TSS concentrations found here do not present a significant concern for salmonid populations when compared to the maximum 30-day average of 30–35 mg L−1, and the daily maximum of 58 mg L−1. Such thresholds are applied by a few US states, North Dakota, South Dakota, and Utah [24], which embrace TSS concentrations as a descriptor for cold water streams.
The scenario would change if more stringent thresholds were established for clear water basins in B. Columbia, such as <25 mg L−1 [94], to sustain salmonid populations. These values state that background levels should not increase over 25 mg L−1, for a daily maximum, or over an additional 5 mg L−1, for a 30-day average. If B. Columbia standards were applied here, flooding moments in Bl would bring TSS peaks (in mg L−1 fall: 45.62 ± 28.73, winter: 64.40 ± 28.33, Table 1) that largely exceed the 25 mg L−1 increment (Bl low-flow mean: 5 ± 3.3 mg L−1) accepted for daily fluctuations. Although the values refer to a single sampling moment across the flooding phase, we cannot exclude that such ranges could be maintained for a longer period, thus exceeding the maximum increment of 5 mg L−1 from 1 to 30 days [94]. The latter situation would be visible not only in Bl, but also in the Sm stream. Given the increasing precipitation intensity under the current climate change scenario, a high vulnerability to suspended solid suffocation is foreseen for those spawning grounds in the context of highly cultivated watersheds [147,148]. Similar studies carried out in watersheds vocated to salmonid production having similar land cover features confirmed how even one or a few flood events can completely silt the spawning grounds [23,84].
5. Conclusions
This study aimed to fill the gaps in our understanding of the relationships between water quality under different hydrological conditions and land usage. To do so, physicochemical responses in water quality across three boreal rivers were evaluated, considering different land uses and hydrological extreme events. We further questioned the possible implications that parameters outside the range can have on the early salmonid ontogenetic phases.
Justification to gather information about land use and find a possible correlation with the water quality is validated by the uni- and multivariate tests that showed marked differences among the three basins, reflecting their land cover. The Bl watershed presented generally high levels of DO, contrasting with the high levels of nutrients, EC, and alkalinities typical of the Sm watershed. Such diversity can be explained by the low percentage of urbanized areas, high shares of forests, and complex cultivation patterns for Bl, in contraposition to the high percentage of uncovered arable land in the Sm watershed and urban areas in the proximity of its watercourse. Sv stations presented high shares of forests but also of arable areas, placing this basin in an intermediate position between the other two.
The present study shows how the consequences of different land covers are reflected in the behavior of the three systems under hydrological extremes. Prolonged low-flow periods amplify concentrations of N-compounds, alkalinity, and EC values, regarded as proxies of organic matter coming from point source pollution. In this regard, the Sm basin seems the most vulnerable to nutrient overloads, given the individual wastewater treatment devices coming from the surrounding households. Non-point source pollution coming from agricultural fields can be negligible during low-flow events, which is the reason why Bl waters globally maintained an oligotrophic signature during low flows, characterized by high DO levels. Conversely, intense rainfall events have the potential to mobilize high quantities of particulate and suspended solids via field leaching from unsheltered soils, in turn increasing the input of exogenous nutrient loads. Furthermore, elevated runoff rates have the potential to impair wastewater treatment plants’ efficacy, thus delivering more nutrients to the watercourse. Such a scenario is reflected by the higher N-compound values for Bl and Sm and TSS peaks for Bl. On the other side, lower values registered in Sv can be attributed to the forest abundance that partially dampened the surface runoff erosive potential.
Salmonids’ ability to reproduce and rear in freshwater ecosystems is inherently linked to the ecological status of the ecosystems. This, in turn, is shaped by the ongoing anthropogenic pressures in the watershed and the current climate change scenario. The three systems displayed different vulnerabilities that may hamper smolt production: intragravel stages are threatened by low DO episodes connected with organic matter decomposition and a high trophic status in Sm waters. TSS peaks in Bl may have the potential to clog the spawning grounds within a single spate event.
Finally, temperatures approaching the upper tolerance limit can hamper juveniles’ fitness in Sv during the summer period. Prolonged low-flow periods or intense spate moments can trigger fluctuations in the physicochemical values, resulting in the appropriate requirements being missed for the most critical freshwater stages of the salmonids’ lifecycle. Therefore, transient hypoxia periods, severe siltation events, and heat waves can impair embryo and juvenile survival during their incubation and rearing periods. We cannot exclude that such weaknesses are meant to be worsened under the ongoing global warming scenario.
This study outlines the sampling strategy recommendations aimed at evaluating the systems’ functioning in view of possible restoration actions.
Specifically, the two-fold recommendations of this study are as follows: 1. Encourage a sampling strategy aimed at capturing water quality variation during moments of hydrological extremes (i.e., floods and low flow). This would allow the practitioners to catch “temporal hot spots” in the physicochemical variation of incubating and rearing environments. These “temporal hot spots” can be characterized by physicochemical parameters that are temporarily out of range but that can compromise an entire brood-year. Therefore, a fine spatial- and temporal-scale resolution sampling can outline the basin-specific vulnerabilities appearing during hydrological extreme moments. 2. Systems respond to hydrological variations in different ways according to their related land use layout. Land cover unbalances trigger watershed-specific responses during moments of hydrological variation. This, in turn, translates into watershed-specific weaknesses that should be differently tackled when considering river restoration and land use improvement projects. Thus, in those contexts with a high presence of urbanized areas near the watercourse, nature-based solutions like artificial wetlands should be developed to trap the main nutrient inputs. This can lead to lowered eutrophication phenomena, preventing hypoxic conditions in the nests where salmonid larvae are incubating. In watersheds having high shares of cultivated areas, cover and permanent crops should be improved, which in some cases can minimize the suspended solids’ delivery to the watercourse, thus preventing siltation events into the spawning grounds. Watershed-scale actions can be nested to remedy actions targeting the spawning areas, such as gravel augmentation and cleaning. We stress, therefore, the importance of representing the overall functioning of a watercourse, considering its related land use management. This can be accomplished by initiating investigations on a watershed scale. Through a holistic understanding of the problems and their underlying causes, practitioners can be provided with robust tools for effective management and restoration strategies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/w16233352/s1, Table S1: PCA variables; Table S2: Assumption tests; Table S3: Univariate tests; Table S4: Post hoc comparisons; Table S5: Correspondence analysis; Table S6: Averages; Table S7: Contingency table; Figure S1: Boxplots—flooding.
Author Contributions
Conceptualization, R.B. and M.B.; methodology, R.B., E.S., N.Č., M.M. and M.B.; validation, N.N. and M.B.; formal analysis, R.B., E.S. and N.Č.; investigation, R.B., N.N. and M.M.; resources, N.N. and M.B.; data curation, R.B. and E.S.; writing—original draft preparation, R.B., E.S. and M.B.; writing—review and editing, N.N., N.Č., M.M. and M.B.; visualization, R.B. and N.Č.; supervision, E.S., N.N. and M.B.; project administration, M.B.; funding acquisition, N.N. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by the Klaipeda University doctorate program. This research benefited from the equipment and framework of the COMP-R Initiative, funded by the “Departments of Excellence” program of the Italian Ministry for University and Research (MUR, 2023–2027).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [R.B.] upon reasonable request.
Acknowledgments
We are grateful to Edgaras Ivanauskas, Andrius Skersonas, and Tobia Politi for their assistance in the field, and Mindaugas Zilius for his assistance during the laboratory analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brooks, R.T. Potential impacts of global climate change on the hydrology and ecology of ephemeral freshwater systems of the forests of the northeastern United States. Clim. Chang. 2009, 95, 469–483. [Google Scholar] [CrossRef]
- Nohara, D.; Kitoh, A.; Hosaka, M.; Oki, T. Impact of climate change on river discharge projected by multimodel ensemble. J. Hydrometeorol. 2006, 7, 1076–1089. [Google Scholar] [CrossRef]
- Stanley, E.H.; Fisher, S.G.; Grimm, N.B. Ecosystem expansion and contraction in streams. BioScience 1997, 47, 427–435. [Google Scholar] [CrossRef]
- Dahm, C.N.; Baker, M.A.; Moore, D.I.; Thibault, J.R. Coupled biogeochemical and hydrological responses of streams and rivers to drought. Freshw. Biol. 2003, 48, 1219–1231. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Bailey, M.J.; Bainbridge, I.P.; Brereton, T.; Dick, J.T.; Drewitt, J.; Dulvy, N.K.; Dusic, N.R.; Freckleton, R.P.; Gaston, K.J. Future novel threats and opportunities facing UK biodiversity identified by horizon scanning. J. Appl. Ecol. 2008, 45, 821–833. [Google Scholar] [CrossRef]
- Wilby, R.; Whitehead, P.; Wade, A.; Butterfield, D.; Davis, R.; Watts, G. Integrated modelling of climate change impacts on water resources and quality in a lowland catchment: River Kennet, UK. J. Hydrol. 2006, 330, 204–220. [Google Scholar] [CrossRef]
- Bernal, S.; von Schiller, D.; Sabater, F.; Martí, E. Hydrological extremes modulate nutrient dynamics in mediterranean climate streams across different spatial scales. Hydrobiologia 2013, 719, 31–42. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Lange, J.; Haensler, A. Runoff generation following a prolonged dry period. J. Hydrol. 2012, 464, 157–164. [Google Scholar] [CrossRef]
- Larned, S.T.; Datry, T.; Arscott, D.B.; Tockner, K. Emerging concepts in temporary-river ecology. Freshw. Biol. 2010, 55, 717–738. [Google Scholar] [CrossRef]
- Poff, N.L.; Zimmerman, J.K.H. Ecological responses to altered flow regimes: A literature review to inform the science and management of environmental flows. Freshw. Biol. 2010, 55, 194–205. [Google Scholar] [CrossRef]
- Woelfle-Erskine, C.; Larsen, L.G.; Carlson, S.M. Abiotic habitat thresholds for salmonid over-summer survival in intermittent streams. Ecosphere 2017, 8, e01645. [Google Scholar] [CrossRef]
- Hosen, J.D.; Aho, K.S.; Appling, A.P.; Creech, E.C.; Fair, J.H.; Hall, R.O., Jr.; Kyzivat, E.D.; Lowenthal, R.S.; Matt, S.; Morrison, J.; et al. Enhancement of primary production during drought in a temperate watershed is greater in larger rivers than headwater streams. Limnol. Oceanogr. 2019, 64, 1458–1472. [Google Scholar] [CrossRef]
- Burke, N. Physical Controls on Salmon Spawning Habitat Quality and Embryo Fitness: An Integrated Analysis. Ph.D. Thesis, University of Southampton, Southampton, UK, 2011. [Google Scholar]
- Greig, S.; Sear, D.; Carling, P. The impact of fine sediment accumulation on the survival of incubating salmon progeny: Implications for sediment management. Sci. Total Environ. 2005, 344, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Conallin, J. The negative impacts of sedimentation on brown trout (Salmo trutta) natural recruitment, and the management of Danish streams. J. Transdiscipl. Environ. Stud. 2004, 3, 2. [Google Scholar]
- Guarch-Ribot, A.; Butturini, A. Hydrological conditions regulate dissolved organic matter quality in an intermittent headwater stream. From drought to storm analysis. Sci. Total Environ. 2016, 571, 1358–1369. [Google Scholar] [CrossRef]
- Malcolm, I.; Middlemas, C.; Soulsby, C.; Middlemas, S.; Youngson, A. Hyporheic zone processes in a canalised agricultural stream: Implications for salmonid embryo survival. Fundam. Appl. Limnol. 2010, 176, 319. [Google Scholar] [CrossRef]
- Pulg, U.; Barlaup, B.T.; Sternecker, K.; Trepl, L.; Unfer, G. Restoration of spawning habitats of brown trout (Salmo trutta) in a regulated chalk stream. River Res. Appl. 2013, 29, 172–182. [Google Scholar] [CrossRef]
- Calles, O.; Nyberg, L.; Greenberg, L. Temporal and spatial variation in quality of hyporheic water in one unregulated and two regulated boreal rivers. River Res. Appl. 2007, 23, 829–842. [Google Scholar] [CrossRef]
- Dumas, J.; Bassenave, J.; Jarry, M.; Barriere, L.; Glise, S. Effects of fish farm effluents on egg-to-fry development and survival of brown trout in artificial redds. J. Fish Biol. 2007, 70, 1734–1758. [Google Scholar] [CrossRef]
- Adjovu, G.E.; Stephen, H.; Ahmad, S. Spatiotemporal Variability in Total Dissolved Solids and Total Suspended Solids along the Colorado River. Hydrology 2023, 10, 125. [Google Scholar] [CrossRef]
- Soulsby, C.; Malcolm, I.; Youngson, A. Hydrochemistry of the hyporheic zone in salmon spawning gravels: A preliminary assessment in a degraded agricultural stream. Regul. Rivers Res. Manag. Int. J. Devoted River Res. Manag. 2001, 17, 651–665. [Google Scholar] [CrossRef]
- Swietlik, W.; Berry, W.; Gardner, T.; Hill, B.; Jha, M.; Kaufmann, P.; Melzian, B.; Norton, D.; Paul, J.; Rubinstein, N. Developing Water Quality Criteria for Suspended and Bedded Sediments (SABS); Potential Approaches; US EPA Office of Water, Office of Science and Technology, Health and Ecological Criteria Division: Washington, DC, USA, 2003.
- Sutherland, A.B.; Meyer, J.L.; Gardiner, E.P. Effects of land cover on sediment regime and fish assemblage structure in four southern Appalachian streams. Freshw. Biol. 2002, 47, 1791–1805. [Google Scholar] [CrossRef]
- Malcolm, I.; Soulsby, C.; Youngson, A.; Hannah, D.; McLaren, I.; Thorne, A. Hydrological influences on hyporheic water quality: Implications for salmon egg survival. Hydrol. Process. 2004, 18, 1543–1560. [Google Scholar] [CrossRef]
- Sternecker, K.; Wild, R.; Geist, J. Effects of substratum restoration on salmonid habitat quality in a subalpine stream. Environ. Biol. Fishes 2013, 96, 1341–1351. [Google Scholar] [CrossRef]
- Severini, E.; Magri, M.; Soana, E.; Bartoli, M.; Faggioli, M.; Celico, F. Irrigation practices affect relationship between reduced nitrogen fertilizer use and improvement of river and groundwater chemistry. Agric. Water Manag. 2023, 289, 108564. [Google Scholar] [CrossRef]
- Jarvie, H.P.; Jürgens, M.D.; Williams, R.J.; Neal, C.; Davies, J.J.; Barrett, C.; White, J. Role of river bed sediments as sources and sinks of phosphorus across two major eutrophic UK river basins: The Hampshire Avon and Herefordshire Wye. J. Hydrol. 2005, 304, 51–74. [Google Scholar] [CrossRef]
- Brinkman, S.F.; Woodling, J.D.; Vajda, A.M.; Norris, D.O. Chronic toxicity of ammonia to early life stage rainbow trout. Trans. Am. Fish. Soc. 2009, 138, 433–440. [Google Scholar] [CrossRef]
- Rubin, J.F.; Glimsäter, C. Egg-to-fry survival of the sea trout in some streams of Gotland. J. Fish Biol. 1996, 48, 585–606. [Google Scholar]
- Binkley, D.; Brown, T.C. Forest practices as nonpoint sources of pollution in North America 1. JAWRA J. Am. Water Resour. Assoc. 1993, 29, 729–740. [Google Scholar] [CrossRef]
- Chapman, D. Critical review of variables used to define effects of fines in redds of large salmonids. Trans. Am. Fish. Soc. 1988, 117, 1–21. [Google Scholar] [CrossRef]
- Bjornn, T.C.; Reiser, D.W. Habitat requirements of salmonids in streams. Am. Fish. Soc. Spec. Publ. 1991, 19, 138. [Google Scholar]
- Billi, P.; Spalevic, V. Suspended sediment yield in Italian rivers. Catena 2022, 212, 106119. [Google Scholar] [CrossRef]
- Gorgoglione, A.; Gregorio, J.; Rios, A.; Alonso, J.; Chreties, C.; Fossati, M. Influence of land use/land cover on surface-water quality of Santa Lucìa river, Uruguay. Sustainability 2020, 12, 4692. [Google Scholar] [CrossRef]
- Van Esbroeck, C.J.; Macrae, M.L.; Brunke, R.I.; McKague, K. Annual and seasonal phosphorus export in surface runoff and tile drainage from agricultural fields with cold temperate climates. J. Great Lakes Res. 2016, 42, 1271–1280. [Google Scholar] [CrossRef]
- Chen, N.; Hong, H. Nitrogen export by surface runoff from a small agricultural watershed in southeast China: Seasonal pattern and primary mechanism. Biogeochemistry 2011, 106, 311–321. [Google Scholar] [CrossRef]
- Maraseni, T.; Mitchell, C. An assessment of carbon sequestration potential of riparian zone of Condamine Catchment, Queensland, Australia. Land Use Policy 2016, 54, 139–146. [Google Scholar] [CrossRef]
- Opperman, J.J.; Lohse, K.A.; Brooks, C.; Kelly, N.M.; Merenlender, A.M. Influence of land use on fine sediment in salmonid spawning gravels within the Russian River Basin, California. Can. J. Fish. Aquat. Sci. 2005, 62, 2740–2751. [Google Scholar] [CrossRef]
- Olyaei, M.A.; Karamouz, M.; Farmani, R. Framework for assessing flood reliability and resilience of wastewater treatment plants. J. Environ. Eng. 2018, 144, 04018081. [Google Scholar] [CrossRef]
- Zouboulis, A.; Tolkou, A. Effect of Climate Change in Wastewater Treatment Plants: Reviewing the Problems and Solutions. In Managing Water Resources Under Climate Uncertainty: Examples from Asia, Europe, Latin America, and Australia; Shrestha, S., Anal, A.K., Salam, P.A., van der Valk, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 197–220. [Google Scholar]
- IPCC. Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, NY, USA, 2014. [Google Scholar]
- Šarauskienė, D.; Akstinas, V.; Kriaučiūnienė, J.; Jakimavičius, D.; Bukantis, A.; Kažys, J.; Povilaitis, A.; Ložys, L.; Kesminas, V.; Virbickas, T. Projection of Lithuanian river runoff, temperature and their extremes under climate change. Hydrol. Res. 2018, 49, 344–362. [Google Scholar] [CrossRef]
- Čerkasova, N.; Mėžinė, J.; Idzelytė, R.; Lesutienė, J.; Ertürk, A.; Umgiesser, G. Exploring variability in climate change projections on the Nemunas River and Curonian Lagoon: Coupled SWAT and SHYFEM modeling approach. Ocean Sci. 2024, 20, 1123–1147. [Google Scholar] [CrossRef]
- Jablonskis, J.; Lasinskas, M. Lithuanian river cadastre (discharges, slopes, capacities). Vilnius Lith. 1962, 3, 640. [Google Scholar]
- Gailiušis, B.; Jablonskis, J.; Kovalenkovienė, M. Lithuanian Rivers: Hydrography and Runoff; Lithuanian Energy Institute: Kaunas, Lithuania, 2001; pp. 1–792. [Google Scholar]
- HELCOM. River Restoration in the Baltic Sea Region: Best Practices and Recommendations for Successful Projects. 2021. Available online: https://retrout.org/2021/08/04/river-restoration-in-the-baltic-sea-region-best-practices-and-recommendations-for-successful-projects/ (accessed on 10 March 2024).
- Nemunas River Basin District; Government of the Republic of Lithuania: Vilnius, Lithuania, 2010.
- List of Water Bodies at Risk; Minister of the Environment of the Republic of Lithuania: Vilnius, Lithuania, 2017. (In Lithuanian)
- Venta River Basin District; Government of the Republic of Lithuania: Vilnius, Lithuania, 2010.
- Kesminas, V. Sea trout and salmon populations and rivers in Lithuania: HELCOM assessment of salmon (Salmo salar) and sea trout (Salmo trutta) populations and habitats in rivers flowing to the Baltic Sea. In Baltic Sea Environment Proceedings; Commission, H., Ed.; Baltic Marine Environment Protection Commission: Helsinki, Finland, 2011. [Google Scholar]
- Jablonskis, J.; Janukėnienė, R. Change of Lithuanian River Runoff; Science: Vilnius, Lithuania, 1978. [Google Scholar]
- Kontautas, A.; Rauckis, U. Some data on sea trout population parameters in Minija River basin. Proc. Klaipėda Univ. 1994, 1, 137–142. [Google Scholar]
- Shi, P.; Zhang, Y.; Li, Z.; Li, P.; Xu, G. Influence of land use and land cover patterns on seasonal water quality at multi-spatial scales. Catena 2017, 151, 182–190. [Google Scholar] [CrossRef]
- Ding, J.; Jiang, Y.; Liu, Q.; Hou, Z.; Liao, J.; Fu, L.; Peng, Q. Influences of the land use pattern on water quality in low-order streams of the Dongjiang River basin, China: A multi-scale analysis. Sci. Total Environ. 2016, 551, 205–216. [Google Scholar] [CrossRef]
- Pinardi, M.; Soana, E.; Severini, E.; Racchetti, E.; Celico, F.; Bartoli, M. Agricultural practices regulate the seasonality of groundwater-river nitrogen exchanges. Agric. Water Manag. 2022, 273, 107904. [Google Scholar] [CrossRef]
- Racchetti, E.; Salmaso, F.; Pinardi, M.; Quadroni, S.; Soana, E.; Sacchi, E.; Severini, E.; Celico, F.; Viaroli, P.; Bartoli, M. Is flood irrigation a potential driver of river-groundwater interactions and diffuse nitrate pollution in agricultural watersheds? Water 2019, 11, 2304. [Google Scholar] [CrossRef]
- Severini, E.; Magri, M.; Soana, E.; Bartoli, M. Unraveling the nexus: Exploring river-groundwater interaction as the primary driver of eutrophication in river ecosystems. J. Hydrol. 2024, 645, 132185. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Houser, J.N.; Scheuerell, M.D.; Smits, A.P. Warmer winters increase the biomass of phytoplankton in a large floodplain river. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG006135. [Google Scholar] [CrossRef]
- Thellman, A.; Jankowski, K.J.; Hayden, B.; Yang, X.; Dolan, W.; Smits, A.P.; O’Sullivan, A.M. The ecology of river ice. J. Geophys. Res. Biogeosci. 2021, 126, e2021JG006275. [Google Scholar] [CrossRef]
- Gran, G. Determination of the equivalence point in potentiometric titrations. Part II. Analyst 1952, 77, 661–671. [Google Scholar] [CrossRef]
- Bendixen, M. A practical guide to the use of correspondence analysis in marketing research. Mark. Res. Line 1996, 1, 16–36. [Google Scholar]
- Nenadic, O.; Greenacre, M. Correspondence analysis in R, with two-and three-dimensional graphics: The ca package. J. Stat. Softw. 2007, 20, 1–13. [Google Scholar]
- Kassambara, A. Practical Guide to Principal Component Methods in R: PCA, M (CA), FAMD, MFA, HCPC, Factoextra. Volume 2. Sthda. 2017. Available online: https://www.sthda.com/english/wiki/practical-guide-to-principal-component-methods-in-r?title=practical-guide-to-principal-component-methods-in-r (accessed on 20 April 2024).
- R Core Team. R: A Language and Environment for Statistical Computing. v 4.4.0. 2023. Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 20 April 2024).
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. v. 0.6.0. 2021. Available online: https://rpkgs.datanovia.com/rstatix/ (accessed on 20 April 2024).
- Kassambara, A. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.4. 2016. Available online: https://rpkgs.datanovia.com/factoextra/index.html (accessed on 20 April 2024).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; van den Brand, T. Package ‘ggplot2’. Create Elegant Data Visualisations Using the Grammar of Graphics. Version 3.5.1. 2016. Available online: https://ggplot2.tidyverse.org/reference/ggplot2-package.html (accessed on 20 April 2024).
- McDowell, R.W.; Hamilton, D.P. Nutrients and eutrophication: Introduction. Mar. Freshw. Res. 2013, 64, iii–vi. [Google Scholar] [CrossRef]
- Wu, T.; Zhu, G.; Zhu, M.; Xu, H.; Zhang, Y.; Qin, B. Use of conductivity to indicate long-term changes in pollution processes in Lake Taihu, a large shallow lake. Environ. Sci. Pollut. Res. 2020, 27, 21376–21385. [Google Scholar] [CrossRef] [PubMed]
- Sager, P.E. Ecological Aspects of Eutrophication. Water Int. 1976, 1, 11–17. [Google Scholar] [CrossRef]
- Sivarajah, B.; Simmatis, B.; Favot, E.J.; Palmer, M.J.; Smol, J.P. Eutrophication and climatic changes lead to unprecedented cyanobacterial blooms in a Canadian sub-Arctic landscape. Harmful Algae 2021, 105, 102036. [Google Scholar] [CrossRef]
- Nika, N. Reproductive Ecology and Success of Sea Trout Salmo trutta L. in a Small Lowland Stream of Western Lithuania. Doctoral Dissertation, Klaipeda University, Coastal Research and Planning Institute, Klaipeda, Lithuania, 2011. [Google Scholar]
- Jeon, I.; Nam, K. Change in the site density and surface acidity of clay minerals by acid or alkali spills and its effect on pH buffering capacity. Sci. Rep. 2019, 9, 9878. [Google Scholar] [CrossRef]
- Kumari, N.; Mohan, C. Basics of Clay Minerals and Their Characteristic Properties; IntechOpen: London, UK, 2021. [Google Scholar]
- Cai, W.-J.; Hu, X.; Huang, W.-J.; Murrell, M.C.; Lehrter, J.C.; Lohrenz, S.E.; Chou, W.-C.; Zhai, W.; Hollibaugh, J.T.; Wang, Y. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 2011, 4, 766–770. [Google Scholar] [CrossRef]
- Sunda, W.G.; Cai, W.-J. Eutrophication induced CO2-acidification of subsurface coastal waters: Interactive effects of temperature, salinity, and atmospheric p CO2. Environ. Sci. Technol. 2012, 46, 10651–10659. [Google Scholar] [CrossRef]
- Sutela, T.; Vehanen, T.; Jounela, P. Response of fish assemblages to water quality in boreal rivers. Hydrobiologia 2010, 641, 1–10. [Google Scholar] [CrossRef]
- Delgado, J.A.; Dillon, M.A.; Sparks, R.T.; Essah, S.Y. A decade of advances in cover crops. J. Soil Water Conserv. 2007, 62, 110A–117A. [Google Scholar]
- Dabney, S.M.; Delgado, J.A.; Meisinger, J.J.; Schomberg, H.H.; Liebig, M.A.; Kaspar, T.; Mitchell, J.; Reeves, W. Using cover crops and cropping systems for nitrogen management. Adv. Nitrogen Manag. Water Qual. 2010, 66, 231–282. [Google Scholar]
- Ross, S.M.; Izaurralde, R.C.; Janzen, H.; Robertson, J.; McGill, W.B. The nitrogen balance of three long-term agroecosystems on a boreal soil in western Canada. Agric. Ecosyst. Environ. 2008, 127, 241–250. [Google Scholar] [CrossRef]
- Heathwaite, A.L.; Johnes, P.J.; Peters, N.E. Trends in nutrients. Hydrol. Process. 1996, 10, 263–293. [Google Scholar] [CrossRef]
- Malcolm, I.A.; Youngson, A.F.; Soulsby, C. Survival of salmonid eggs in a degraded gravel-bed stream: Effects of groundwater–surface water interactions. River Res. Appl. 2003, 19, 303–316. [Google Scholar] [CrossRef]
- Kato, T.; Kuroda, H.; Nakasone, H. Runoff characteristics of nutrients from an agricultural watershed with intensive livestock production. J. Hydrol. 2009, 368, 79–87. [Google Scholar] [CrossRef]
- Yang, Q.; Tian, H.; Li, X.; Ren, W.; Zhang, B.; Zhang, X.; Wolf, J. Spatiotemporal patterns of livestock manure nutrient production in the conterminous United States from 1930 to 2012. Sci. Total Environ. 2016, 541, 1592–1602. [Google Scholar] [CrossRef]
- Finn, R.N. The physiology and toxicology of salmonid eggs and larvae in relation to water quality criteria. Aquat. Toxicol. 2007, 81, 337–354. [Google Scholar] [CrossRef]
- Kincheloe, J.W.; Wedemeyer, G.A.; Koch, D.L. Tolerance of developing salmonid eggs and fry to nitrate exposure. Bull. Environ. Contam. Toxicol. 1979, 23, 575–578. [Google Scholar] [CrossRef]
- Crisp, T. Trout and Salmon: Ecology, Conservation and Rehabilitation; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Greig, S.; Sear, D.; Carling, P. A field-based assessment of oxygen supply to incubating Atlantic salmon (Salmo salar) embryos. Hydrol. Process. Int. J. 2007, 21, 3087–3100. [Google Scholar] [CrossRef]
- Williams, E.; Eddy, F. Effect of nitrite on the embryonic development of Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 1989, 46, 1726–1729. [Google Scholar] [CrossRef]
- Smialek, N.; Pander, J.; Geist, J. Environmental threats and conservation implications for Atlantic salmon and brown trout during their critical freshwater phases of spawning, egg development and juvenile emergence. Fish. Manag. Ecol. 2021, 28, 437–467. [Google Scholar] [CrossRef]
- Peterson, R.; Daye, P.; Metcalfe, J. Inhibition of Atlantic salmon (Salmo salar) hatching at low pH. Can. J. Fish. Aquat. Sci. 1980, 37, 770–774. [Google Scholar] [CrossRef]
- Singleton, H. Ambient Water Quality Guidelines (Criteria) for Turbidity, Suspended and Benthic Sediments; British Columbia Ministry of Water, Land and Air Protection: Victoria, BC, Canada, 2001.
- Peña-Guerrero, M.D.; Nauditt, A.; Muñoz-Robles, C.; Ribbe, L.; Meza, F. Drought impacts on water quality and potential implications for agricultural production in the Maipo River Basin, Central Chile. Hydrol. Sci. J. 2020, 65, 1005–1021. [Google Scholar] [CrossRef]
- Vybernaite-Lubiene, I.; Zilius, M.; Saltyte-Vaisiauske, L.; Bartoli, M. Recent trends (2012–2016) of N, Si, and P export from the Nemunas River Watershed: Loads, unbalanced stoichiometry, and threats for downstream aquatic ecosystems. Water 2018, 10, 1178. [Google Scholar] [CrossRef]
- Liu, Y.; Su, B.; Mu, H.; Zhang, Y.; Chen, L.; Wu, B. Effects of point and nonpoint source pollution on urban rivers: From the perspective of pollutant composition and toxicity. J. Hazard. Mater. 2023, 460, 132441. [Google Scholar] [CrossRef]
- Mosley, L.M. Drought impacts on the water quality of freshwater systems; review and integration. Earth-Sci. Rev. 2015, 140, 203–214. [Google Scholar] [CrossRef]
- Povilaitis, A. Phosphorus trends in Lithuanian Rivers affected by agricultural non-point pollution. Environ. Res. Eng. Manag. 2004, 4, 17–27. [Google Scholar]
- Trentman, M.T.; Tank, J.L.; Shepherd, H.A.; Marrs, A.J.; Welsh, J.R.; Goodson, H.V. Characterizing bioavailable phosphorus concentrations in an agricultural stream during hydrologic and streambed disturbances. Biogeochemistry 2021, 154, 509–524. [Google Scholar] [CrossRef]
- Wong, C.H.; Barton, G.W.; Barford, J.P. The nitrogen cycle and its application in wastewater treatment. In The Handbook of Water and Wastewater Microbiology; Mara, D., Horan, N., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 427–440. [Google Scholar]
- Zhou, H.; Li, X.; Xu, G.; Yu, H. Overview of strategies for enhanced treatment of municipal/domestic wastewater at low temperature. Sci. Total Environ. 2018, 643, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Bach, H.; Christensen, N.; Kristensen, P. The State of the Environment in Denmark, 2001; NERI Technical Report; Ministry of the Environment: Copenhagen, Denmark, 2002.
- Schepers, J.S.; Vavricka, E.J.; Andersen, D.R.; Wittmuss, H.D.; Schuman, G.E. Agricultural runoff during a drought period. Water Pollut. Control Fed. 1980, 52, 711–719. [Google Scholar]
- Luke, S.H.; Luckai, N.J.; Burke, J.M.; Prepas, E.E. Riparian areas in the Canadian boreal forest and linkages with water quality in streams. Environ. Rev. 2007, 15, 79–97. [Google Scholar] [CrossRef]
- Bauer, A.; Weert, S. Status of nutrient bookkeeping in the Baltic Sea Countries. Rep. Doc. Texte 2015, 95, 2015. [Google Scholar]
- Green, P.A.; Vörösmarty, C.J.; Meybeck, M.; Galloway, J.N.; Peterson, B.J.; Boyer, E.W. Pre-industrial and contemporary fluxes of nitrogen through rivers: A global assessment based on typology. Biogeochemistry 2004, 68, 71–105. [Google Scholar] [CrossRef]
- Lawlor, P.A.; Helmers, M.J.; Baker, J.L.; Melvin, S.W.; Lemke, D.W. Nitrogen application rate effects on corn yield and nitrate-nitrogen concentration and loss in subsurface drainage. In Proceedings of the 2005 ASAE Annual Meeting, Alor Setar, Malaysia, 20–22 August 2005; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2005. [Google Scholar]
- Szewczyk, C.J.; Smith, E.M.; Benitez-Nelson, C.R. Temperature sensitivity of oxygen demand varies as a function of organic matter source. Front. Mar. Sci. 2023, 10, 1133336. [Google Scholar] [CrossRef]
- White, J.; Khamis, K.; Dugdale, S.; Jackson, F.; Malcolm, I.; Krause, S.; Hannah, D. Drought impacts on river water temperature: A process-based understanding from temperate climates. Hydrol. Process. 2023, 37, e14958. [Google Scholar] [CrossRef]
- Haapala, H.; Sepponen, P.; Meskus, E. Effect of spring floods on water acidity in the Kiiminkijoki area, Finland. Oikos 1975, 26, 26–31. [Google Scholar] [CrossRef]
- Brils, J. Sediment monitoring and the European water framework directive. Ann. Dell’‘istituto Super. Di Sanita 2008, 44, 218. [Google Scholar]
- Nore, A. The Effect of Turbulence on Oxygen Uptake of Water. Master’s Thesis, NTNU, Trondheim, Norway, 2022. [Google Scholar]
- Alderdice, D.; Wickett, W.; Brett, J. Some effects of temporary exposure to low dissolved oxygen levels on Pacific salmon eggs. J. Fish. Board Can. 1958, 15, 229–250. [Google Scholar] [CrossRef]
- Wickett, W.P. The oxygen supply to salmon eggs in spawning beds. J. Fish. Board Can. 1954, 11, 933–953. [Google Scholar] [CrossRef]
- Washington State Department of Ecology. Evaluating Criteria for the Protection of Freshwater Aquatic Life in Washington’s Surface Water Quality Standards: Dissolved Oxygen. 2002; p. 90. Available online: https://apps.ecology.wa.gov/publications/SummaryPages/0010071.html (accessed on 20 April 2024).
- Matthews, K.; Berg, N. Rainbow trout responses to water temperature and dissolved oxygen stress in two southern California stream pools. J. Fish Biol. 1997, 50, 50–67. [Google Scholar] [CrossRef]
- Spoor, W. Distribution of fingerling brook trout, Salvelinus fontinalis (Mitchill), in dissolved oxygen concentration gradients. J. Fish Biol. 1990, 36, 363–373. [Google Scholar] [CrossRef]
- Bams, R. Adaptations of sockeye salmon associated with incubation in stream gravels. In Symposium on Salmon and Trout in Streams; University of British Columbia: Vancouver, BC, Canada, 1969; p. 87. [Google Scholar]
- Claire, E.W.; Phillips, R.W. The stonefly Acroneuria pacifica as a potential predator on salmonid embryos. Trans. Am. Fish. Soc. 1968, 97, 50–52. [Google Scholar] [CrossRef]
- Heywood, M.; Walling, D. The sedimentation of salmonid spawning gravels in the Hampshire Avon catchment, UK: Implications for the dissolved oxygen content of intragravel water and embryo survival. Hydrol. Process. Int. J. 2007, 21, 770–788. [Google Scholar] [CrossRef]
- McDaniels, T.; Wilmot, S.; Healey, M.; Hinch, S. Vulnerability of Fraser River sockeye salmon to climate change: A life cycle perspective using expert judgments. J. Environ. Manag. 2010, 91, 2771–2780. [Google Scholar] [CrossRef]
- Massa, F.; Bagliniere, J.; Prunet, P.; Grimaldi, C. Egg-to-Fry Survival of Brown Trout (Salmo trutta) and Chemical Environment in the Redd. Cybium Int. J. Ichthyol. 2000, 24, 129–140. [Google Scholar]
- Burkhalter, D.E.; Kaya, C.M. Effects of prolonged exposure to ammonia on fertilized eggs and sac fry of rainbow trout (Salmo gairdneri). Trans. Am. Fish. Soc. 1977, 106, 470–475. [Google Scholar] [CrossRef]
- Isaak, D.J.; Young, M.K. Cold-water habitats, climate refugia, and their utility for conserving salmonid fishes. Can. J. Fish. Aquat. Sci. 2023, 80, 1187–1206. [Google Scholar] [CrossRef]
- Crisp, D. Environmental requirements of common riverine European salmonid fish species in fresh water with particular reference to physical and chemical aspects. Hydrobiologia 1996, 323, 201–221. [Google Scholar] [CrossRef]
- Elliott, J. Some aspects of thermal stress on freshwater teleosts. Stress Fish 1981, 1981, 209–245. [Google Scholar]
- Elliott, J.; Hurley, M. A functional model for maximum growth of Atlantic salmon parr, Salmo salar, from two populations in northwest England. Funct. Ecol. 1997, 11, 592–603. [Google Scholar] [CrossRef]
- Davis, J.C. Minimal dissolved oxygen requirements of aquatic life with emphasis on Canadian species: A review. J. Fish. Board Can. 1975, 32, 2295–2332. [Google Scholar] [CrossRef]
- Martin, B.T.; Pike, A.; John, S.N.; Hamda, N.; Roberts, J.; Lindley, S.T.; Danner, E.M. Phenomenological vs. biophysical models of thermal stress in aquatic eggs. Ecol. Lett. 2017, 20, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Geist, D.R.; Hanrahan, T.P.; Arntzen, E.V.; McMichael, G.A.; Murray, C.J.; Chien, Y.-J. Physicochemical characteristics of the hyporheic zone affect redd site selection by chum salmon and fall Chinook salmon in the Columbia River. N. Am. J. Fish. Manag. 2002, 22, 1077–1085. [Google Scholar] [CrossRef]
- Burril, S.E.; Zimmerman, C.E.; Finn, J.E. Characteristics of Fall Chum Salmon Spawning Habitat on a Mainstem River in Interior Alaska. 2010. Available online: https://pubs.usgs.gov/publication/ofr20101164 (accessed on 9 March 2024).
- Stanley, J.G.; Trial, J.G. Habitat Suitability Index Models: Nonmigratory Freshwater Life Stages of Atlantic Salmon; US Department of the Interior, National Biological Service: Washington, DC, USA, 1995; Volume 3.
- McLaughlin, B.C.; Ackerly, D.D.; Klos, P.Z.; Natali, J.; Dawson, T.E.; Thompson, S.E. Hydrologic refugia, plants, and climate change. Glob. Chang. Biol. 2017, 23, 2941–2961. [Google Scholar] [CrossRef]
- Bean, J.R.; Wilcox, A.C.; Woessner, W.W.; Muhlfeld, C.C. Multiscale hydrogeomorphic influences on bull trout (Salvelinus confluentus) spawning habitat. Can. J. Fish. Aquat. Sci. 2015, 72, 514–526. [Google Scholar] [CrossRef]
- Larsen, L.G.; Woelfle-Erskine, C. Groundwater is key to salmonid persistence and recruitment in intermittent Mediterranean-climate streams. Water Resour. Res. 2018, 54, 8909–8930. [Google Scholar] [CrossRef]
- USEPA. 1999 Update of Ambient Water Quality Criteria for Ammonia; USEPA: Washington, DC, USA, 1999.
- EIFAC. Water Quality Criteria for European Freshwater Fish; Report on Ammonia and Inland Fisheries; FAO: Rome, Italy, 1970. [Google Scholar]
- Russo, R.C.; Thurston, R.V. Toxicity of ammonia, nitrite and nitrate to fishes. Aquac. Water Qual. 1991, 3, 58–89. [Google Scholar]
- Eddy, F. Ammonia in estuaries and effects on fish. J. Fish Biol. 2005, 67, 1495–1513. [Google Scholar] [CrossRef]
- Emerson, K.; Russo, R.C.; Lund, R.E.; Thurston, R.V. Aqueous ammonia equilibrium calculations: Effect of pH and temperature. J. Fish. Board Can. 1975, 32, 2379–2383. [Google Scholar] [CrossRef]
- Randall, D.J.; Tsui, T. Ammonia toxicity in fish. Mar. Pollut. Bull. 2002, 45, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Calamari, D.; Marchetti, R.; Vailati, G. Effects of Long-Term Exposure to Ammonia on the Developmental Stages of Rainbow Trout(Salmo gairdneri Richardson). Int. Explor. Mer. 1981, 178, 81–86. [Google Scholar]
- Ip, Y.; Chew, S.; Randall, D. Ammonia toxicity, tolerance, and excretion. Fish Physiol. 2001, 20, 109–148. [Google Scholar]
- Chen, B.; Li, Y. Numerical modeling of biofilm growth at the pore scale. In Proceedings of the 1999 Conference on Hazardous Waste Research, St. Louis, MI, USA, 24–27 May 1999; pp. 215–226. [Google Scholar]
- Battin, T.J.; Kaplan, L.A.; Newbold, J.D.; Cheng, X.; Hansen, C. Effects of current velocity on the nascent architecture of stream microbial biofilms. Appl. Environ. Microbiol. 2003, 69, 5443–5452. [Google Scholar] [CrossRef] [PubMed]
- McKean, J.; Tonina, D. Bed stability in unconfined gravel bed mountain streams: With implications for salmon spawning viability in future climates. J. Geophys. Res. Earth Surf. 2013, 118, 1227–1240. [Google Scholar] [CrossRef]
- Neachell, E. Environmental flows: Saving rivers in the third millennium, by A.H. Arthington. 2012. University of California Press: Berkeley, 424. (ISBN 978-0-520-27369-6) Price: £52.00. River Res. Appl. 2014, 30, 132–133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).