Impact of Microplastic on Freshwater Sediment Biogeochemistry and Microbial Communities Is Polymer Specific
Abstract
1. Introduction
2. Methods
2.1. Microcosm Design and Set-Up
2.2. Material Additions
2.3. Oxygen and Nutrient Flux Measurements
2.4. Sediment Properties
2.5. Potential Denitrification
2.6. Microbial Community Structure
2.7. Statistical Analysis
3. Results
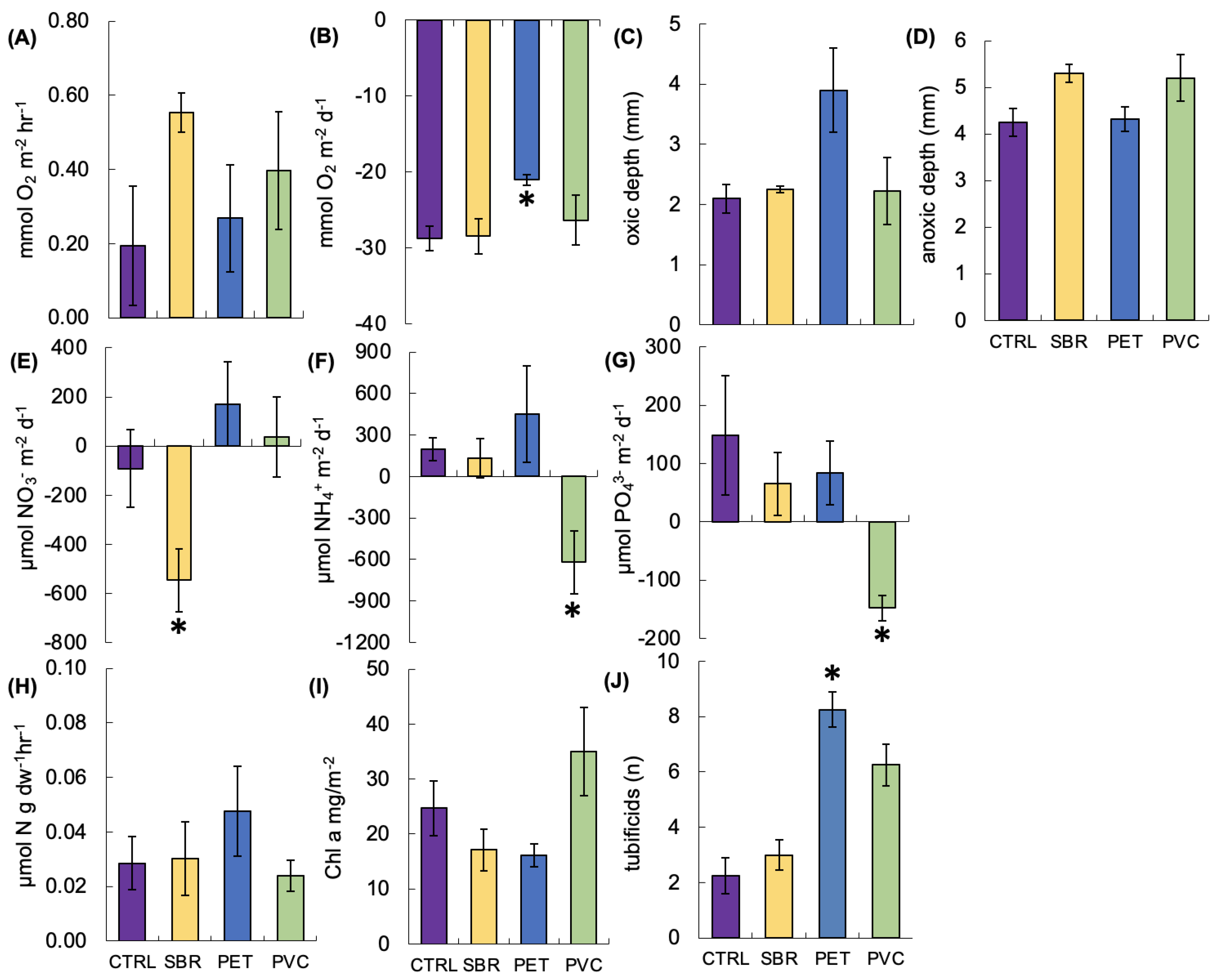
3.1. Nutrient Fluxes and Sediment Properties
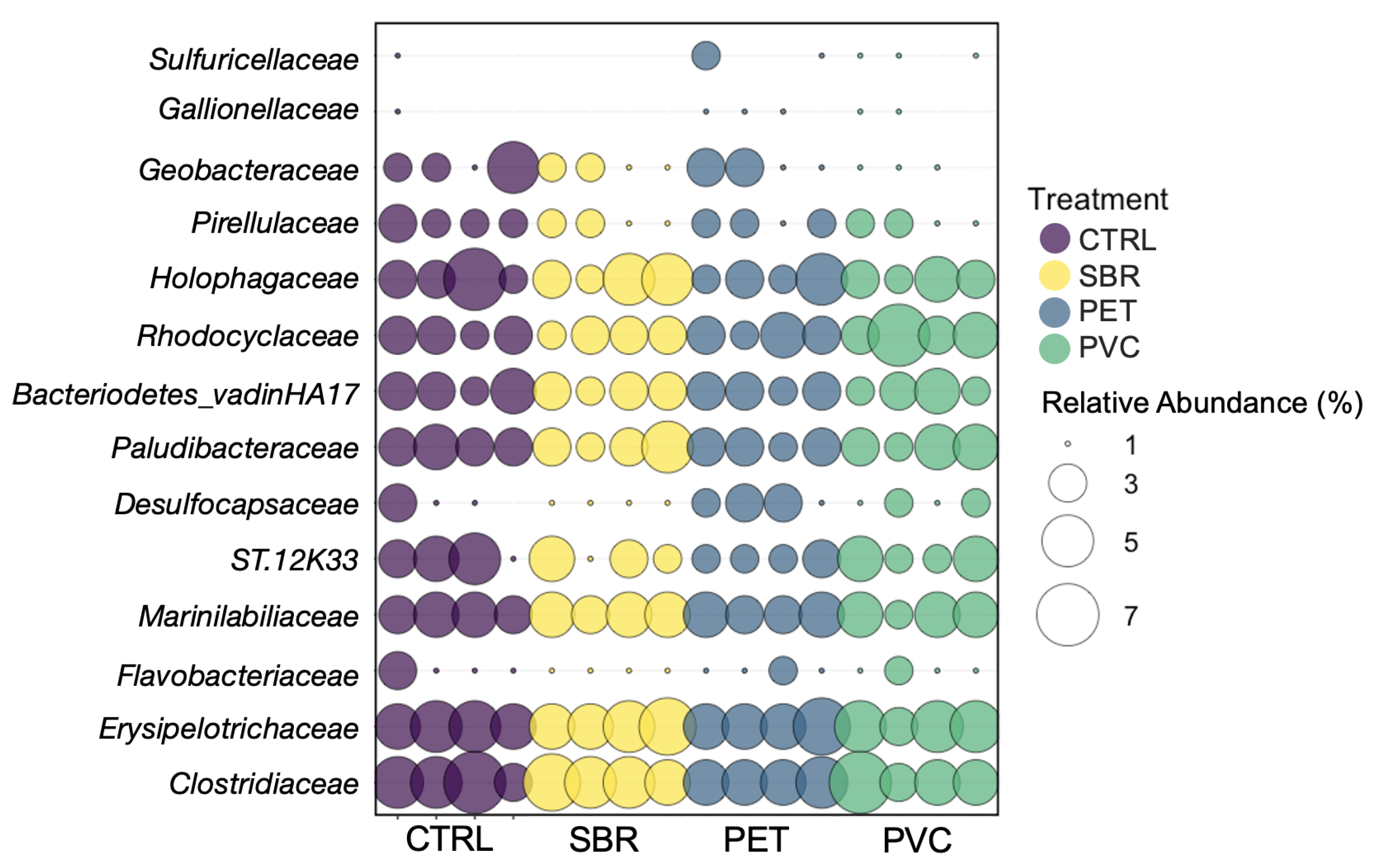
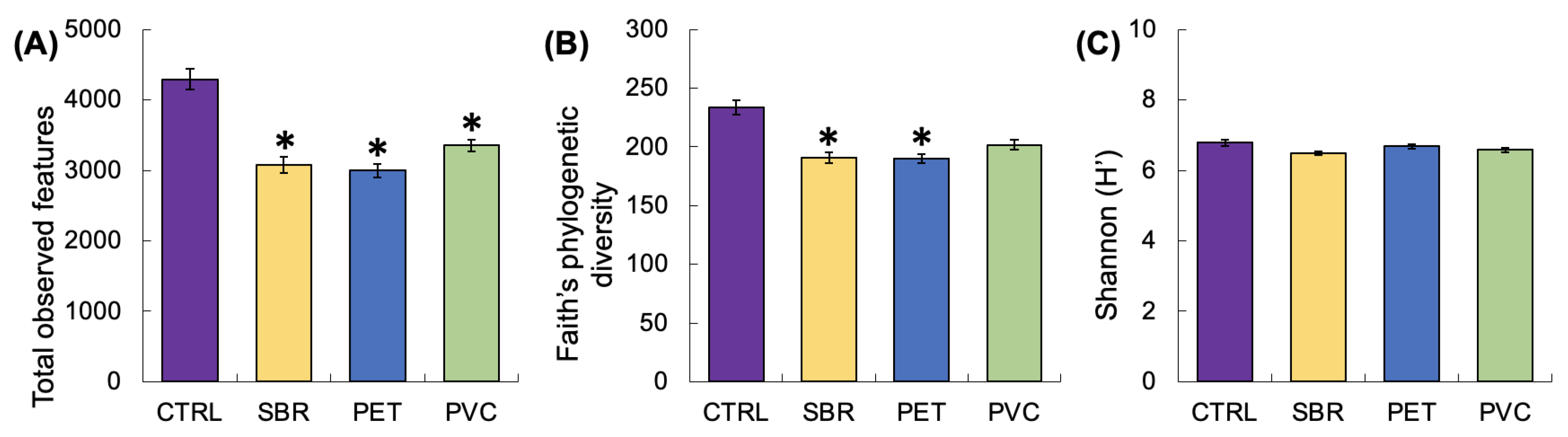
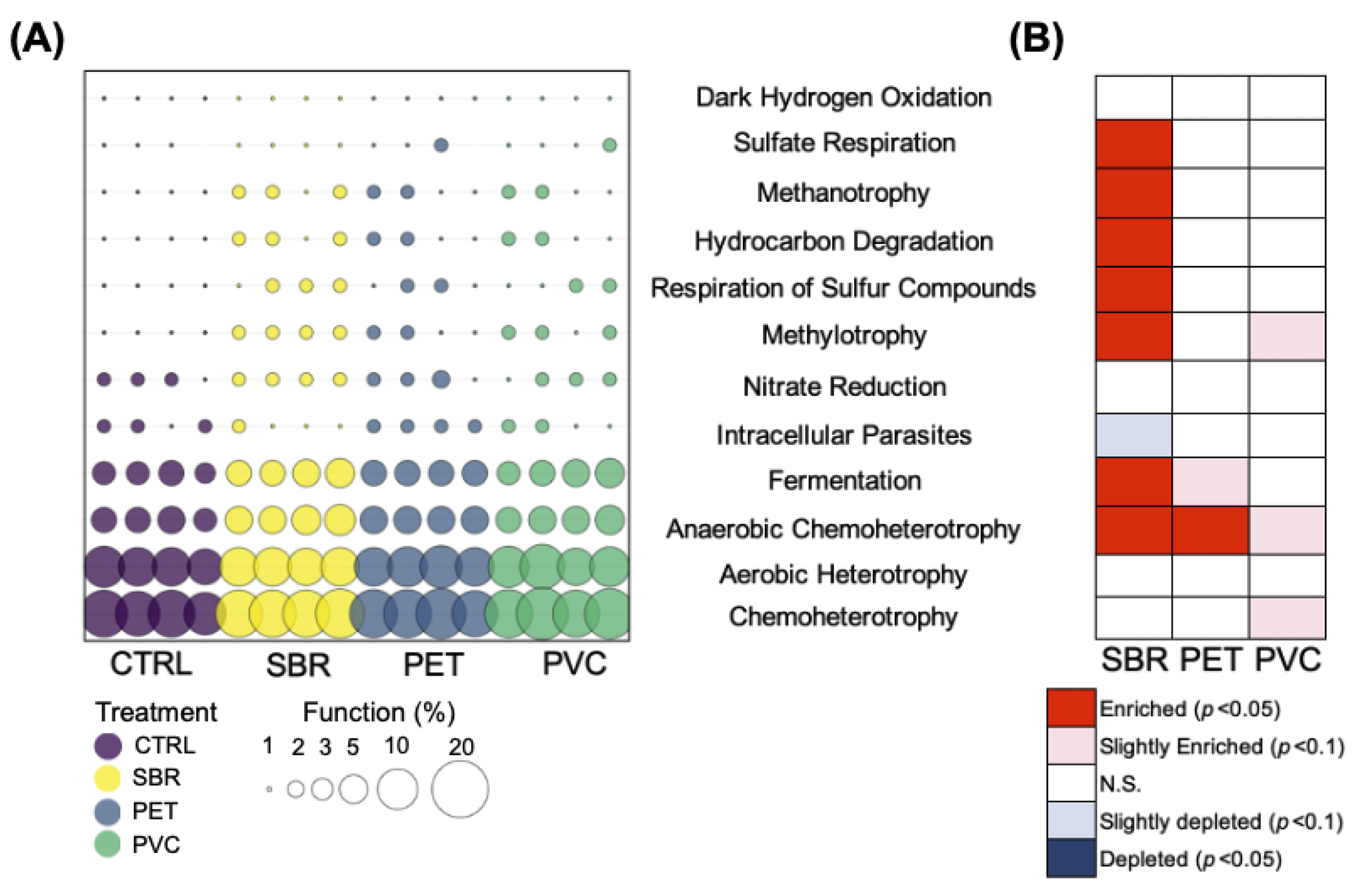
3.2. Microbial Community Structure: Diversity and Function
4. Discussion
4.1. PET Impacts Ecosystem Metabolism
4.2. PVC Impacts Nutrient Cycling
4.3. SBR Promotes Unique Microbial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’ambrières, W. Plastics recycling worldwide: Current overview and desirable changes. J. Field Actions 2019, 19, 12–21. [Google Scholar]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Brandon, J.A.; Jones, W.; Ohman, M.D. Multidecadal increase in plastic particles in coastal ocean sediments. Sci. Adv. 2019, 5, eaax0587. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, J.N.; Mitrovic, S.M. Microplastic pollution in estuaries across a gradient of human impact. Environ. Pollut. 2019, 247, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, M.; Trihadiningrum, Y.; Lestari, P. Microplastic pollution in the sediment of Jagir Estuary, Surabaya City, Indonesia. Mar. Pollut. Bull. 2020, 150, 110790. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Gui, X.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Microplastics in the soil-groundwater environment: Aging, migration, and co-transport of contaminants—A critical review. J. Hazard. Mater. 2021, 419, 126455. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Corsi, S.R.; Mason, S.A. Plastic Debris in 29 Great Lakes Tributaries: Relations to Watershed Attributes and Hydrology. Environ. Sci. Technol. 2016, 50, 10377–10385. [Google Scholar] [CrossRef]
- Great Lakes Commission. About the Lakes. Available online: https://www.glc.org/about/ (accessed on 11 January 2023).
- Hoffman, M.J.; Hittinger, E. Inventory and transport of plastic debris in the Laurentian Great Lakes. Mar. Pollut. Bull. 2017, 115, 273–281. [Google Scholar] [CrossRef]
- Daily, J.; Hoffman, M.J. Modeling the three-dimensional transport and distribution of multiple microplastic polymer types in Lake Erie. Mar. Pollut. Bull. 2020, 154, 111024. [Google Scholar] [CrossRef]
- Daily, J.; Tyler, A.C.; Hoffman, M.J. Modeling three-dimensional transport of microplastics and impacts of biofouling in Lake Erie and Lake Ontario. J. Great Lakes Res. 2022, 48, 1180–1190. [Google Scholar] [CrossRef]
- Driedger, A.G.; Dürr, H.H.; Mitchell, K.; Van Cappellen, P. Plastic debris in the Laurentian Great Lakes: A review. J. Great Lakes Res. 2015, 41, 9–19. [Google Scholar] [CrossRef]
- Cable, R.N.; Beletsky, D.; Beletsky, R.; Wigginton, K.; Locke, B.W.; Duhaime, M.B. Distribution and modeled transport of plastic pollution in the Great Lakes, the world’s largest freshwater resource. Front. Environ. Sci. 2017, 5, 45. [Google Scholar] [CrossRef]
- Zbyszewski, M.; Corcoran, P.L. Distribution and degradation of fresh water plastic particles along the beaches of Lake Huron, Canada. Water Air Soil Pollut. 2011, 220, 365–372. [Google Scholar] [CrossRef]
- Corcoran, P.L.; Norris, T.; Ceccanese, T.; Walzak, M.J.; Helm, P.A.; Marvin, C.H. Hidden plastics of Lake Ontario, Canada and their potential preservation in the sediment record. Environ. Pollut. 2015, 204, 17–25. [Google Scholar] [CrossRef]
- Ballent, A.; Corcoran, P.L.; Madden, O.; Helm, P.A.; Longstaffe, F.J. Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar. Pollut. Bull. 2016, 110, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.Y.; Corcoran, P.L.; Helm, P.A. Factors in fl uencing microplastic abundances in nearshore, tributary and beach sediments along the Ontario shoreline of Lake Erie. J. Great Lakes Res. 2018, 44, 1002–1009. [Google Scholar] [CrossRef]
- Kuntz, K.L.; Tyler, A.C. Bioturbating invertebrates enhance decomposition and nitrogen cycling in urban stormwater ponds. J. Urban Ecol. 2018, 4, juy015. [Google Scholar] [CrossRef]
- Vinebrooke, R.D.; Cottingham, K.L.; Norberg, J.; Scheffer, M.; Dodson, S.I.; Maberly, S.C.; Sommer, U. Impacts of multiple stressors on biodiversity and ecosystem functioning: The role of species co-tolerance. Oikos 2004, 104, 451–457. [Google Scholar] [CrossRef]
- Hadley, K.R.; Paterson, A.M.; Hall, R.I.; Smol, J.P. Effects of multiple stressors on lakes in south-central Ontario: 15 years of change in lakewater chemistry and sedimentary diatom assemblages. Aquat. Sci. 2013, 75, 349–360. [Google Scholar] [CrossRef]
- Jackson, M.C.; Loewen, C.J.; Vinebrooke, R.D.; Chimimba, C.T. Net effects of multiple stressors in freshwater ecosystems: A meta-analysis. Glob. Chang. Biol. 2016, 22, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Ponte, S.; Moore, E.A.; Border, C.T.; Babbitt, C.W.; Christina, A. Science of the Total Environment Fullerene toxicity in the benthos with implications for freshwater ecosystem services. Sci. Total Environ. 2019, 687, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Sun, Y.; Wang, X.; Wang, J. Microplastics reduce soil microbial network complexity and ecological deterministic selection. Environ. Microbiol. 2022, 24, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef] [PubMed]
- Oberbeckmann, S.; Osborn, A.M.; Duhaime, M.B. Microbes on a bottle: Substrate, season and geography influence community composition of microbes colonizing marine plastic debris. PLoS ONE 2016, 11, e0159289. [Google Scholar] [CrossRef]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Kreikemeyer, B.; Labrenz, M. Environmental factors support the formation of specific bacterial assemblages on microplastics. Front. Microbiol. 2018, 8, 2709. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Labrenz, M. Marine Microbial Assemblages on Microplastics: Diversity, Adaptation, and Role in Degradation. Annu. Rev. Mar. Sci. 2020, 12, 209–232. [Google Scholar] [CrossRef]
- Rosato, A.; Barone, M.; Negroni, A.; Brigidi, P.; Fava, F.; Xu, P.; Candela, M.; Zanaroli, G. Microbial colonization of different microplastic types and biotransformation of sorbed PCBs by a marine anaerobic bacterial community. Sci. Total Environ. 2020, 705, 135790. [Google Scholar] [CrossRef] [PubMed]
- Bowley, J.; Baker-Austin, C.; Porter, A.; Hartnell, R.; Lewis, C. Oceanic Hitchhikers – Assessing Pathogen Risks from Marine Microplastic. Trends Microbiol. 2021, 29, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Marsay, K.S.; Ambrosino, A.C.; Koucherov, Y.; Davidov, K.; Figueiredo, N.; Yakovenko, I.; Itzahri, S.; Martins, M.; Sobral, P.; Oren, M. The geographical and seasonal effects on the composition of marine microplastic and its microbial communities: The case study of Israel and Portugal. Front. Microbiol. 2023, 14, 1089926. [Google Scholar] [CrossRef] [PubMed]
- Mußmann, M.; Ribot, M.; von Schiller, D.; Merbt, S.N.; Augspurger, C.; Karwautz, C.; Winkel, M.; Battin, T.J.; Martí, E.; Daims, H. Colonization of freshwater biofilms by nitrifying bacteria from activated sludge. FEMS Microbiol. Ecol. 2013, 85, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.H.; Gao, J.F.; Wang, Z.Q.; Zhao, Y.F.; Zhang, D. Behavior of nitrogen, phosphorus and antibiotic resistance genes under polyvinyl chloride microplastics pressures in an aerobic granular sludge system. J. Clean. Prod. 2020, 256, 120402. [Google Scholar] [CrossRef]
- Kelly, J.J.; London, M.G.; McCormick, A.R.; Rojas, M.; Scott, J.W.; Hoellein, T.J. Wastewater treatment alters microbial colonization of microplastics. PLoS ONE 2021, 16, e0244443. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Gong, Z.; Wang, Z.; Liu, H.; Chen, G.; Guo, G. Elucidating degradation properties, microbial community, and mechanism of microplastics in sewage sludge under different terminal electron acceptors conditions. Bioresour. Technol. 2022, 346, 126624. [Google Scholar] [CrossRef]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef]
- Rochman, C.M.; Browne, M.A.; Underwood, A.J.; Van Franeker, J.A.; Thompson, R.C.; Amaral-Zettler, L.A. The ecological impacts of marine debris: Unraveling the demonstrated evidence from what is perceived. Ecology 2016, 97, 302–312. [Google Scholar] [CrossRef]
- Mendoza, L.M.R.; Jones, P.R. Characterisation of microplastics and toxic chemicals extracted from microplastic samples from the North Pacific Gyre. Environ. Chem. 2015, 12, 611. [Google Scholar] [CrossRef]
- Schönlau, C.; Larsson, M.; Lam, M.M.; Engwall, M.; Giesy, J.P.; Rochman, C.; Kärrman, A. Aryl hydrocarbon receptor-mediated potencies in field-deployed plastics vary by type of polymer. Environ. Sci. Pollut. Res. 2019, 26, 9079–9088. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Tyler, A.C.; Hoffman, M.J.; Savka, M.A.; Hudson, A.O. Is Plastic Pollution in Aquatic and Terrestrial Environments a Driver for the Transmission of Pathogens and the Evolution of Antibiotic Resistance? Environ. Sci. Technol. 2019, 53, 1744–1745. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Aqeel, M.; Noman, A.; Hashem, M.; Mostafa, Y.S.; Abdulaziz, H.; Alhaithloul, S.; Alghanem, S.M. Chemosphere Linking effects of microplastics to ecological impacts in marine environments. Chemosphere 2021, 264, 128541. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Liu, Y.; Li, L.; Li, Y.; Vogts, A.; Luo, Y.; Waniek, J.J. Structural and Functional Characteristics of Microplastic Associated Biofilms in Response to Temporal Dynamics and Polymer Types. Bull. Environ. Contam. Toxicol. 2021, 53, 1744–1745. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, J.; Li, D.; Dai, H.; Zhao, Y. Co-occurrence of microplastics and triclosan inhibited nitrification function and enriched antibiotic resistance genes in nitrifying sludge. J. Hazard. Mater. 2020, 399, 123049. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zhou, S.; Zhang, L.; Ding, S. The effects of three different microplastics on enzyme activities and microbial communities in soil. Water Environ. Res. 2021, 93, 24–32. [Google Scholar] [CrossRef]
- Ya, H.; Xing, Y.; Zhang, T.; Lv, M.; Jiang, B. LDPE microplastics affect soil microbial community and form a unique plastisphere on microplastics. Appl. Soil Ecol. 2022, 180, 104623. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Z.; Peng, Y.; Zhang, Z.; Fan, Z.; Wang, J.; Wang, X. Microbes drive metabolism, community diversity, and interactions in response to microplastic-induced nutrient imbalance. Sci. Total Environ. 2023, 877, 162885. [Google Scholar] [CrossRef]
- Frère, L.; Maignien, L.; Chalopin, M.; Huvet, A.; Rinnert, E.; Morrison, H.; Kerninon, S.; Cassone, A.L.; Lambert, C.; Reveillaud, J.; et al. Microplastic bacterial communities in the Bay of Brest: Influence of polymer type and size. Environ. Pollut. 2018, 242, 614–625. [Google Scholar] [CrossRef]
- Wagner, M.; Lambert, S. Freshwater Microplastics; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; Volume 58. [Google Scholar] [CrossRef]
- Hu, H.; Jin, D.; Yang, Y.; Zhang, J.; Ma, C.; Qiu, Z. Distinct profile of bacterial community and antibiotic resistance genes on microplastics in Ganjiang River at the watershed level. Environ. Res. 2021, 200, 111363. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; van der Plaats, R.Q.; van der Wielen, P.W.; Bauerlein, P.S.; de Roda Husman, A.M. Riverine microplastic and microbial community compositions: A field study in the netherlands. Water Res. 2021, 192, 116852. [Google Scholar] [CrossRef] [PubMed]
- Di Pippo, F.; Crognale, S.; Levantesi, C.; Vitanza, L.; Sighicelli, M.; Pietrelli, L.; Di Vito, S.; Amalfitano, S.; Rossetti, S. Plastisphere in lake waters: Microbial diversity, biofilm structure, and potential implications for freshwater ecosystems. Environ. Pollut. 2022, 310, 119876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yi, M.; Lu, P. Effects of pyrene on the structure and metabolic function of soil microbial communities. Environ. Pollut. 2022, 305, 119301. [Google Scholar] [CrossRef] [PubMed]
- Hoellein, T.; Rojas, M.; Pink, A.; Gasior, J.; Kelly, J. Anthropogenic litter in urban freshwater ecosystems: Distribution and microbial interactions. PLoS ONE 2014, 9, e98485. [Google Scholar] [CrossRef] [PubMed]
- Seeley, M.E.; Song, B.; Passie, R.; Hale, R.C. Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat. Commun. 2020, 11, 2372. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wei, R.; Luo, W.; Hu, L.; Li, B.; Di, Y.; Shi, H. Microplastic pollution in water and sediment in a textile industrial area. Environ. Pollut. 2019, 258, 113658. [Google Scholar] [CrossRef]
- Lenaker, P.L.; Baldwin, A.K.; Corsi, S.R.; Mason, S.A.; Reneau, P.C.; Scott, J.W. Vertical Distribution of Microplastics in the Water Column and Surficial Sediment from the Milwaukee River Basin to Lake Michigan. Environ. Sci. Technol. 2019, 53, 12227–12237. [Google Scholar] [CrossRef]
- Peller, J.R.; Eberhardt, L.; Clark, R.; Nelson, C.; Kostelnik, E.; Iceman, C. Tracking the distribution of microfiber pollution in a southern Lake Michigan watershed through the analysis of water, sediment and air. Environ. Sci. Process. Impacts 2019, 21, 1549–1559. [Google Scholar] [CrossRef]
- Peller, J.; Nevers, M.B.; Byappanahalli, M.; Nelson, C.; Ganesh, B.; Anne, M.; Kostelnik, E.; Keller, M.; Johnston, J.; Shidler, S. Sequestration of micro fi bers and other microplastics by green algae, Cladophora, in the US Great Lakes. Environ. Pollut. 2021, 276, 116695. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, K.; Yeerken, S.; Geng, S.; Liu, D.; Dai, Z.; Xie, F.; Zhou, X.; Wang, Q. Effect evaluation of microplastics on activated sludge nitrification and denitrification. Sci. Total Environ. 2020, 707, 135953. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Green, D.S.; Boots, B.; Sigwart, J.; Jiang, S.; Rocha, C. Effects of conventional and biodegradable microplastics on a marine ecosystem engineer (Arenicola marina) and sediment nutrient cycling. Environ. Pollut. 2016, 208, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, X.; Xia, Y.; Tao, R.; Zhang, M.; Mei, Y.; Qu, M. Simulation of the effects of microplastics on the microbial community structure and nitrogen cycle of paddy soil. Sci. Total Environ. 2022, 818, 151768. [Google Scholar] [CrossRef] [PubMed]
- Panko, J.M.; Kreider, M.L.; McAtee, B.L.; Marwood, C. Chronic toxicity of tire and road wear particles to water- and sediment-dwelling organisms. Ecotoxicology 2013, 22, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Hüffer, T.; Klöckner, P.; Wehrhahn, M.; Hofmann, T.; Reemtsma, T. Tire wear particles in the aquatic environment—A review on generation, analysis, occurrence, fate and effects. Water Res. 2018, 139, 83–100. [Google Scholar] [CrossRef]
- Tamis, J.E.; Koelmans, A.A.; Dröge, R.; Kaag, N.H.B.M.; Keur, M.C.; Tromp, P.C.; Jongbloed, R.H. Environmental risks of car tire microplastic particles and other road runoff pollutants. Microplast. Nanoplast. 2021, 1, 10. [Google Scholar] [CrossRef]
- Chibwe, L.; Parrott, J.L.; Shires, K.; Khan, H.; Clarence, S.; Lavalle, C.; Sullivan, C.; O’Brien, A.M.; Silva, A.O.D.; Muir, D.C.; et al. A Deep Dive into the Complex Chemical Mixture and Toxicity of Tire Wear Particle Leachate in Fathead Minnow. Environ. Toxicol. Chem. 2022, 41, 1144–1153. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms, 4th ed.; Technical Report EPA-821-R-02-013; EPA: Washington, DC, USA, 2002; 335p. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/short-term-chronic-freshwater-wet-manual_2002.pdf (accessed on 11 January 2024).
- National Centers for Environmental Information (NCEI). Climate Data Online. Available online: https://www.ncei.noaa.gov/cdo-web/ (accessed on 11 January 2024).
- Rani, M.; Shim, W.J.; Han, G.M.; Jang, M.; Al-Odaini, N.A.; Song, Y.K.; Hong, S.H. Qualitative analysis of additives in plastic marine debris and its new products. Arch. Environ. Contam. Toxicol. 2015, 69, 352–366. [Google Scholar] [CrossRef]
- Tyler, A.; McGlathery, K.; Anderson, I. Macroalgae Mediation of Dissolved Organic Nitrogen Fluxes in a Temperate Coastal Lagoon. Estuar. Coast. Shelf Sci. 2001, 53, 155–168. [Google Scholar] [CrossRef]
- McGlathery, K.J.; Anderson, I.C.; Tyler, A.C. Magnitude and variability of benthic and pelagic metabolism in a temperate coastal lagoon. Mar. Ecol. Prog. Ser. 2001, 216, 1–15. [Google Scholar] [CrossRef]
- Doane, T.A.; Horwáth, W.R. Spectrophotometric determination of nitrate with a single reagent. Anal. Lett. 2003, 36, 2713–2722. [Google Scholar] [CrossRef]
- Solorzano, L. Determination of Ammonia in Natural Waters by the Phenolhypochlorite Method 1 1 This research was fully supported by U.S. Atomic Energy Commission Contract No. ATS (11-1) GEN 10, P.A. 20. Limnol. Oceanogr. 1969, 14, 799–801. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Bogue, J.P.; Smith, L.F.; Lipsett, L. A Practical Handbook. J. High. Educ. 1957, 28, 405. [Google Scholar] [CrossRef]
- Lorenzen, C.J. Determination of Chlorophyll and Pheo-pigments: Spectrophotometric Equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Jarvis, S.C.; Hatch, D.J.; Lovell, R.D. An improved soil core incubation method for the field measurement of denitrification and net mineralization using acetylene inhibition. Nutr. Cycl. Agroecosyst. 2001, 59, 219–225. [Google Scholar] [CrossRef]
- Groffman, P.; Holland, E.; Myrold, D.D.; Robertson, G.P.; Zou, X. Denitrification. In Standard Soil Methods for Long-Term Ecological Research; Robertson, G.P., Bledsoe, C.S., Coleman, D.C., Sollins, P., Eds.; Oxford Press: New York, NY, USA, 1999; pp. 272–288. ISBN 9780195120837. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Bolyen, E.; Ram Rideout, J.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Sankaran, K.; Fukuyama, J.A.; McMurdie, P.J.; Holmes, S.P. Bioconductor Workflow for Microbiome Data Analysis: From raw reads to community analyses. F1000Research 2016, 5, 1492. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Robeson, M.S.; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible sequence taxonomy reference database management. PLoS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio Team: Boston, MA, USA, 2022. [Google Scholar]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.S.; Schmidt, P.J.; Tremblay, B.J.; Emelko, M.B.; Müller, K.M. Enhancing diversity analysis by repeatedly rarefying next generation sequencing data describing microbial communities. Sci. Rep. 2021, 11, 22302. [Google Scholar] [CrossRef]
- Wickham, H. Data analysis. In ggplot2; Springer: Berlin/Heidelberg, Germany, 2016; pp. 189–201. [Google Scholar]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Spanjer, A.R.; Rosen, M.R.; Thom, T. Microplastics in Lake Mead National Recreation Area, USA: Occurrence and biological uptake. PLoS ONE 2020, 15, e0228896. [Google Scholar] [CrossRef]
- Silva, C.J.; Machado, A.L.; Campos, D.; Rodrigues, A.C.; Patrício Silva, A.L.; Soares, A.M.; Pestana, J.L. Microplastics in freshwater sediments: Effects on benthic invertebrate communities and ecosystem functioning assessed in artificial streams. Sci. Total Environ. 2022, 804, 150118. [Google Scholar] [CrossRef]
- Nava, V.; Leoni, B. A critical review of interactions between microplastics, microalgae and aquatic ecosystem function. Water Res. 2021, 188, 116476. [Google Scholar] [CrossRef] [PubMed]
- López-Rojo, N.; Pérez, J.; Alonso, A.; Correa-Araneda, F.; Boyero, L. Microplastics have lethal and sublethal effects on stream invertebrates and affect stream ecosystem functioning. Environ. Pollut. 2020, 259, 113898. [Google Scholar] [CrossRef] [PubMed]
- Marchant, D.J.; Martínez Rodríguez, A.; Francelle, P.; Jones, J.I.; Kratina, P. Contrasting the effects of microplastic types, concentrations and nutrient enrichment on freshwater communities and ecosystem functioning. Ecotoxicol. Environ. Saf. 2023, 255, 114834. [Google Scholar] [CrossRef] [PubMed]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Faith, D.P.; Lozupone, C.A.; Nipperess, D.; Knight, R. The cladistic basis for the phylogenetic diversity (PD) measure links evolutionary features to environmental gradients and supports broad applications of microbial ecology’s “phylogenetic beta diversity” framework. Int. J. Mol. Sci. 2009, 10, 4723–4741. [Google Scholar] [CrossRef] [PubMed]
- Demars, B.O.L.; Kemp, J.L.; Marteau, B.; Friberg, N.; Thornton, B. Stream Macroinvertebrates and Carbon Cycling in Tangled Food Webs. Ecosystems 2021, 24, 1944–1961. [Google Scholar] [CrossRef]
- Frost, P.C.; Cross, W.F.; Benstead, J.P. Ecological stoichiometry in freshwater benthic ecosystems: An introduction. Freshw. Biol. 2005, 50, 1781–1785. [Google Scholar] [CrossRef]
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The Role of Benthic Invertebrate Species in Freshwater Ecosystems. BioScience 1999, 49, 119. [Google Scholar] [CrossRef]
- Covich, A.P.; Austen, M.C.; Bärlocher, F.; Chauvet, E.; Cardinale, B.J.; Biles, C.L.; Inchausti, P.; Dangles, O.; Solan, M.; Gessner, M.O.; et al. The Role of Biodiversity in the Functioning of Freshwater and Marine Benthic Ecosystems. BioScience 2004, 54, 767. [Google Scholar] [CrossRef]
- Prather, C.M.; Pelini, S.L.; Laws, A.; Rivest, E.; Woltz, M.; Bloch, C.P.; Del Toro, I.; Ho, C.; Kominoski, J.; Newbold, T.A.S.; et al. Invertebrates, ecosystem services and climate change. Biol. Rev. 2013, 88, 327–348. [Google Scholar] [CrossRef]
- Benke, A.C.; Huryn, A.D. Benthic invertebrate production—Facilitating answers to ecological riddles in freshwater ecosystems. J. N. Am. Benthol. Soc. 2010, 29, 264–285. [Google Scholar] [CrossRef]
- Wall Freckman, D.; Blackburn, T.H.; Brussaard, L.; Hutching, P.; Palmer, M.A.; Snelgrove, P. Linking Biodiversity and Ecosystem functioning of soils and sediments. Ambio 1997, 26, 556–562. [Google Scholar]
- Chomiak, K.M.; Eddingsaas, N.C.; Tyler, A.C. Direct and Indirect Impacts of Disposable Face Masks and Gloves on Freshwater Benthic Fauna and Sediment Biogeochemistry. Environ. Sci. Technol. Water 2023, 3, 51–59. [Google Scholar] [CrossRef]
- Kim, D.; Cui, R.; Moon, J.; Kwak, J.I.; An, Y.J. Soil ecotoxicity study of DEHP with respect to multiple soil species. Chemosphere 2019, 216, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Weir, S.M.; Wooten, K.J.; Smith, P.N.; Salice, C.J. Phthalate ester leachates in aquatic mesocosms: Implications for ecotoxicity studies of endocrine disrupting compounds. Chemosphere 2014, 103, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Q.; Wang, C.; Yang, Z.; Wang, J.; Zhu, L.; Zhang, D.; Wang, J. Ecotoxicological response of zebrafish liver (Danio rerio) induced by di-(2-ethylhexyl) phthalate. Ecol. Indic. 2022, 143, 109388. [Google Scholar] [CrossRef]
- Liang, J.; Ning, X.; Kong, M.; Liu, D.; Wang, G.; Cai, H.; Sun, J.; Zhang, Y.; Lu, X.; Yuan, Y. Elimination and ecotoxicity evaluation of phthalic acid esters from textile-dyeing wastewater. Environ. Pollut. 2017, 231, 115–122. [Google Scholar] [CrossRef]
- Cluzard, M.; Kazmiruk, T.N.; Kazmiruk, V.D.; Bendell, L.I. Intertidal Concentrations of Microplastics and Their Influence on Ammonium Cycling as Related to the Shellfish Industry. Arch. Environ. Contam. Toxicol. 2015, 69, 310–319. [Google Scholar] [CrossRef]
- Huang, S.; Guo, T.; Feng, Z.; Li, B.; Cai, Y.; Ouyang, D.; Gustave, W.; Ying, C.; Zhang, H. Polyethylene and polyvinyl chloride microplastics promote soil nitrification and alter the composition of key nitrogen functional bacterial groups. J. Hazard. Mater. 2023, 453, 131391. [Google Scholar] [CrossRef]
- Shen, H.; Sun, Y.; Duan, H.; Ye, J.; Zhou, A.; Meng, H.; Zhu, F.; He, H.; Gu, C. Effect of PVC microplastics on soil microbial community and nitrogen availability under laboratory-controlled and field-relevant temperatures. Appl. Soil Ecol. 2023, 184, 104794. [Google Scholar] [CrossRef]
- Lin, S.; Boegman, L.; Rao, Y.R.; Weis, J.S.; Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M.; Imhof, H.K.; Laforsch, C.; et al. PVC additives: A global review PVC compounds account for the greatest volume of plastics additives. Ron Babinsky, director Additives & Specialties with Townsend Polymers & Information, reviews the global market for PVC additives. Environ. Pollut. 2021, 6, 778–788. [Google Scholar] [CrossRef]
- Walter, R.K.; Lin, P.H.; Edwards, M.; Richardson, R.E. Investigation of factors affecting the accumulation of vinyl chloride in polyvinyl chloride piping used in drinking water distribution systems. Water Res. 2011, 45, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson, Å.; Dave, G. Science of the Total Environment Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Filella, M. The role of titanium dioxide on the behaviour and fate of plastics in the aquatic environment. Sci. Total. Environ. 2023, 869, 161727. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.N.A.; Shah, Z.; Hussain, M.; Khan, M. Hazardous Effects of Titanium Dioxide Nanoparticles in Ecosystem. Bioinorg. Chem. Appl. 2017, 2017, 4101735. [Google Scholar] [CrossRef] [PubMed]
- Simonin, M.; Richaume, A.; Guyonnet, J.P.; Dubost, A.; Martins, J.M.; Pommier, T. Titanium dioxide nanoparticles strongly impact soil microbial function by affecting archaeal nitrifiers. Sci. Rep. 2016, 6, 33643. [Google Scholar] [CrossRef] [PubMed]
- Kostka, J.E.; Prakash, O.; Overholt, W.A.; Green, S.J.; Freyer, G.; Canion, A.; Delgardio, J.; Norton, N.; Hazen, T.C.; Huettel, M. Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the deepwater horizon oil spill. Appl. Environ. Microbiol. 2011, 77, 7962–7974. [Google Scholar] [CrossRef]
- Jurelevicius, D.; Alvarez, V.M.; Marques, J.M.; Lima, L.R.F.d.S.; Dias, F.d.A.; Seldin, L. Bacterial community response to petroleum hydrocarbon amendments in freshwater, marine, and hypersaline water-containing microcosms. Appl. Environ. Microbiol. 2013, 79, 5927–5935. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum Hydrocarbon-Degrading Bacteria for the Remediation of Oil Pollution Under Aerobic Conditions: A Perspective Analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef]
- Armada, D.; Llompart, M.; Celeiro, M.; Garcia-Castro, P.; Ratola, N.; Dagnac, T.; de Boer, J. Global evaluation of the chemical hazard of recycled tire crumb rubber employed on worldwide synthetic turf football pitches. Sci. Total Environ. 2022, 812, 152542. [Google Scholar] [CrossRef]
- Cao, G.; Wang, W.; Zhang, J.; Wu, P.; Zhao, X.; Yang, Z.; Hu, D.; Cai, Z. New Evidence of Rubber-Derived Quinones in Water, Air, and Soil. Environ. Sci. Technol. 2022, 56, 4142–4150. [Google Scholar] [CrossRef] [PubMed]
- Armada, D.; Martinez-Fernandez, A.; Celeiro, M.; Dagnac, T.; Llompart, M. Assessment of the bioaccessibility of PAHs and other hazardous compounds present in recycled tire rubber employed in synthetic football fields. Sci. Total Environ. 2023, 857, 159485. [Google Scholar] [CrossRef] [PubMed]
- Fohet, L.; Andanson, J.M.; Charbouillot, T.; Malosse, L.; Leremboure, M.; Delor-Jestin, F.; Verney, V. Time-concentration profiles of tire particle additives and transformation products under natural and artificial aging. Sci. Total Environ. 2023, 859, 160150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.N.; Hu, X.; Gonzalez, M.; Rideout, C.A.; Hobby, G.C.; Fisher, M.F.; McCormick, C.J.; Dodd, M.C.; Kim, K.E.; Tian, Z.; et al. Screening p-Phenylenediamine Antioxidants, Their Transformation Products, and Industrial Chemical Additives in Crumb Rubber and Elastomeric Consumer Products. Environ. Sci. Technol. 2023, 57, 2779–2791. [Google Scholar] [CrossRef] [PubMed]
- Hiki, K.; Asahina, K.; Kato, K.; Yamagishi, T.; Omagari, R.; Iwasaki, Y.; Watanabe, H.; Yamamoto, H. Acute Toxicity of a Tire Rubber-Derived Chemical, 6PPD Quinone, to Freshwater Fish and Crustacean Species. Environ. Sci. Technol. Lett. 2021, 8, 779–784. [Google Scholar] [CrossRef]
- Tian, Z.; Zhao, H.; Peter, K.T.; Gonzalez, M.; Wetzel, J.; Wu, C.; Hu, X.; Prat, J.; Mudrock, E.; Hettinger, R.; et al. A ubiquitous tire rubber-derived chemical induces acute mortality in coho salmon. Science 2022, 371, 185–189, Erratum in Science 2022, 375, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Varshney, S.; Gora, A.H.; Siriyappagouder, P.; Kiron, V.; Olsvik, P.A. Toxicological effects of 6PPD and 6PPD quinone in zebrafish larvae. J. Hazard. Mater. 2022, 424, 127623. [Google Scholar] [CrossRef]
- Brinkmann, M.; Montgomery, D.; Selinger, S.; Miller, J.G.; Stock, E.; Alcaraz, A.J.; Challis, J.K.; Weber, L.; Janz, D.; Hecker, M.; et al. Acute Toxicity of the Tire Rubber-Derived Chemical 6PPD-quinone to Four Fishes of Commercial, Cultural, and Ecological Importance. Environ. Sci. Technol. Lett. 2022, 9, 333–338. [Google Scholar] [CrossRef]
- Sadiktsis, I.; Bergvall, C.; Johansson, C.; Westerholm, R. Automobile Tires—A Potential Source of Highly Carcinogenic Dibenzopyrenes to the Environment. Environ. Sci. Technol. 2012, 46, 3326–3334. [Google Scholar] [CrossRef]
- Torma, A.; Raghavan, D. Biodesulfurization of Rubber Materials; Technical Report; EG and G Idaho, Inc.: Idaho Falls, ID, USA, 1990. [Google Scholar]

| Variable | Plastic Type | |
|---|---|---|
| F/X | p | |
| GPP | 1.32 | 0.31 |
| NEM | 4.97 | 0.02 |
| Oxic Depth | 3.38 | 0.05 |
| Anoxic Depth | 2.82 | 0.08 |
| Daily NO | 7.91 * | 0.04 |
| Daily NH | 8.93 | 0.03 |
| Daily PO | 3.93 * | 0.03 |
| PDNF | 2.84 | 0.58 |
| Chl a | 2.84 | 0.08 |
| Worm Count | 5.59 | 0.01 |
| Variable | Light | CTRL | SBR | PET | PVC |
|---|---|---|---|---|---|
| NO | Light | −43.8 ± 18.3 | −24.6 ± 14.0 | 26.4 ± 9.7 | −23.6 ± 12.5 |
| Dark | 52.1 ± 17.5 | −20.2 ± 27.7 | −19.9 ± 23.1 | 36.8 ± 24.4 | |
| NH | Light | −7.1 ± 12.6 | 21.3 ± 11.6 | 9.1 ± 20.0 | −68.0 ± 31.7 |
| Dark | 29.9 ± 13.7 | −16.5 ± 8.7 | 30.7 ± 10.2 | 33.2 ± 24.3 | |
| PO | Light | 11.6 ± 9.1 | 6.1 ± 5.4 | 9.7 ± 4.6 | −2.9 ± 1.3 |
| Dark | 3.9 ± 3.3 | 1.7 ± 4.5 | 0.5 ± 4.6 | −7.0 ± 1.3 |
| Variable | Component 1 | Component 2 |
|---|---|---|
| NEM | 0.42 | 0.28 |
| GPP | −0.07 | 0.44 |
| Oxic Depth | 0.45 | 0.33 |
| Daily NO | 0.23 | 0.30 |
| Daily NH | 0.38 | −0.37 |
| Daily PO | 0.23 | −0.38 |
| PDNF | 0.18 | −0.20 |
| Chl a | −0.18 | 0.45 |
| Worm Count | 0.54 | 0.07 |
| Diversity Index | F | p |
|---|---|---|
| Total observed features | 7.06 | 0.005 |
| Faith’s phylogenetic diversity | 4.76 | 0.02 |
| Shannon index (H’) | 0.83 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chomiak, K.M.; Owens-Rios, W.A.; Bangkong, C.M.; Day, S.W.; Eddingsaas, N.C.; Hoffman, M.J.; Hudson, A.O.; Tyler, A.C. Impact of Microplastic on Freshwater Sediment Biogeochemistry and Microbial Communities Is Polymer Specific. Water 2024, 16, 348. https://doi.org/10.3390/w16020348
Chomiak KM, Owens-Rios WA, Bangkong CM, Day SW, Eddingsaas NC, Hoffman MJ, Hudson AO, Tyler AC. Impact of Microplastic on Freshwater Sediment Biogeochemistry and Microbial Communities Is Polymer Specific. Water. 2024; 16(2):348. https://doi.org/10.3390/w16020348
Chicago/Turabian StyleChomiak, Kristina M., Wendy A. Owens-Rios, Carmella M. Bangkong, Steven W. Day, Nathan C. Eddingsaas, Matthew J. Hoffman, André O. Hudson, and Anna Christina Tyler. 2024. "Impact of Microplastic on Freshwater Sediment Biogeochemistry and Microbial Communities Is Polymer Specific" Water 16, no. 2: 348. https://doi.org/10.3390/w16020348
APA StyleChomiak, K. M., Owens-Rios, W. A., Bangkong, C. M., Day, S. W., Eddingsaas, N. C., Hoffman, M. J., Hudson, A. O., & Tyler, A. C. (2024). Impact of Microplastic on Freshwater Sediment Biogeochemistry and Microbial Communities Is Polymer Specific. Water, 16(2), 348. https://doi.org/10.3390/w16020348







