Abstract
The olive mill wastewater (OMW) treatment process is modeled and optimized through new design of experiments (DOE). The first step of the process is coagulation–flocculation using three coagulants (modeled with the mixture design) followed by photo-degradation (modelled with the full factorial design). Based on this methodology, we successfully established a direct correlation between the system’s composition during the coagulation–flocculation step and the conditions of the photo-catalytic degradation step. Three coagulants are used in this study, Fe3+ solution, lime, and cactus juice, and two parameters are considered for the photo-degradation conditions: dilution and catalyst mass. Utilizing a sophisticated quadratic model, the analysis of the two observed responses reveals the ideal parameters for achieving maximum efficiency in coagulation–flocculation and photo-degradation processes. This is attained using a quasi-equal mixture of limewater and cactus juice, exclusively. To achieve an optimal photo-catalytic degradation, it is essential to maintain a minimal dilution rate while employing an elevated concentration of TiO2. It was found that the experimental tests validations were in good concordance with the mathematical predictions (a decolorization of 92.57 ± 0.90% and an organic degradation of 96.19 ± 0.97%).
1. Introduction
The implementation of any industrial process requires optimization. In this context, the application of the design of experiments (DOE) methodology emerges as an exceptionally robust statistical and mathematical approach, consisting of four main steps: design of the experiments, fitting the model, verifying the model fit quality, and optimization of the studied response. DOE aims to simultaneously study the responses to experimental parameters, overcoming the limitations of traditional experimental approaches that involve parameter exchanges one-by-one, which are often time-consuming and fail to reveal complex variable–response interactions. But even this, DOE, in a simplistic approach, is mostly carried out individually for multi-stage processes. The mixture design, for example, is regularly used for the conception of many real mixtures composed of at least two components to optimize the studied response versus the composition adjustments. Meanwhile, full factorial design creates experimental points using all possible combinations of the levels of the factors in each complete trial or replication of the experiments. Consequently, the synergy between compositional variables and process factors cannot be exhibited. In fact, DOE can be a very useful tool in industrial applications such in the olive oil industry.
The global production of olive oil is experiencing a significant upsurge because of its numerous virtues, as well as its significance as a foodstuff with associated health benefits. According to the international olive oil council, the international production of this product reached 3,398,000 tons in 2022. In the season of 2022/2023, the prices reached a maximum level, with an increase of 40% in the fourth week of March compared with the same period of the previous year. This production is no longer limited to the Mediterranean Basin. Many emergent countries are marking their presence in this field, like Chile (21,000 tons), Australia (19,500 tons), the USA (15,500 tons), and Saudi Arabia (5000 tons). The classical producers like Tunisia (240,000 tons), Turkey (235,000 tons), and Morocco (200,000 tons), classed after the European union, are working on increasing their production. Despite these important contributions to the national economy, it is important to highlight the environmental impact of this industry. It generates approximately 5.4 × 106 m3 of olive mill wastewater (OMW) per year [1]. This quantity can vary depending on different characteristics, such the variety of olive and maturity, climate and soil conditions, and the oil extraction method [2]. For the traditional pressing system involving three-phase centrifugation and continuous de-pitted extraction, the amount of wastewater produced surpasses 47 kg for every 100 kg of olives [3,4]. This agro-industrial effluent presents significant challenges in terms of weight and management complexity [5,6,7]. It comprises a dark, malodorous, and turbid aqueous liquid containing emulsified grease and a high organic content [8]. More than 80% of its composition includes dissolved sugar, phenols, nitrogen, alcohols, organic acids, pectins, and tannins. Other inorganic materials are also found [9]. The most phytotoxic effect of OMW is related to its high concentration of total phenolic compounds and residual fats responsible for the inhibition of microbial activity [10,11,12,13]. Regarding the large quantities produced in short periods of time, the waste generated by this sector significantly contributes to greenhouse gas emissions [14,15]. Particular attention should be considered for its disposal [16], which is regulated by Norm NT106-02.
Certainly, the recovery of valuable compounds such as specific polyphenols, phenols, proteins, fats, cellulose, and lignin from these by-products and their conversion into higher-value products is one of the most efficient alternatives to manage this source of pollution [14,17,18,19]. But according to Donner et al. [20], the approach of managing this problem via a circular economy is still complex to apply to certain Mediterranean countries like Tunisia and Morocco, as well as France. On the other hand, it is important to relate this question to the context of regions where a scarcity of water constitutes the greatest challenge. In the current Mediterranean context, water recovery constitutes the first priority [21]. Working on saving a greater quantity of water from the oil production process would be the best response to the needs of these highly productive regions. Promising outcomes are being obtained for recovering the best quality and quantity of water through the use of advanced oxidative processes and biological processes [10,11,13,18,22,23]. However, high prices and imposing installation of these processes can sometimes be limiting to their widespread use. Direct evaporation is still the most widely used technique, even though its ecological drawbacks cause the diffusion of bad odors [24,25,26]. Coagulation-flocculation can be a more effective technology regarding its ease of application and cost-effectiveness. It is frequently used, and it remains a subject for further improvement [27,28,29,30]. This technology allows for good removal of suspended solids. The addition of coagulants serves to neutralize the negative charges of oil particles and to decrease the electrostatic repulsion of the electric double layer. Thus, the agglomeration of particles is favorable, and they glide at the interface [31,32]. Commonly, the most widely used coagulants can be metallic, such as aluminum and iron; inorganic, such as lime; or organic, such as polyacrylamidenatural compounds including Moringa oleifera seeds and cactus [24]. Recently, studies have been more oriented towards the use of a mixture of coagulants to improve the performance of this process [33]. Nevertheless, this technology is still unsatisfactory for obtaining water of acceptable quality. Coagulation–flocculation requires complementary treatments. It generally constitutes the first step of a two- or three-stage treatment process. It can be followed by biological or oxidative treatments, or even a combination of these two processes [33,34,35]. Among the advanced oxidative processes, sunlight photo-catalysis shows strong potential for the degradation of organic pollutants due to its low cost and low energy consumption [36].
In this study, a new, green, and low-cost OMW treatment process was designed consisting of two technologies: coagulation–flocculation and sunlight photo-catalysis. In our approach, a combination of coagulant mixture design and a full factorial design was chosen to optimize OMW treatments.
2. Materials and Methods
2.1. Reagents and Materials
Olive mill effluent was received (twenty-four hours after the olive trituration process) from an oil mill.
Cactus rackets were sourced from a local farm at El Manar region (Tunisia). After being rinsed with water and peeled, the pulp was ground into a greenish-yellow liquid and then used without further dilution.
Ferric chloride (99%, Merck) and lime solutions were prepared at a concentration of 1.5 g · L−1 and 30 g · L−1, respectively.
The TiO2 catalyst was synthesized using the sol-gel method, involving the mixing of titanium isopropoxide with isopropanol and acetyl acetate to form the sol. A solution of 0.1 mol · L−1 nitric acid was used to induce gelation. The resultant gel was calcinated for three hours at 673 K.
2.2. Solid Characterization
The morphology of the solid was investigated using a high-resolution scanning electron microscope from Thermo Fisher Scientific (SEM, 5 kV; 30 kV). The elemental composition was determined using a coupled Energy-Dispersion X-ray (EDX), extending the capabilities of SEM in semi-quantitative detection mode, with a 30 kV acceleration voltage. To conduct X-ray diffraction (XRD), a nickel monochromatic and CuK radiation-based automatic Philips Panalytical diffractometer were used. Comparisons were made between the calculated reticular distances and the Joint Committee on Powder Diffraction Standards (JCPDS) provided with the software used in the Philips Panalytical X-PERT PRO, Dy993 XRPD.
IR spectra were collected using a Perkin Elmer PRAGON 1000 PC FTIR spectrophotometer across a range from 4000 to 400 cm−1. Samples pellets for the FTIR analysis were made by diluting the materials with KBr.
UV-visible spectra in the range of 200–900 nm were recorded using a Perkin Elmer spectrophotometer type Instrument lambda 45 linked to an integration sphere type RSA-PE-20 with a speed of 960 nm · min−1 and an aperture of 4 nm.
2.3. Physico-Chemical Analysis
Prior to use, the OMW was characterized by the following measurements after homogenization. Each measurement was conducted thrice.
The pH was measured according to the description of Rondinini [37]. The dry matter (DM) and ash content (AC), both expressed in g · L−1, were determined as described by Paredes et al. [38]. The chemical oxygen demand (COD, expressed in gO2 · L−1) and the spectrophotometer absorbance (288 nm) were determined following the method adopted by ref. [39].
As described in the previous works of Raissi et al. [40], measuring the decolorization of the solution involves applying an RBG color evaluation. Subsequently, the absorbance is determined using Equation (1):
where the color intensity of the solution is denoted as “I”, while the color intensity of water, used as a reference, is represented by “I0”. This method is also employed for analyzing treated samples and the OMW.
The decolorization (%D) and organic degradation (%OD), representing distinct responses in this study, were accomplished using the following formulas (Equations (2) and (3), respectively):
2.4. Design of Experiments
The primary objective of the design of experiments (DOE) is to accurately identify the optimal response(s) (dependent variable(s)) depending on multiple factors (as independent variables) simultaneously. This goal could be achieved in a minimum number of experimentally designed runs by using predefined statistical and mathematical design(s) involving model(s) that could predict the experimental response(s). Additionally, we could statistically analyze the quality of the model fit and assess the significance of the influence of each factor and each interaction between them. Mixture design is generally used to identify the optimal response(s) which depend(s) on the composition of the tested mixture, typically consisting of at least two compounds. Nevertheless, the 22 full factorial design is commonly employed for analyzing response(s) that could be affected by two factors, each having two levels.
Given our objective to maximize the decolorization (%) and organic degradation (%) of the OMW through a two-step process involving coagulation–flocculation and photo-catalysis, we opted to examine all the independent variables that could influence the responses. To achieve this, we employed two specific designs: (1) a simplex-lattice mixture design to ascertain the optimal composition of the flocculant–coagulant used in treating the studied OMW, consisting of lime water (A), Fe3+ solution (B), and cactus juice (C)—the materials chosen for this study; and (2) a 22 full factorial design to govern the photo-catalysis process, influenced by two factors, each with two levels: the mass of TiO2 (X1) as a catalyst added to the OMW (with masses of 20 or 40 mg) and the dilution factor (X2) (at ratios of 1/10 or 2/10). Indeed, using these parameters, the studied process could be classified as green and low-cost.
As shown in Figure 1, the coagulation was carried out by mixing 50 mL of OMW—for which we measured the initial hue and phenol concentration—vigorously with the same volume (50 mL) of a mixture composed of lime water (A), Fe3+ solution (B), and cactus juice (C). The mixtures were prepared following the volume ratios specified in columns 2, 3, and 4 of Table 1 using solutions A, B, and C, respectively. The corresponding amount/percentage (and volume) of each mixture composed of those three solutions are presented in Table 1. The photo-catalytic test was carried out on the liquid phase after decantation followed by 1/10 or 2/10 dilution (presenting two levels of factor X1) and using 20 or 40 mg of TiO2 (presenting two levels of factor X2).
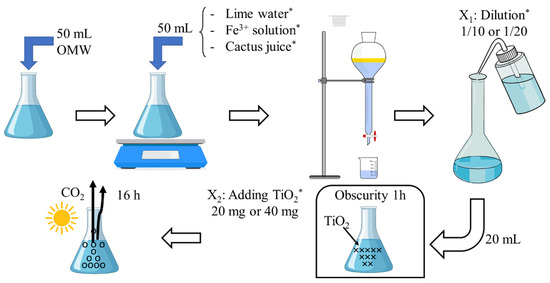
Figure 1.
Diagram of the two-step process treatment of OMW. Notes: * Conditions were defined by means of design of experiments (shown in Table 1).

Table 1.
Combined design of experiments: mixture design (simplex-lattice; A, B, and C) and full factorial design (22; X1, and X2).
After one hour, the mixing operation was halted, and the mixture was transferred into a cylinder for decantation. Over the course of approximately 5 days, decantation was allowed to occur, ensuring a complete separation between the aqueous solution and the sludge. After successful separation, the aqueous solution was diluted with water at a ratio of 1 or 2 mL per 10 mL, describing the first process factor labeled as X1. The associated high (1 mL/10 mL) and low (2 mL/10 mL) levels were indicated in Table 1 as coded levels: −1 and +1, respectively. Figure 2 shows the positions of all the experiments presented in Table 1.
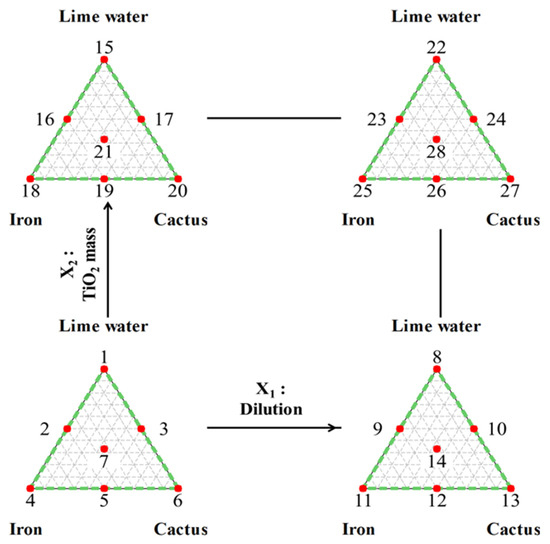
Figure 2.
Implemented experimental approach. Combination: simplex-lattice mixture and 22 designs. Notes: Numbers from 1 to 28 represent the runs conditions and positions of design of experiments presented in Table 1.
A volume of 20 mL from the resulting mixture was combined with an appropriate amount of the TiO2 catalyst for every experimental condition listed in Table 1. Depending on the level selected, the mass changed: 20 mg for the low level (X2 = −1, Table 1) and 40 mg for the high level (X2 = +1, Table 1). The solution was then left in dark for one hour, and after that, it was subjected to sunlight exposure for 16 h, during which time the CO2 emissions were tracked.
The use of Minitab® 16.2.1 Software significantly enhanced the efficacy of designing the experiments with 28 trials, as outlined in Table 1, Figure 1 and Figure 2, as well as for deriving the model coefficients. This comprehensive software package was used to proficiently carry out a wide array of statistical tests. It not only computed the optimal responses and corresponding conditions but also seamlessly generated detailed visual representations of the various graphical elements.
We selected decolorization (%) and phenolic compound degradation (%) as responses because of their high significance within this research. This choice underscored the impact of the treatment on the interplay between the effectiveness of decolorization and the reduction in organic substances simultaneously. Equation (4) provides the formulation of the quadratic model that characterized both outcomes. This model functioned in relation to the constituents of the mixture, namely lime water (A), Fe3+ solution (B), and cactus juice (C), as well as the variables of the process, denoted as X1 (Dilution) and X2 (TiO2 mass):
where:
- : the modeled response expressed as a decolorization or organic degradation;
- xA, xB and xC: proportions of lime water (A), Fe3+ solution (B), and cactus juice (C), respectively; they can take the values of 0, 1/3, 1/2, or 1.
- X1: the coded value of the dilution factor (independent variable) in the second process;
- X2: the coded value of the TiO2 mass as second factor (independent variable) in the second process;
- : model coefficients.
After the determination of the coefficients for both responses, a statistical study was conducted in order to discuss the fitting quality of the model and determine the responses’ maximum simultaneously. The optimization was achieved using the determined model given in Equation (4) with the help of the optimizer tool in the software Minitab® 16.2.1. The corresponding algorithm was implemented to maximize the while using this concept of desirability. The validation of the obtained optimum involving the maximum responses within the same time was conducted experimentally in triplicate using the determined level of each factor.
3. Results and Discussion
3.1. Catalyst Characterization
Figure 3 presents the X-Ray diffraction (XRD) spectrum of TiO2, displaying discernible peaks at 2θ (degree) values of 25.3°, 36.9°, 37.9°, 48.1°, 53.9°, 54.9°, and 62.9°. These peaks are in concordance with the patterns found in the [JCPDS card no. 21-1272] for the anatase phase of TiO2. Specifically, these peaks correspond to the (101), (103), (004), (112), (200), (105), (211), and (204) crystallographic planes. Additionally, smaller peaks were evident at 2θ angles of 26.9°, 36.1°, 44.9°, 62.7°, and 69.0°, which aligned well with the presence of the rutile phase. The existence of peaks at 25.3° and 48.1° serves as confirmation of the anatase structure of TiO2, in agreement with prior studies [41].
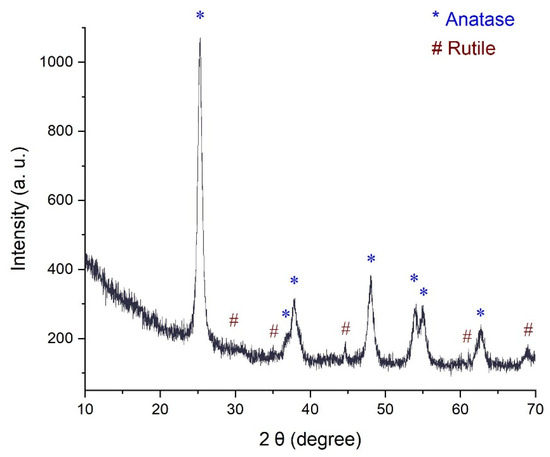
Figure 3.
X-Ray diffraction pattern of the catalyst TiO2.
SEM was used to describe the morphology of the solid, and EDX spectroscopy was used to identify its elemental composition. Field emission scanning electron microscopy (FE-SEM) was used to examine the morphology of TiO2, as shown in Figure 4a, with micro-particles that are noticeably enlarged in size ( µm). This micrograph shows pebble-like microscopic structures that agglomerate to form larger rocky and non-porous structures. The solid has distinct fissures that are caused by the surface tension that builds up on the pores during evacuation, causing their shrinkage. The EDX results reveal a purity of the solid formed exclusively of titanium and oxygen with residual carbon (Figure 4b).
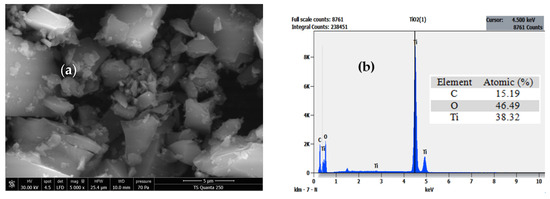
Figure 4.
Field emission scanning electron microscopy image (a) and X-Ray diffraction pattern (b) of the catalyst TiO2.
In Figure 5, the IR transmission spectra of TiO2 reveals distinct bands positioned at 435 and 526 cm−1, aligning with the Ti-O stretching and Ti-O-Ti bridging stretching modes inherent in the crystalline lattice of TiO2, as previously reported [42,43]. Notably, peaks are also discernible at 795 and 956 cm−1, characteristic of Ti-O-Ti interactions [44]. The presence of bands at 1065 and 1224 cm−1 can be ascribed to organic compounds, specifically C-O-C and C-OH functional groups in sol-gel prepared solids, consistent with findings from prior studies [45,46].
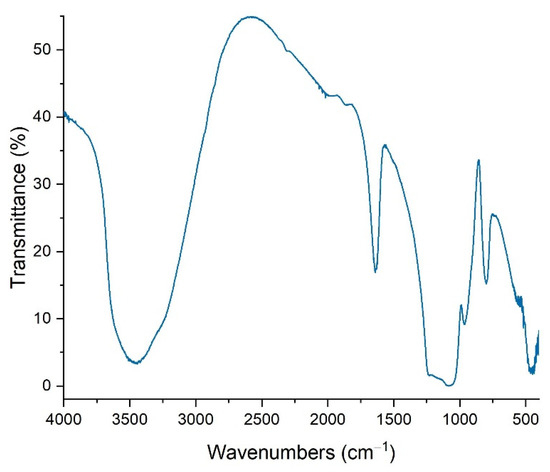
Figure 5.
FTIR spectra of TiO2.
The broad bands evident at 3401 and 1630 cm−1, frequently observed in the spectra of anatase nanocrystalline TiO2, are attributed to the stretching modes of Ti-OH bonds. Additionally, the band spanning between 3292 and 3445 cm−1 corresponds to O–H asymmetrical and symmetrical stretching vibrations; a phenomenon that is well-documented in the literature [47,48]. Furthermore, the band situated at 1640 cm−1 corresponds to the deformation vibration δH–O–H, indicating substantial adsorption of water molecules, in accordance with previous research [47,48].
The optical band gap of the titanium dioxide catalyst was determined utilizing the Kubelka–Munk method, which relies on the calculation of F(R). F(R) is directly proportional to the extinction coefficient (α) and is determined from the Equation (5):
where R represents the reflectance.
Considering an indirect allowed transition for TiO2, the band gap energy value could be deduced by plotting (F(R) × hν)1/2 against hν, where h is the Planck constant and ν is the frequency [49].
Figure 6 elucidates the band edge properties of this semiconductor. With a discernible value of approximately 3.2 eV, this measurement is consistent with findings from prior studies [47,48].
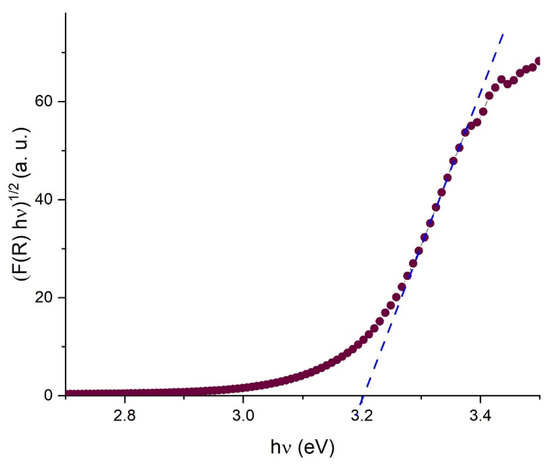
Figure 6.
Transformed spectrum reflectance plot for band gap energy determination. The intersection of the blue line with x-axis indicates the band gap energy value.
Our decision to utilize titanium dioxide (TiO2), synthesized through the sol-gel process, is based on its proven efficacy, as documented in the existing literature (Table 2). This material, when harnessed in the treatment of OMW, consistently exhibited a commendable level of efficiency, particularly when subjected to the stimulating effects of UV light. While its activity under such circumstances is firmly established, the expanding ongoing scientific exploration is now set on a course to amplify its capabilities even further. The focal point of this activity revolves around optimizing this material to harness the abundant energy of sunlight for excitation, a leap that promises to significantly broaden its practicality and effectiveness in various applications.

Table 2.
TiO2 active photo-catalyst.
3.2. OMW Characterization
OMW ranks among the most perilous effluents, presenting substantial environmental risks due to its complex, pollutant-rich composition. Because of the high levels of contaminants such as phenolic chemicals and solid suspension, it is also one of the hardest to treat [5,6]. These compounds are estimated to be present in concentrations of approximately 0.26 to 10.70 g · L−1, expressed as the gallic acid content [23]. Phenolic compounds are estimated to be present in concentrations of approximately 6.92 g · L−1 in the treated OMW [53]. The measured pH is approximately 4.62 ± 0.01. This parameter varies between 4.60 and 6.50 in the literature [49]. The parameters of the studied effluent in this work are COD, ash content, and dry matter. The COD was found to be equal to 52.1 ± 0.02 gO2 · L−1, while the ash content and the dry matter were equal to 17.5 ± 0.8 g · L−1 and 108.0 ± 0.5 g · L−1, respectively. These values are within the range of those reported in refs. [54,55,56].
3.3. Coagulation–Flocculation Trials
Given the importance of the effluent’s dry matter concentration, coagulation appears to be the most suitable initial treatment option. This method makes it easier to turn solid suspensions into materials that have been composted or pyrolyzed. Recent studies in this field have revealed a clear trend toward the acceptance of natural ingredients as important participants in the coagulation process, which is consistent with the growing interest in environmentally friendly elements. Among the natural coagulants, notable mentions include chitosane [57], seeds of Moringa oleifera [27,58], and cactus [59]. For this study, we opted to use cactus as our flocculent agent; a choice informed by its affordability within the Tunisian context [60] and its straightforward cultivation process.
According to Ndabigengesere and Narasiah [61], the cationic concentration has considerable effects on coagulation, while the anionic impact appears insignificant. In light of this, our exploration delved into the potential enhancement of the cactus’s efficacy when used in combination with lime and ferric solutions. Notably, the utilization of metals for odor reduction, as highlighted by Lagoudianaki et al. [62], reinforced the positive contributions of such elements. The final pH showed differences after the coagulation procedure and decantation, ranging from 5.92 with cactus juice alone to 11.67 when lime alone. A synthesis of Fe3+ solution, lime, and cactus juice yielded minimal sludge generation (approximately 48%), while the maximal production of sludge (70%) was observed when relying solely on lime.
The findings after the coagulation–flocculation step, presented in Table 3, demonstrate the variations in the percentage of decolorization and organic compound degradation. The values outlined in Table 3 were derived from the supernatant without any dilution. The coagulation–flocculation using a ternary mixture achieved a phenolic compound degradation of 56.7%, whereas the degradation was higher, reaching 85.4% while employing lime only.

Table 3.
Decolorization percentage and organic degradation after the coagulation–flocculation step.
In terms of decolorization, different trends were observed, with the Fe3+ solution yielding the lowest result of 14.6%, while the mixture of cactus and lime achieved the highest decolorization rate of 59.9%. The use of lime alone resulted in a decolorization rate of 53.8%. These results highlight the need for adding lime to the photodegradation process throughout, as well as during the coagulation–flocculation step.
The role of lime in coagulation–flocculation may not be highly effective, but it still contributes significantly to the overall process.
However, this treatment remained insufficient, necessitating further processing for the liquid fraction due to its lingering harmful compounds, particularly soluble polyphenols. Herein, photo-catalysis with sunlight emerges as an economic and effective method that is prominent for its efficiency in effluent treatment. Employing TiO2 and allowing for a 1 h adsorption period followed by roughly 16 h of daylight in August, the resultant mixtures attained a final pH of approximately 8.50. The COD of the central data point was 7.64 ± 0.02 gO2 · L−1. In terms of gallic acid, the phenolic compounds were remarkably decreased to 0.2 ± 0.01 10−5 mg · L−1.
This streamlined operational process boasts energy efficiency, capitalizing on the direct and passive utilization of the abundant solar energy accessible within the Mediterranean region. A significant operational challenge encountered in this approach involved ensuring optimal light exposure to the active centers. This goal was achieved through the procedure of flocculation and coagulation, which reduced the suspended matter and solids.
In the quest to model and optimize this dual-stage process, we devised an experimental design that combined a mixture design with a full factorial one. The synergistic application of these two designs enabled an in-depth exploration into the interplay between mixture formulations and independent physic-chemical factors.
3.4. Design of Experiments Results
Table 1 reports the experimental matrix derived from the combination of two kinds of design: a simplex-lattice mixture and 22 full factorial designs. All the experiences are regrouped in Figure S1 (Supplementary Materials) before the start and after the achievement of the photo-catalytic process.
This tabulation effectively showcases the intricate interrelationship between the 28 distinct experimental conditions and the two designated responses: decolorization (%) and organic degradation (%).
The decolorization values exhibited a range stretching from 36.25% to 99.59%, with a central tendency characterized by a mean of 73.79 ± 20.52%. Similarly, the spectrum of phenolic compound degradation showed spans between 95.76% and 99.36%, with the average resting at 97.59 ± 1.09%. These outcomes served as a compelling validation of the process’s efficacy, particularly in terms of phenolic compound degradation.
Table 4 compiles the results of the t-tests for both the decolorization and degradation of phenolic compounds, as well as each coefficient of the model in Equation (4). With regard to decolorization, the results unequivocally underline the sole significant yet negative influence, a consequence of the interaction between the proportion of Fe3+ solution (B) and dilution (X1) (p < 0.05). This seemingly counterintuitive effect can be attributed to the original yellow–brown color of the Fe3+ (III) solution due to the hexaaquairon (III) ion [Fe(H2O)6]3+ upon undergoing chemical changes [63]. Conversely, in the context of the phenolic compound degradation response, six terms stand out with significant effects (p < 0.05): the relative proportions of the constituent components, along with three linear terms representing the interaction between these component proportions and dilution (X1) (p < 0.05). This confluence of findings distinctly underscores the pivotal role played by these factors in driving the degradation of phenolic compounds.

Table 4.
Statistical parameters and model coefficients obtained for the two responses: decolorization and organic degradation.
The fitting quality of the model describing the experimental value of decolorization (%), with its coefficients shown in the first column of Table 4, was determined based on four statistical criteria: a high coefficient of correlation (R2 = 81.70%), an adjusted coefficient of correlation (R2adj = 50.59%) very close to 51%, a low RMSE (14.42%—unit of decolorization), and a level of significance (p = 0.062, very close to the critical value of significance—0.05). These results allow us to accept the model’s fitting of the experimental data of decolorization and to adopt it in this work. The fitting of the model applied to phenolic compound degradation appears to be more powerful than the model applied to decolorization, as evident from the statistical parameters. Specifically, the regression for phenolic compound degradation boasts a substantial level of significance (p = 0.001 < 0.01), a strong coefficient of correlation (R2 = 93.71%), a high adjusted coefficient of correlation (R2adj = 83.03%), and a low RMSE (0.448%—unit of phenolic compound degradation) [27].
Based on the component proportions and process factors, Figure 7 shows the progression of iso-responses for decolorization (Figure 7a) and organic compound degradation (Figure 7b). This graph demonstrates that the decolorization and decomposition of organic compounds are both enhanced by high lime concentrations (>40%).
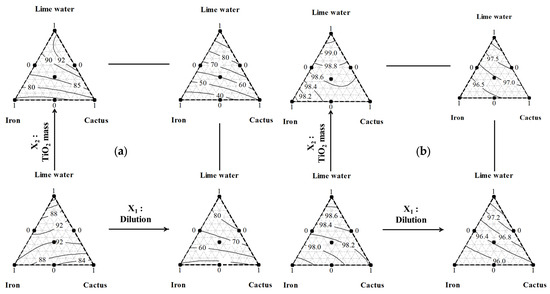
Figure 7.
Iso-responses of OMW’s decolorization (a) and organic degradation (b) after treatment utilizing a mixture (composed of lime water, Fe3+ solution, and cactus juice) as the coagulant–flocculant and 2 process variables, the dilution rate and TiO2 mass, as factors influencing the photo-catalysis process.
As evident in Figure 7, the positive effect of lime is distinctly observed across all considered dilution levels (X1) and TiO2 catalyst masses (X2). Moreover, the negative effect of the factor dilution rate (X2) can be clearly observed in Figure 7a for decolorization and in Figure 7b for the organic compound degradation. This is valid for all the factor TiO2 mass (X1) levels and all the mixtures. Additionally, the graph demonstrates that the optimal decolorization (>92%) and phenolic compound degradation (>98.5%) percentages are attainable through the application of a low dilution rate (X1) and either a low or high TiO2 catalyst mass (X2). This outcome is achievable by employing a ternary mixture composed predominantly of lime in conjunction with the three utilized components, as emphasized by the research of Karatasios et al. [64].
The contribution of lime to the enhancement of the water treatment process is intricately tied to the significant influence of the solution pH, as expounded by Dong et al., in 2015 [65]. At the heart of this effect lies calcium hydroxide, the primary constituent of lime, which acts as a potent alkaline entity that is able to release two hydroxide ions . Consequently, the emergence of the highly reactive OH˙ radical results from augmentation in an alkaline environment. In a classical scenario, the generation of this radical stems from the interaction of water with the positive hole on the valence band, denoted as , within TiO2, as depicted in Equation (6) followed by Equation (7). In an alkaline medium, the direct interaction of the positive hole with OH˙ also contributes to the creation of this active species in alignment with the findings elucidated in Equation (8), as highlighted in the investigations conducted by Karatasios et al. [64].
Electron-hole pair formation: TiO2 + hv → e− (CB) + h+ (VB)
Water splitting by photo-hole to produce OH˙ radicals: h+ + H2O → H+ OH˙
Direct OH˙ radicals’ production on a photo-hole: h+ + OH− → OH˙
The Minitab® 16.2.1 Software optimizer was used to find the settings that would maximize both responses simultaneously, as shown in Figure 7, Figure 8 and Figure 9. This is an important phase, since it finds the ideal levels of each process variable as well as the ideal composition of the ternary mixture. The outcomes of this pure mathematical optimization reaffirm that the inclusion of iron holds no practical advantage. The apex of coagulation–flocculation efficiency is realized through a blend comprising 48.48% lime and 51.52% cactus juice. Similarly, the paramount outcome in photo-catalytic performance necessitates a low dilution rate and a high mass of TiO2, as illustrated in Figure 8. Moreover, the desirability to achieve maximum responses was 0.931 and 0.987 for decolorization and organic degradation, respectively (Figure 8). Due to this high desirability, the outcomes for both responses could be considered as highly satisfactory and convergent.
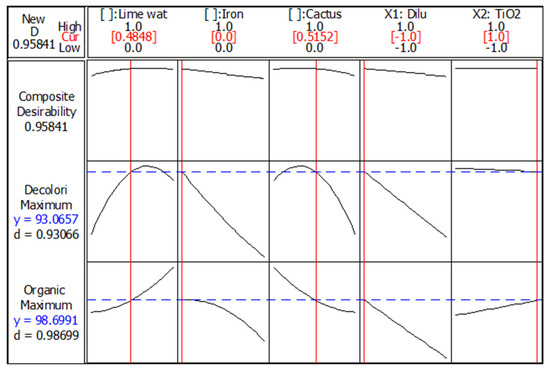
Figure 8.
Optimization plot. Dash blue lines indicate the optimum responses simultaneously (written in blue in the right); Continuous red line is useful to determine level of each factor giving the optimum responses (written in red in the top).
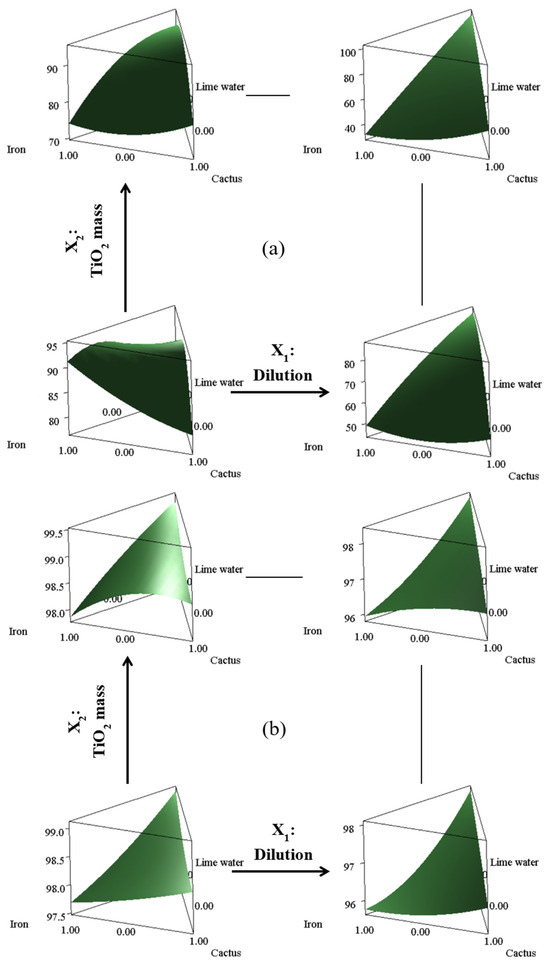
Figure 9.
Three-dimensional response surface plot of OMW’s decolorization (a) and organic degradation (b) depending on the different variables of the studied two-step process.
4. Conclusions
According to the calculated prediction deduced for this particular combination, as displayed in Figure 9, an optimum result indicating a decolorization of 93.06% and an organic degradation of 98.70% can be achieved. To validate this computed optimum, a series of experimental trials was conducted in triplicate, yielding a decolorization of 92.57 ± 0.90% and an organic degradation of 96.19 ± 0.97%. These results indicate that the experimental outcomes are replicable given to the low standard deviations and close to the mathematical optimization calculus.
An alternative combination of factors that stands as a strong contender against the determined optimum can be found in the experimental conditions specified in run 15 of Table 1. This scenario involves the utilization of 100% lime, coupled with a low dilution rate and a high TiO2 mass, culminating in a remarkable decolorization rate of 99.59% and an organic degradation rate of 99.36%. However, despite important achievements in terms of water quality enhancement, this particular solution falls short when considering the magnitude and quality of the generated sludge. Notably, it is worth mentioning that the use of lime as the sole coagulant agent results in the production of the highest quantity of sludge, reaching a substantial value of 69.9%.
Coagulation–flocculation, recognized for its simplicity and cost-effectiveness, brings satisfactory outcomes when dealing with particle-charged wastewater. Its effectiveness when used alone, meanwhile, could occasionally fall short of expectations. Therefore, the goal of our work was to develop a two-stage method combining coagulation–flocculation with sunlight photo-catalysis to increase the effectiveness of this procedure.
A combination of mixture and complete factorial design approaches was used to enhance this original tandem process, focusing on decolorization and phenolic compound degradation by adjusting the composition of the coagulant and the photo-catalytic conditions, respectively. Among the prevalent families of coagulants, metals, lime, and organic agents held our primary focus. Applying the efficiency of the mixture design technique, we methodically investigated the effects caused by iron, lime, and cactus in the area of coagulant composition. We also simultaneously investigated photo-catalytic testing while modifying the mass of the TiO2 catalyst and dilution rate at two different levels. Guided by this comprehensive optimization, we pinpointed better conditions: the omission of Fe3+ solution and the inclusion of 48.48% lime water and 51.52% cactus juice, coupled with a low dilution rate and a high TiO2 catalyst mass. Subsequent experimental testing, conducted independently in triplicate, yielded a remarkable decolorization rate of 92.57 ± 0.90% and an organic degradation rate of 96.19 ± 0.97%, close to the mathematical predictions of 93.06% decolorization and 98.70% organic degradation.
A significant decolorization rate of 99.59% and an organic degradation rate of 99.36% were obtained when we investigated the possibility of coagulation using 100% lime. However, this high efficiency came at the cost of generating a considerable amount of sludge, prompting thoughtful consideration from a practical standpoint.
In conclusion, the two-step process involving coagulation–flocculation and sunlight photo-catalysis, optimized by the combination of mixture and full factorial designs methodologies, led to successes in decolorization and organic degradation.
The best conditions we identified, alongside the alternative employment of 100% lime, stress the effectiveness of our treatment approach. However, when assessing the 100% lime approach’s overall viability, one must take into account the considerable sludge output generated. The proposed process can be implemented directly within the olive oil plant on site, avoiding the transport step to the wastewater treatment plant. The first step of the process is coagulation–flocculation, which can be conducted using cheap and local Tunisian compounds: cactus juice and lime. The next step is also feasible on-site, since in Tunisia, sunlight is predominant year-round. Therefore, the photocatalysis step is also possible. With this two-stage process, we showed in this study that it is possible to treat olive oil mill wastewater, thus reducing its negative environmental impact while using local and cheap compounds via a green and low-cost process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16020327/s1, Figure S1: Photo of the different experiences of the DOE (a) before sunlight exposure and (b) after 24 h of the photo-catalytic test. C: Cactus; L: Lime; I: Iron; I + L + C: Iron + Lime + Cactus; L + C: Lime + Cactus; I + C: Iron + Cactus; I + L: Iron + Lime; X1: dilution rate and X2: TiO2 Catalyst mass.
Author Contributions
Conceptualization, F.F., S.R., K.K., C.M., L.K. and B.H.; methodology, F.F., S.R., K.K., C.M., L.K. and B.H.; formal analysis, F.F., S.R. and B.H.; investigation, F.F., S.R., K.K., C.M., L.K. and B.H.; writing—original draft, F.F., S.R., K.K., C.M., L.K. and B.H.; writing—review and editing F.F., S.R., K.K., C.M., L.K. and B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-RG23057).
Data Availability Statement
All the data analyzed during this study are included in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hodaifa, G.; Gallardo, P.A.R.; García, C.A.; Kowalska, M.; Seyedsalehi, M. Chemical oxidation methods for treatment of real industrial olive oil mill wastewater. J. Taiwan Inst. Chem. Eng. 2019, 97, 247–254. [Google Scholar] [CrossRef]
- Sierra, J.; Martı, E.; Montserrat, G.; Cruanas, R.; Garau, M.A. Characterisation and evolution of a soil affected by olive oil mill wastewater disposal. Sci. Total Environ. 2001, 279, 207–214. [Google Scholar] [CrossRef]
- Cappelletti, G.M.; Ioppolo, G.; Nicoletti, G.M.; Russo, C. Energy requirement of extra virgin olive oil production. Sustainability 2014, 6, 4966–4974. [Google Scholar] [CrossRef]
- Doula, M.K.; Moreno-Ortego, J.L.; Tinivella, F.; Inglezakis, V.J.; Sarris, A.; Komnitsas, K. Olive Mill Waste: Recent Advances for the Sustainable Development of Olive Oil Industry. In Olive Mill Waste: Recent Advances for Sustainable Management; Galanakis, C.M., Ed.; Academic Press: London, UK, 2017; pp. 29–56. [Google Scholar]
- Elkadri, A.; Sahnoun, H.; Elfkih, S.; Abichou, M. Assessing the sustainability of olive mill wastewater storage tank locations in Tunisia. Euro-Mediterr. J. Environ. Integr. 2023, 8, 255–273. [Google Scholar] [CrossRef]
- Kovačević, M.; Stjepanović, N.; Trigui, S.; Hackenberger, K.D.; Lončarić, Z.; Glavaš, O.G.; Kallel, A.; Hackenberger, B.K. Assessment of adverse effects of olive mill wastewater and olive mill waste contaminated soil on springtail Folsomia candida. Chemosphere 2022, 300, 134651. [Google Scholar] [CrossRef] [PubMed]
- Souilem, S.; El-Abbassi, A.; Kiai, H.; Hafidi, A.; Sayadi, S.; Galanakis, C.M. Olive Oil Production Sector: Environmental Effects and Sustainability Challenges. In Olive Mill Waste: Recent Advances for Sustainable Management; Galanakis, C.M., Ed.; Academic Press: London, UK, 2017; pp. 1–28. [Google Scholar]
- Bombino, G.; Andiloro, S.; Folino, A.; Lucas-Borja, M.E.; Zema, D.A. Short-term effects of olive oil mill wastewater application on soil water repellency. Agric. Water Manag. 2021, 244, 106563. [Google Scholar] [CrossRef]
- Bouaouine, O.; Bourven, I.; Khalil, F.; Baudu, M. Reuse of olive mill wastewater as a bioflocculant for water treatment processes. J. Clean. Prod. 2020, 246, 119031. [Google Scholar] [CrossRef]
- Amor, C.; Lucas, M.S.; García, J.; Dominguez, J.R.; De Heredia, J.B.; Peres, J.A. Combined treatment of olive mill wastewater by Fenton’s reagent and anaerobic biological process. J. Environ. Sci. Health Part A 2015, 50, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Khoufi, S.; Louhichi, A.; Sayadi, S. Optimization of anaerobic co-digestion of olive mill wastewater and liquid poultry manure in batch condition and semi-continuous jet-loop reactor. Bioresour. Technol. 2015, 182, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sanghamitra, P.; Mazumder, D.; Mukherjee, S. Treatment of wastewater containing oil and grease by biological method—A review. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2021, 56, 394–412. [Google Scholar] [CrossRef]
- Borja, R.; Rincón, B.; Raposo, F.; Alba, J.; Martín, A. A study of anaerobic digestibility of two-phases olive mill solid waste (OMSW) at mesophilic temperature. Process Biochem. 2002, 38, 733–742. [Google Scholar] [CrossRef]
- Velasco-Munoz, J.F.; Mendoza, J.M.F.; Aznar-Sánchez, J.A.; Gallego-Schmid, A. Circular economy implementation in the agricultural sector: Definition, strategies, and indicators. Resour. Conserv. Recycl. 2021, 170, 105618. [Google Scholar] [CrossRef]
- Antonelli, M.; Basile, L.; Gagliardi, F.; Riccaboni, A.; Isernia, P. The AGRIFOODMED Delphi Final Report. Trends, Challenges and Policy Options for Water Management, Farming Systems and Agri-Food Value Chains in 2020–2030; PRIMA Document. 2019. Available online: http://www.primaitaly.it/wp-content/uploads/2019/06/AGRIFOODMED-Delphi-Final-Report.pdf (accessed on 13 January 2024).
- Arvanitoyannis, I.S.; Kassaveti, A. Olive Oil Waste Management: Treatment Methods and Potential Uses of Treated Waste. In Food Science and Technology, Waste Management for the Food Industries; Arvanitoyannis, I.S., Ed.; Academic Press: Cambridge, MA, USA, 2008; Chapter 8; pp. 453–568. ISBN 9780123736543. [Google Scholar] [CrossRef]
- Donner, M.; Verniquet, A.; Broeze, J.; Kayser, K.; De Vries, H. Critical success and risk factors for circular business models valorizing agricultural waste and by-products. Resour. Conserv. Recycl. 2021, 165, 105236. [Google Scholar] [CrossRef]
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R.Z.; Almalki, W.H. Treatment technologies for olive mill wastewater with impacts on plants. Environ. Res. 2023, 216, 114399. [Google Scholar] [CrossRef] [PubMed]
- Soto, E.R.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging opportunities for the effective valorisation of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Donner, M.; Erraach, Y.; López-i-Gelats, F.; Manuel-I Martin, J.; Yatribi, T.; Radić, I.; El Hadad-Gauthier, F. Circular bioeconomy for olive oil waste and by-product valorisation: Actors’ strategies and conditions in the Mediterranean area. J. Environ. Manag. 2022, 321, 115836. [Google Scholar] [CrossRef] [PubMed]
- Fersi, C.; Ben Salah, I.; Medimagh, R. Circular Economy in Tunisia. In Circular Economy: Recent Trends in Global Perspective; Ghosh, S.K., Ghosh, S.K., Eds.; Springer Nature: Singapore, 2021; Chapter 4. [Google Scholar] [CrossRef]
- Jarboui, R.; Hadrich, B.; Gharsallah, N.; Ammar, E. Olive mill wastewater disposal in evaporation ponds in Sfax (Tunisia): Moisture content effect on microbiological and physical chemical parameters. Biodegradation 2009, 20, 845–858. [Google Scholar] [CrossRef]
- Khoufi, S.; Aloui, F.; Sayadi, S. Pilot scale hybrid process for olive mill wastewater treatment and reuse. Chem. Eng. Process. 2009, 48, 643–650. [Google Scholar] [CrossRef]
- Achak, M.; Boumya, W.; Elamraoui, S.; Asdiou, N.; Taoufik, N.; Barka, N.; Aboulkas, A.; Lamy, E. Performance of olive mill wastewater treatment using hybrid system combining sand filtration and vertical flow constructed wetlands. J. Water Process Eng. 2023, 53, 103737. [Google Scholar] [CrossRef]
- Martínez-Gallardo, M.R.; López, M.J.; Jurado, M.M.; Suárez-Estrella, F.; López-González, J.A.; Sáez, J.A.; Moral, R.; Moreno, J. Bioremediation of Olive Mill Wastewater sediments in evaporation ponds through in situ composting assisted by bioaugmentation. Sci. Total Environ. 2020, 703, 135537. [Google Scholar] [CrossRef] [PubMed]
- El Hassani, F.Z.; El Karkouri, A.; Errachidi, F.; Merzouki, M.; Benlemlih, M. The impact of Olive Mill Wastewater spreading on soil and plant in arid and semi-arid areas. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100798. [Google Scholar] [CrossRef]
- Rifia, S.K.; Souabia, S.; El Felsb, L.; Driouicha, A.; Nassrid, I.; Haddajia, C.; Hafidi, M. Optimization of coagulation process for treatment of olive oil mill wastewater using Moringa oleifera as a natural coagulant, CCD combined with RSM for treatment optimization. Process Saf. Environ. Prot. 2022, 162, 406–418. [Google Scholar] [CrossRef]
- Gandiwa, B.I.; Moyo, L.B.; Ncube, S.; Mamvura, T.A.; Mguni, L.L.; Hlabangana, N. Optimisation of using a blend of plant based natural and synthetic coagulants for water treatment: (Moringa Oleifera-Cactus Opuntia-alum blend). S. Afr. J. Chem. Eng. 2020, 34, 158–164. [Google Scholar] [CrossRef]
- El Shahawy, A.; Hassan, S.; Ebrahiem, E.E.; El Kersh, I. Organic pollutants removal from olive mill wastewater by coagulation and electrocoagulation: Application of Box-Behnken design (BBD). Desalin. Water Treat. 2019, 148, 102–118. [Google Scholar] [CrossRef]
- Vuppala, S.; Shaik, R.U.; Stoller, M. Multi-response optimization of coagulation and flocculation of olive mill wastewater: Statistical approach. Appl. Sci. 2021, 11, 2344. [Google Scholar] [CrossRef]
- Villaseñor-Basulto, D.L.; Astudillo-Sánchez, P.D.; del Real-Olvera, J.; Bandala, E.R. Wastewater treatment using Moringa oleifera Lam seeds: A review. J. Water Process Eng. 2018, 23, 151–164. [Google Scholar] [CrossRef]
- Zhang, Z. The flocculation mechanism and treatment of oily wastewater by flocculation. Water Sci. Technol. 2017, 76, 2630–2637. [Google Scholar] [CrossRef]
- Sher, F.; Malik, A.; Liu, H. Industrial polymer effluent treatment by chemical coagulation and flocculation. J. Environ. Chem. Eng. 2013, 1, 684–689. [Google Scholar] [CrossRef]
- Aouan, B.; Alehyen, S.; Fadil, M.; El Alouani, M.; Saufi, H.; El Herradi, E.H.; El Makhoukhi, F.; Taibi, M. Development and optimization of geopolymer adsorbent for water treatment: Application of mixture design approach. J. Environ. Manag. 2023, 338, 117853. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M. Role of coagulation/flocculation as a pretreatment option to reduce colloidal/bio-colloidal fouling in tertiary filtration of textile wastewater: A review and future outlooks. Front. Environ. Sci. 2023, 11, 1142227. [Google Scholar] [CrossRef]
- Fan, X.; Liu, X.; Wang, Y. Low-cost and resource-efficient monolithic photocatalyst with enhanced solar light utilization for the photocatalytic treatment of organic wastewater. Chemosphere 2023, 312, 137052. [Google Scholar] [CrossRef] [PubMed]
- Rondinini, S. pH measurments in non-aqueous and aqueous-organic solvents-definition standard procedures. Anal. Bioanal. Chem. 2002, 374, 813–816. [Google Scholar] [CrossRef]
- Paredes, C.; Cegarra, J.; Roig, A.; Sánchez-Monedero, M.A.; Bernal, M.P. Characterization of olive mill wastewater (alpechin) and its sludge for agricultural purposes. Bioresour. Technol. 1999, 67, 111–115. [Google Scholar] [CrossRef]
- Nassar, N.N.; Arar, L.A.; Marei, N.N.; Abu Ghanim, M.M.; Dwekat, M.S.; Sawalha, S.H. Treatment of olive mill based wastewater by means of magnetic nanoparticles: Decolourization, dephenolization and COD removal. Environ. Nanotechnol. Monit. Manag. 2014, 1–2, 14–23. [Google Scholar] [CrossRef]
- Raissi, S.; Fakhfakh, F. Analytical validation of smartphone spectroscopic technic used in an educational kinetic study. Int. J. Anal. Chem. 2023, 2023, 3729633. [Google Scholar] [CrossRef]
- Ba-Abbad, M.; Kadhum, A.; Mohamad, A.; Takriff, M.S.; Sopian, K. Synthesis and catalytic activity of TiO2 nanoparticles for photochemical oxidation of concentrated chlorophenols under direct solar radiation. Int. J. Electrochem. Sci. 2012, 7, 4871–4888. [Google Scholar] [CrossRef]
- Wang, G.; Xu, L.; Zhang, J.; Yin, T.; Han, D. Enhanced photocatalytic activity of TiO2 powders (P25) via calcination treatment. Int. J. Photoenergy 2012, 2012, 265760. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Wong, S.P. Photocatalytic efficiency of TiO2-biomass loaded mixture for wastewater treatment. J. Chem. 2018, 2018, 4314969. [Google Scholar] [CrossRef]
- Qourzal, S.; Barka, N.; Tamimi, M.; Assabbane, A.; Nounah, A.; Ihlal, A.; Ait-Ichou, Y. Sol–gel synthesis of TiO2–SiO2 photocatalyst for β-naphthol photodegradation. Mater. Sci. Eng. C 2009, 29, 1616–1620. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and raman characterization of TiO2 nanoparticles coated with polyethylene glycol as carrier for 2-methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, N.; Li, J.; Xia, Q.; Meng, J.; Dingb, J.; Lu, J. Synthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Adv. 2018, 8, 34241–34251. [Google Scholar] [CrossRef] [PubMed]
- Bezrodna, Y.; Puchkovska, G.; Shymanovska, V.; Baran, J.; Ratajczak, H. IR-analysis of H-bonded H2O on the pure TiO2 surface. J. Mol. Struct. 2004, 700, 175. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L. NH4Cl-assisted low temperature synthesis of anatase TiO2 nanostructures from Ti powder. J. Mater. Lett. 2009, 63, 1797–1799. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- El Hajjouji, H.; Barje, F.; Pinelli, E.; Bailly, J.R.; Richard, C.; Winterton, P.; Revel, J.C.; Hafidi, M. Photochemical UV/TiO2 treatment of olive mill wastewater (OMW). Bioresour. Technol. 2008, 99, 7264–7269. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Z.; Guo, H.; Yang, H.; Zhang, G.; Ji, S.; Zeng, T. Low-temperature synthesis of anatase TiO2 nanoparticles with tunable surface charges for enhancing photocatalytic activity. PLoS ONE 2014, 9, e114638. [Google Scholar] [CrossRef] [PubMed]
- Barba-Nieto, I.; Caudillo-Flores, U.; Fernández-García, M.; Kubacka, A. Sunlight-operated TiO2-based photocatalysts. Molecules 2022, 25, 4008. [Google Scholar] [CrossRef]
- Speltini, F.; Maraschi, A.; Sturini, M.; Caratto, V.; Ferretti, M.; Profumo, A. Sorbents coupled to solar light TiO2-based photocatalysts for olive mill wastewater treatment. Int. J. Photoenergy 2016, 2016, 8793841. [Google Scholar] [CrossRef]
- Hanafi, F.; Belaoufi, A.; Mountadar, M.; Assobhei, O. Augmentation of biodegradability of olive mill wastewater by electrochemical pre-treatment: Effect on phytotoxicity and operating cost. J. Hazard. Mater. 2011, 190, 94–99. [Google Scholar] [CrossRef]
- Al-Essa, K. Activation of Jordanian bentonite by hydrochloric acid and its potential for olive mill wastewater enhanced treatment. J. Chem. 2018, 2018, 8385692. [Google Scholar] [CrossRef]
- Elayadi, F.; Achak, M.; Beniich, N.; El Adlouni, C. Factorial design for optimizing and modeling the removal of organic pollutants from olive mill wastewater using a novel low-cost bioadsorbent. Water Air Soil Pollut. 2020, 231, 351. [Google Scholar] [CrossRef]
- Patel, H.; Vashi, R.T. Comparison of naturally prepared coagulants for removal of COD and color from textile wastewater. Glob. Nest J. 2013, 15, 522–528. [Google Scholar]
- Rasheed, F.A.; Alkaradaghi, K.; Al-Ansari, N. The potential of Moringa oleifera seed in water coagulation-flocculation technique to reduce water turbidity. Water Air Soil Pollut. 2023, 234, 250. [Google Scholar] [CrossRef]
- Oladoja, N.A. Advances in the quest for substitute for synthetic organic polyelectrolytes as coagulant aid in water and wastewater treatment operations. Sustain. Chem. Pharm. 2016, 3, 47–58. [Google Scholar] [CrossRef]
- Louhaichi, M.; Hamdeni, I.; Slim, S.; Hassen, S.; Harberg, S.; Gouhis, F. Economic valuation of cactus pear production in semi- arid regions of Tunisia. Acta Hortic. 2022, 1343, 97–102. [Google Scholar] [CrossRef]
- Ndabigengesere, K.; Narasiah, S. Influence of operating parameters on turbidity removal by coagulation with Moringa oleifera seeds. Environ. Technol. 1996, 17, 1103–1112. [Google Scholar] [CrossRef]
- Yazdanbakhsh, A.; Mehdipour, F.; Eslami, A.; Maleksari, H.S.; Ghanbari, F. The combination of coagulation, acid cracking and Fenton-like processes for olive oil mill wastewater treatment: Phytotoxicity reduction and biodegradability augmentation. Water Sci. Technol. 2015, 71, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Miliordos, E.; Xantheas, S.S. Ground and Excited States of the [Fe(H2O)6]2+ and [Fe(H2O)6]3+ Clusters: Insight into the Electronic Structure of the [Fe(H2O)6]2+–[Fe(H2O)6]3+ Complex. J. Chem. Theory Comput. 2015, 11, 1549–1563. [Google Scholar] [CrossRef]
- Karatasios, I.; Katsiotis, M.S.; Likodimos, V.; Kontos, A.I.; Papavassiliou, G.; Falaras, P.; Kilikoglou, V. Photo-induced carbonation of lime-TiO2 mortars. Appl. Catal. B Environ. 2010, 95, 78–86. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).