Temporal and Spatial Distribution of Phytoplankton and Role of Environment Factors in the Shending River Backwater in the Danjiangkou Reservoir Area
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Samples and Data
2.3. Sample Analysis
2.4. Remote Sensing Estimation of Chlorophyll-a (Chl-a) Concentration
2.5. Characterization of Phytoplankton Community Structure
2.6. Statistical Analysis
3. Results
3.1. Remote Sensing Estimation Results
3.2. Phytoplankton Community
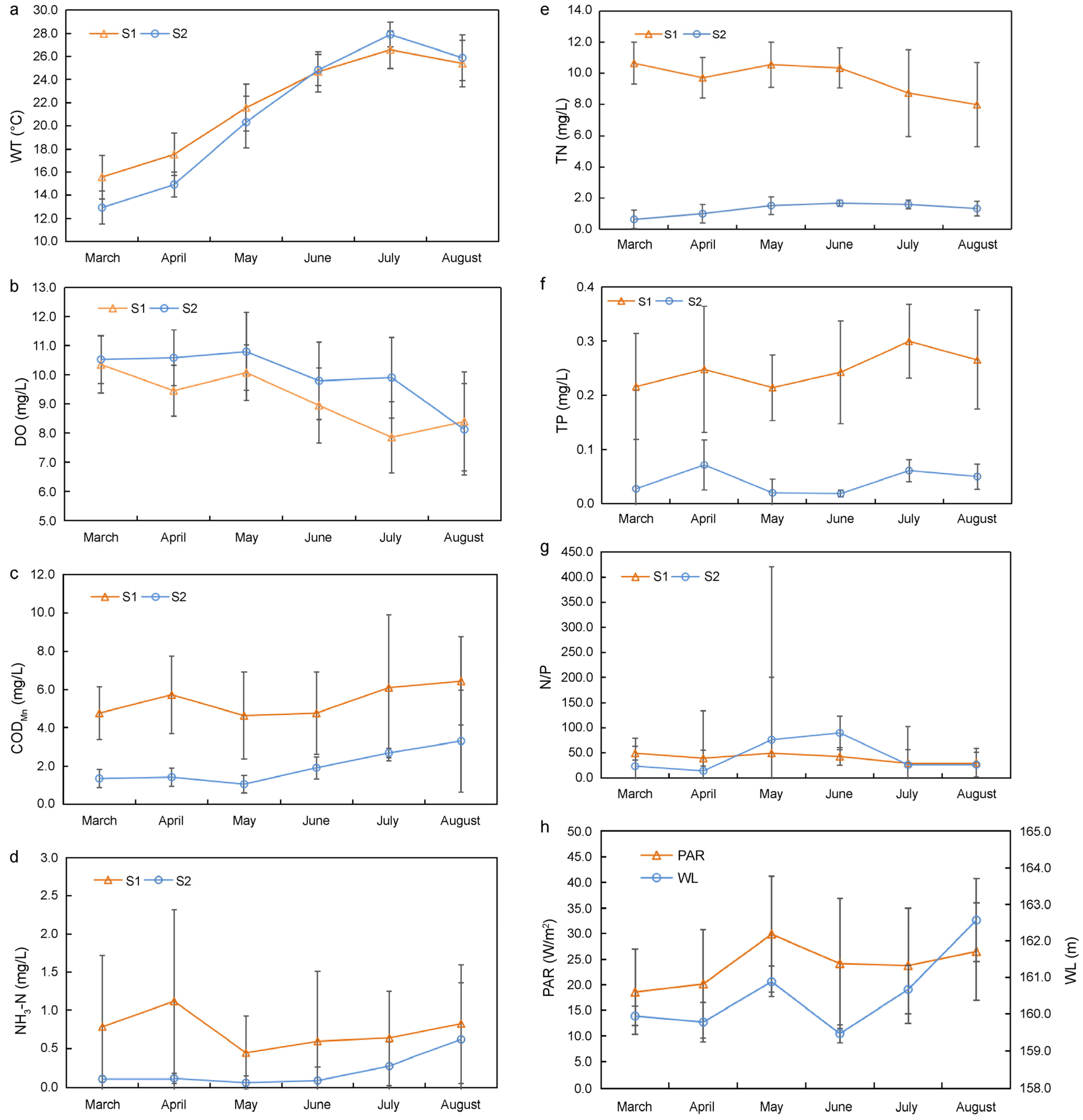
3.3. Environmental Factors
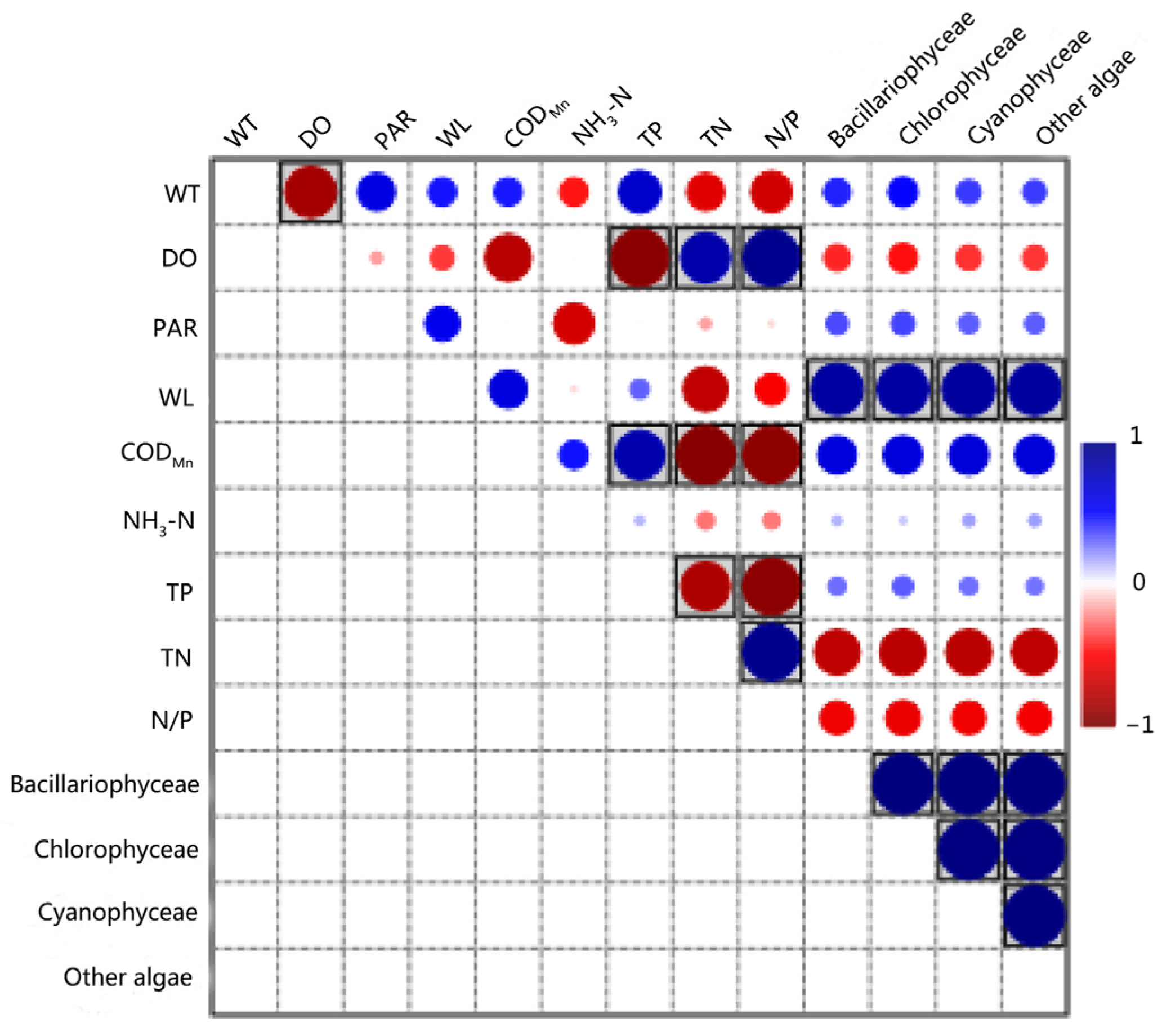
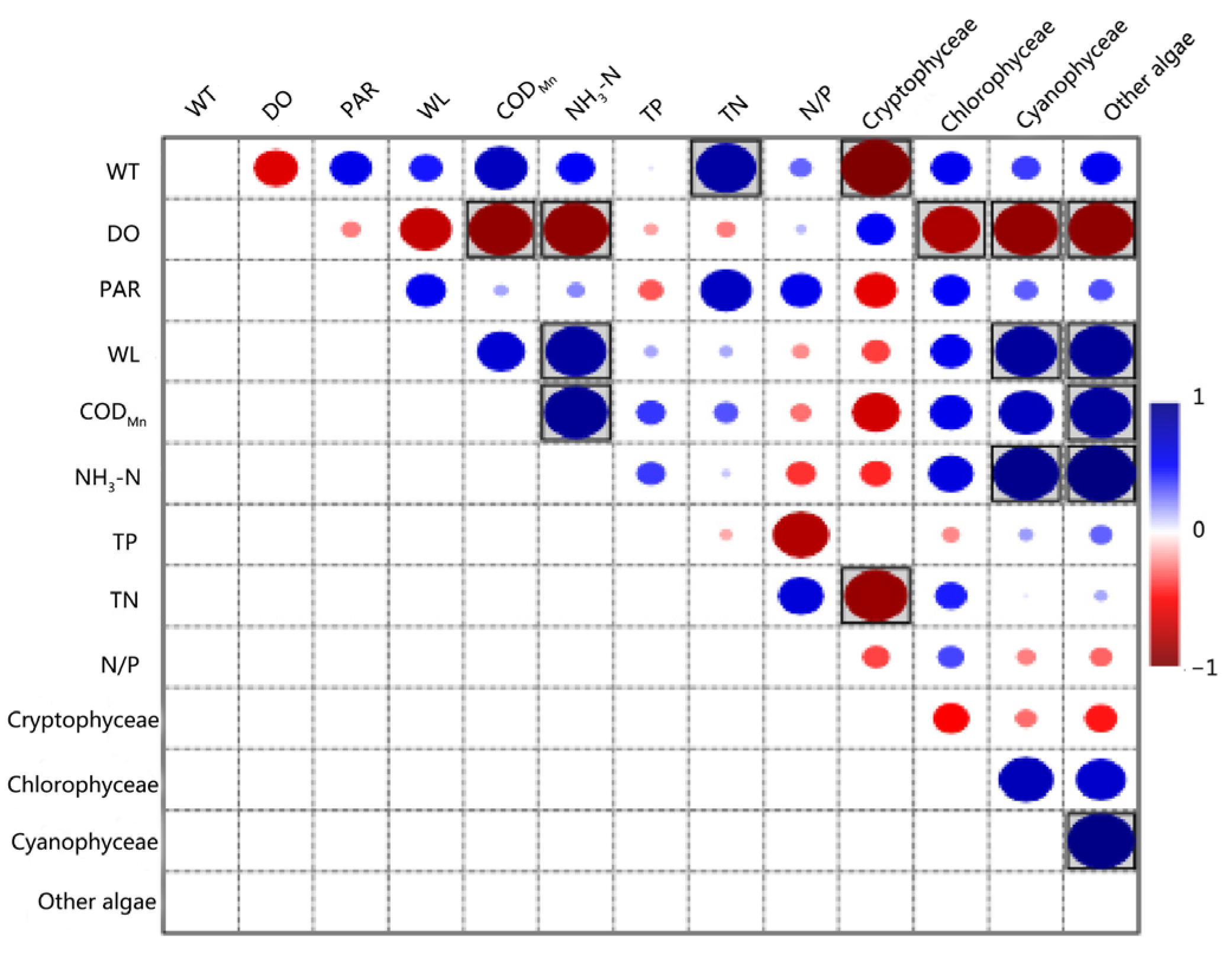
3.4. Results of the Pearson Correlation Analysis
3.5. Results of the Mantel Test
4. Discussion
4.1. Overall Spatiotemporal Distribution Characteristics in the Shending River Backwater Area
4.2. Phytoplankton Community in the Backwater of the Shending River in Spring and Summer
4.3. Environmental Variables Impacting the Proliferation of Phytoplankton in the Shending River Backwater Area
4.4. Suggestions on Water Bloom Control in the Shending River Backwater Area
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, Y.; Hu, M.; Wang, Y.; Zhang, H.; He, C.; Wang, Y.; Wang, D.; Wu, X.; Zhuang, Y.; Hong, S.; et al. Identifying key drivers of harmful algal blooms in a tributary of the Three Gorges Reservoir between different seasons: Causality based on data-driven methods. Environ. Pollut. 2022, 297, 118759. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Chen, J.; Wang, J.; Jiao, S.; Chen, F. Temporal and spatial distribution of phytoplankton functional groups and role of environment factors in a deep subtropical reservoir. J. Oceanol. Limnol. 2018, 36, 761–771. [Google Scholar] [CrossRef]
- Tian, Y.; Jiang, Y.; Liu, Q.; Xu, D.; Liu, Y.; Song, J. The impacts of local and regional factors on the phytoplankton community dynamics in a temperate river, northern China. Ecol. Indic. 2021, 123, 107352. [Google Scholar] [CrossRef]
- Zhang, Y.; Ban, X.; Li, E.; Wang, Z.; Xiao, F. Evaluating ecological health in the middle-lower reaches of the Hanjiang River with cascade reservoirs using the Planktonic index of biotic integrity (P-IBI). Ecol. Indic. 2020, 114, 106282. [Google Scholar] [CrossRef]
- Minaudo, C.; Abonyi, A.; Leitao, M.; Lancon, A.M.; Floury, M.; Descy, J.P.; Moatar, F. Long-term impacts of nutrient control, climate change, and invasive clams on phytoplankton and cyanobacteria biomass in a large temperate river. Sci. Total Environ. 2021, 756, 144074. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.R.A.; Cardoso, M.M.L.; Selmeczy, G.B.; Padisák, J.; Becker, V. Phytoplankton functional responses induced by extreme hydrological events in a tropical reservoir. Hydrobiologia 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Abirhire, O.; Davies, J.M.; Imtiazy, N.; Hunter, K.; Emmons, S.; Beadle, J.; Hudson, J. Response of phytoplankton community composition to physicochemical and meteorological factors under different hydrological conditions in Lake Diefenbaker. Sci. Total Environ. 2023, 856 Pt 2, 159–210. [Google Scholar] [CrossRef] [PubMed]
- Daines, S.J.; Clark, J.R.; Lenton, T.M. Multiple environmental controls on phytoplankton growth strategies determine adaptive responses of the N:P ratio. Ecol. Lett. 2014, 17, 414–425. [Google Scholar] [CrossRef]
- Zhang, H.K.; Min, F.L.; Cui, H.R.; Peng, X.; Zhang, X.Y.; Zhang, S.X.; Li, Z.X.; Ge, F.J.; Zhang, L.; Wu, Z.B.; et al. Characteristics of Phytoplankton Communities and Key Impact Factors in Three Types of Lakes in Wuhan. Huan Jing Ke Xue 2023, 44, 2093–2102. [Google Scholar] [CrossRef]
- Serre-Fredj, L.; Chasselin, L.; Jolly, O.; Jacqueline, F.; Claquin, P. Colimitation assessment of phytoplankton growth using a resource use efficiency approach in the Bay of Seine (French-English Channel). J. Environ. Manag. 2022, 306, 114487. [Google Scholar] [CrossRef]
- Paver, S.F.; Hayek, K.R.; Gano, K.A.; Fagen, J.R.; Brown, C.T.; Davis-Richardson, A.G.; Crabb, D.B.; Rosario-Passapera, R.; Giongo, A.; Triplett, E.W.; et al. Interactions between specific phytoplankton and bacteria affect lake bacterial community succession. Environ. Microbiol. 2013, 15, 2489–2504. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mou, X.; Cao, H.; Struewing, I.; Allen, J.; Lu, J. Co-occurring microorganisms regulate the succession of cyanobacterial harmful algal blooms. Environ. Pollut. 2021, 288, 117682. [Google Scholar] [CrossRef]
- Rosinska, J.; Kozak, A.; Dondajewska, R.; Goldyn, R. Cyanobacteria blooms before and during the restoration process of a shallow urban lake. J. Environ. Manag. 2017, 198 Pt 1, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Znachor, P.; Nedoma, J.; Hejzlar, J.; Seďa, J.; Komárková, J.; Kolář, V.; Mrkvička, T.; Boukal, D.S. Changing environmental conditions underpin long-term patterns of phytoplankton in a freshwater reservoir. Sci. Total Environ. 2020, 710, 135626. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Lv, T.; Wang, L.; Li, Y.; Han, C.; Yu, W.; Yan, Z.; Ma, X.; Zhao, H.; Zuo, Z.; et al. The spatiotemporal characteristics of water quality and phytoplankton community in a shallow eutrophic lake: Implications for submerged vegetation restoration. Sci. Total Environ. 2022, 821, 153460. [Google Scholar] [CrossRef]
- Cheng, W.; Hwang, S.; Guo, Q.; Qian, L.; Liu, W.; Yu, Y.; Liu, L.; Tao, Y.; Cao, H. The Special and General Mechanism of Cyanobacterial Harmful Algal Blooms. Microorganisms 2023, 11, 987. [Google Scholar] [CrossRef]
- Wang, Z.; Akbar, S.; Sun, Y.; Gu, L.; Zhang, L.; Lyu, K.; Huang, Y.; Yang, Z. Cyanobacterial dominance and succession: Factors, mechanisms, predictions, and managements. J. Environ. Manag. 2021, 297, 113281. [Google Scholar] [CrossRef] [PubMed]
- Van de Waal, D.B.; Gsell, A.S.; Harris, T.; Paerl, H.W.; de Senerpont Domis, L.N.; Huisman, J. Hot summers raise public awareness of toxic cyanobacterial blooms. Water Res. 2023, 249, 120817. [Google Scholar] [CrossRef]
- Dokulil, M.T.; Teubner, K. Cyanobacterial dominance in lakes. Hydrobiologia 2000, 438, 1–12. [Google Scholar] [CrossRef]
- Foysal, M.J.; Timms, V.; Neilan, B.A. Dynamics of the benthic and planktic microbiomes in a Planktothrix-dominated toxic cyanobacterial bloom in Australia. Water Res. 2023, 249, 120980. [Google Scholar] [CrossRef]
- Lefler, F.W.; Barbosa, M.; Zimba, P.V.; Smyth, A.R.; Berthold, D.E.; Laughinghouse, H.D. Spatiotemporal diversity and community structure of cyanobacteria and associated bacteria in the large shallow subtropical Lake Okeechobee (Florida, United States). Front. Microbiol. 2023, 14, 1219261. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Climate. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Yan, G.; Wang, X.; Huang, D.; Li, L. Spatiotemporal Distribution Pattern of Phytoplankton Community and Its Main Driving Factors in Dongting Lake, China—A Seasonal Study from 2017 to 2019. Water 2022, 14, 1674. [Google Scholar] [CrossRef]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony formation in the cyanobacterium Microcystis. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1399–1420. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lu, N.; Gu, L.; Sun, Y.; Zhang, L.; Huang, Y.; Chen, Y.; Yang, Z. Daphnia enhances relative reproductive allocation in response to toxic microcystis: Changes in the performance of parthenogenetic and sexual reproduction. Environ. Pollut. 2020, 259, 113890. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhu, B.; Struewing, I.; Xu, N.; Duan, S. Nitrogen-phosphorus-associated metabolic activities during the development of a cyanobacterial bloom revealed by metatranscriptomics. Sci. Rep. 2019, 9, 2480. [Google Scholar] [CrossRef]
- He, P.; Cai, X.; Chen, K.; Fu, X. Identification of small RNAs involved in nitrogen fixation in Anabaena sp. PCC 7120 based on RNA-seq under steady state conditions. Ann. Microbiol. 2020, 70, 4. [Google Scholar] [CrossRef]
- Yu, X.P.; Chen, Y.W.; Liu, J.F.; Wang, J.J.; Xu, G.F.; Zou, H.Y.; Chen, N.; Xia, Y. Analysis on the Current Situation of Phytoplankton in the Typical River-Lake Ecotone of Lake Poyang. Huan Jing Ke Xue 2023, 44, 4954–4964. [Google Scholar] [CrossRef]
- Chen, S.; He, H.; Zong, R.; Liu, K.; Yan, M.; Xu, L.; Miao, Y. Geographical Patterns of Algal Communities Associated with Different Urban Lakes in China. Int. J. Environ. Res. Public. Health 2020, 17, 1009. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, L.; Li, Y.; Lin, Q.; He, C.; Huang, S.; Li, H.; Zhang, X.; Liu, B.; Ge, F.; et al. The changing characteristics of phytoplankton community and biomass in subtropical shallow lakes: Coupling effects of land use patterns and lake morphology. Water Res. 2021, 200, 117235. [Google Scholar] [CrossRef]
- Mao, Z.; Gu, X.; Cao, Y.; Luo, J.; Zeng, Q.; Chen, H.; Jeppesen, E. Pelagic energy flow supports the food web of a shallow lake following a dramatic regime shift driven by water level changes. Sci. Total Environ. 2021, 756, 143642. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Shi, K.; Zhang, M.; Han, T.; Lai, L.; Zhan, P. Sensitivity of phytoplankton to climatic factors in a large shallow lake revealed by column-integrated algal biomass from long-term satellite observations. Water Res. 2021, 207, 117786. [Google Scholar] [CrossRef] [PubMed]
- Li, X.T.; Liu, B.; Wang, Y.M.; Yang, Y.G.; Liang, R.F.; Peng, F.J.; Xue, S.D.; Zhu, Z.X.; Li, K.F. Hydrodynamic and environmental characteristics of a tributary bay influenced by backwater jacking and intrusions from a main reservoir. Hydrol. Earth Sys. Sc. 2020, 24, 5057–5076. [Google Scholar] [CrossRef]
- Liro, M. Dam reservoir backwater as a field-scale laboratory of human-induced changes in river biogeomorphology: A review focused on gravel-bed rivers. Sci. Total Environ. 2019, 651, 2899–2912. [Google Scholar] [CrossRef] [PubMed]
- Holbach, A.; Bi, Y.; Yuan, Y.; Wang, L.; Zheng, B.; Norra, S. Environmental water body characteristics in a major tributary backwater of the unique and strongly seasonal Three Gorges Reservoir, China. Environ. Sci. Proc. Imp. 2015, 17, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Bouwman, A.F.; Yu, Z.; Liu, J. Implications of eutrophication for biogeochemical processes in the Three Gorges Reservoir, China. Reg. Environ. Chang. 2018, 19, 55–63. [Google Scholar] [CrossRef]
- Yang, Z.; Cheng, B.; Xu, Y.; Liu, D.; Ma, J.; Ji, D. Stable isotopes in water indicate sources of nutrients that drive algal blooms in the tributary bay of a subtropical reservoir. Sci. Total Environ. 2018, 634, 205–213. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Z.; Guo, J.; Liu, J.; Wu, S. Cyanobacteria in a tributary backwater area in the Three Gorges Reservoir, China. Inland. Waters 2016, 6, 77–88. [Google Scholar] [CrossRef]
- Zhu, K.; Bi, Y.; Hu, Z. Responses of phytoplankton functional groups to the hydrologic regime in the Daning River, a tributary of Three Gorges Reservoir, China. Sci. Total Environ. 2013, 450, 169–177. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, J.; Sun, F.; Zhang, F.; Chen, Y.; Ding, C.; Shi, J.; Li, Y.; Yao, L. Planktonic fungal community structures and their relationship to water quality in the Danjiangkou Reservoir, China. Sci. Rep. 2018, 8, 10596. [Google Scholar] [CrossRef]
- Li, S.; Shi, W.; You, M.; Zhang, R.; Kuang, Y.; Dang, C.; Sun, W.; Zhou, Y.; Wang, W.; Ni, J. Antibiotics in water and sediments of Danjiangkou Reservoir, China: Spatiotemporal distribution and indicator screening. Environ. Pollut. 2019, 246, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, Y.; Li, W.; Guo, X.; Xiao, C.; Zhao, T. High-throughput sequencing of diatom community, its spatial and temporal variation and interrelationships with physicochemical factors in Danjiangkou Reservoir. Water 2022, 14, 1609. [Google Scholar] [CrossRef]
- Xin, X.; Zhang, H.; Lei, P.; Tang, W.; Yin, W.; Li, J.; Zhong, H.; Li, K. Algal blooms in the middle and lower Han River: Characteristics, early warning and prevention. Sci. Total Environ. 2020, 706, 135293. [Google Scholar] [CrossRef] [PubMed]
- Han, B.P.; Liu, Z. Tropical and sub-tropical reservoir limnology in China: Theory and practice. In River Basin Environments and Ecological Succession in Danjiangkou Reservoir; Zheng, J., Han, D., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 211–241. [Google Scholar]
- Ostle, C.; Paxman, K.; Graves, C.A.; Arnold, M.; Artigas, L.F.; Atkinson, A.; Aubert, A.; Baptie, M.; Bear, B.; Bedford, J.; et al. The Plankton Lifeform Extraction Tool: A digital tool to increase the discoverability and usability of plankton time-series data. Earth Syst. Sci. Data 2021, 13, 5617–5642. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, J.; Wang, X.C.; Zhang, Y.; Liu, H.; Zhou, B.; Pan, P.; Liu, Y.; Ding, F. Relationship between phytoplankton community and environmental factors in landscape water with high salinity in a coastal city of China. Environ. Sci. Pollut. Res. Int. 2018, 25, 28460–28470. [Google Scholar] [CrossRef] [PubMed]
- Galván, K.; Fleeger, J.W.; Fry, B. Stable isotope addition reveals dietary importance of phytoplankton and microphytobenthos to saltmarsh infauna. Mar. Ecol. Prog. Ser. 2008, 359, 37–49. [Google Scholar] [CrossRef]
- GB 3838-2002; Environmental Quality Standard for Surface Water. The State Environmental Protection Administration (SEPA) and the General Administration of Quality Supervision, Inspection and Quarantine(AQSIQ) of the People’s Republic of China: Beijing, China, 2002.
- Weng, J.; Xu, H. Atlas of Common Freshwater Algae in China; Science Technology Press: Shanghai, China, 2010. [Google Scholar]
- Thuesen, E.V.; Ruggiero, M.A.; Gordon, D.P.; Orrell, T.M.; Bailly, N.; Bourgoin, T.; Brusca, R.C.; Cavalier-Smith, T.; Guiry, M.D.; Kirk, P.M. A Higher Level Classification of All Living Organisms. PLoS ONE 2015, 10, e0130114. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Fresh Water Phytoplankton; Cambridge University Press: London, UK, 1984. [Google Scholar]
- Johson, R.W.; Harris, R.C. Remote sensing for water quality and biological measurements in coastal waters. Photogramm. Eng. Remote Sens. 1980, 46, 77–85. [Google Scholar]
- Cheng, C.; Wei, Y.; Lv, G.; Xu, N. Remote sensing estimation of chlorophyll-a concentration in Taihu Lake considering spatial and temporal variations. Environ. Monit. Assess. 2019, 191, 84. [Google Scholar] [CrossRef]
- Kaneko, H.; Endo, H.; Henry, N.; Berney, C.; Mahé, F.; Poulain, J.; Labadie, K.; Beluche, O.; El Hourany, R.; Chaffron, S.; et al. Predicting global distributions of eukaryotic plankton communities from satellite data. ISME Commun. 2023, 3, 101. [Google Scholar] [CrossRef]
- Tuygun, G.T.; Salgut, S.; Elçi, A. Long-term spatial-temporal monitoring of eutrophication in Lake Burdur using remote sensing data. Water Sci. Technol. 2023, 87, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Makwinja, R.; Inagaki, Y.; Sagawa, T.; Obubu, J.P.; Habineza, E.; Haaziyu, W. Monitoring trophic status using in situ data and Sentinel-2 MSI algorithm: Lesson from Lake Malombe, Malawi. Environ. Sci. Pollut. Res. 2023, 30, 29755–29772. [Google Scholar] [CrossRef] [PubMed]
- Molkov, A.A.; Fedorov, S.V.; Pelevin, V.V.; Korchemkina, E.N. Regional models for high-resolution retrieval of chlorophyll a and TSM concentrations in the Gorky Reservoir by Sentinel-2 imagery. Remote Sens. 2019, 11, 1215. [Google Scholar] [CrossRef]
- Sakuno, Y.; Maeda, A.; Mori, A.; Ono, S.; Ito, A. A simple red tide monitoring method using Sentinel-2 data for sustainable management of brackish lake Koyama-ike. Water 2019, 11, 1044. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Ma, D.; Yin, H.; Wu, C. Remote Sensing Estimation of Water Quality Parameters in Gaoyou Lakebased on Sentinel-2 MSIData. Environ. Sci. Manag. 2022, 47, 77–81. [Google Scholar] [CrossRef] [PubMed]
- HANG, X.; CAO, Y.; HANG, R.R.; XU, M.; HUO, Y.; SUN, L.X.; ZHU, S.H. Estimation model of chlorophyll-a concentration in Taihu Lake based on random forest algcrithm and Gaofen Observations. Meteor. Mon. 2021, 47, 1525–1536. [Google Scholar] [CrossRef]
- MA, C. Remote sensing inversion of chlorophyll a, suspended solids and salt in Songnen Plain. Water Sav. Irrig. 2022, 7, 95–101. [Google Scholar]
- SL88-1994; Determination of Chlorophyll (Spectrophotometric Method). Ministry of Water Resources of the People’s Republic of China: Beijing, China, 1995.
- Lampitt, R.S.; Wishner, K.F.; Turley, C.M.; Angel, M.V. Marine snow studies in the Northeast Atlantic Ocean: Distribution, composition and role as a food source for migrating plankton. Mar. Biol. 1993, 116, 702. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- He, R.; Luo, H.; He, N.; Chen, W.; Yang, F.; Huang, W.; Li, N.; Sun, L.; Peng, S. Phytoplankton Communities and Their Relationship with Environmental Factors in the Waters around Macau. Int. J. Environ. Res. Public. Health 2022, 19, 7788. [Google Scholar] [CrossRef]
- Xu, Y.; Xiang, Z.; Rizo, E.Z.; Naselli-Flores, L.; Han, B.P. Combination of linear and nonlinear multivariate approaches effectively uncover responses of phytoplankton communities to environmental changes at regional scale. J. Environ. Manag. 2022, 305, 114399. [Google Scholar] [CrossRef]
- Faquim, R.C.P.; Machado, K.B.; Teresa, F.B.; Oliveira, P.H.F.; Granjeiro, G.F.; Galli Vieira, L.C.; Nabout, J.C. Shortcuts for biomonitoring programs of stream ecosystems: Evaluating the taxonomic, numeric, and cross-taxa congruence in phytoplankton, periphyton, zooplankton, and fish assemblages. PLoS ONE 2021, 16, e0258342. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P. Is the Mantel correlogram powerful enough to be useful in ecological analysis? A simulation study. Ecology 2012, 93, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- OECD. Eutrophication of Waters Monitoring, Assessment and Control; Techical Report OECD; Organization for Economic Cooperation and Development, Environmental Directorate, OECD: Paris, France, 1982; pp. 75–85. [Google Scholar]
- Tan, X.; Xia, X.; Cheng, X.; Zhang, Q. Temporal and spatial pattern of phytoplankton community and its biodiversity indices in the Danjiangkou Reservoir. Huanjing Kexue 2011, 32, 2875–2882. [Google Scholar]
- Li, N.; Wang, J.; Yin, W.; Jia, H.; Xu, J.; Hao, R.; Zhong, Z.; Shi, Z. Linking water environmental factors and the local watershed landscape to the chlorophyll a concentration in reservoir bays. Sci. Total Environ. 2021, 758, 143617. [Google Scholar] [CrossRef] [PubMed]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG-model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar] [CrossRef]
- Zhang, N.; Zang, S. Characteristics of phytoplankton distribution for assessment of water quality in the Zhalong Wetland, China. Int. J. Env. Sci. Technol. 2015, 12, 3657–3664. [Google Scholar] [CrossRef]
- Gao, M.; Li, Y.; Li, Y.; Sun, A.; Tian, S.; Zhang, C.; Geng, Y.; Li, L. Characteristics of Phytoplankton Community Structure and Their Relationships with Environmental Factors in Autumn in Qinhe River Basin of Jincheng Region. Huan Jing Ke Xue 2022, 43, 4576–4586. [Google Scholar] [CrossRef]
- Fai, P.B.A.; Kenko, D.B.N.; Tchamadeu, N.N.; Mbida, M.; Korejs, K.; Riegert, J. Use of multivariate analysis to identify phytoplankton bioindicators of stream water quality in the monomodal equatorial agroecological zone of Cameroon. Environ. Monit. Assess. 2023, 195, 788. [Google Scholar] [CrossRef]
- Kuang, Q.; Ming, M.; Yu, H.; Jie, Z. Study on the evaluation and treatment of lake eutrophication by means of algae biology. J. Saf. Environ./Anquan Yu Huanjing Xuebao 2005, 5, 87–91. [Google Scholar]
- Zhang, L.; Wang, Z.; Wang, N.; Gu, L.; Sun, Y.; Huang, Y.; Chen, Y.; Yang, Z. Mixotrophic Ochromonas Addition Improves the Harmful Microcystis-Dominated Phytoplankton Community in In Situ Microcosms. Env. Sci. Technol. 2020, 54, 4609–4620. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, H.; Wang, S.; Wang, Y.; Zhang, R.; Hu, X.; Ye, J. Effect of propionamide on the growth of Microcystis flos-aquae colonies and the underlying physiological mechanisms. Sci. Total Environ. 2018, 630, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.M.; Zhang, J.Y.; Guan, R.; Hale, L.; Chen, N.; Li, M.; Lu, Z.H.; Ge, Q.Y.; Yang, Y.F.; Zhou, J.Z.; et al. Alternate succession of aggregate-forming cyanobacterial genera correlated with their attached bacteria by co-pathways. Sci. Total Environ. 2019, 688, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, D.; Xiao, B.; Dauta, A.; Liu, Y. Microcystin-RR induces physiological stress and cell death in the cyanobacterium Aphanizomenon sp. DC01 isolated from Lake Dianchi, China. Fundam. Appl. Limnol. 2008, 173, 111. [Google Scholar] [CrossRef]
- Leflaive, J.P.; Ten-Hage, L. Algal and cyanobacterial secondary metabolites in freshwaters: A comparison of allelopathic compounds and toxins. Freshw. Biol. 2007, 52, 199–214. [Google Scholar] [CrossRef]
- Cao, H.; Kong, F.; Luo, L.; Shi, X.; Zhang, X. Effects of Wind and Wind-Induced Waves on Vertical Phytoplankton Distribution and Surface Blooms of Microcystis aeruginosa in Lake Taihu. J. Freshw. Ecol. 2006, 21, 231–238. [Google Scholar] [CrossRef]
- Walsby, A.E.; Hayes, P.K.; Boje, R.; Stal, L.J. The selective advantage of buoyancy provided by gas vesicles for planktonic cyanobacteria in the Baltic Sea. New Phytol. 1997, 136, 407–417. [Google Scholar] [CrossRef]
- Maberly, S.C.; Chao, A.; Finlay, B.J. Seasonal Patterns of Phytoplankton Taxon Richness in Lakes: Effects of Temperature, Turnover and Abundance. Protist 2022, 173, 125925. [Google Scholar] [CrossRef]
- Yang, J.; Wang, F.; Lv, J.; Liu, Q.; Nan, F.; Liu, X.; Xu, L.; Xie, S.; Feng, J. Interactive effects of temperature and nutrients on the phytoplankton community in an urban river in China. Environ. Monit. Assess. 2019, 191, 688. [Google Scholar] [CrossRef]
- Reynolds, C.S. Phytoplankton periodicity: The interactions of form, function and environmental variability. Freshw. Biol. 1984, 14, 111–142. [Google Scholar] [CrossRef]
- Schabhuttl, S.; Hingsamer, P.; Weigelhofer, G.; Hein, T.; Weigert, A.; Striebel, M. Temperature and species richness effects in phytoplankton communities. Oecologia 2013, 171, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.S. Ecology of Phytoplankton; Cambridge University Press: Cambridge, CA, USA, 2006. [Google Scholar]
- Zhong, Y.; Su, Y.; Zhang, D.; She, C.; Chen, N.; Chen, J.; Yang, H.; Balaji-Prasath, B. The spatiotemporal variations in microalgae communities in vertical waters of a subtropical reservoir. J. Environ. Manag. 2022, 317, 115379. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Fulton, R.S., 3rd; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Fao, G. Hypothesis on cyanobacteria bloom-forming mechanism in large shallow eutrophic lakes. Acta Ecol. Sin./Shengtai Xuebao 2005, 25, 589–596. [Google Scholar]
- Li, T.; Fan, X.; Cai, M.; Jiang, Y.; Wang, Y.; He, P.; Ni, J.; Mo, A.; Peng, C.; Liu, J. Advances in investigating microcystin-induced liver toxicity and underlying mechanisms. Sci. Total Environ. 2023, 905, 167. [Google Scholar] [CrossRef]









| Band/Band Combination | Correlation Coefficient | Band/Band Combination | Correlation Coefficient |
|---|---|---|---|
| B2 | −0.059 | B8/B6 | −0.131 |
| B3 | −0.090 | B8/B7 | −0.268 |
| B4 | −0.0723 | B7/B6 | 0.274 |
| B5 | 0.010 | B7/B5 | 0.175 |
| B6 | 0.018 | B6/B5 | 0.095 |
| B7 | 0.036 | B8a/B5 | 0.162 |
| B8 | 0.032 | B8a/B6 | 0.279 |
| B8a | 0.041 | B8a/B7 | 0.161 |
| 2.5 × (B8 − B4)/(B8 + 6 × B4 − 7.5 × B2 + 1) | 0.312 | B8a/B8 | 0.386 |
| (B8 − B4)/(B8 + B4) | 0.271 | (1/B4 − 1/B5) × B6 | 0.598 |
| B8 − B4 | 0.319 | (1/B4 − 1/B5) × B7 | 0.539 |
| B8/B4 | 0.252 | (1/B4 − 1/B5) × B8 | 0.718 |
| B8/B5 | 0.030 | (1/B4 − 1/B5) × B8a | 0.502 |
| Season | Mean (μg/L) | 5% Trimmed Mean (μg/L) | Min (μg/L) | Max (μg/L) | STD (μg/L) |
|---|---|---|---|---|---|
| Spring (From March to May) | 28.77 | 27.47 | 14.67 | 121.14 | 9.63 |
| Summer (From June to August) | 75.26 | 63.96 | 1.21 | 499.57 | 69.23 |
| Autumn (From September to November) | 48.61 | 41.26 | 13.84 | 496.67 | 46.70 |
| Winter (From December to February) | 16.93 | 16.21 | 10.81 | 135.35 | 5.57 |
| Class | Dominant Species | Dominance Values (Y) | |
|---|---|---|---|
| S1 | S2 | ||
| Bacillariophyceae | Cyclotella meneghiniana | 0.039 | 0.033 |
| Synedra sp. | 0.024 | - | |
| Cyanophyceae | Dactylococcopsis acicularis | 0.137 | - |
| Microcystis aeruginosa | - | 0.175 | |
| Oscillatoria tenuis | - | 0.116 | |
| Chlorophyceae | Scenedesmus sp. | 0.023 | 0.049 |
| Chlorella vulgaris | 0.038 | - | |
| Dinophyceae | Peridinium bipes | 0.033 | |
| Cryptophyceae | Chroomonas acuta | - | 0.082 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Luo, L.; Mei, L.; Zeng, H. Temporal and Spatial Distribution of Phytoplankton and Role of Environment Factors in the Shending River Backwater in the Danjiangkou Reservoir Area. Water 2024, 16, 326. https://doi.org/10.3390/w16020326
Wang B, Luo L, Mei L, Zeng H. Temporal and Spatial Distribution of Phytoplankton and Role of Environment Factors in the Shending River Backwater in the Danjiangkou Reservoir Area. Water. 2024; 16(2):326. https://doi.org/10.3390/w16020326
Chicago/Turabian StyleWang, Bo, Lingxi Luo, Liyong Mei, and Hui Zeng. 2024. "Temporal and Spatial Distribution of Phytoplankton and Role of Environment Factors in the Shending River Backwater in the Danjiangkou Reservoir Area" Water 16, no. 2: 326. https://doi.org/10.3390/w16020326
APA StyleWang, B., Luo, L., Mei, L., & Zeng, H. (2024). Temporal and Spatial Distribution of Phytoplankton and Role of Environment Factors in the Shending River Backwater in the Danjiangkou Reservoir Area. Water, 16(2), 326. https://doi.org/10.3390/w16020326






