Characteristics of Greenhouse Gas Emissions from Constructed Wetlands Vegetated with Myriophyllum aquatic: The Effects of Influent C/N Ratio and Microbial Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction and Operation of SFCWs
2.2. Sample Collection and Analysis
2.3. Illumina MiSeq Sequencing Analysis
2.4. Statistical Analysis
3. Results and Discussion
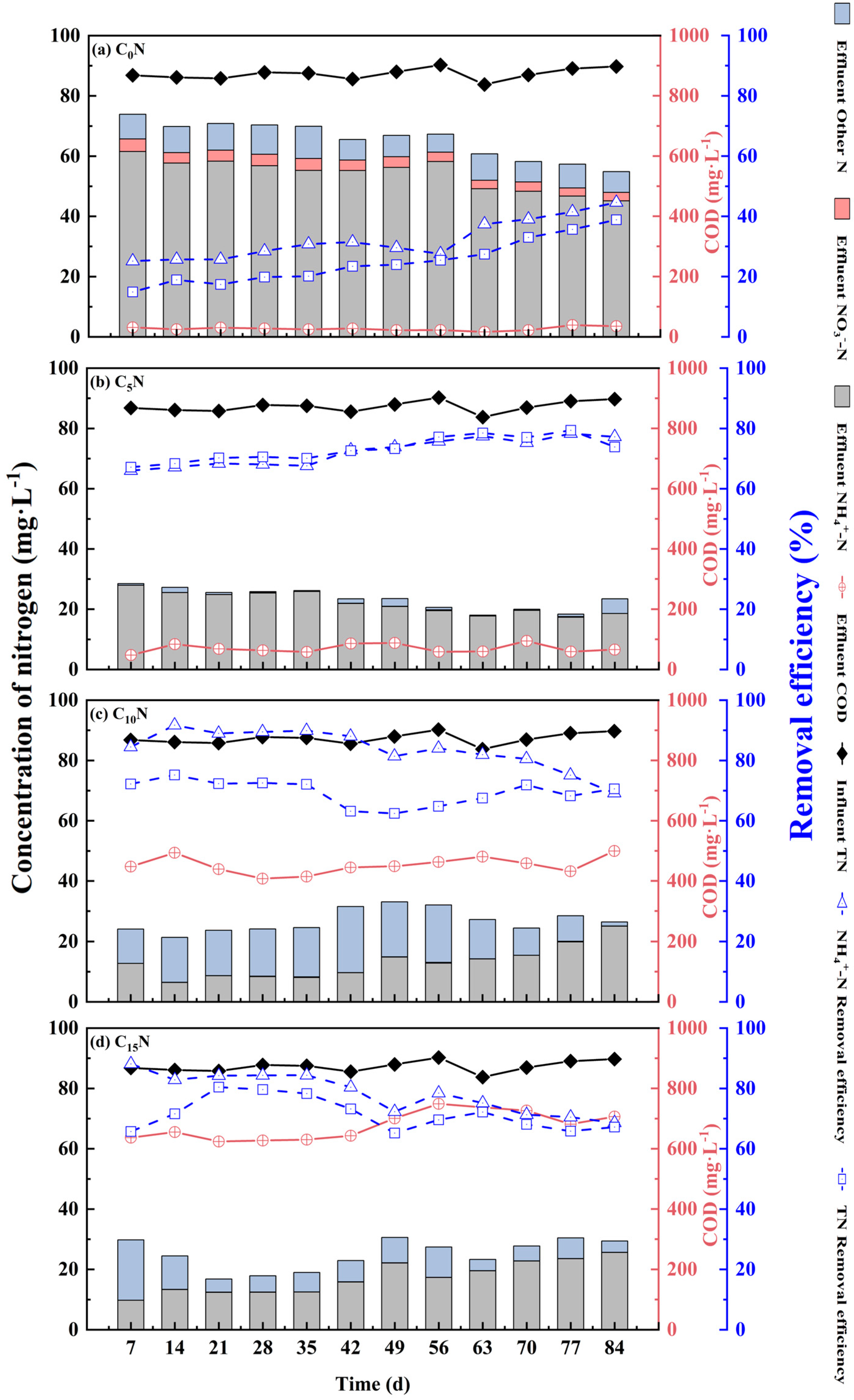
3.1. Effect of Influent C/N Ratio on Pollutant Removal from SFCWs
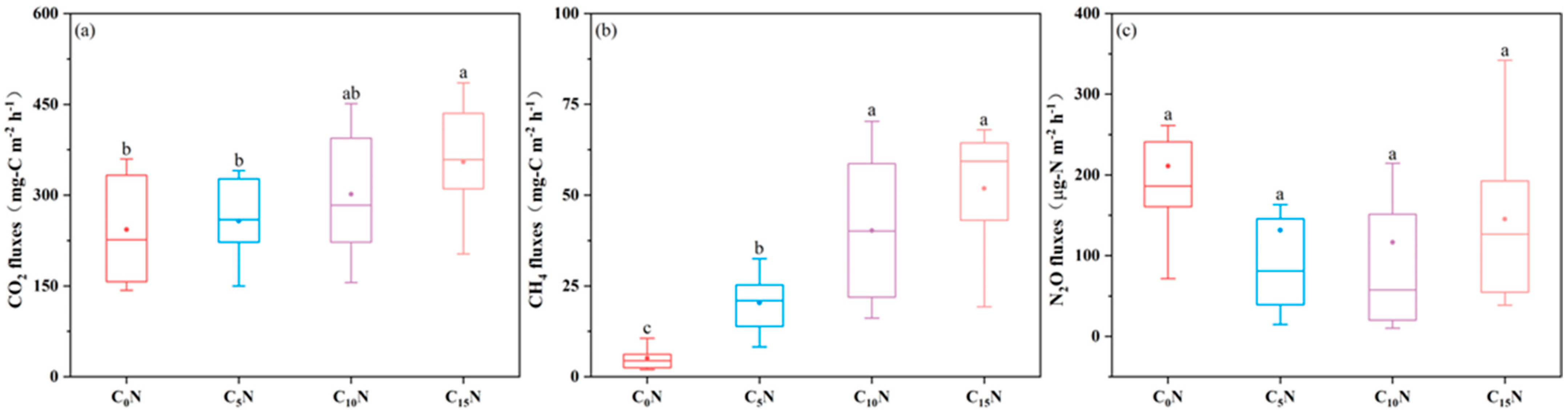
3.2. Effect of C/N Ratio on GHG Emissions from SFCWs
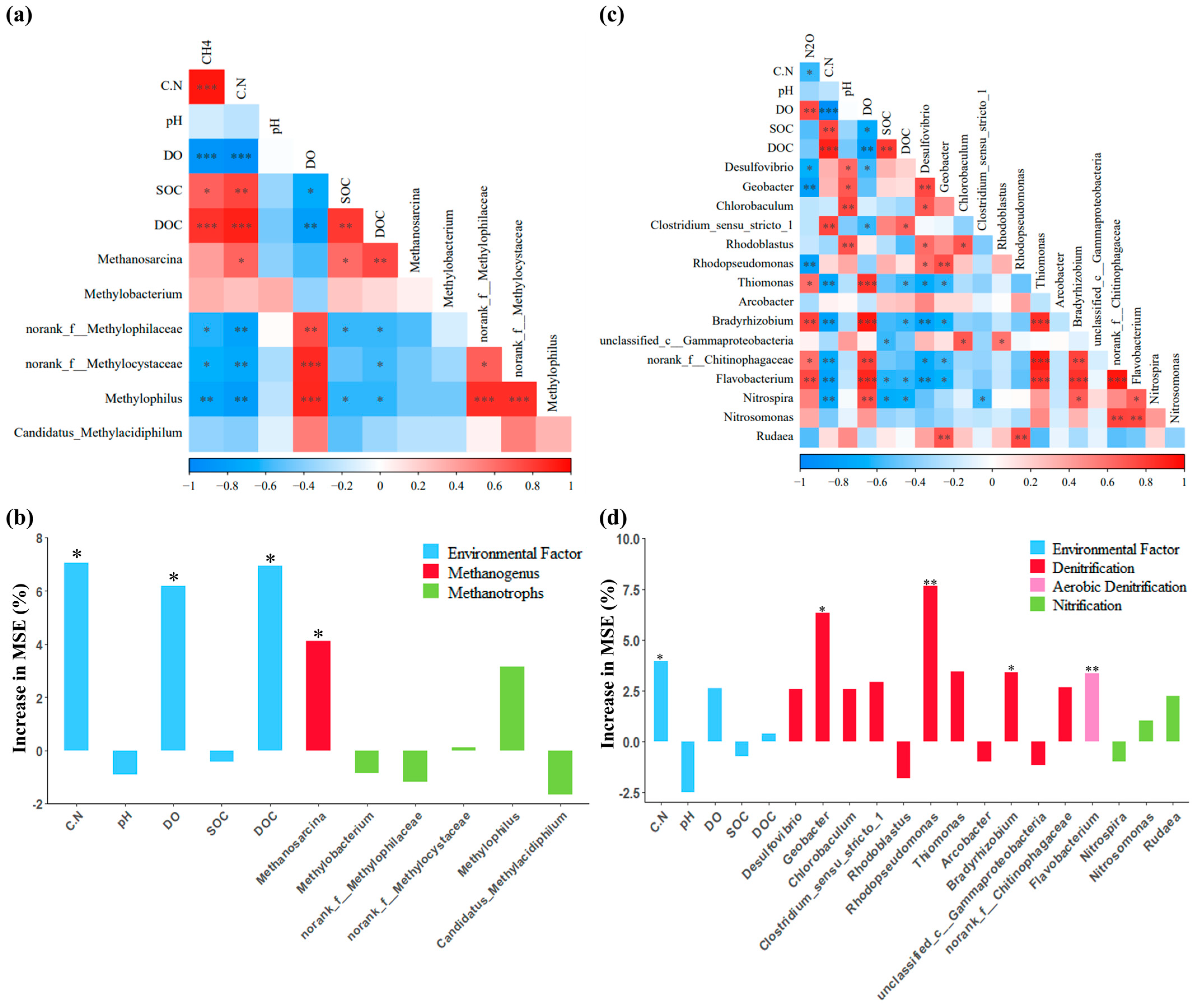
3.3. Effect of C/N Ratio on Microbial Community from SFCWs
3.4. Drivers of CH4 and N2O Emissions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.Y.; Xu, Y.X.; Chai, B.B. Effect of Temperature on Microorganisms and Nitrogen Removal in a Multi-Stage Surface Flow Constructed Wetland. Water 2023, 15, 1265. [Google Scholar] [CrossRef]
- Maltais-Landry, G.; Maranger, R.; Brisson, J.; Chazarenc, F. Greenhouse gas production and efficiency of planted and artificially aerated constructed wetlands. Environ. Pollut. 2009, 157, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.X.; Jiang, C.C.; Xu, S.J.; Yu, X.J.; Yin, X.L.; Wang, J.L.; Maihaiti, M.; Wang, C.; Zheng, X.X.; Zhuang, X.L. Greenhouse Gases Emissions of Constructed Wetlands: Mechanisms and Affecting Factors. Water 2023, 15, 2871. [Google Scholar] [CrossRef]
- Wu, H.M.; Wang, R.G.; Yan, P.H.; Wu, S.B.; Chen, Z.B.; Zhao, Y.Q.; Chen, C.; Hu, Z.; Zhuang, L.L.; Guo, Z.Z. Constructed wetlands for pollution control. Nat. Rev. Earth. Environ. 2023, 4, 218–234. [Google Scholar] [CrossRef]
- Jahangir, M.M.; Richards, K.G.; Healy, M.G.; Gill, L.; Müller, C.; Johnston, P.; Fenton, O. Carbon and nitrogen dynamics and greenhouse gas emissions in constructed wetlands treating wastewater: A review. Hydrol. Earth Syst. Sci. 2016, 20, 109–123. [Google Scholar] [CrossRef]
- Maucieri, C.; Barbera, A.C.; Vymazal, J.; Borin, M. A review on the main affecting factors of greenhouse gases emission in constructed wetlands. Agric. For. Meteorol. 2017, 236, 175–193. [Google Scholar] [CrossRef]
- Xu, D.; Sun, H.M.; Wang, J.; Wang, N.; Zuo, Y.J.; Mosa, A.A.; Yin, X.Q. Global trends and current advances regarding greenhouse gases in constructed wetlands: A bibliometric-based quantitative review over the last 40 years. Ecol. Eng. 2023, 193, 107018. [Google Scholar] [CrossRef]
- Zhi, W.; Ji, G.D. Quantitative response relationships between nitrogen transformation rates and nitrogen functional genes in a tidal flow constructed wetland under C/N ratio constraints. Water Res. 2023, 64, 32–41. [Google Scholar] [CrossRef]
- Li, H.F.; Liu, F.; Luo, P.; Chen, X.; Chen, J.L.; Huang, Z.R.; Peng, J.W.; Xiao, R.L.; Wu, J.S. Stimulation of optimized influent C:N ratios on nitrogen removal in surface flow constructed wetlands: Performance and microbial mechanisms. Sci. Total Environ. 2019, 694, 133575. [Google Scholar] [CrossRef]
- Niu, T.T.; Zhu, H.; Shutes, B.; Yu, J.; He, C.G.; Hou, S.N.; Cui, H.; Yan, B.X. Wastewater treatment performance and gaseous emissions in MFC-CWs affected by influent C/N ratios. Chem. Eng. J. 2023, 461, 141876. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Yan, B.X.; Shutes, B.; Tian, L.P.; Wen, H.Y. Optimal influent COD/N ratio for obtaining low GHG emissions and high pollutant removal efficiency in constructed wetlands. J. Clean. Prod. 2020, 267, 122003. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, S.B.; Wang, R.G.; Wu, H.M. Nitrogen removal in response to the varying C/N ratios in subsurface flow constructed wetland microcosms with biochar addition. Environ. Sci. Pollut. Res. 2019, 26, 3382–3391. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.D.; Dai, N.; Hu, X.J.; Cheng, C.; Xie, H.J.; Hu, Z.; Liang, S.; Zhang, J. New insights into the effects of wetland plants on nitrogen removal pathways in constructed wetlands with low C/N ratio wastewater: Contribution of partial denitrification-anammox. Water Res. 2023, 243, 120277. [Google Scholar] [CrossRef]
- Li, M.; Wu, H.M.; Zhang, J.; Ngo, H.H.; Guo, W.S.; Kong, Q. Nitrogen removal and nitrous oxide emission in surface flow constructed wetlands for treating sewage treatment plant effluent: Effect of C/N ratios. Bioresour. Technol. 2017, 240, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.O.; Tian, Y.M.; Liu, H.; Zhao, X.H.; Peng, S. The influence of incorporating microbial fuel cells on greenhouse gas emissions from constructed wetlands. Sci. Total Environ. 2019, 656, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, H.; Li, B.; Wang, D.; Zhao, Y.J.; Zheng, Z. Effects of influent C/N ratios on CO2 and CH4 emissions from vertical subsurface flow constructed wetlands treating synthetic municipal wastewater. J. Hazard. Mater. 2012, 203, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q.L. Methane emissions from wetlands: Biogeochemical, microbial, and modeling perspectives from local to global scales. Global. Chang. Biol. 2013, 19, 1325–1346. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.L.; Chen, J.D.; Wang, G.L.; Chen, H.F.; Huang, J.J.; Li, Y.F.; Wang, W.M.; Song, F.M.; Ma, Y.J.; Wang, Q. Recent advances in constructed wetlands methane reduction: Mechanisms and methods. Front. Microbiol. 2023, 14, 1106332. [Google Scholar] [CrossRef]
- Mander, Ü.; Tournebize, J.; Espenberg, N.; Chaumont, C.; Torga, R.; Garnier, J.; Muhel, M.; Maddison, M.; Lebrun, J.; Uher, E. High denitrification potential but low nitrous oxide emission in a constructed wetland treating nitrate-polluted agricultural run-off. Sci. Total Environ. 2021, 779, 146614. [Google Scholar] [CrossRef]
- Lyu, W.L.; Huang, L.; Xiao, G.Q.; Chen, Y.C. Effects of carbon sources and COD/N ratio on N2O emissions in subsurface flow constructed wetlands. Bioresour. Technol. 2017, 245, 171–181. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, S.N.; Wang, Y.; Li, Y.; Xiao, R.L.; Li, H.F.; He, Y.; Zhang, M.M.; Wang, D.; Li, X. Nitrogen removal and mass balance in newly-formed Myriophyllum aquaticum mesocosm during a single 28-day incubation with swine wastewater treatment. J. Environ. Manag. 2016, 166, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Liu, F.; Zhang, S.N.; Li, H.F.; Yao, R.; Jiang, Q.W.; Xiao, R.L.; Wu, J.S. Nitrogen removal and recovery from lagoon-pretreated swine wastewater by constructed wetlands under sustainable plant harvesting management. Bioresour. Technol. 2018, 258, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.N.; Liu, F.; Xiao, R.L.; Li, Y.; He, Y.; Wu, J.S. Effects of vegetation on ammonium removal and nitrous oxide emissions from pilot-scale drainage ditches. Aquat. Bot. 2016, 130, 37–44. [Google Scholar] [CrossRef]
- Du, Y.Y.; Pan, K.X.; Yu, C.C.; Luo, B.; Gu, W.L.; Sun, H.Y.; Min, Y.; Liu, D.; Geng, Y.; Han, W.J. Plant diversity decreases net global warming potential integrating multiple functions in microcosms of constructed wetlands. J. Clean. Prod. 2018, 184, 718–726. [Google Scholar] [CrossRef]
- Shen, Z.Q.; Zhou, Y.X.; Liu, J.; Xiao, Y.; Cao, R.; Wu, F.P. Enhanced removal of nitrate using starch/PCL blends as solid carbon source in a constructed wetland. Bioresour. Technol. 2015, 175, 239–244. [Google Scholar] [CrossRef]
- Huang, L.; Wang, N.; Deng, C.R.; Liang, Y.K.; Wang, Q.H.; Liu, M.L.; Chen, Y.C. Interactive effect of carbon source with influent COD/N on nitrogen removal and microbial community structure in subsurface flow constructed wetlands. J. Environ. Manag. 2019, 250, 109491. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.H.; Song, X.S. High-effective denitrification of low C/N wastewater by combined constructed wetland and biofilm-electrode reactor (CW–BER). Bioresour. Technol. 2016, 203, 245–251. [Google Scholar] [CrossRef]
- Xu, J.; Huang, X.X.; Luo, P.; Zhang, M.M.; Li, H.F.; Gong, D.L.; Liu, F.; Xiao, R.L.; Wu, J.S. Root exudates release from Myriophyllum aquaticum and effects on nitrogen removal by constructed wetlands. J. Clean. Prod. 2022, 375, 134095. [Google Scholar] [CrossRef]
- Zhu, H.; Yan, B.X.; Xu, Y.Y.; Guan, J.N.; Liu, S.Y. Removal of nitrogen and COD in horizontal subsurface flow constructed wetlands under different influent C/N ratios. Ecol. Eng. 2014, 63, 58–63. [Google Scholar] [CrossRef]
- Rhaman, M.E.; Halmi, M.I.E.B.; Samad, M.Y.B.A.; Uddin, M.K.; Mahmud, K.; Shukor, M.Y.A.; Abdullah, S.R.S.; Shamsuzzaman, S.M. Design, Operation and Optimization of Constructed Wetland for Removal of Pollutant. Int. J. Environ. Res. Public Health 2020, 17, 8839. [Google Scholar] [CrossRef]
- Mander, Ü.; Dotro, G.; Ebie, Y.; Towprayoon, S.; Chiemchaisri, C.; Nogueira, S.F.; Jamsranjav, B.; Kasak, K.; Truu, J.; Tournebize, J. Greenhouse gas emission in constructed wetlands for wastewater treatment: A review. Ecol. Eng. 2014, 66, 19–35. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Zhang, Y.J.; Ge, Z.G.; Hu, C.W.; Zhang, H. Effects of influent C/N ratios on wastewater nutrient removal and simultaneous greenhouse gas emission from the combinations of vertical subsurface flow constructed wetlands and earthworm eco-filters for treating synthetic wastewater. Environ. Sci.-Proc. Imp. 2014, 16, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.T.; Xue, H.P.; Wang, M.X.; Feng, X.J.; Lee, H. The role of microbial electrogenesis in regulating methane and nitrous oxide emissions from constructed wetland-microbial fuel cell. Int. J. Hydrogen Energy 2022, 47, 27279–27292. [Google Scholar] [CrossRef]
- Wu, H.M.; Zhang, J.; Ngo, H.H.; Guo, W.S.; Liang, S. Evaluating the sustainability of free water surface flow constructed wetlands: Methane and nitrous oxide emissions. J. Clean. Prod. 2017, 147, 152–156. [Google Scholar] [CrossRef]
- Liu, S.T.; Feng, X.J.; Li, X.N. Bioelectrochemical approach for control of methane emission from wetlands. Bioresour. Technol. 2017, 241, 812–820. [Google Scholar] [CrossRef]
- Zhou, X.; Liang, C.L.; Jia, L.X.; Feng, L.K.; Wang, R.G.; Wu, H.M. An innovative biochar-amended substrate vertical flow constructed wetland for low C/N wastewater treatment: Impact of influent strengths. Bioresour. Technol. 2018, 247, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, H.; Yan, B.X.; Shutes, B.; Xing, D.F.; Banuelos, G.; Chen, R.; Wang, X.Y. Greenhouse gas emissions and wastewater treatment performance by three plant species in subsurface flow constructed wetland mesocosms. Chemosphere 2020, 239, 124795. [Google Scholar] [CrossRef]
- Xu, G.M.; Li, Y.; Hou, W.H.; Wang, S.; Kong, F.L. Effects of substrate type on enhancing pollutant removal performance and reducing greenhouse gas emission in vertical subsurface flow constructed wetland. J. Environ. Manag. 2021, 280, 111674. [Google Scholar] [CrossRef]
- Mu, X.Y.; Lv, X.Y.; Liu, W.; Qiu, C.H.; Ma, Y.; Zhang, S.H.; Jeppesen, E. Biofilms attached to Myriophyllum spicatum play a dominant role in nitrogen removal in constructed wetland mesocosms with submersed macrophytes: Evidence from 15N tracking, nitrogen budgets and metagenomics analyses. Environ. Pollut. 2020, 266, 115203. [Google Scholar] [CrossRef]
- Jia, W.; Yang, Y.C.; Yang, L.Y.; Gao, Y. High-efficient nitrogen removal and its microbiological mechanism of a novel carbon self-sufficient constructed wetland. Sci. Total Environ. 2021, 775, 145901. [Google Scholar] [CrossRef]
- Chen, D.Y.; Gu, X.S.; Zhu, W.Y.; He, S.B.; Huang, J.C.; Zhou, W.L. Electrons transfer determined greenhouse gas emissions in enhanced nitrogen-removal constructed wetlands with different carbon sources and carbon-to-nitrogen ratios. Bioresour. Technol. 2019, 285, 121313. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Sun, T.Y.; Li, H.J.; He, Q.; Pavlostathis, S.G.; Zhang, J. New insights in correlating greenhouse gas emissions and microbial carbon and nitrogen transformations in wetland sediments based on genomic and functional analysis. J. Environ. Manag. 2021, 297, 113280. [Google Scholar] [CrossRef]
- Bernardes, F.S.; Herrera, P.G.; Chiquito, G.M.; Morales, M.F.; Castro, A.P.; Paulo, P.L. Relationship between microbial community and environmental conditions in a constructed wetland system treating greywater. Ecol. Eng. 2019, 139, 105581. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, S.; Lu, Y.H. Biogeographic distribution of bacterial, archaeal and methanogenic communities and their associations with methanogenic capacity in Chinese wetlands. Sci. Total Environ. 2018, 622, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.H.; Chen, J.Q.; Li, W.; Mei, J.; Yang, Y.; Chang, J.J. Greenhouse gas emissions from constructed wetlands are mitigated by biochar substrates and distinctly affected by tidal flow and intermittent aeration modes. Environ. Pollut. 2021, 271, 116328. [Google Scholar] [CrossRef]
- Le Mer, J.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Guerrero-Cruz, S.; Vaksmaa, A.; Horn, M.A.; Niemann, H.; Pijuan, M.; Ho, A. Methanotrophs: Discoveries, environmental relevance, and a perspective on current and future applications. Front. Microbiol. 2021, 12, 678057. [Google Scholar] [CrossRef]
- Wang, J.W.; Long, Y.N.; Yu, G.L.; Wang, G.L.; Zhou, Z.Y.; Li, P.Y.; Zhang, Y.M.; Yang, K.; Wang, S.T. A review on microorganisms in constructed wetlands for typical pollutant removal: Species, function, and diversity. Front. Microbiol. 2022, 13, 845725. [Google Scholar] [CrossRef]
- Tan, X.; Yang, Y.L.; Liu, Y.W.; Li, X.; Zhu, W.B. Quantitative ecology associations between heterotrophic nitrification-aerobic denitrification, nitrogen-metabolism genes, and key bacteria in a tidal flow constructed wetland. Bioresour. Technol. 2021, 337, 125449. [Google Scholar] [CrossRef]
- Daims, H.; Lücker, S.; Wagner, M. A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef]
- Huang, L.; Gao, X.; Guo, J.S.; Ma, X.X.; Liu, M. A review on the mechanism and affecting factors of nitrous oxide emission in constructed wetlands. Environ. Earth Sci. 2013, 68, 2171–2180. [Google Scholar] [CrossRef]
- Fu, C.X.; Li, J.; Lv, X.M.; Song, W.; Zhang, X.L. Operation performance and microbial community of sulfur-based autotrophic denitrification sludge with different sulfur sources. Environ. Geochem. Health 2020, 42, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Z.; Zhang, X.W.; Yang, S.Y.; Xu, Z.H.; Feng, C.Y.; Zhao, F.X. Enhanced nitrogen removal driven by S/Fe2+ cycle in a novel hybrid constructed wetland. J. Clean. Prod. 2023, 426, 139113. [Google Scholar] [CrossRef]
- Pishgar, R.; Dominic, J.A.; Sheng, Z.Y.; Tay, J.H. Denitrification performance and microbial versatility in response to different selection pressures. Bioresour. Technol. 2019, 281, 72–83. [Google Scholar] [CrossRef]
- Chen, M.L.; Chang, L.; Zhang, J.M.; Guo, F.C.; Vymazal, J.; He, Q.; Chen, Y. Global nitrogen input on wetland ecosystem: The driving mechanism of soil labile carbon and nitrogen on greenhouse gas emissions. Environ. Sci. Ecotechnol. 2020, 4, 100063. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Trinh, X.T.; Chen, Q.R.; Wang, C.; Wang, H.H.; Zhou, Q.H.; Xu, D.; Wu, Z.B. Enhancement of microbial nitrogen removal pathway by vegetation in Integrated Vertical-Flow Constructed Wetlands (IVCWs) for treating reclaimed water. Bioresour. Technol. 2018, 249, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Orellana, L.H.; Rodriguez-R, L.M.; Higgins, S.; Chee-Sanford, J.C.; Sanford, R.A.; Ritalahti, K.M.; Löffler, F.E.; Konstantinidis, K.T. Detecting nitrous oxide reductase (nosZ) genes in soil metagenomes: Method development and implications for the nitrogen cycle. Mbio 2014, 5, e01193. [Google Scholar] [CrossRef]
- Peng, Y.Y.; He, S.B.; Gu, X.S.; Yan, P.; Tang, L. Zero-valent iron coupled plant biomass for enhancing the denitrification performance of ecological floating bed. Bioresour. Technol. 2021, 341, 125820. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Xu, D.; Bai, G.L.; Cao, T.T.; Liu, W.; Hu, Z.; Chen, D.S.; Qiu, D.R.; Wu, Z.B. Changes of microbial community structure during the initial stage of biological clogging in horizontal subsurface flow constructed wetlands. Bioresour. Technol. 2021, 337, 125405. [Google Scholar] [CrossRef]


| C/N | CH4 Flux (g·m−2·d−1) | N2O Flux (mg·m−2·d−1) | GWP (g·m−2·d−1) | GWP/TN | GWP/COD |
|---|---|---|---|---|---|
| 0:1 | 0.17 ± 0.03 | 7.95 ± 1.67 | 6.12 ± 0.80 c | 11.79 ± 1.37 b | |
| 5:1 | 0.65 ± 0.07 | 4.96 ± 1.94 | 18.25 ± 2.13 b | 12.85 ± 1.60 b | 2.39 ± 0.31 b |
| 10:1 | 1.29 ± 0.18 | 4.39 ± 1.56 | 34.77 ± 4.87 a | 25.58 ± 3.39 a | 3.87 ± 0.57 a |
| 15:1 | 1.66 ± 0.16 | 5.48 ± 1.08 | 42.91 ± 4.15 a | 30.68 ± 3.05 a | 4.36 ± 0.50 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Li, H.; Du, X.; Cai, Y.; Peng, J.; Zhang, S.; Liu, F. Characteristics of Greenhouse Gas Emissions from Constructed Wetlands Vegetated with Myriophyllum aquatic: The Effects of Influent C/N Ratio and Microbial Responses. Water 2024, 16, 308. https://doi.org/10.3390/w16020308
Wang B, Li H, Du X, Cai Y, Peng J, Zhang S, Liu F. Characteristics of Greenhouse Gas Emissions from Constructed Wetlands Vegetated with Myriophyllum aquatic: The Effects of Influent C/N Ratio and Microbial Responses. Water. 2024; 16(2):308. https://doi.org/10.3390/w16020308
Chicago/Turabian StyleWang, Biaoyi, Hongfang Li, Xiaonan Du, Yixiang Cai, Jianwei Peng, Shunan Zhang, and Feng Liu. 2024. "Characteristics of Greenhouse Gas Emissions from Constructed Wetlands Vegetated with Myriophyllum aquatic: The Effects of Influent C/N Ratio and Microbial Responses" Water 16, no. 2: 308. https://doi.org/10.3390/w16020308
APA StyleWang, B., Li, H., Du, X., Cai, Y., Peng, J., Zhang, S., & Liu, F. (2024). Characteristics of Greenhouse Gas Emissions from Constructed Wetlands Vegetated with Myriophyllum aquatic: The Effects of Influent C/N Ratio and Microbial Responses. Water, 16(2), 308. https://doi.org/10.3390/w16020308






