Adsorption and Kinetics Modelling for Chromium (Cr6+) Uptake from Contaminated Water by Quaternized Date Palm Waste
Abstract
1. Introduction
2. Materials and Methods
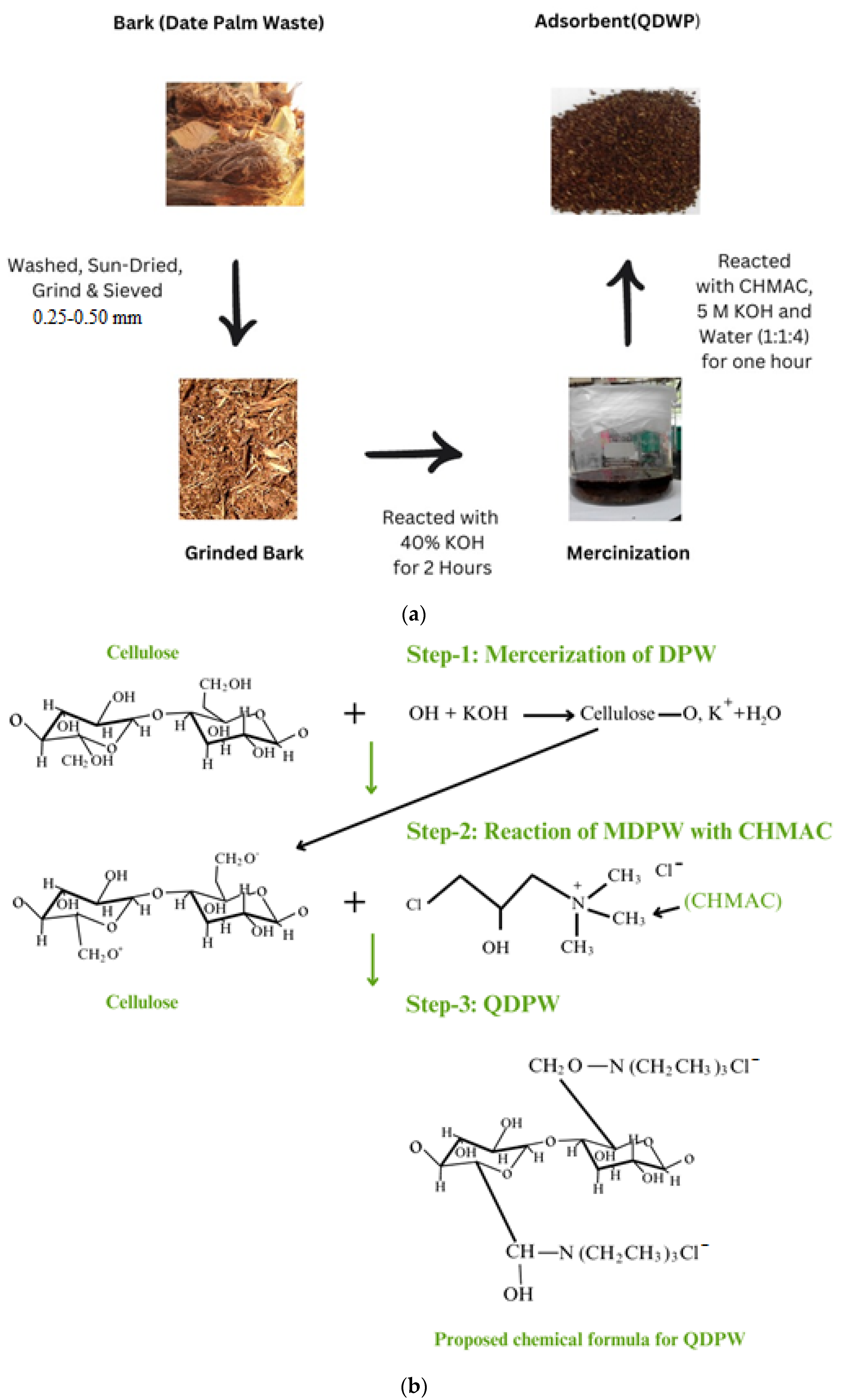
2.1. Synthesis of Quaternized Date Palm Waste
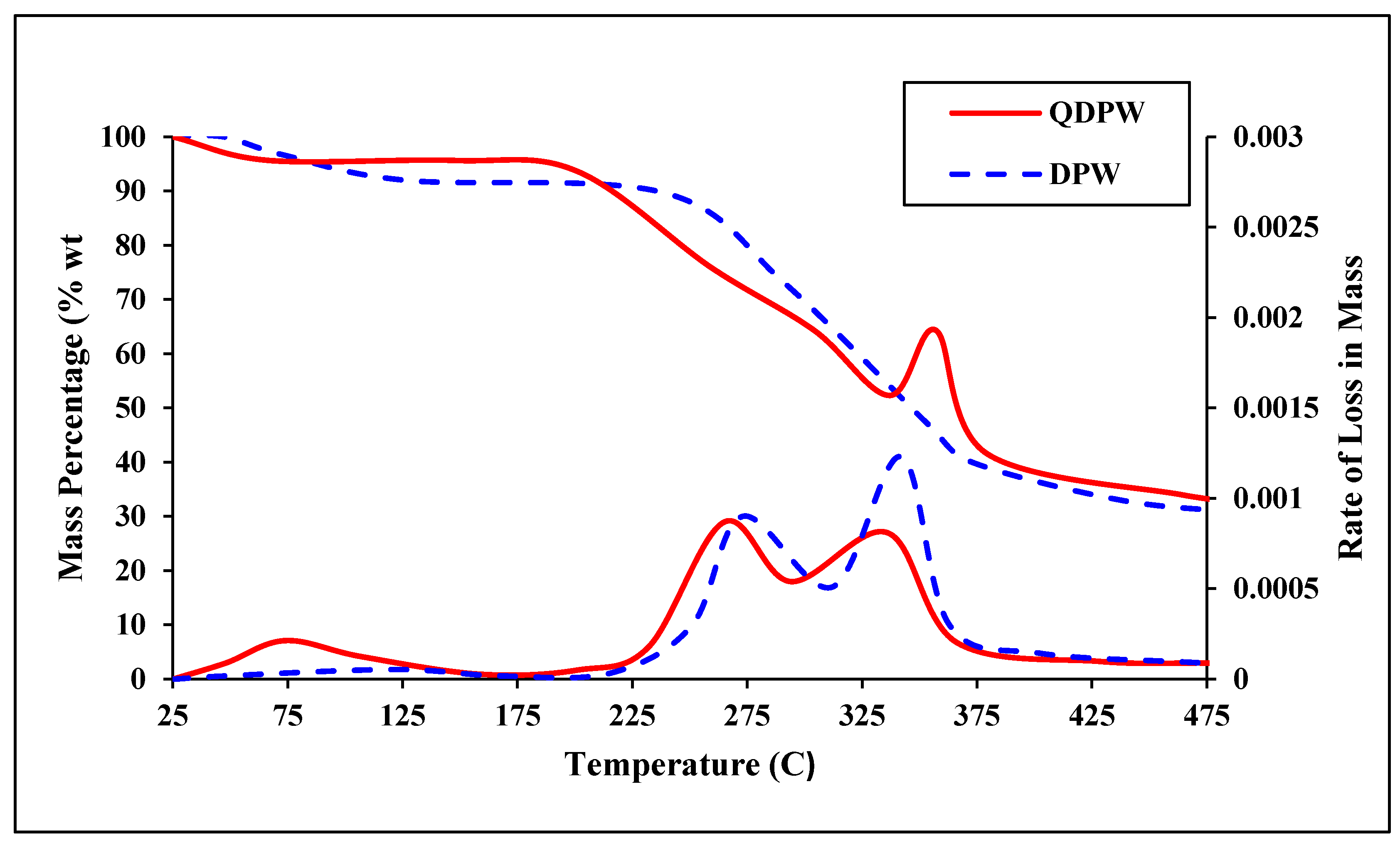
2.2. Characterization of DPW and QDPW
2.3. Preparation of Chromium Solution for Isotherms and Kinetics
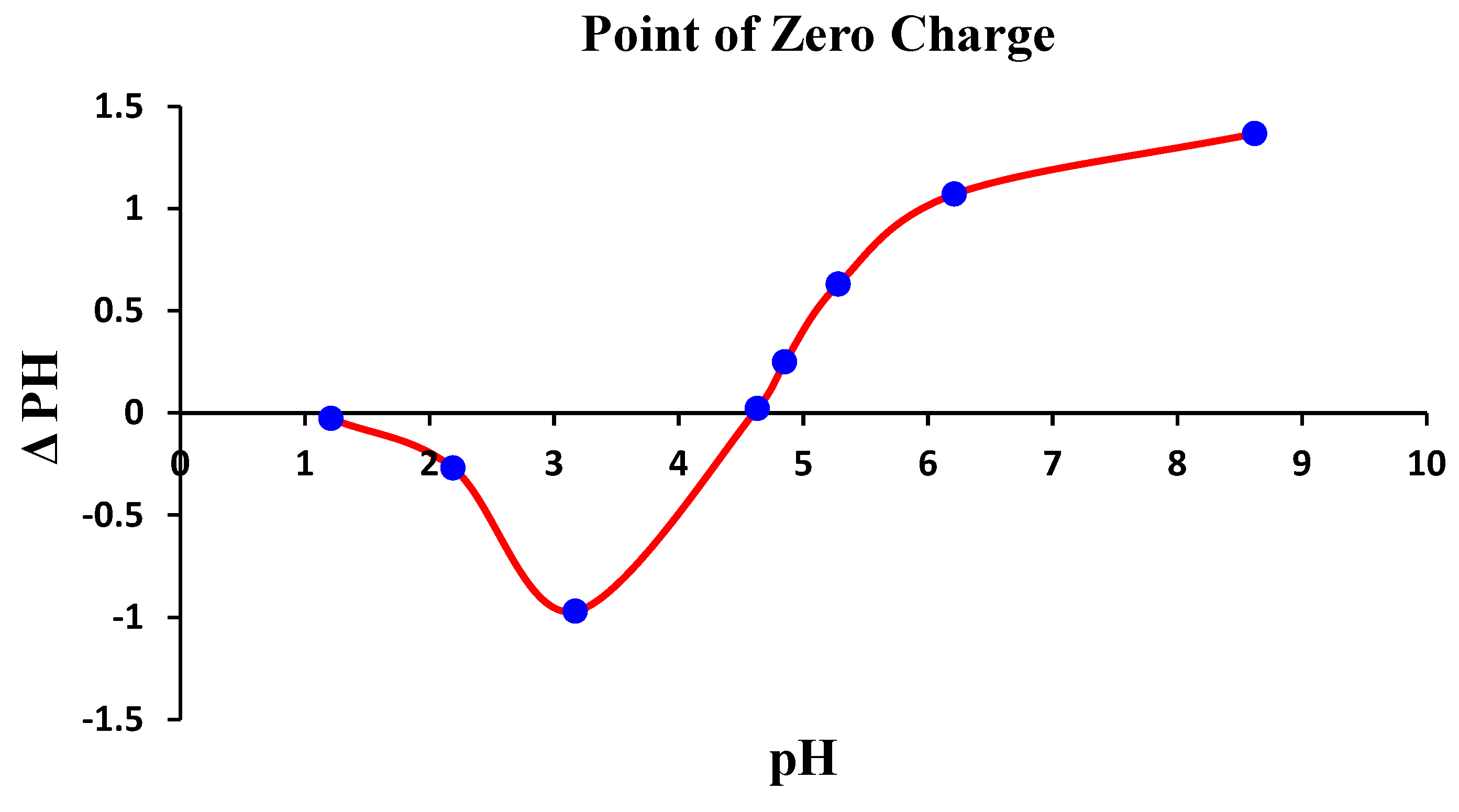
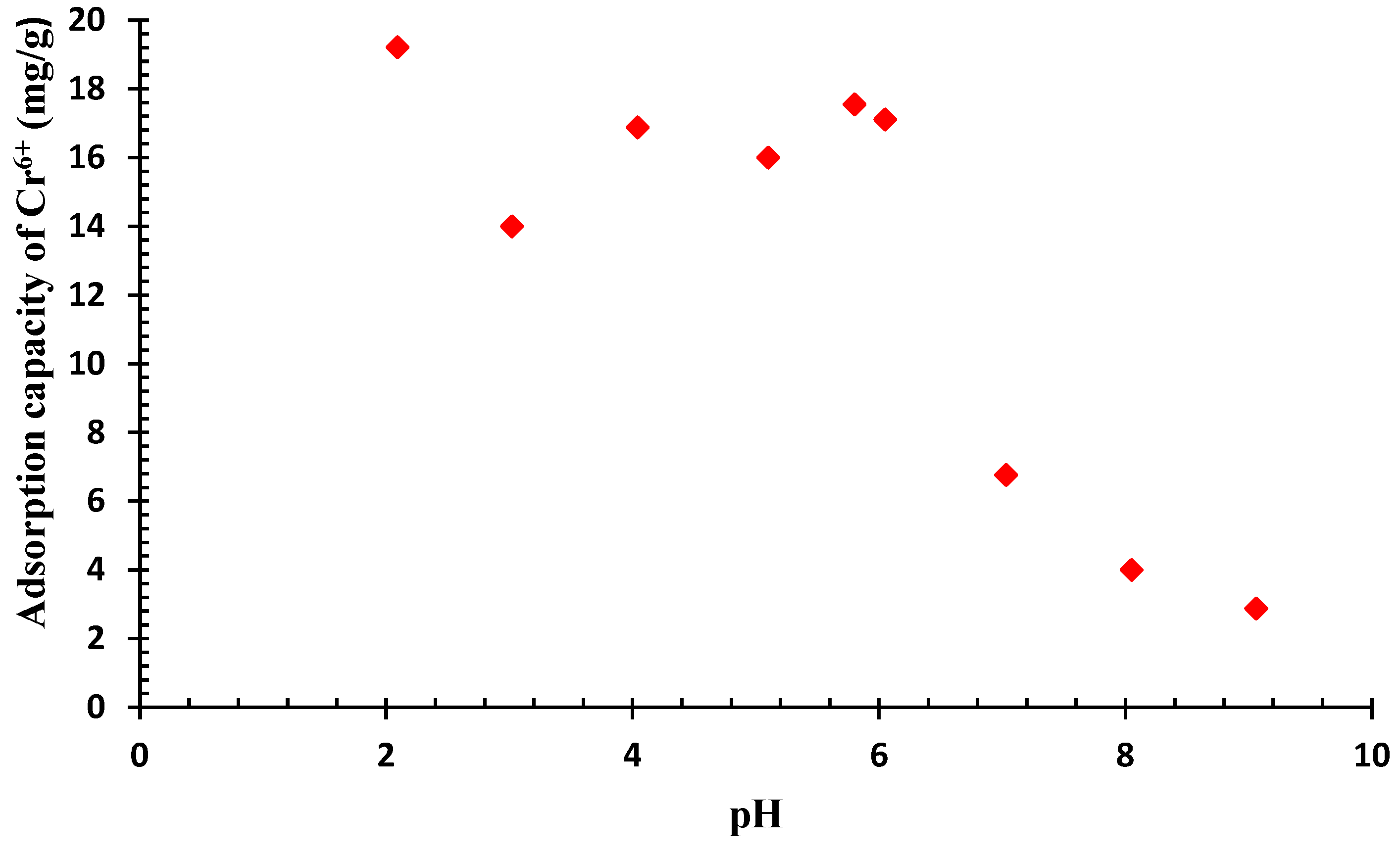
2.4. Impact of pHpzc and pH on the Process
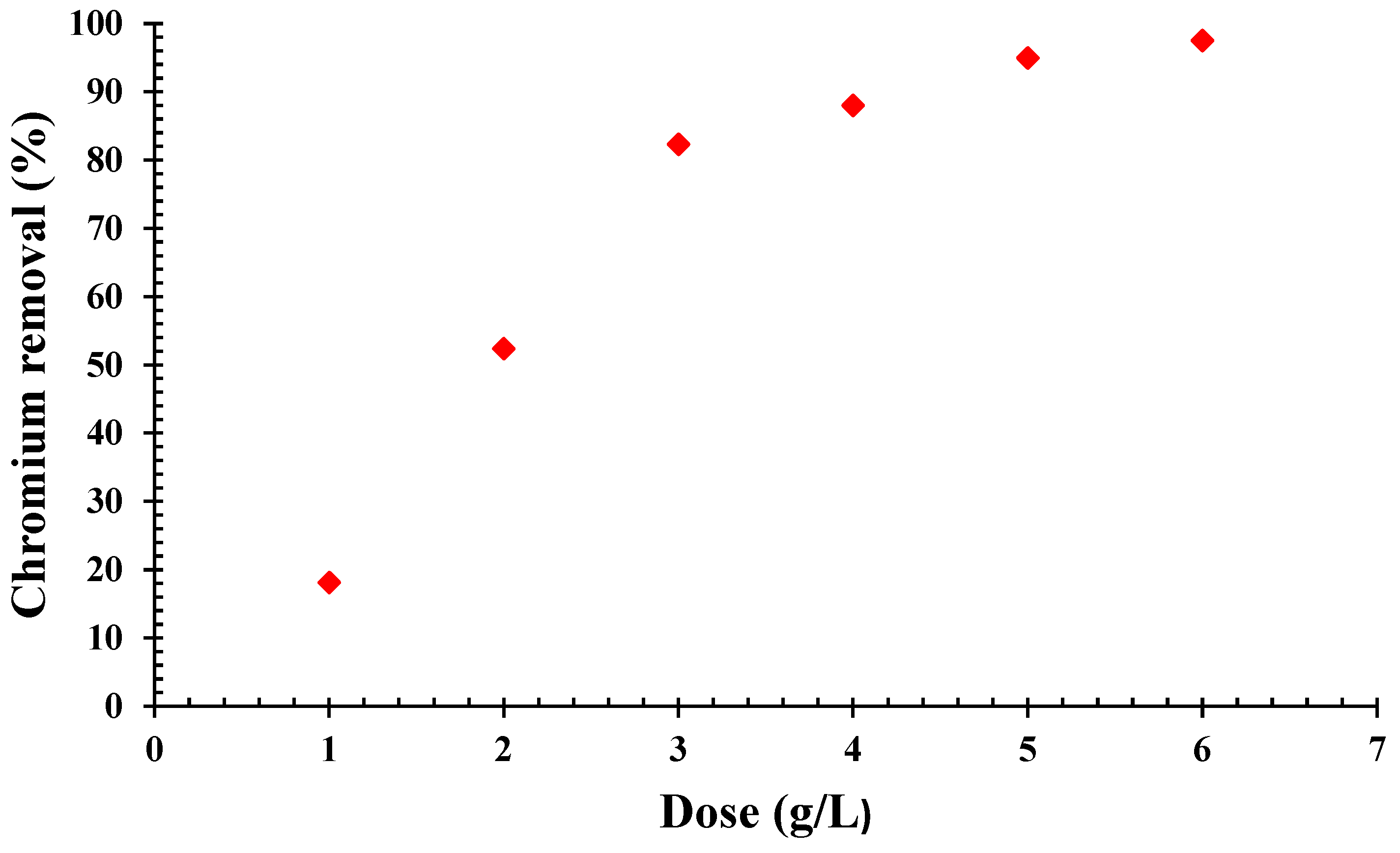
2.5. Impact of Dosage and Initial Concentration
2.6. Adsorption Dynamics of Chromium Removal on QDPW
2.6.1. Kinetic Modeling for Chromium Removal on QDPW
2.6.2. Adsorption Isotherms
3. Results and Discussion
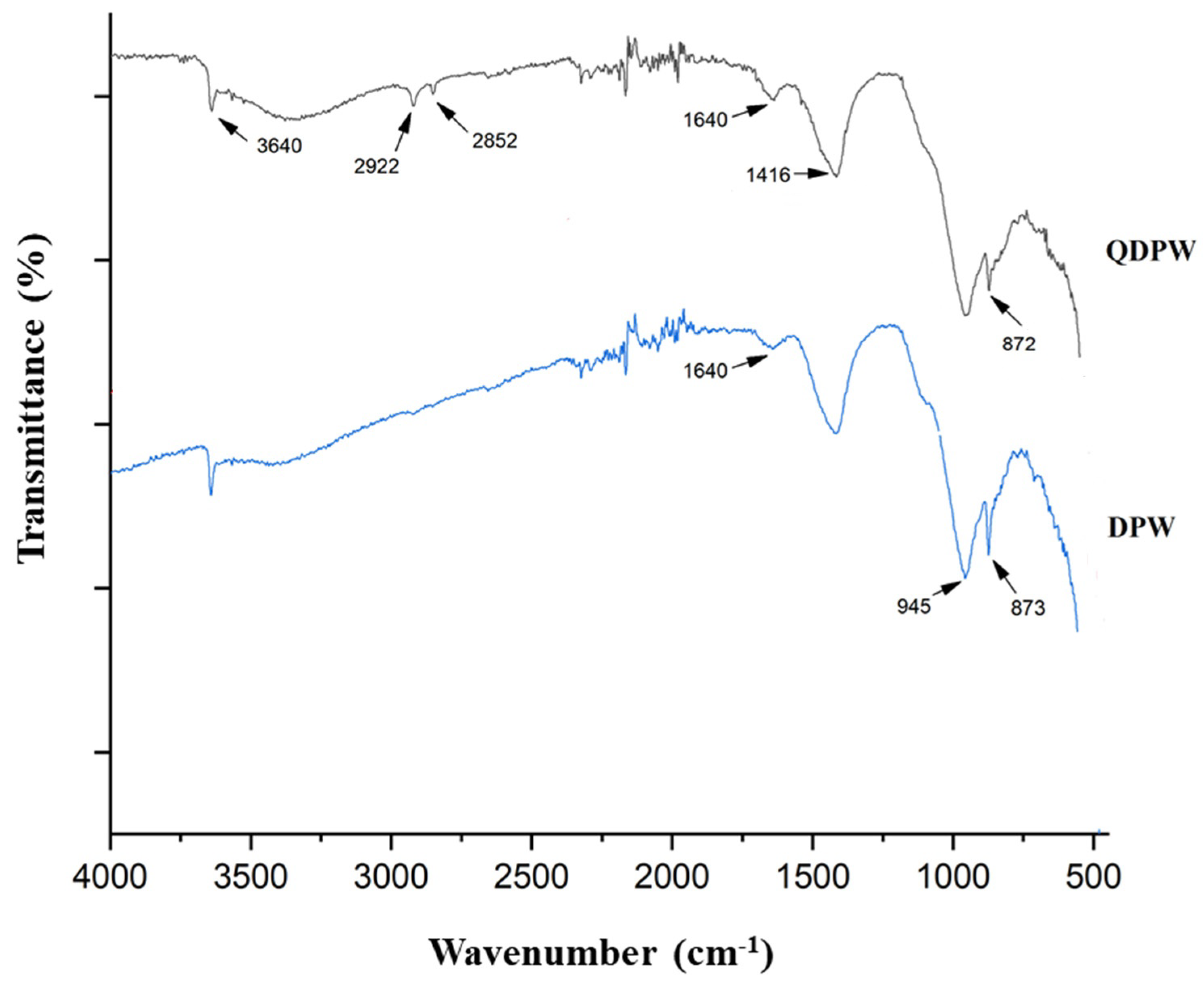
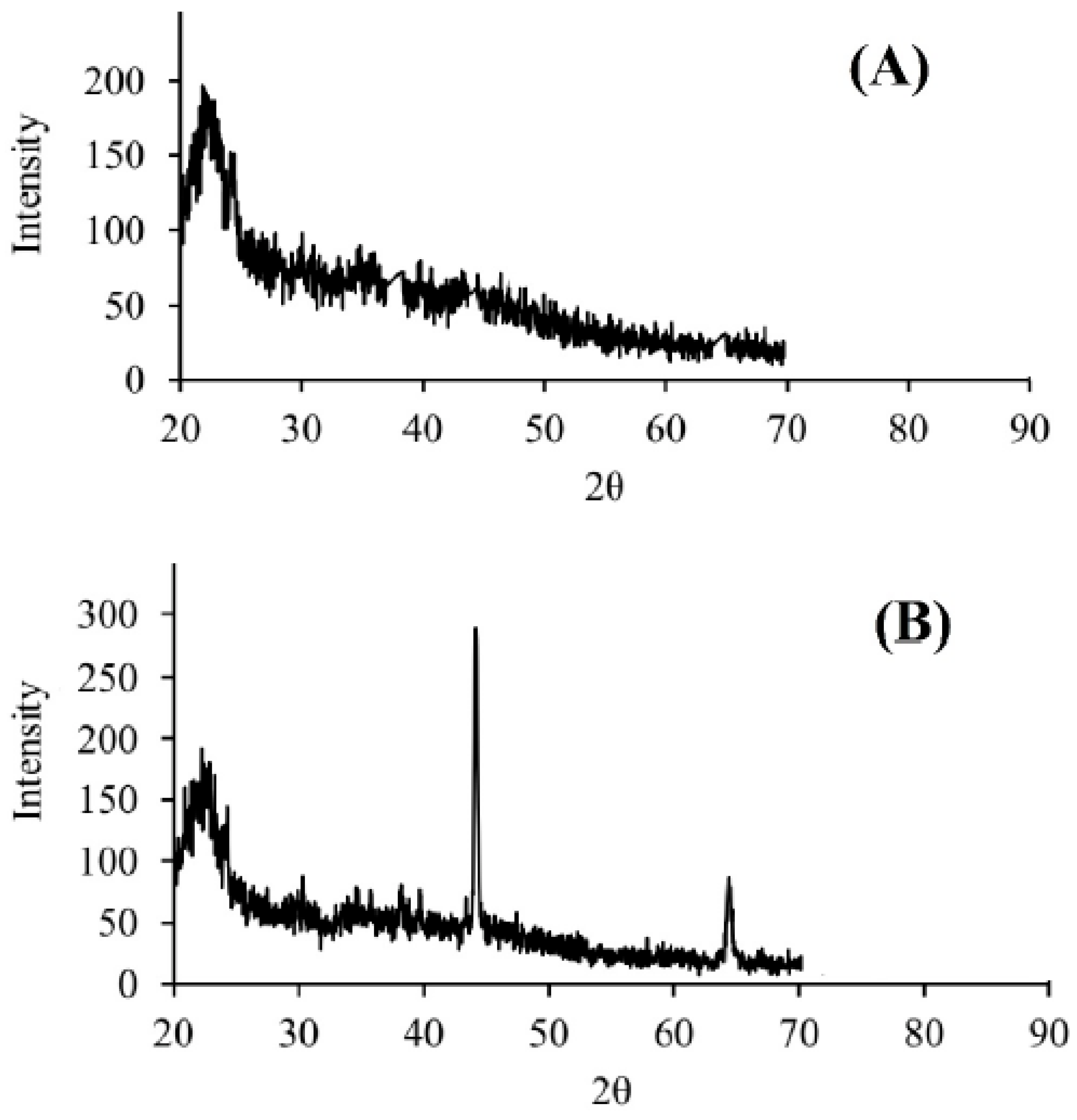
3.1. Characterization of DPW and QDPW
3.2. Analysis of pHpzc and pH onto QDPW for Chromium
3.3. Dynamics of QDPW Dosage
3.4. Influence of Initial Concentration
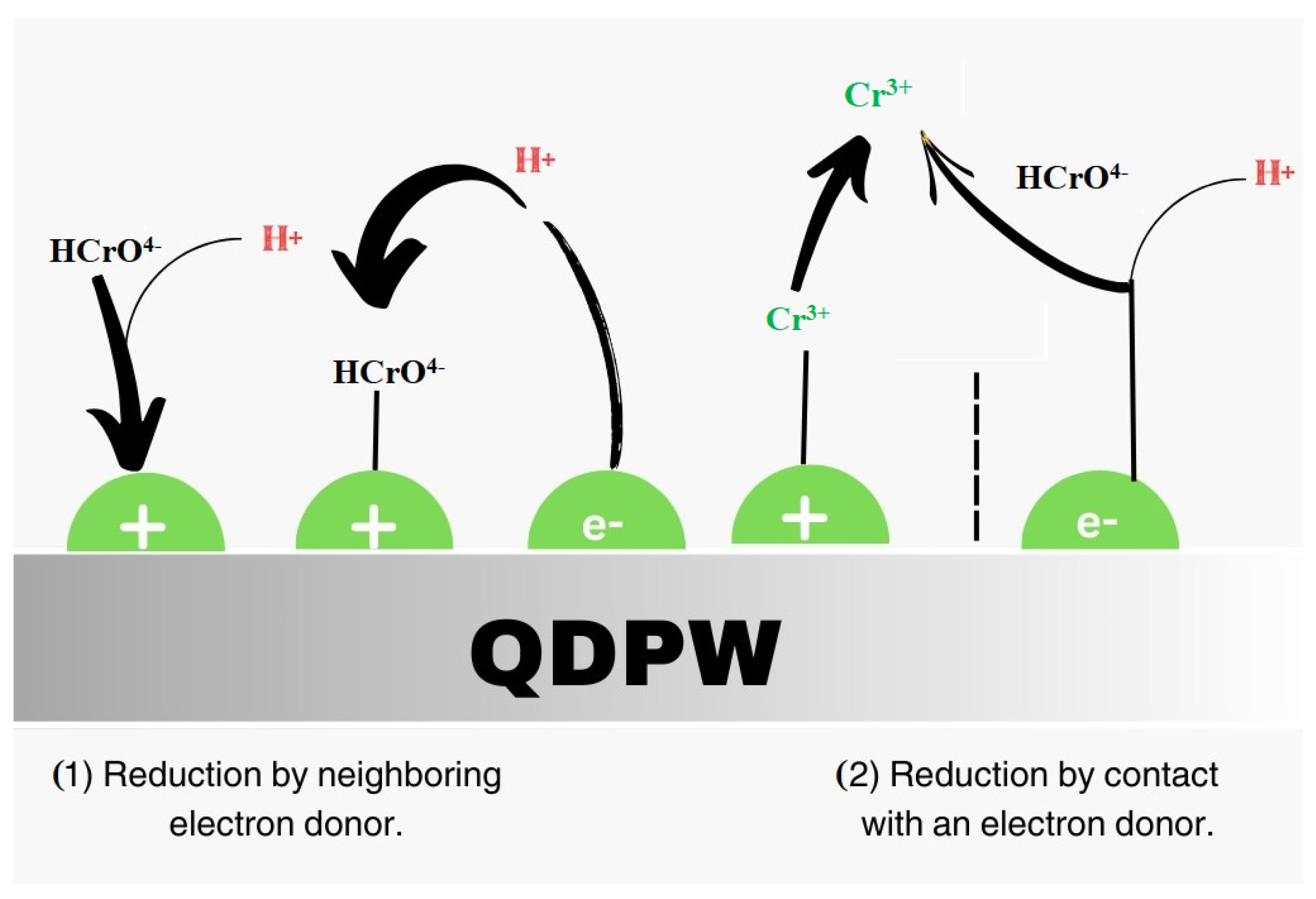
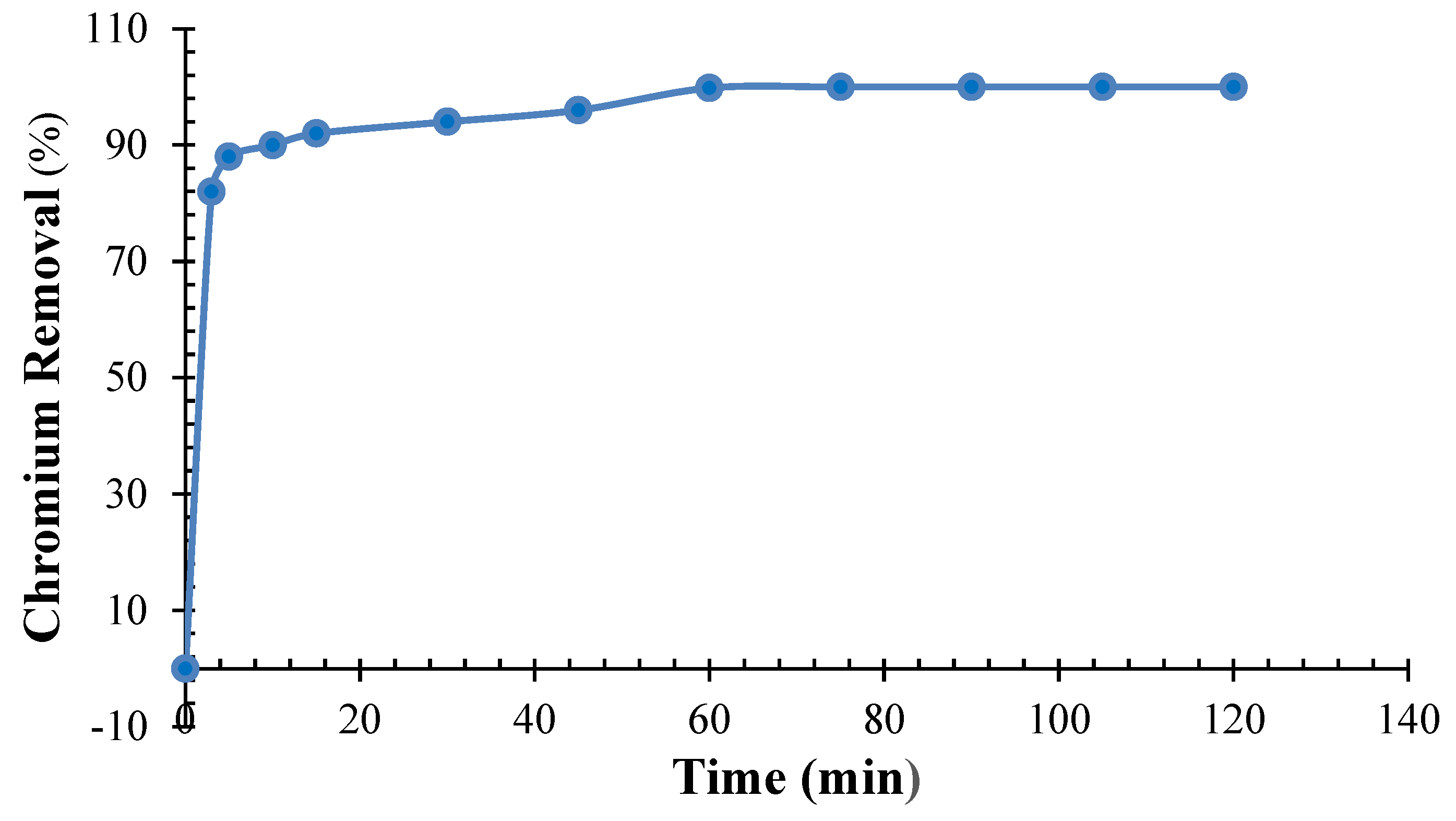
3.5. Impact of Chromium Kinetics onto QDPW
3.6. Kinetics Dynamics of Chromium onto QDPW
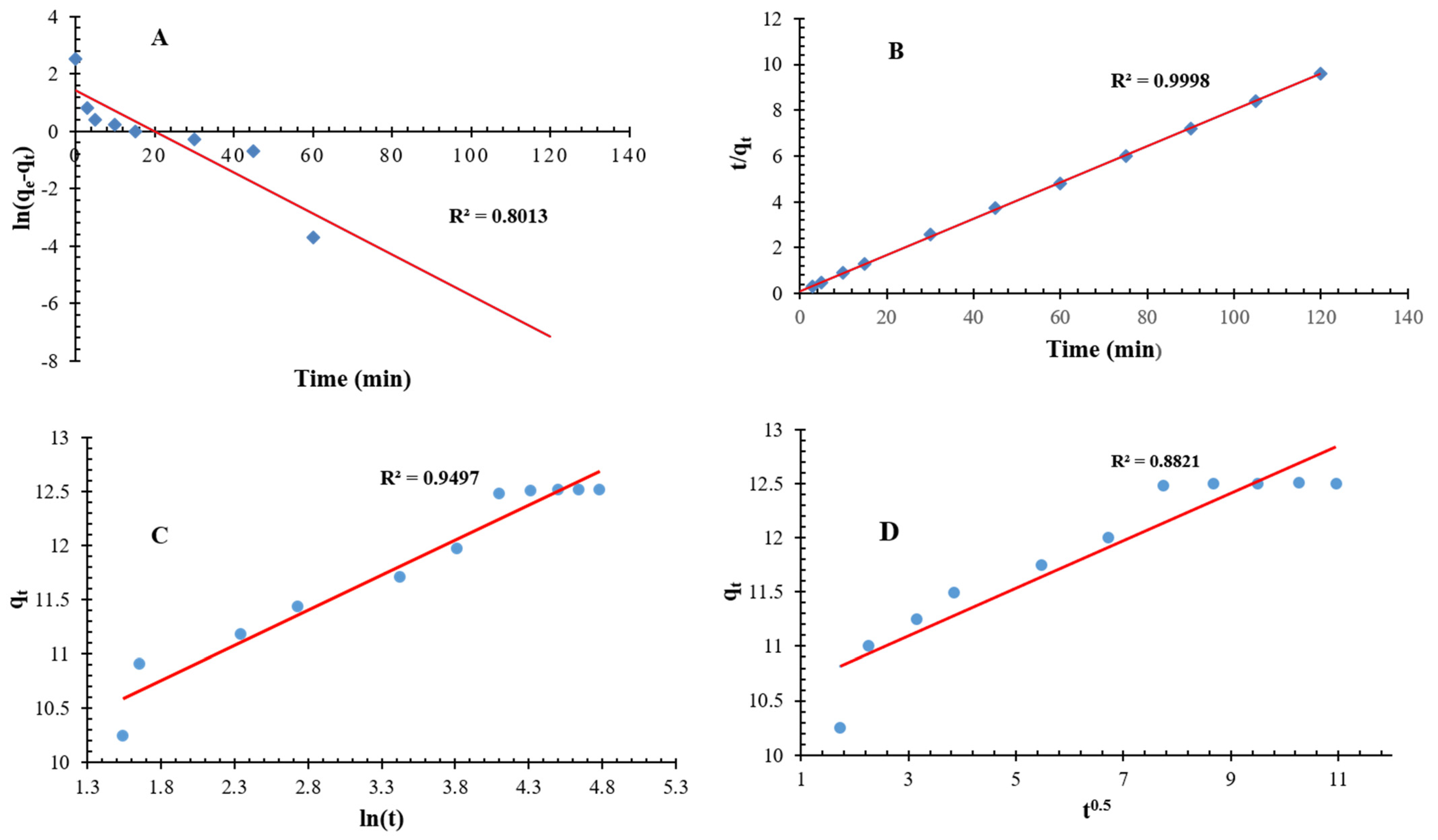
3.6.1. Pseudo-Order Kinetic Dynamics
3.6.2. Elovich Kinetic Model
3.6.3. Intra-Particle Diffusion Model
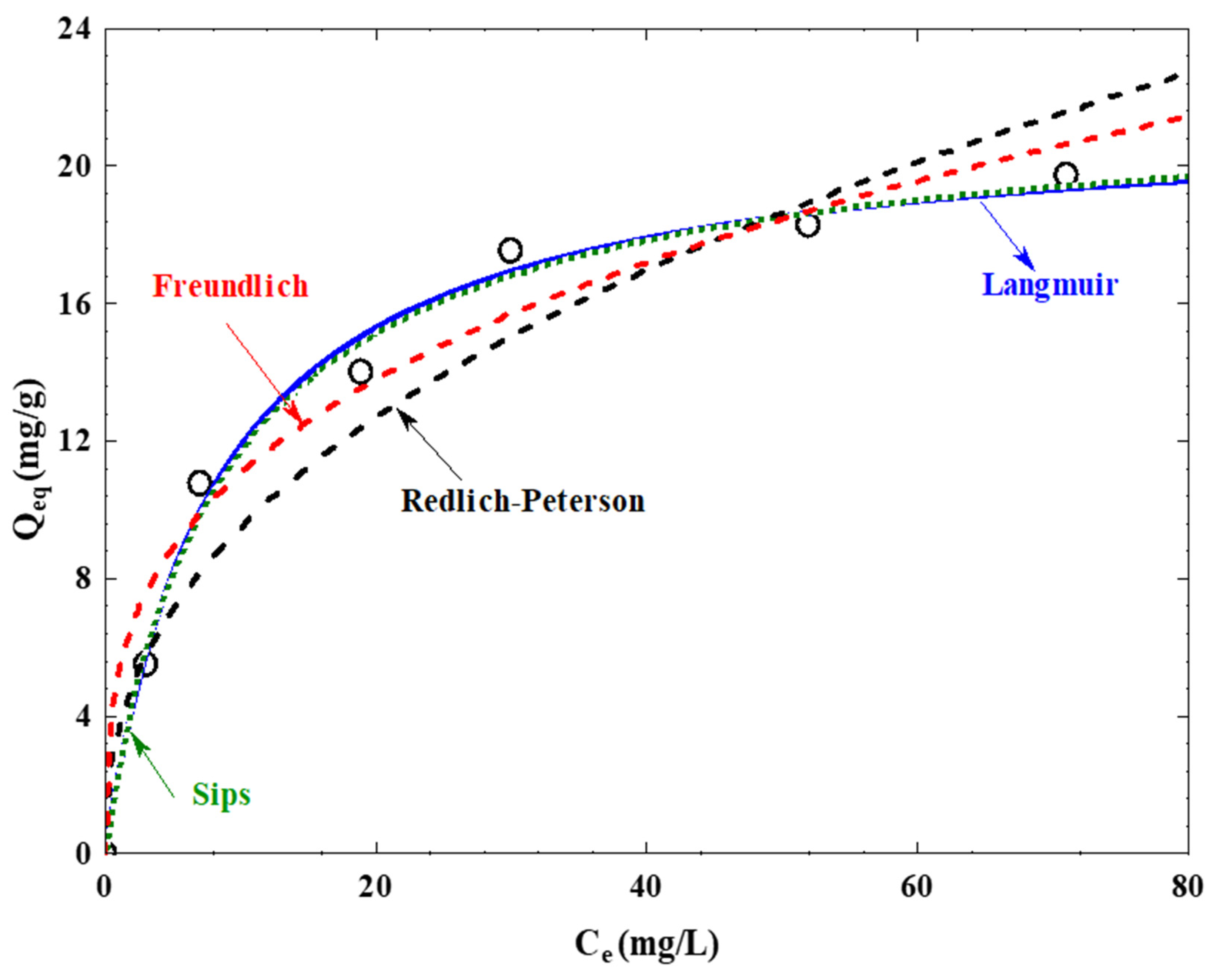
3.7. Analysis of Adsorption Isotherms
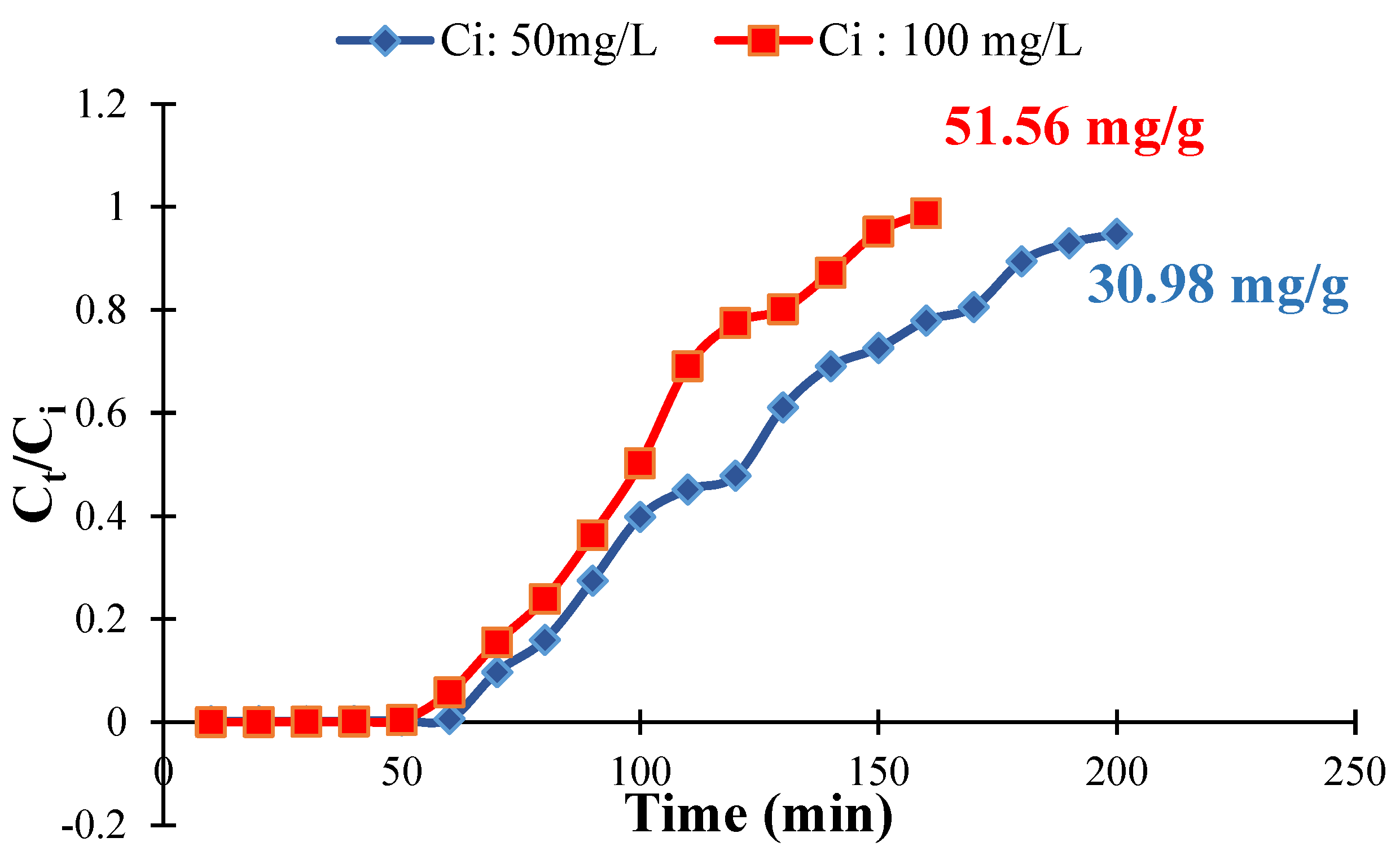
3.8. Column Study for Chromium Sorption onto QDPW
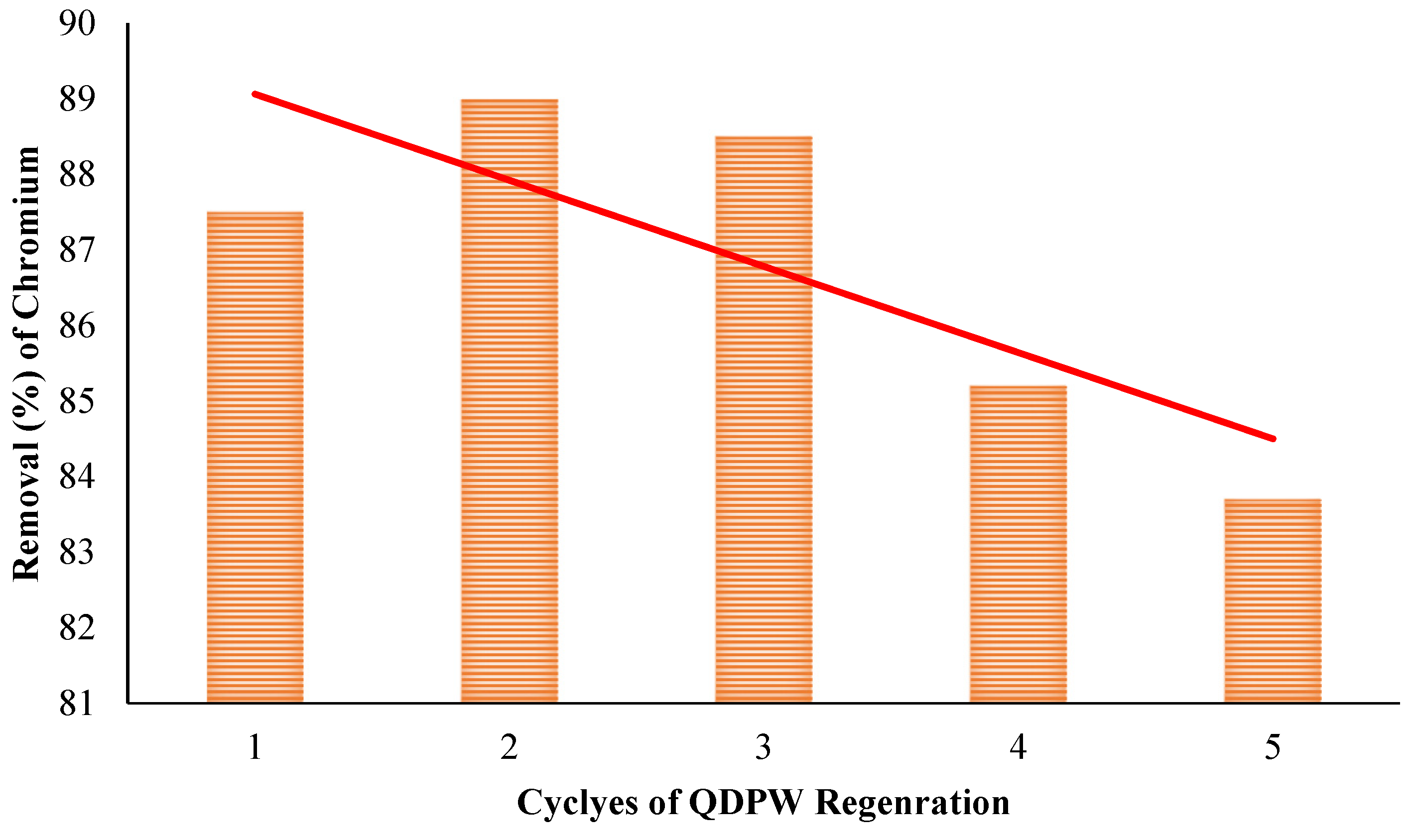
3.9. Regeneration and Reusability of QDPW
3.10. Comparison Study for Chromium Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, W.; Ding, C.; Wang, X.; Wu, Z.; Sun, Y.; Yu, S.; Hayat, T.; Wang, X. Competitive Sorption of As (V) and Cr (VI) on Carbonaceous Nanofibers. Chem. Eng. J. 2016, 293, 311–318. [Google Scholar] [CrossRef]
- Shang, J.; Zong, M.; Yu, Y.; Kong, X.; Du, Q.; Liao, Q. Removal of Chromium (VI) from Water Using Nanoscale Zerovalent Iron Particles Supported on Herb-Residue Biochar. J. Environ. Manag. 2017, 197, 331–337. [Google Scholar] [CrossRef]
- Zou, H.; Zhao, J.; He, F.; Zhong, Z.; Huang, J.; Zheng, Y.; Zhang, Y.; Yang, Y.; Yu, F.; Bashir, M.A.; et al. Ball Milling Biochar Iron Oxide Composites for the Removal of Chromium (Cr (VI)) from Water: Performance and Mechanisms. J. Hazard. Mater. 2021, 413, 125252. [Google Scholar] [CrossRef]
- Atieh, M.A. Removal of Chromium (VI) from Polluted Water Using Carbon Nanotubes Supported with Activated Carbon. Procedia Environ. Sci. 2011, 4, 281–293. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ayati, A.; Ghanbari, S.; Orooji, Y.; Tanhaei, B.; Karimi, F.; Alizadeh, M.; Rouhi, J.; Fu, L.; Sillanpää, M. Recent Advances in Removal Techniques of Cr (VI) Toxic Ion from Aqueous Solution: A Comprehensive Review. J. Mol. Liq. 2021, 329, 115062. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st Addendum; WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 2 July 2023).
- Alrowais, R.; Bashir, M.T.; Sikandar, M.A.; Khan, M.A. Synthesis and Performance Evaluation of Olive Fruit Waste Resin for Removal of Fluoride from Aqueous Solution: Batch and Column Modeling. Desalination Water Treat. 2022, 252, 261–275. [Google Scholar] [CrossRef]
- Pipíška, M.; Valica, M.; Partelová, D.; Horník, M.; Lesný, J.; Hostin, S. Removal of Synthetic Dyes by Dried Biomass of Freshwater Moss Vesicularia Dubyana: A Batch Biosorption Study. Environments 2018, 5, 10. [Google Scholar] [CrossRef]
- Bakar, A.H.B.A.; Koay, Y.S.; Ching, Y.C.; Abdullah, L.C.; Choong, T.S.Y.; Alkhatib, M.; Mobarekeh, M.N.; Zahri, N.A.M. Removal of Fluoride Using Quaternized Palm Kernel Shell as Adsorbents: Equilibrium Isotherms and Kinetics Studies. BioResources 2016, 11, 4485–4511. [Google Scholar]
- Geng, J.; Liang, Q.; Yu, W.; Chen, W.; Lu, G.; Luo, H. Enhanced Removal of Cr (VI) from Aqueous Solutions by Polymer-Mediated Nitrogen-Rich Reduced Graphene Oxide. J. Hazard. Mater. 2022, 436, 129184. [Google Scholar] [CrossRef]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent Advances in Hexavalent Chromium Removal from Aqueous Solutions by Adsorptive Methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef]
- Rehman, I.U.; Zahoor, M.; Bashir, M.T.; Ali, J.; Khan, F.A.; Sher, M. Removal of Cu2+ from Aqueous Solution by Activated Carbon Prepared from Sawdust and Nutshells. Desalination Water Treat. 2018, 126, 171–180. [Google Scholar] [CrossRef]
- Sergeev, V.; Balandinsky, D.; Romanov, G.; Rukin, G. Sorption Treatment of Water from Chromium Using Biochar Material. Arab J. Basic Appl. Sci. 2023, 30, 299–306. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Selvasembian, R.; Ashiq, A.; Gunarathne, V.; Ekanayake, A.; Perera, V.O.; Wijesekera, H.; Mia, S.; Ahmad, M.; Vithanage, M.; et al. A Systematic Review on Adsorptive Removal of Hexavalent Chromium from Aqueous Solutions: Recent Advances. Sci. Total Environ. 2022, 809, 152055. [Google Scholar] [CrossRef]
- Bashir, M.; Salmiaton, A.; Idris, A.; Harun, R. Monitoring Kinetic and Thermodynamic Parameters of Fluoride Adsorption from Aqueous Solution by Pks-Based Anion Resins. J. Mech. Eng. Sci. 2018, 12, 3624–3632. [Google Scholar] [CrossRef]
- Low, W.-P.; Lim, W.-J.; Lee, H.-P.; Rahman, N.A. Removal of Copper, Chromium, and Nickel Ions from Aqueous Solution by Using Different Pre-Treated Orange Peel. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2023; Volume 1205, p. 12013. [Google Scholar]
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Fluoride Removal from Water by Adsorption—A Review. Chem. Eng. J. 2011, 171, 811–840. [Google Scholar] [CrossRef]
- Yadav, A.K.; Abbassi, R.; Gupta, A.; Dadashzadeh, M. Removal of Fluoride from Aqueous Solution and Groundwater by Wheat Straw, Sawdust and Activated Bagasse Carbon of Sugarcane. Ecol. Eng. 2013, 52, 211–218. [Google Scholar] [CrossRef]
- Bashir, M.T.; Ali, S.; Idris, A.; Harun, R. Kinetic and Thermodynamic Study of Nitrate Adsorption from Aqueous Solution by Lignocellulose-Based Anion Resins. Desalination Water Treat. 2017, 62, 20136. [Google Scholar] [CrossRef]
- Marshall, W.E.; Wartelle, L.H. An Anion Exchange Resin from Soybean Hulls. Journal of Chemical Technology & Biotechnology: International Research in Process. Environ. Clean Technol. 2004, 79, 1286–1292. [Google Scholar]
- de Lima, A.C.A.; Nascimento, R.F.; de Sousa, F.F.; Josue Filho, M.; Oliveira, A.C. Modified Coconut Shell Fibers: A Green and Economical Sorbent for the Removal of Anions from Aqueous Solutions. Chem. Eng. J. 2012, 185, 274–284. [Google Scholar] [CrossRef]
- Bashir, M.; Salmiaton, A.; Nourouzi, M.; Azni, I.; Harun, R. Fluoride Removal by Chemical Modification of Palm Kernel Shell-Based Adsorbent: A Novel Agricultural Waste Utilization Approach. Asian J. Microbiol. Biotechnol. Environ. Sci. 2015, 17, 533–542. [Google Scholar] [CrossRef]
- Alrowais, R.; Bashir, M.T.; Sikandar, M.A.; Khan, M.H.; Alwushayh, B.; Ghazy, A.; Uddin, A.; Iqbal, J. Synthesis and Characterization of Nanometal Oxide-Biochar Derived from Date Palm Waste for Adsorption of Manganese and Iron from Contaminated Water. Water 2023, 15, 3603. [Google Scholar] [CrossRef]
- Bashir, M.T. Nitrate and Fluoride Adsorption from Aqueous Solution by Chemically Modified Palm Kernel Shells; Universiti Putra Malaysia: Serdang, Malaysia, 2016. [Google Scholar]
- Weber Jr, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Ho, Y.-S. Second-Order Kinetic Model for the Sorption of Cadmium onto Tree Fern: A Comparison of Linear and Non-Linear Methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
- Daffalla, S. Adsorption of Chromium (VI) from Aqueous Solution Using Palm Leaf-Derived Biochar: Kinetic and Isothermal Studies. Separations 2023, 10, 260. [Google Scholar] [CrossRef]
- Olalekan, A.P.; Olatunya, A.; Ekiti, A.; Dada, A.O. Langmuir, Freundlich, Temkin and Dubinin—Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Singha, B.; Naiya, T.K.; Bhattacharya, A.K.; Das, S.K.; Ftir, K.; Vi, C.; Straw, R.; Roots, H.; Dust, S. Cr (VI) Ions Removal from Aqueous Solutions Using Natural Adsorbents–FTIR Studies. J. Environ. Prot. 2011, 2, 729–735. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Cr (VI) and Cr (III) Removal from Aqueous Solution by Raw and Modified Lignocellulosic Materials: A Review. J. Hazard. Mater. 2010, 180, 1–19. [Google Scholar] [CrossRef]
- Xu, X.; Gao, B.-Y.; Tan, X.; Yue, Q.-Y.; Zhong, Q.-Q.; Li, Q. Characteristics of Amine-Crosslinked Wheat Straw and Its Adsorption Mechanisms for Phosphate and Chromium (VI) Removal from Aqueous Solution. Carbohydr. Polym. 2011, 84, 1054–1060. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Chen, R.; Yu, P.; Guo, S.; Wang, X. Adsorption and Reduction of Chromium (VI) from Aqueous Solution Using Polypyrrole/Calcium Rectorite Composite Adsorbent. Water Res. 2019, 160, 148–157. [Google Scholar] [CrossRef]
- Keränen, A.; Leiviskä, T.; Hormi, O.; Tanskanen, J. Removal of Nitrate by Modified Pine Sawdust: Effects of Temperature and Co-Existing Anions. J. Environ. Manag. 2015, 147, 46–54. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of Porous MgO-Biochar Nanocomposites for Removal of Phosphate and Nitrate from Aqueous Solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, Q.; Li, Q.; Gao, B.; Han, S.; Yu, H. The Regeneration Characteristics of Various Red Mud Granular Adsorbents (RMGA) for Phosphate Removal Using Different Desorption Reagents. J. Hazard. Mater. 2010, 182, 309–316. [Google Scholar] [CrossRef]
- Chandana, L.; Krushnamurty, K.; Suryakala, D.; Subrahmanyam, C. Low-Cost Adsorbent Derived from the Coconut Shell for the Removal of Hexavalent Chromium from Aqueous Medium. Mater. Today Proc. 2020, 26, 44–51. [Google Scholar] [CrossRef]
- López-Téllez, G.; Barrera-Díaz, C.E.; Balderas-Hernández, P.; Roa-Morales, G.; Bilyeu, B. Removal of Hexavalent Chromium in Aquatic Solutions by Iron Nanoparticles Embedded in Orange Peel Pith. Chem. Eng. J. 2011, 173, 480–485. [Google Scholar] [CrossRef]
- Kar, S.; Equeenuddin, S.M. Adsorption of Hexavalent Chromium Using Natural Goethite: Isotherm, Thermodynamic and Kinetic Study. J. Geol. Soc. India 2019, 93, 285–292. [Google Scholar] [CrossRef]
- Espinoza-Sanchez, M.A.; Arevalo-Nino, K.; Quintero-Zapata, I.; Castro-Gonzalez, I.; Almaguer-Cantu, V. Cr (VI) Adsorption from Aqueous Solution by Fungal Bioremediation Based Using Rhizopus sp. J. Environ. Manag. 2019, 251, 109595. [Google Scholar] [CrossRef]
- Niam, A.C.; Fenelon, E.; Ningsih, E.; Mirzayanti, Y.W.; Kristanti, E. High-Efficiency Adsorption of Hexavalent Chromium from Aqueous Solution by Samanea Saman Activated Carbon. Adsorpt. Sci. Technol. 2022, 2022, 8960379. [Google Scholar] [CrossRef]
- Tripathy, S.; Sahu, S.; Patel, R.K.; Panda, R.B.; Kar, P.K. Efficient Removal of Cr (VI) by Polyaniline Modified Biochar from Date (Phoenix Dactylifera) Seed. Groundw. Sustain. Dev. 2021, 15, 100653. [Google Scholar] [CrossRef]
- Gupta, G.K.; Mondal, M.K. Mechanism of Cr (VI) Uptake onto Sagwan Sawdust Derived Biochar and Statistical Optimization via Response Surface Methodology. Biomass Convers. Biorefinery 2023, 13, 709–725. [Google Scholar] [CrossRef]
- Sallau, A.B.; Aliyu, S.; Ukuwa, S. Biosorption of Chromium (VI) from Aqueous Solution by Corn Cob Powder. Int. J. Environ. Bioenergy 2012, 4, 131–140. [Google Scholar]
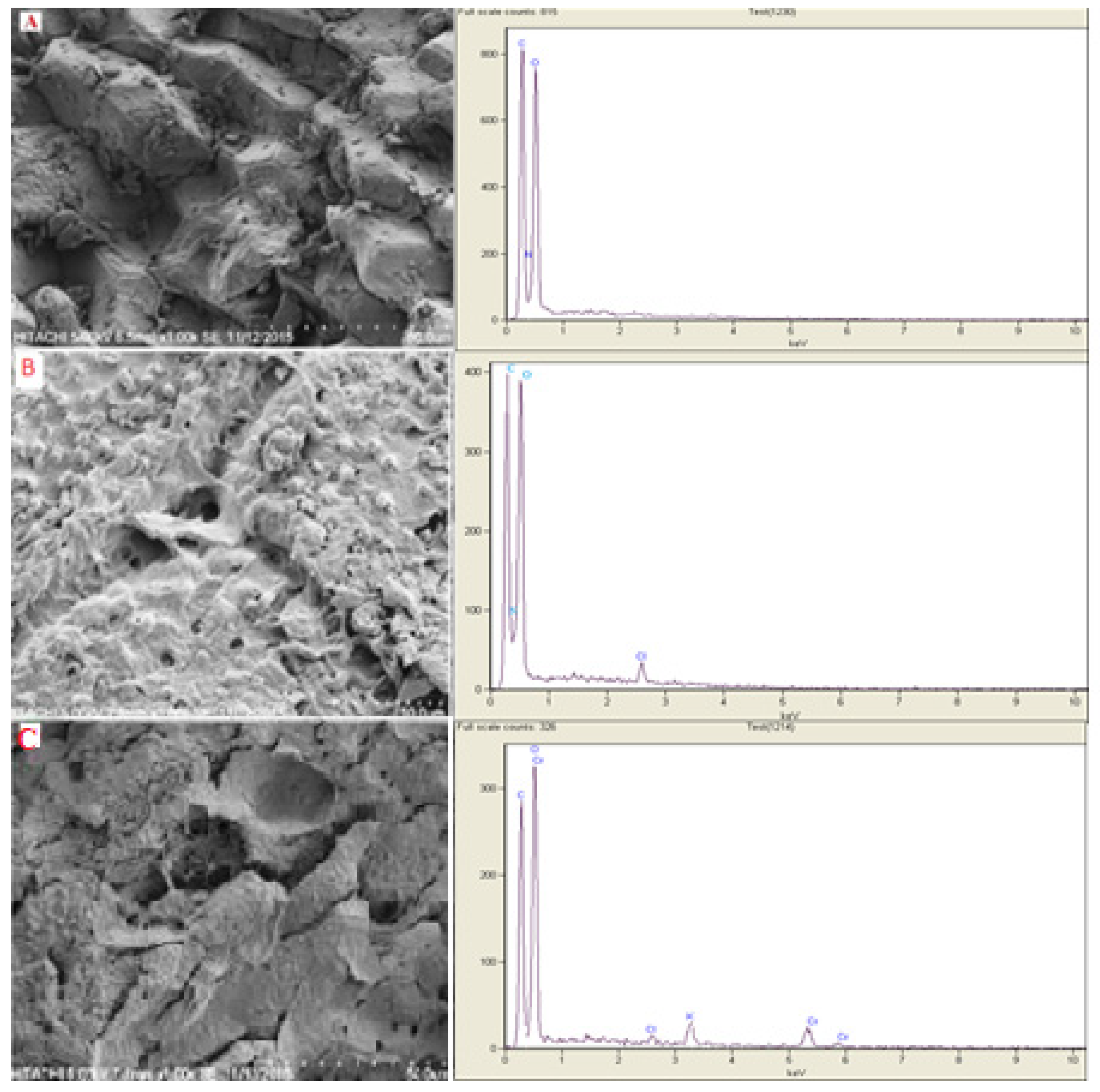

| Elements Weight (%) | DPW | QDPW | Cr-Loaded QDPW |
|---|---|---|---|
| C | 28.53 | 27.04 | 25.91 |
| N | 2.16 | 3.41 | - |
| O | 69.81 | 61.91 | 68.33 |
| Cr | - | - | 2.95 |
| Materials | BET Area (m2·g−1) | Pore Size (nm) | Mesopore dAv. (nm) | Macropre dAv. (nm) | VAv. (cm3·g−1) |
|---|---|---|---|---|---|
| DPW | 0.69 | 13.674 | 13.34 | 90.34 | 2.36 |
| QDPW | 1.368 | 16.675 | 10.36 | 98.68 | 5.71 |
| Materials | Carbon (wt.%) | Hydrogen (wt.%) | Nitrogen (wt.%) | Oxygen (wt.%) |
|---|---|---|---|---|
| DPW | 51.96 | 5.23 | 2.92 | 39.83 |
| QDPW | 48.52 | 6.86 | 3.73 | 43.67 |
| Material (wt.%) | DPW (wt.%) | QDPW (wt.%) |
|---|---|---|
| Hemicellulose | 29.87 | 20.15 |
| Cellulose | 28.96 | 25.85 |
| Lignin | 49.75 | 38.25 |
| Extractives | 12.88 | 9.63 |
| Volatile matter | 54.49 | 53.25 |
| Fixed carbon | 33.05 | 30.15 |
| Moisture | 5.78 | 9.05 |
| Ash | 6.68 | 7.55 |
| Pseudo 1st Order Model | K1 (min−1) | qe (mg/g) | R2 |
| 0.1829 | 0.403 | 0.801 | |
| Pseudo 2nd Order Model | K2 (g/mg·min) | qe (mg/g) | R2 |
| 0.0611 | 12.64 | 0.999 | |
| Elovich Kinetic Model | α (mg/g·min) | β (g/mg) | R2 |
| 0.4550 | 1.701 | 0.949 | |
| Intra-particle Diffusion Model | Ki mg/g·min0.5 | C (mg/g) | R2 |
| 0.1360 | 4.850 | 0.882 |
| Langmuir | Freundlich | Redlich-Peterson | Sips | |
|---|---|---|---|---|
| Isotherm Parameters | Q0 = 22.22 mg/g | QS = 22.26 mg/g | ||
| KL = 0.1122 L/mg | KF = 4.30 mg/g | AR = 2.95 mg/g | KS = 0.131 L/mg | |
| n = 3.145 | BR = 0.168 L/mg | β = 0.931 | ||
| g = 0.952 | ||||
| R2 | 0.989 | 0.923 | 0.981 | 0.983 |
| RMSE (mg/g) | 0.6471 | 1.2502 | 0.6245 | 0.6378 |
| Adsorbent | Max. Conc. (mg/L) | pH | Qmax (mg/g) | Reference |
|---|---|---|---|---|
| QDPW | 150 | 7 | 22.22 | This Study |
| Coconut Shell | 30 | 2 | 14.62 | [36] |
| Orange peel pith | 10 | 1 | 5.37 | [37] |
| Palm Leave Biochar | 250 | 2 | 14.97 | [27] |
| Natural goethite | 25 | 2 | 0.727 | [38] |
| Fungal Rhizopus sp. | 300 | 2 | 8.06 | [39] |
| Samanea saman AC | 30 | 5 | 0.2893 | [40] |
| Date seed biochar | 100 | 5 | 27.3 | [41] |
| Sagwan sawdust-derived biochar | 100 | 2 | 9.62 | [42] |
| Orange peel pith | 10 | 1 | 5.37 | [37] |
| Corn cob powder | 10 | 3 | 10 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrowais, R.; Bashir, M.T.; Khan, A.A.; Bashir, M.; Abbas, I.; Abdel Daiem, M.M. Adsorption and Kinetics Modelling for Chromium (Cr6+) Uptake from Contaminated Water by Quaternized Date Palm Waste. Water 2024, 16, 294. https://doi.org/10.3390/w16020294
Alrowais R, Bashir MT, Khan AA, Bashir M, Abbas I, Abdel Daiem MM. Adsorption and Kinetics Modelling for Chromium (Cr6+) Uptake from Contaminated Water by Quaternized Date Palm Waste. Water. 2024; 16(2):294. https://doi.org/10.3390/w16020294
Chicago/Turabian StyleAlrowais, Raid, Muhammad Tariq Bashir, Aftab Ahmad Khan, Manahil Bashir, Inam Abbas, and Mahmoud M. Abdel Daiem. 2024. "Adsorption and Kinetics Modelling for Chromium (Cr6+) Uptake from Contaminated Water by Quaternized Date Palm Waste" Water 16, no. 2: 294. https://doi.org/10.3390/w16020294
APA StyleAlrowais, R., Bashir, M. T., Khan, A. A., Bashir, M., Abbas, I., & Abdel Daiem, M. M. (2024). Adsorption and Kinetics Modelling for Chromium (Cr6+) Uptake from Contaminated Water by Quaternized Date Palm Waste. Water, 16(2), 294. https://doi.org/10.3390/w16020294










