Enhanced Adsorption Capacity of Methylene Blue Dye onto Kaolin through Acid Treatment: Batch Adsorption and Machine Learning Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Adsorbents
2.1.1. Purification of Natural Kaolinite
2.1.2. Acid Activation of Kaolinite
2.2. Characterization of Adsorbents
2.3. Methylene Blue Adsorption Experiments
2.3.1. Batch Adsorption Experiment of Methylene Blue
2.3.2. Effect of Various Parameters on MB Adsorption on DD3 and Treated-DD3
2.3.3. Application of Treated-DD3 for Treating Real Effluent
2.3.4. Gaussian Process Regression Coupled with Particle Swarm Optimization (GPR–PSO)
- (1)
- Data were prepared and examined;
- (2)
- The database was divided into three parts: 222 points for training, 47 points for evaluating model performance (test), and 47 points for verifying model results (validation);
- (3)
- Ten kernels’ functions, including Exponential, Square Exponential, Matern32, Matern52, Rational Quadratic, Ard Exponential, Ard Square Exponential, Ardmatern32, Ardmatern52 and Ard Rational Quadratic, were optimized with four basis functions—Constant, Linear, Zero and Pure Quadratic;
- (4)
- The PSO algorithm’s iteration count and search agents were optimized to determine the best parameters (Kernel Scale [sigmaM, sigmaF] and sigma) for each kernel function.
2.3.5. Statistical Evaluation Criteria
3. Results and Discussion
3.1. Characterization of DD3 and Treated-DD3 Kaolinites
3.1.1. Chemical Composition of the Kaolinites
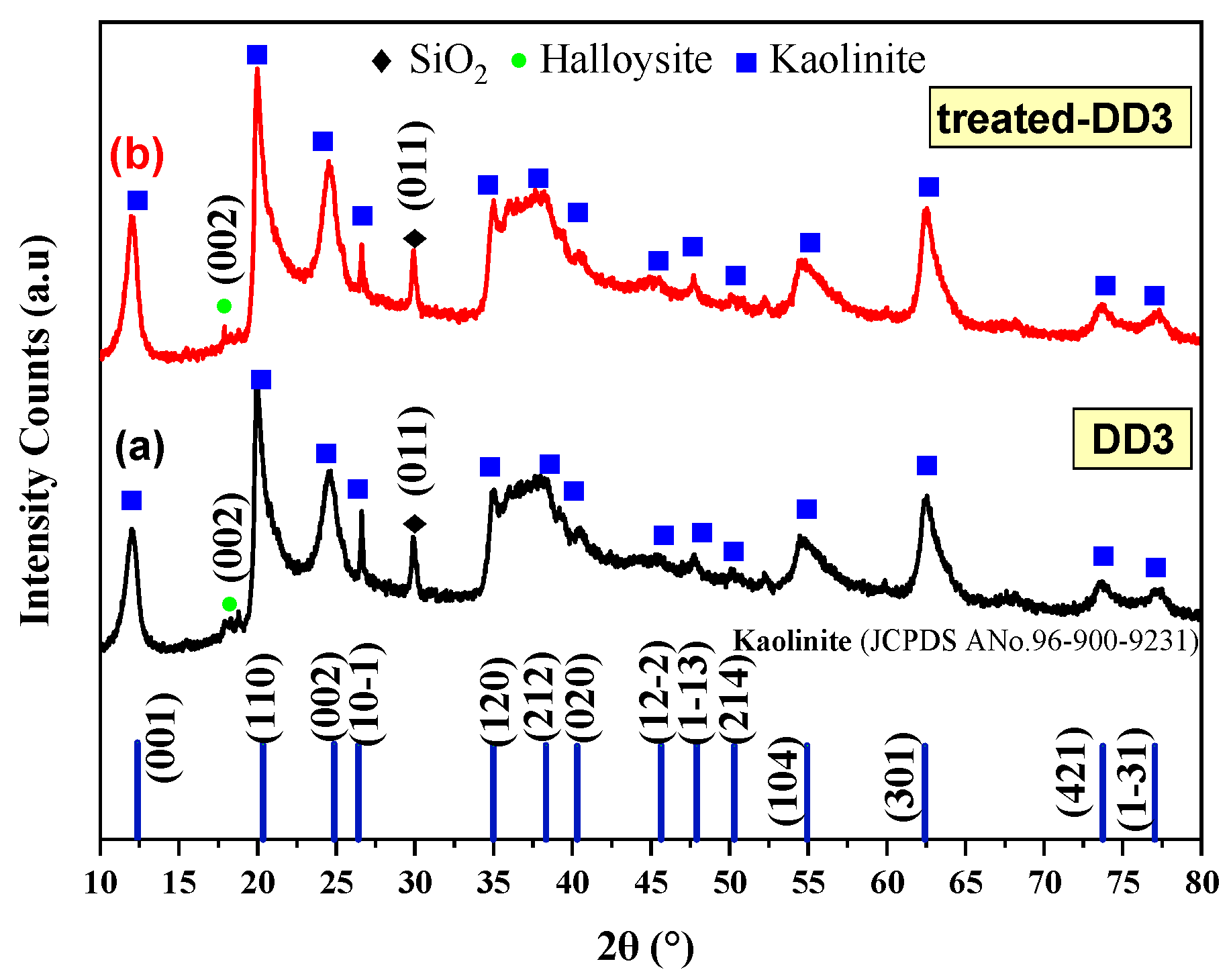
3.1.2. Crystal Structure of the Kaolinites
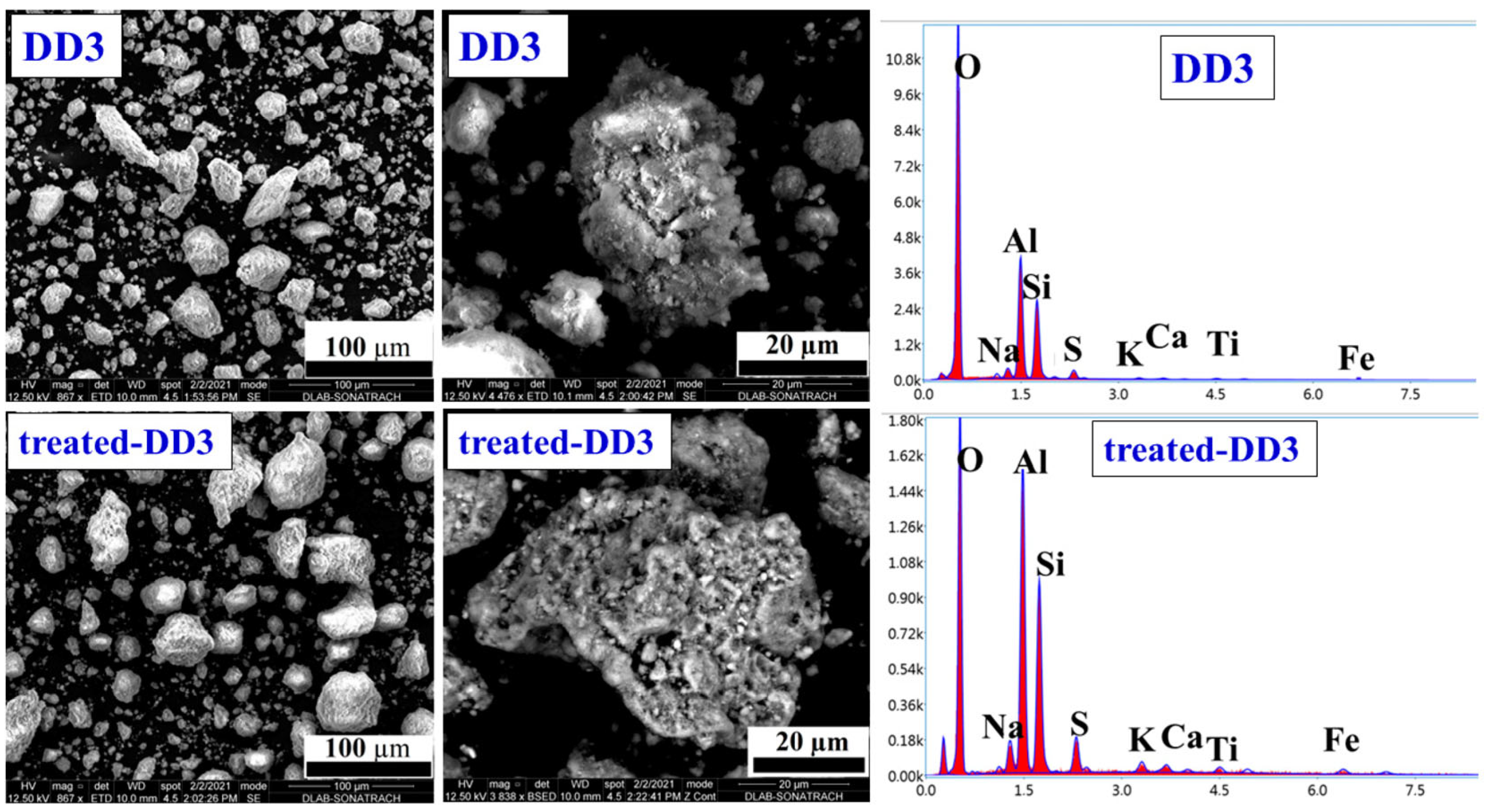
3.1.3. Surface Morphology and Element Component of the Kaolinites
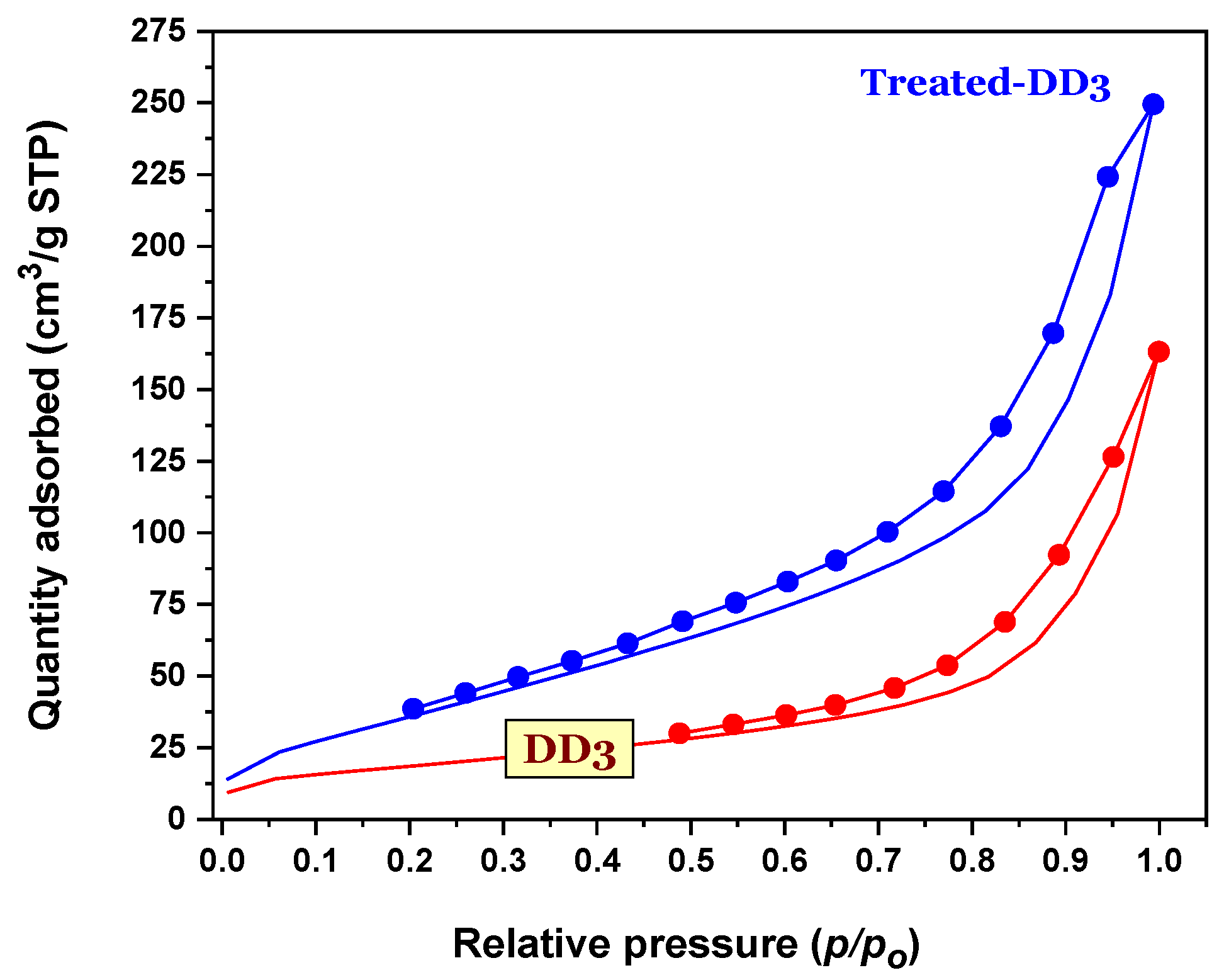
3.1.4. Textural Property of the Kaolinites
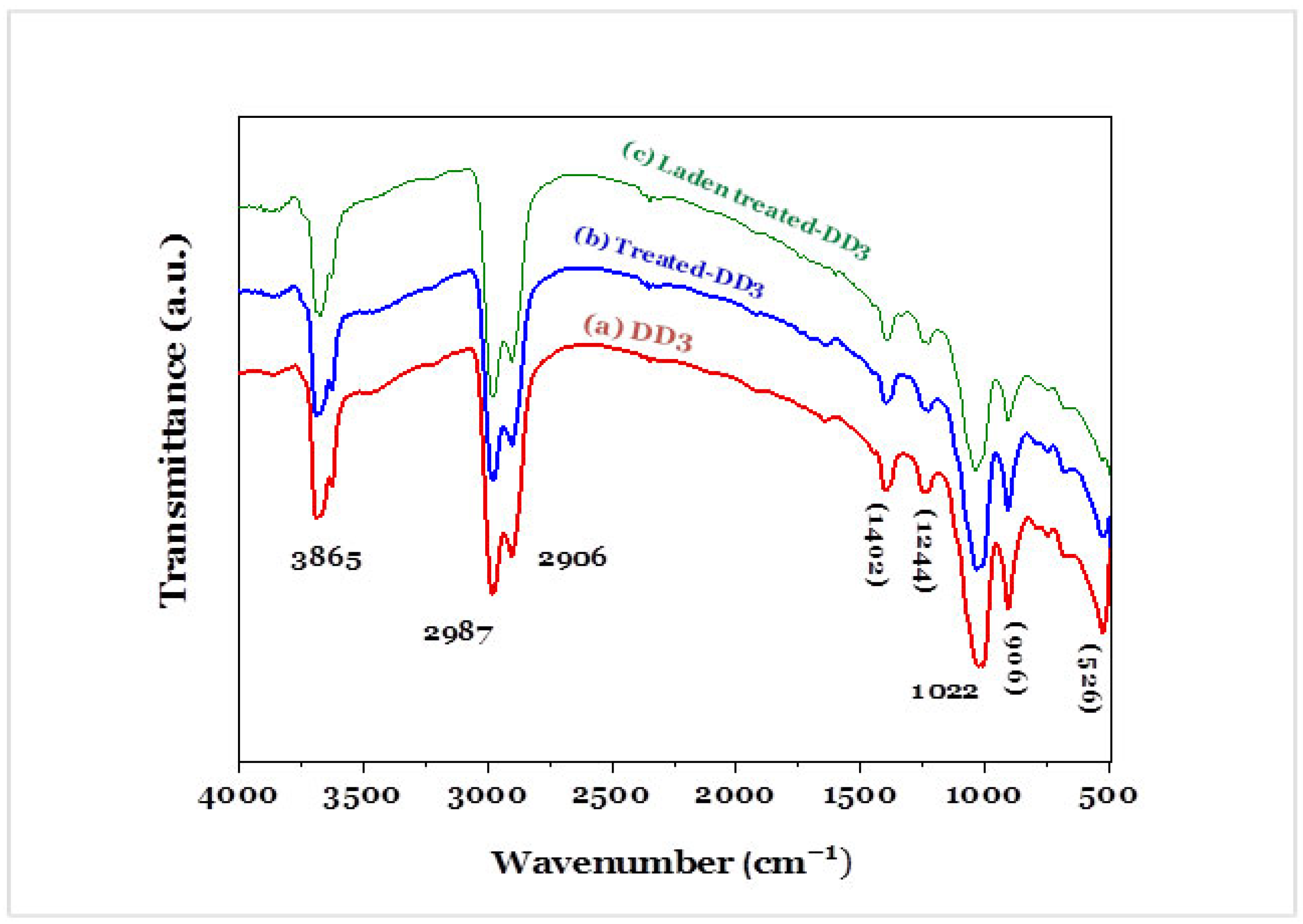
3.1.5. Functional Group on the Surface of Kaolinites
3.2. Adsorption of MB Dye by DD3 and Treated-DD3
3.2.1. Effect of Adsorbent Dose, Solution pH, and Ionic Strength on Adsorption Processes
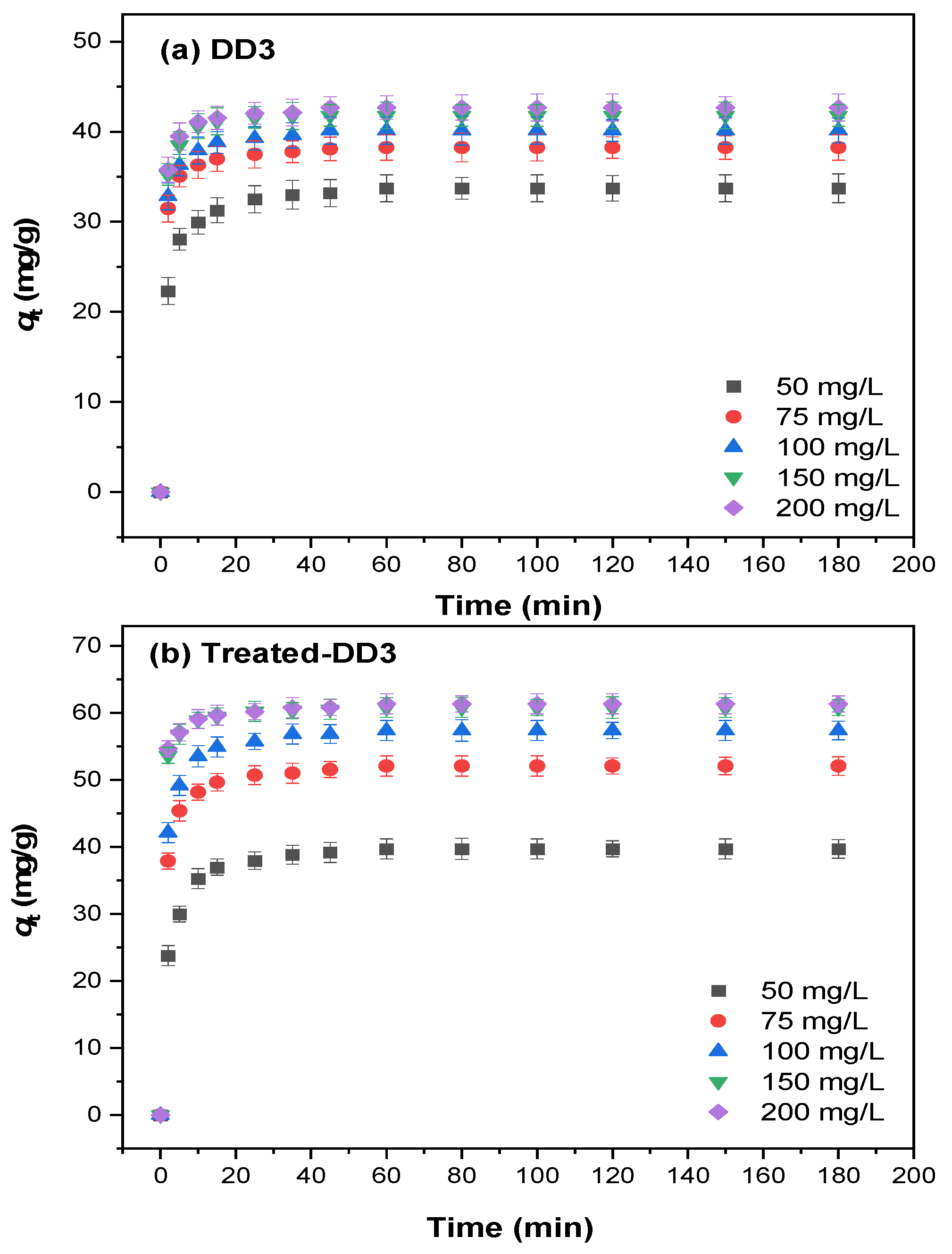
3.2.2. Adsorption Kinetics
3.2.3. Adsorption Isotherms
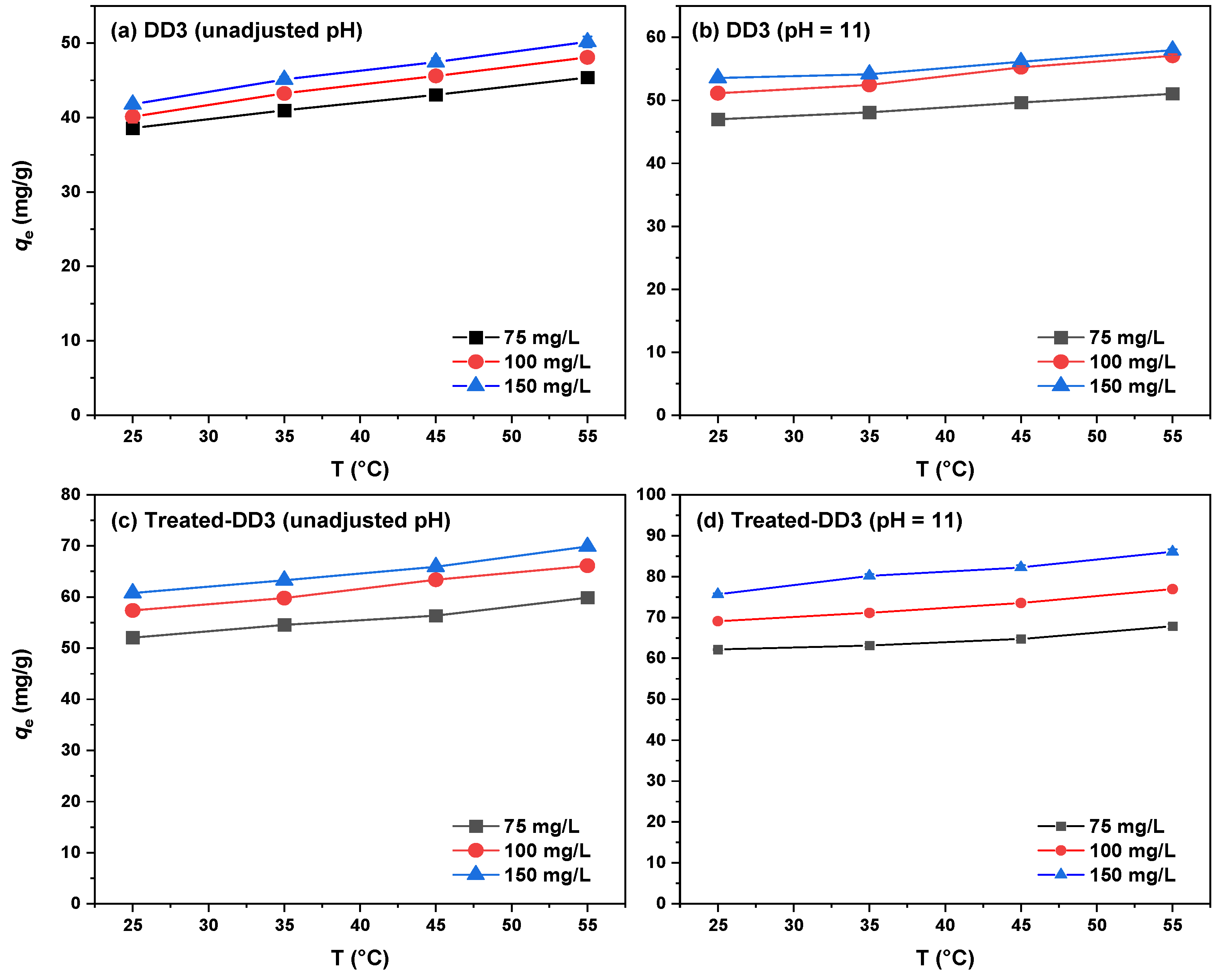
3.2.4. Adsorption Thermodynamics
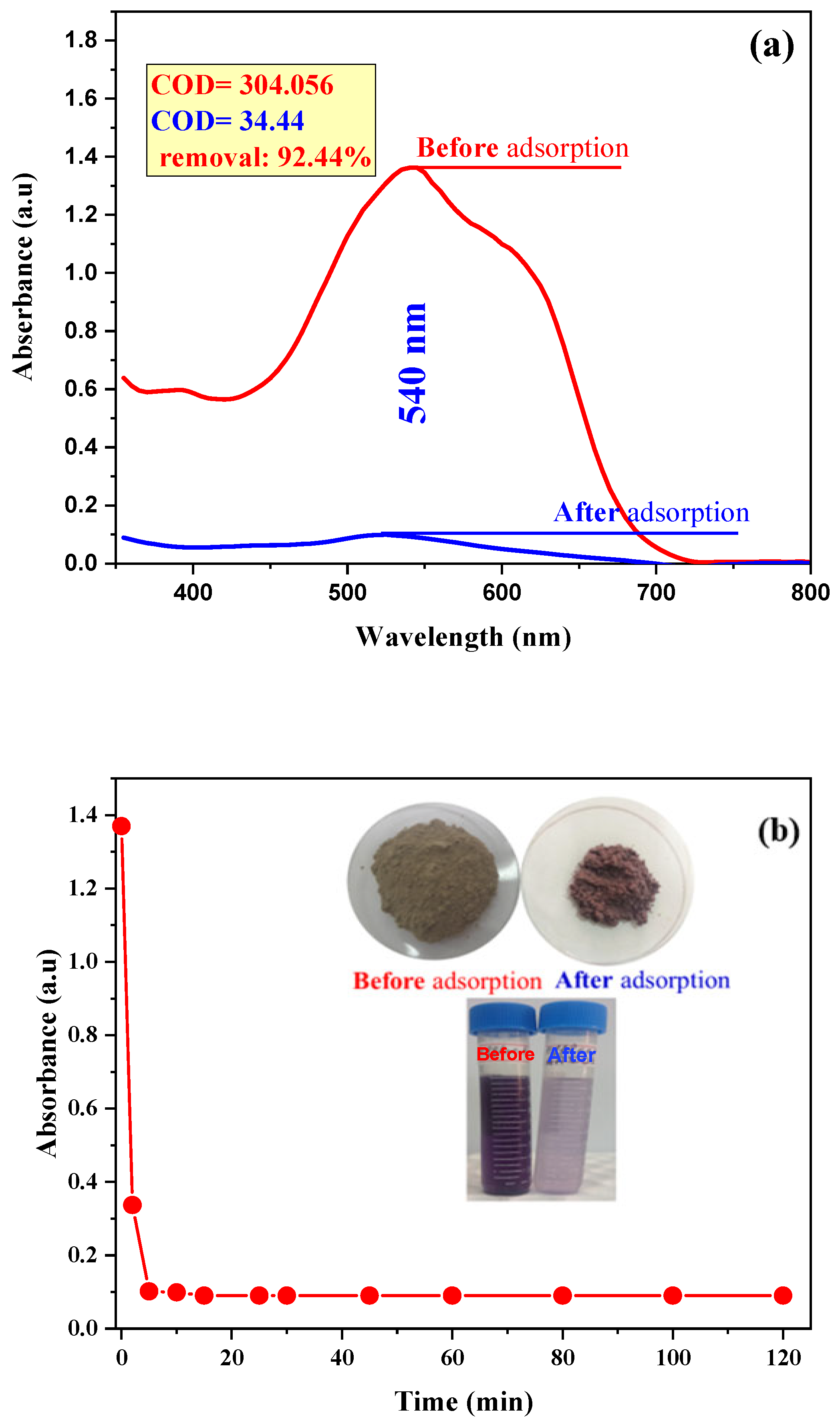
3.2.5. Dye Removal Efficiency by Treated-DD3 under Real BM-Wastewater
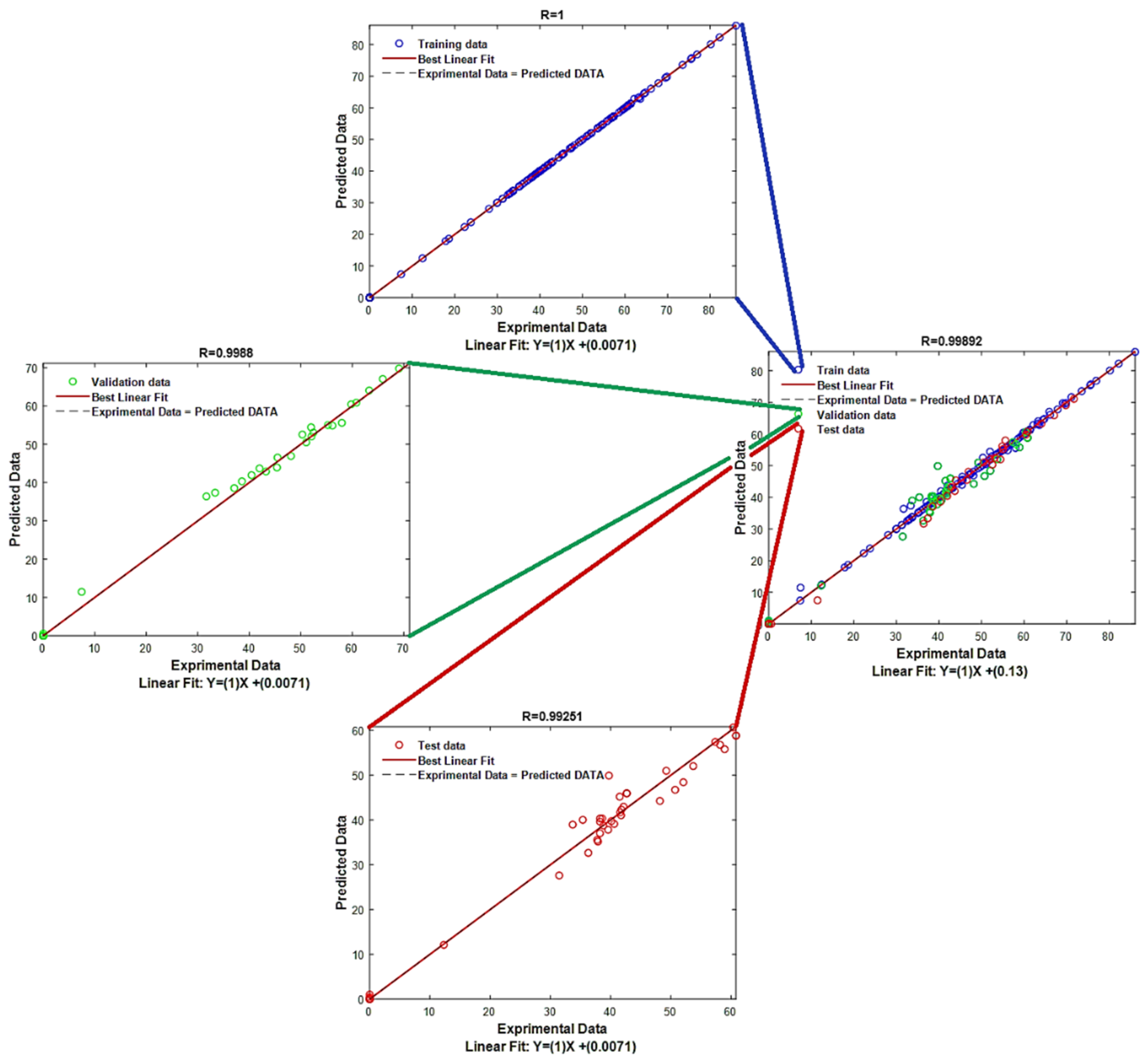
3.3. Gaussian Process Regression
3.4. Interface for Optimization and Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.-C.; Assadi, A.A.; Amrane, A.; Mouni, L. Comparison of Four Plant-Based Bio-Coagulants Performances against Alum and Ferric Chloride in the Turbidity Improvement of Bentonite Synthetic Water. Water 2022, 14, 3324. [Google Scholar] [CrossRef]
- Zen, S.; Berrichi, F. Adsorption of Tannery Anionic Dyes by Modified Kaolin from Aqueous Solution. Desalination Water Treat. 2014, 57, 6024–6032. [Google Scholar] [CrossRef]
- Fei, F.; Gao, Z.; Wu, H.; Wurendaodi, W.; Zhao, S.; Asuha, S. Facile Solid-State Synthesis of Fe3O4/Kaolinite Nanocomposites for Enhanced Dye Adsorption. J. Solid State Chem. 2020, 291, 121655. [Google Scholar] [CrossRef]
- Cheikh, S.; Imessaoudene, A.; Bollinger, J.-C.; Hadadi, A.; Amar, M.; Bouzaza, A.; Assadi, A.A.; Amrane, A.; Zamouche, M.; El Jery, A.; et al. Complete Elimination of the Ciprofloxacin Antibiotic from Water by the Combination of Adsorption-Photocatalysis Process Using Natural Hydroxyapatite and TiO2. Catalysts 2023, 13, 336. [Google Scholar] [CrossRef]
- Hajjaji, W.; Andrejkovičová, S.; Pullar, R.; Tobaldi, D.; Galindo, A.; Jammousi, F.; Rocha, F.; Labrincha, J.A. Effective Removal of Anionic and Cationic Dyes by Kaolinite and TiO2/Kaolinite Composites. Clay Miner. 2016, 51, 19–27. [Google Scholar] [CrossRef]
- Kausar, A.; Naeem, K.; Tariq, M.; Nazli, Z.-I.-H.; Bhatti, H.; Jubeen, F.; Nazir, A. Preparation and Characterization of Chitosan/Clay Composite for Direct Rose FRN Dye Removal from Aqueous Media: Comparison of Linear and Non-Linear Regression Methods. J. Mater. Res. Technol. 2018, 8, 1161–1174. [Google Scholar] [CrossRef]
- Lertcumfu, N.; Jaita, P.; Thammarong, S.; Lamkhao, S.; Tandorn, S.; Randorn, C.; Tunkasiri, T.; Rujijanagul, G. Influence of Graphene Oxide Additive on Physical, Microstructure, Adsorption, and Photocatalytic Properties of Calcined Kaolinite-Based Geopolymer Ceramic Composites. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125080. [Google Scholar] [CrossRef]
- Bouras, D.; Mecif, A.; Harabi, A.; Barille, R.; Mahdjoub, A.; Zaabat, M. Economic and Ultrafast Photocatalytic Degradation of Orange II Using Ceramic Powders. Catalysts 2021, 11, 733. [Google Scholar] [CrossRef]
- Shikuku, V.; Mishra, T. Adsorption Isotherm Modeling for Methylene Blue Removal onto Magnetic Kaolinite Clay: A Comparison of Two-Parameter Isotherms. Appl. Water Sci. 2021, 11, 103. [Google Scholar] [CrossRef]
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.-C.; Cheikh, S.; Assadi, A.A.; Amrane, A.; Kebir, M.; Mouni, L. Parametrical Study for the Effective Removal of Mordant Black 11 from Synthetic Solutions: Moringa Oleifera Seeds’ Extracts Versus Alum. Water 2022, 14, 4109. [Google Scholar] [CrossRef]
- Adeyemo-Oloidi, A.; Adeoye, I.; Bello, O. Adsorption of Dyes Using Different Types of Clay: A Review. Appl. Water Sci. 2015, 20, 543–568. [Google Scholar] [CrossRef]
- González-Crisostomo, J.; López-Juárez, R.; Yocupicio-Gaxiola, R.; Villanueva, E.; Zavala-Flores, E.; Petranovskii, V. Chabazite Synthesis and Its Exchange with Ti, Zn, Cu, Ag and Au for Efficient Photocatalytic Degradation of Methylene Blue Dye. Int. J. Mol. Sci. 2022, 23, 1730. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.-C.; Cheikh, S.; Manseri, A.; Mouni, L. Dual Valorization of Potato Peel (Solanum tuberosum) as a Versatile and Sustainable Agricultural Waste in Both Bioflocculation of Eriochrome Black T and Biosorption of Methylene Blue. J. Polym. Environ. 2023, 31, 2983–2998. [Google Scholar] [CrossRef]
- Oussalah, A.; Boukerroui, A.; Amina, A.; Djellouli, B. Cationic and Anionic Dyes Removal by Low-Cost Hybrid Alginate/Natural Bentonite Composite Beads: Adsorption and Reusability Studies. Int. J. Biol. Macromol. 2018, 124, 854–862. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A. Mesoporous Iraqi Red Kaolin Clay as an Efficient Adsorbent for Methylene Blue Dye: Adsorption Kinetic, Isotherm and Mechanism Study. Surf. Interfaces 2019, 18, 100422. [Google Scholar] [CrossRef]
- Oussalah, A.; Boukerroui, A. Alginate-Bentonite Beads for Efficient Adsorption of Methylene Blue Dye. Euro-Mediterr. J. Environ. Integr. 2020, 5, 31. [Google Scholar] [CrossRef]
- Bouchelkia, N.; Tahraoui, H.; Amrane, A.; Belkacemi, H.; Bollinger, J.-C.; Bouzaza, A.; Zoukel, A.; Zhang, J.; Mouni, L. Jujube Stones Based Highly Efficient Activated Carbon for Methylene Blue Adsorption: Kinetics and Isotherms Modeling, Thermodynamics and Mechanism Study, Optimization via Response Surface Methodology and Machine Learning Approaches. Process Saf. Environ. Prot. 2022, 170, 513–535. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Bassyouni, M.; Zoromba, M.; Alshehri, A. Removal of Dyes from Waste Solutions by Anodic Oxidation on an Array of Horizontal Graphite Rods Anodes. Ind. Eng. Chem. Res. 2018, 58, 1004–1018. [Google Scholar] [CrossRef]
- Ma, J.; Tang, X.; He, Y.; Fan, Y.; Chen, J.; Yu, H. Robust Stable MoS2/GO Filtration Membrane for Effective Removal of Dyes and Salts from Water with Enhanced Permeability. Desalination 2020, 480, 114328. [Google Scholar] [CrossRef]
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.-C.; Bouzaza, A.; Amrane, A.; Tahraoui, H.; Mouni, L. Aleppo Pine Seeds (Pinus halepensis Mill.) as a Promising Novel Green Coagulant for the Removal of Congo Red Dye: Optimization via Machine Learning Algorithm. J. Environ. Manag. 2023, 331, 117286. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.C.; Bassin, J.P.; Sant’Anna, G.L.; Dezotti, M. Ozonation of the Dye Reactive Red 239 and Biodegradation of Ozonation Products in a Moving-Bed Biofilm Reactor: Revealing Reaction Products and Degradation Pathways. Int. Biodeterior. Biodegrad. 2019, 144, 104742. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Albayati, T.M.; Alrubaye, J.M. A Hybrid Adsorption Membrane Process for Removal of Dye from Synthetic and Actual Wastewater. Chem. Eng. Process. Process Intensif. 2020, 157, 108113. [Google Scholar] [CrossRef]
- Mishra, S.; Cheng, L.; Maiti, A. The Utilization of Agro-Biomass/Byproducts for Effective Bio-Removal of Dyes from Dyeing Wastewater: A Comprehensive Review. J. Environ. Chem. Eng. 2021, 9, 104901. [Google Scholar] [CrossRef]
- Joseph, J.; Radhakrishnan, R.C.; Johnson, J.K.; Joy, S.P.; Thomas, J. Ion-Exchange Mediated Removal of Cationic Dye-Stuffs from Water Using Ammonium Phosphomolybdate. Mater. Chem. Phys. 2020, 242, 122488. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic Degradation of Dyes Using Semiconductor Photocatalysts to Clean Industrial Water Pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Chedri Mammar, A.; Mouni, L.; Bollinger, J.-C.; Belkhiri, L.; Bouzaza, A.; Assadi, A.A.; Belkacemi, H. Modeling and Optimization of Process Parameters in Elucidating the Adsorption Mechanism of Gallic Acid on Activated Carbon Prepared from Date Stones. Sep. Sci. Technol. 2020, 55, 3113–3125. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Chen, J.; Liu, Q. Hierarchical Structure Kaolinite Nanospheres with Remarkably Enhanced Adsorption Properties for Methylene Blue. Nanoscale Res. Lett. 2019, 14, 104. [Google Scholar] [CrossRef]
- Zhang, L.; Min, F.; Chen, J.; Liu, C.; Wang, T. New Insights into the Interaction between Monomers from Acrylamide-Based Polymeric Flocculants and Montmorillonite: A DFT Study. J. Mol. Liq. 2022, 365, 120171. [Google Scholar] [CrossRef]
- Pajak, M. Adsorption Capacity of Smectite Clay and Its Thermal and Chemical Modification for Two Anionic Dyes: Comparative Study. Water Air Soil Pollut. 2021, 232, 83. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Bollinger, J.-C.; Belkhiri, L.; Tiri, A.; Bouzaza, A.; El Jery, A.; Assadi, A.; Amrane, A.; Mouni, L. Zeolite Waste Characterization and Use as Low-Cost, Ecofriendly, and Sustainable Material for Malachite Green and Methylene Blue Dyes Removal: Box–Behnken Design, Kinetics, and Thermodynamics. Appl. Sci. 2022, 12, 7587. [Google Scholar] [CrossRef]
- Sandollah, N.A.S.M.; Ghazali, S.A.I.S.M.; Ibrahim, W.N.W.; Rusmin, R. Adsorption-Desorption Profile of Methylene Blue Dye on Raw and Acid Activated Kaolinite. Indones. J. Chem. 2020, 20, 755. [Google Scholar] [CrossRef]
- Sternik, D.; Galaburda, M.V.; Bogatyrov, V.; Oranska, O.I.; Charmas, B.; Gun’ko, V. Novel Porous Carbon/Clay Nanocomposites Derived from Kaolinite/Resorcinol-Formaldehyde Polymer Blends: Synthesis, Structure and Sorption Properties. Appl. Surf. Sci. 2020, 525, 146361. [Google Scholar] [CrossRef]
- Achraf, H.; Gharibi, E.; Nasri, H.; El Ouahabi, M. Thermodynamics and Kinetics of the Removal of Methylene Blue from Aqueous Solution by Raw Kaolin. SN Appl. Sci. 2020, 2, 277. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from Aqueous Solutions by Adsorption on Kaolin: Kinetic and Equilibrium Studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Nandi, B.; Goswami, A.; Purkait, M. Removal of Cationic Dyes from Aqueous Solutions by Kaolin: Kinetic and Equilibrium Studies. Appl. Clay Sci. 2009, 42, 583–590. [Google Scholar] [CrossRef]
- Hamdi, M.; Doğan, M.; Alkan, M. Removal of Cationic Dyes by Kaolinite. Microporous Mesoporous Mater. 2009, 122, 20–27. [Google Scholar] [CrossRef]
- Tehrani Bagha, A.; Nikkar, H.; Mahmoodi, N.M.; Menger, F.M. The Sorption of Cationic Dyes onto Kaolin: Kinetic, Isotherm and Thermodynamic Studies. Desalination 2011, 266, 274–280. [Google Scholar] [CrossRef]
- Sarma, G.; Gupta, S.; Bhattacharyya, K. Removal of Hazardous Basic Dyes from Aqueous Solution by Adsorption onto Kaolinite and Acid-Treated Kaolinite: Kinetics, Isotherm and Mechanistic Study. SN Appl. Sci. 2019, 1, 211. [Google Scholar] [CrossRef]
- Ding, Y.; Jin, Y.; Yao, B.; Khan, A. Artificial Intelligence Based Simulation of Cd (II) Adsorption Separation from Aqueous Media Using a Nanocomposite Structure. J. Mol. Liq. 2021, 344, 117772. [Google Scholar] [CrossRef]
- Khan, H.; Hussain, S.; Hussain, S.F.; Gul, S.; Ahmad, A.; Ullah, S. Multivariate Modeling and Optimization of Cr (VI) Adsorption onto Carbonaceous Material via Response Surface Models Assisted with Multiple Regression Analysis and Particle Swarm Embedded Neural Network. Environ. Technol. Innov. 2021, 24, 101952. [Google Scholar] [CrossRef]
- Khiam, G.K.; Karri, R.R.; Mubarak, N.M.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Rahman, M.E. Modelling and Optimization for Methylene Blue Adsorption Using Graphene Oxide/Chitosan Composites via Artificial Neural Network-Particle Swarm Optimization. Mater. Today Chem. 2022, 24, 100946. [Google Scholar] [CrossRef]
- Cristóbal, A.G.; Castelló, R.; Martin-Luengo, M.; Vizcayno, C. Zeolites Prepared from Calcined and Mechanically Modified Kaolins: A Comparative Study. Appl. Clay Sci. 2010, 49, 239–246. [Google Scholar] [CrossRef]
- Al-Ghouti, M.; Khraisheh, M.; Ahmad, M.; Allen, S. Adsorption Behaviour of Methylene Blue onto Jordanian Diatomite: A Kinetic Study. J. Hazard. Mater. 2008, 165, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Vafaei, F.; Karaca, S.; Khataee, A. Adsorption of a Cationic Dye from Aqueous Solution Using Turkish Lignite: Kinetic, Isotherm, Thermodynamic Studies and Neural Network Modeling. J. Ind. Eng. Chem. 2014, 20, 2615–2624. [Google Scholar] [CrossRef]
- Doğan, M.; Abak, H.; Alkan, M. Adsorption of Methylene Blue onto Hazelnut Shell: Kinetics, Mechanism and Activation Parameters. J. Hazard. Mater. 2008, 164, 172–181. [Google Scholar] [CrossRef]
- Rida, K.; Sarra, B.; Selma, H. Adsorption of Methylene Blue from Aqueous Solution by Kaolin and Zeolite. Appl. Clay Sci. 2013, 83–84, 99–105. [Google Scholar] [CrossRef]
- Yavuz, Ö.; Saka, C. Surface Modification with Cold Plasma Application on Kaolin and Its Effects on the Adsorption of Methylene Blue. Appl. Clay Sci. 2013, 85, 96–102. [Google Scholar] [CrossRef]
- Rahmani, S.; Zeynizadeh, B.; Karami, S. Removal of Cationic Methylene Blue Dye Using Magnetic and Anionic- Cationic Modified Montmorillonite: Kinetic, Isotherm and Thermodynamic Studies. Appl. Clay Sci. 2019, 184, 105391. [Google Scholar] [CrossRef]
- Tahraoui, H.; Belhadj, A.-E.; Triki, Z.; Boudella, N.; Seder, S.; Amrane, A.; Zhang, J.; Moula, N.; Tifoura, A.; Ferhat, R.; et al. Mixed Coagulant-Flocculant Optimization for Pharmaceutical Effluent Pretreatment Using Response Surface Methodology and Gaussian Process Regression. Process Saf. Environ. Prot. 2022, 169, 909–927. [Google Scholar] [CrossRef]
- Tahraoui, H.; Belhadj, A.-E.; Amrane, A.; Houssein, E.H. Predicting the Concentration of Sulfate Using Machine Learning Methods. Earth Sci. Inform. 2022, 15, 1023–1044. [Google Scholar] [CrossRef]
- Zamouche, M.; Tahraoui, H.; Laggoun, Z.; Mechati, S.; Chemchmi, R.; Kanjal, M.I.; Amrane, A.; Hadadi, A.; Mouni, L. Optimization and Prediction of Stability of Emulsified Liquid Membrane (ELM): Artificial Neural Network. Processes 2023, 11, 364. [Google Scholar] [CrossRef]
- Bousselma, A.; Abdessemed, D.; Tahraoui, H.; Amrane, A. Umjetna Inteligencija i Matematičko Modeliranje Kinetike Sušenja Prethodno Obrađenih Cjelovitih Plodova Marelice. Kem. U Ind. Časopis Kemičara I Kem. Inženjera Hrvat. 2021, 70, 651–667. [Google Scholar]
- Zamouche, M.; Chermat, M.; Kermiche, Z.; Tahraoui, H.; Kebir, M.; Bollinger, J.-C.; Amrane, A.; Mouni, L. Predictive Model Based on K-Nearest Neighbor Coupled with the Gray Wolf Optimizer Algorithm (KNN_GWO) for Estimating the Amount of Phenol Adsorption on Powdered Activated Carbon. Water 2023, 15, 493. [Google Scholar] [CrossRef]
- Rao, B.; Dai, H.; Gao, L.; He, F.; Zhang, M.; Gan, F.; Zhang, Q.; Liu, M.; Yin, Z. A Novel Combined Metallurgy-Beneficiation Method for the Facile and Low-Cost Comprehensive Resource Utilization of Low-Grade Kaolin Solid Wastes. J. Environ. Manag. 2023, 345, 118650. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, A.A. Physiochemical Characterization of Ethiopian Mined Kaolin Clay through Beneficiation Process. Adv. Mater. Sci. Eng. 2023, 2023, 9104807. [Google Scholar] [CrossRef]
- Misrar, W.; Loutou, M.; Saadi, L.; Waqif, M.; Mansori, M. Modification of Ceramic Membranes Porosity Using Layer Deposition of Kaolinite and Palygorskite. J. Aust. Ceram. Soc. 2023, 59, 995–1012. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Temesgen, F. Synthesis and Characterization of Ethiopian Kaolin for the Removal of Basic Yellow (BY 28) Dye from Aqueous Solution as a Potential Adsorbent. Heliyon 2020, 6, e04975. [Google Scholar] [CrossRef]
- Aljohani, N.S.; Kavil, Y.N.; Al-Farawati, R.K.; Alelyani, S.S.; Orif, M.I.; Shaban, Y.A.; Al-Mhyawi, S.R.; Aljuhani, E.H.; Salam, M.A. The Effective Adsorption of Arsenic from Polluted Water Using Modified Halloysite Nanoclay. Arab. J. Chem. 2023, 16, 104652. [Google Scholar] [CrossRef]
- Felhi, M.; Tlili, A.; Gaied, M.; Montacer, M. Mineralogical Study of Kaolinitic Clays from Sidi El Bader in the Far North of Tunisia. Appl. Clay Sci. 2008, 39, 208–217. [Google Scholar] [CrossRef]
- Sing, K. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Mellouk, S.; Cherifi, S.; Sassi, M.; Marouf-Khelifa, K.; Bengueddach, A.; Khelifa, A. Intercalation of Halloysite from Djebel Debagh (Algeria) and Adsorption of Copper Ions. Appl. Clay Sci. 2009, 44, 230–236. [Google Scholar] [CrossRef]
- Khan, T.A.; Khan, E.A.; Shahjahan. Removal of Basic Dyes from Aqueous Solution by Adsorption onto Binary Iron-Manganese Oxide Coated Kaolinite: Non-Linear Isotherm and Kinetics Modeling. Appl. Clay Sci. 2015, 107, 70–77. [Google Scholar] [CrossRef]
- Omer, O.S.; Hussein, M.A.; Hussein, B.H.; Mgaidi, A. Adsorption Thermodynamics of Cationic Dyes (Methylene Blue and Crystal Violet) to a Natural Clay Mineral from Aqueous Solution between 293.15 and 323.15 K. Arab. J. Chem. 2017, 11, 615–623. [Google Scholar] [CrossRef]
- Munir, M.; Nazar, M.; Zafar, M.; Zubair, M.; Ashfaq, M.; Hosseini-Bandegharaei, A.; Khan, S.; Ahmad, A. Effective Adsorptive Removal of Methylene Blue from Water by Didodecyldimethylammonium Bromide-Modified Brown Clay. ACS Omega 2020, 5, 16711–16721. [Google Scholar] [CrossRef]
- Errais, E.; Duplay, J.; Darragi, F.; M’Rabet, I.; Aubert, A.; Huber, F.; Morvan, G. Efficient Anionic Dye Adsorption on Natural Untreated Clay: Kinetic Study and Thermodynamic Parameters. Desalination 2011, 275, 74–81. [Google Scholar] [CrossRef]
- Luckham, P.; Rossi, S. The Colloidal and Rheological Properties of Bentonite Suspensions. Adv. Colloid Interface Sci. 1999, 82, 43–92. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, H.; Liu, G.; Qiao, J.; Wang, J.; Haiying, L.; Yang, L. Methylene Blue Adsorption onto Swede Rape Straw (Brassica napus L.) Modified by Tartaric Acid: Equilibrium, Kinetic and Adsorption Mechanisms. Bioresour. Technol. 2012, 125, 138–144. [Google Scholar] [CrossRef]
- Sheshmani, S.; Ashori, A.; Hasanzadeh, S. Removal of Acid Orange 7 from Aqueous Solution Using Magnetic Graphene/Chitosan: A Promising Nano-Adsorbent. Int. J. Biol. Macromol. 2014, 68, 218–224. [Google Scholar] [CrossRef]
- Lima, É.C.; Adebayo, M.A.; Machado, F.M. Kinetic and Equilibrium Models of Adsorption. In Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications; Springer: Berlin/Heidelberg, Germany, 2015; pp. 33–69. [Google Scholar]
- Fernandes, J.; Rodrigues, A.M.; Menezes, R.; Neves, G. Adsorption of Anionic Dye on the Acid-Functionalized Bentonite. Materials 2020, 13, 3600. [Google Scholar] [CrossRef]
- Nguyen, V.; Pho, Q. Preparation of Chitosan Coated Magnetic Hydroxyapatite Nanoparticles and Application for Adsorption of Reactive Blue 19 and Ni2+ Ions. Sci. World J. 2014, 2014, 273082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiaoyun, W.; Honghong, L.; Tianshe, T.; Wu, W. Adsorption Behavior of Th(IV) onto Illite: Effect of Contact Time, pH Value, Ionic Strength, Humic Acid and Temperature. Appl. Clay Sci. 2016, 127–128, 35–43. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Kul, A.; Gökırmak Söğüt, E.; Caliskan, N. Adsorption of neutral red dye from aqueous solutions by natural adsorbent: An equilibrium, kinetic and thermodynamic study. Commun. Fac. Sci. Univ. Ank. Ser. B Chem. Chem. Eng. 2021, 63, 27–60. [Google Scholar]
- Mustapha, S.; Ndamitso, M.; Abdulkareem, A.; Tijani Oladejo, J.; Mohammed, A.; Shuaib, D.T. Potential of Using Kaolin as a Natural Adsorbent for the Removal of Pollutants from Tannery Wastewater. Heliyon 2019, 5, e02923. [Google Scholar] [CrossRef]
- Tran, H.; You, S.-J.; Chao, H.-P. Thermodynamic Parameters of Cadmium Adsorption onto Orange Peel Calculated from Various Methods: A Comparison Study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Cheikh, S.; Imessaoudene, A.; Bollinger, J.-C.; Manseri, A.; Bouzaza, A.; Hadadi, A.; Hamri, N.; Amrane, A.; Mouni, L. Adsorption Behavior and Mechanisms of the Emerging Antibiotic Pollutant Norfloxacin on Eco-Friendly and Low-Cost Hydroxyapatite: Integrated Experimental and Response Surface Methodology Optimized Adsorption Process. J. Mol. Liq. 2023, 392, 123424. [Google Scholar] [CrossRef]
- Agarwal, S.; Singh, A.P.; Mathur, S. Removal of COD and Color from Textile Industrial Wastewater Using Wheat Straw Activated Carbon: An Application of Response Surface and Artificial Neural Network Modeling. Environ. Sci. Pollut. Res. 2023, 30, 41073–41094. [Google Scholar] [CrossRef]

| Component | DD3 | Treated-DD3 |
|---|---|---|
| SiO2 | 41.13 | 37.73 |
| Al2O3 | 35.92 | 35.49 |
| Fe2O3 | 0.92 | 0.98 |
| MgO | 0.03 | 0.08 |
| CaO | 0.20 | 0.39 |
| Na2O | 0.32 | 0.37 |
| K2O | 0.27 | 0.26 |
| NiO | 0.04 | 0.05 |
| BaO | 0.05 | 0.07 |
| P2O5 | 0.05 | 0.05 |
| SO3 | 0.74 | 0.71 |
| MnO | 0.71 | 0.92 |
| LOI * | 19.56 | 22.58 |
| Unit | DD3 | Treated-DD3 | |
|---|---|---|---|
| SBET | m2/g | 67.60 | 155.1 |
| VTot | cm3/g | 0.252 | 0.386 |
| Dp | nm | 14.94 | 9.95 |
| CEC | meq/100 g | 14.36 | 9.83 |
| pHPZC | — | 6.70 | 3.85 |
| Co (mg/L) | qe (exp) (mg/g) | PFO Model | PSO Model | ||||
|---|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (1/min) | R2 | qe (mg/g) | k2 g/(mg × min) | R2 | ||
| 1. DD3 50 | 33.70 | 32.84 | 0.504 | 0.980 | 33.94 | 0.027 | 0.999 |
| 75 | 38.23 | 37.64 | 0.871 | 0.993 | 38.31 | 0.058 | 0.999 |
| 100 | 40.11 | 39.46 | 0.852 | 0.991 | 40.19 | 0.052 | 0.999 |
| 150 | 41.79 | 41.40 | 0.921 | 0.994 | 42.03 | 0.060 | 0.999 |
| 2. Treated-DD3 50 | 39.71 | 38.78 | 0.379 | 0.977 | 40.30 | 0.016 | 0.999 |
| 75 | 52.07 | 51.03 | 0.627 | 0.987 | 52.36 | 0.024 | 0.999 |
| 100 | 57.37 | 56.28 | 0.628 | 0.985 | 57.75 | 0.022 | 0.999 |
| 150 | 60.80 | 60.14 | 1.091 | 0.995 | 60.85 | 0.057 | 0.999 |
| Unit | DD3 | Treated-DD3 | |
|---|---|---|---|
| 1. Langmuir model Qmax | mg/g | 44.48 | 64.58 |
| KL | L/mg | 0.148 | 0.163 |
| R2 | — | 0.999 | 0.999 |
| 2. Freundlich model KF | (mg/g)/(mg/L)1/n | 24.16 | 31.48 |
| 1/n | — | 0.116 | 0.141 |
| R2 | — | 0.937 | 0.866 |
| T (K) | KC | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° [J/(mol × K)] |
|---|---|---|---|---|
| 1. DD3 | ||||
| Unadjusted pH 298 | 47,626 | −26.69 | 8.40 | 117.98 |
| 308 | 56,933 | −28.04 | ||
| 318 | 61,501 | −29.15 | ||
| 328 | 65,397 | −30.24 | ||
| pH = 11 298 | 55,971 | −27.09 | 3.69 | 103.34 |
| 308 | 59,492 | −28.15 | ||
| 318 | 62,371 | −29.19 | ||
| 328 | 64,069 | −30.18 | ||
| 2. Treated-DD3 | ||||
| Unadjusted pH 298 | 52,238 | −26.91 | 14.05 | 137.50 |
| 308 | 66,401 | −28.43 | ||
| 318 | 70,162 | −29.50 | ||
| 328 | 91,154 | −31.14 | ||
| pH = 11 298 | 73,185 | −27.75 | 27.09 | 182.87 |
| 308 | 84,492 | −29.05 | ||
| 318 | 94,267 | −30.28 | ||
| 328 | 217,671 | −33.51 |
| PSO | Max_Iteration = 100 SearchAgents_no = 30 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kernel Function | Basis Function | Kernel Scale | Sigma | R/R2/adj-R2 | RMSE/MAE | |||||||
| Sigma M | Sigma F | Train | Test | VAL | ALL | Train | Test | VAL | ALL | |||
| Ard Exponential | Constant | 0.0296 17.6066 10.1373 75.1580 233.048 11.5636 | 21.3603 | 0.3506 | 1.0000 1.0000 1.0000 | 0.9925 0.9851 0.9828 | 0.9988 0.9976 0.9972 | 0.9989 0.9978 0.9978 | 0.0745 0.0258 | 2.5985 1.7202 | 1.3942 0.7975 | 1.1390 0.3926 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamri, N.; Imessaoudene, A.; Hadadi, A.; Cheikh, S.; Boukerroui, A.; Bollinger, J.-C.; Amrane, A.; Tahraoui, H.; Tran, H.N.; Ezzat, A.O.; et al. Enhanced Adsorption Capacity of Methylene Blue Dye onto Kaolin through Acid Treatment: Batch Adsorption and Machine Learning Studies. Water 2024, 16, 243. https://doi.org/10.3390/w16020243
Hamri N, Imessaoudene A, Hadadi A, Cheikh S, Boukerroui A, Bollinger J-C, Amrane A, Tahraoui H, Tran HN, Ezzat AO, et al. Enhanced Adsorption Capacity of Methylene Blue Dye onto Kaolin through Acid Treatment: Batch Adsorption and Machine Learning Studies. Water. 2024; 16(2):243. https://doi.org/10.3390/w16020243
Chicago/Turabian StyleHamri, Nadia, Ali Imessaoudene, Amina Hadadi, Sabrina Cheikh, Abdelhamid Boukerroui, Jean-Claude Bollinger, Abdeltif Amrane, Hichem Tahraoui, Hai Nguyen Tran, Abdelrahman O. Ezzat, and et al. 2024. "Enhanced Adsorption Capacity of Methylene Blue Dye onto Kaolin through Acid Treatment: Batch Adsorption and Machine Learning Studies" Water 16, no. 2: 243. https://doi.org/10.3390/w16020243
APA StyleHamri, N., Imessaoudene, A., Hadadi, A., Cheikh, S., Boukerroui, A., Bollinger, J.-C., Amrane, A., Tahraoui, H., Tran, H. N., Ezzat, A. O., Al-Lohedan, H. A., & Mouni, L. (2024). Enhanced Adsorption Capacity of Methylene Blue Dye onto Kaolin through Acid Treatment: Batch Adsorption and Machine Learning Studies. Water, 16(2), 243. https://doi.org/10.3390/w16020243









