Effect of Rice–Carp Coculture on Phytoplankton and Microzooplankton Community Composition in Paddy Water during Different Rice Growth Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Collection
2.2. Measurement of Nutrients in the Water
2.3. Planktonic DNA Extraction, PCR Amplification and Sequencing
2.4. Data Processing
2.5. Statistical Analysis
3. Results
3.1. Annotation of Plankton
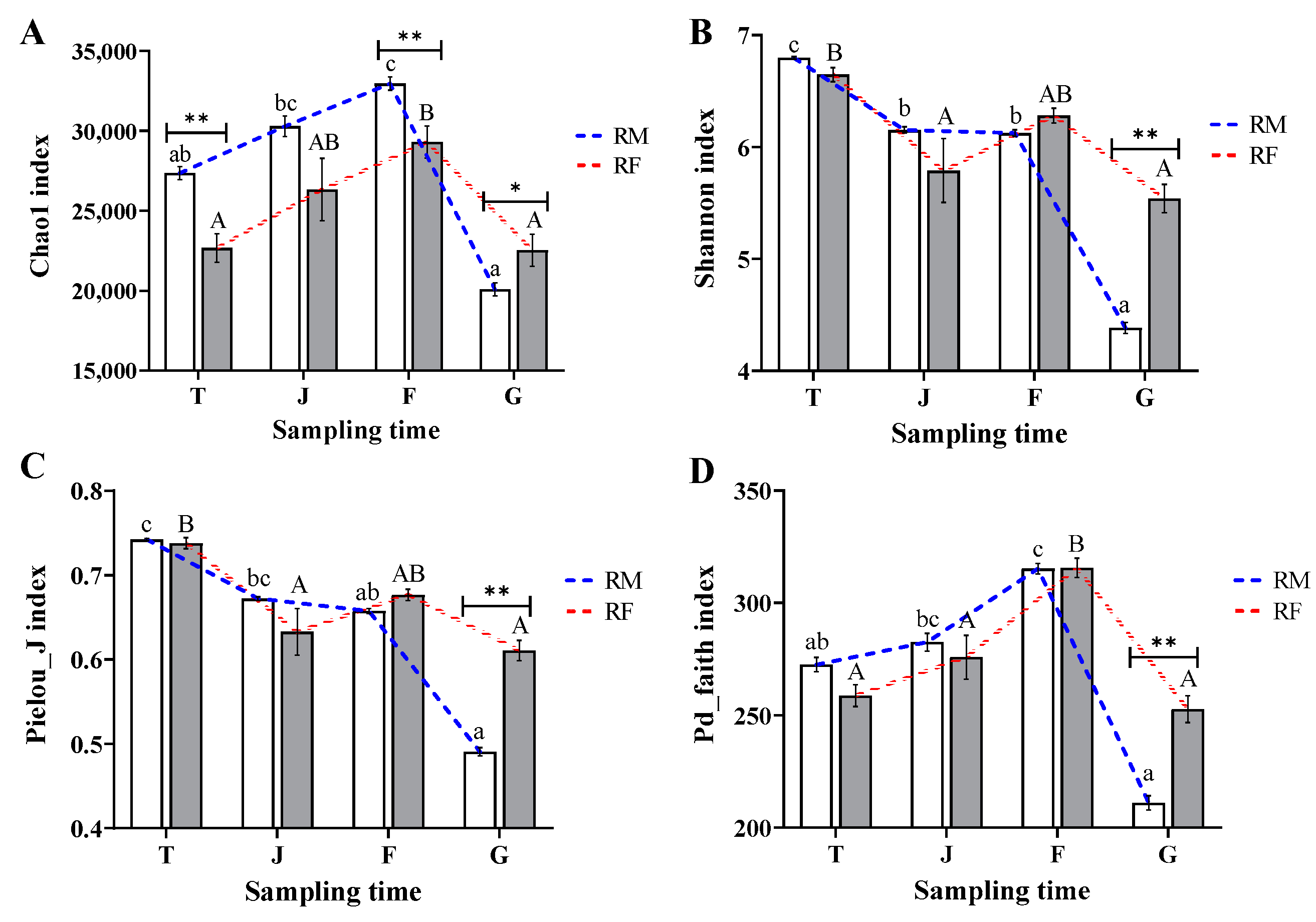
3.2. Differences in Alpha Diversity of Phytoplankton and Microzooplankton
3.3. Differences in Beta Diversity of Phytoplankton and Microzooplankton
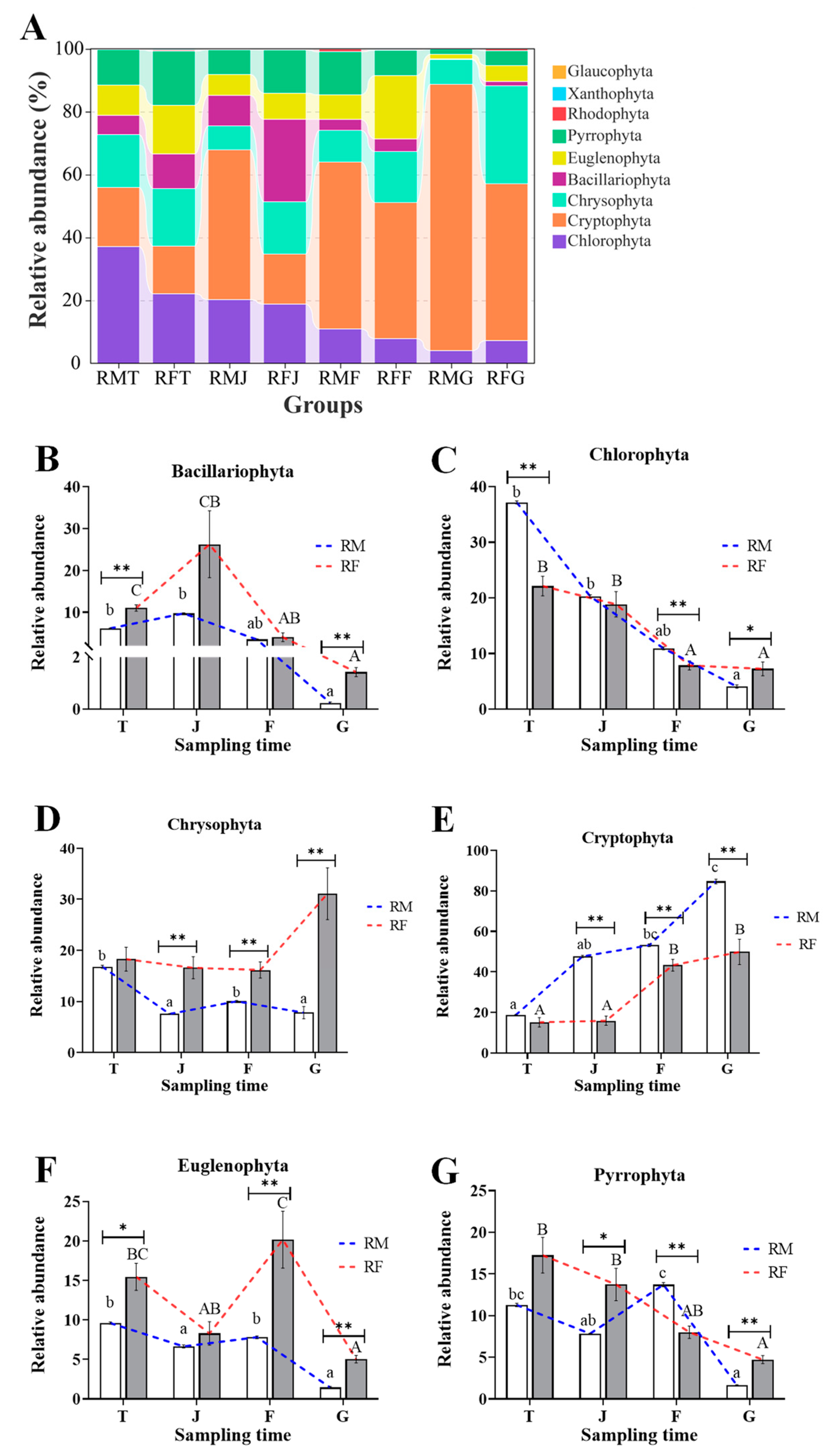
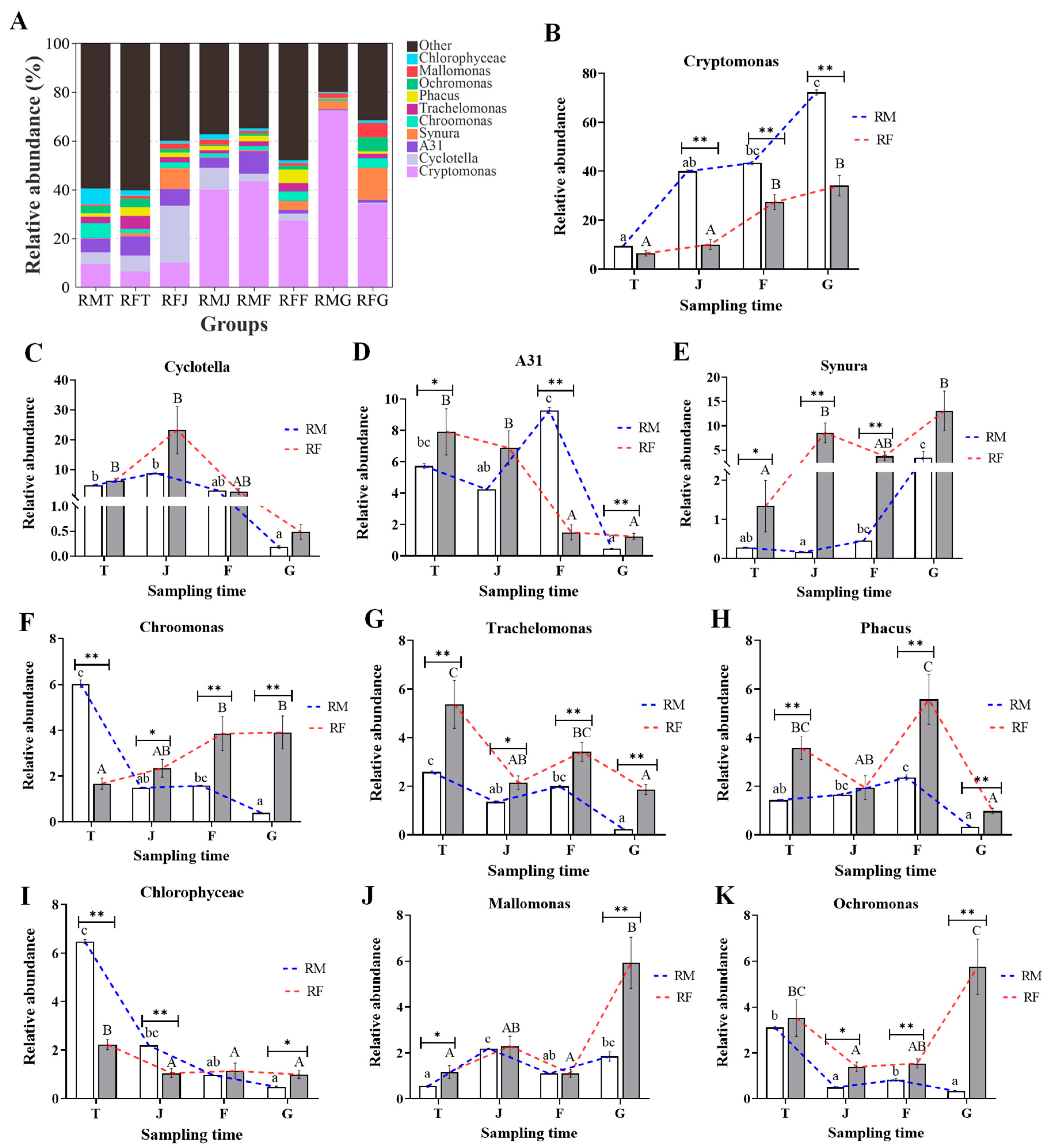
3.4. Differences in Compositions of Phytoplankton
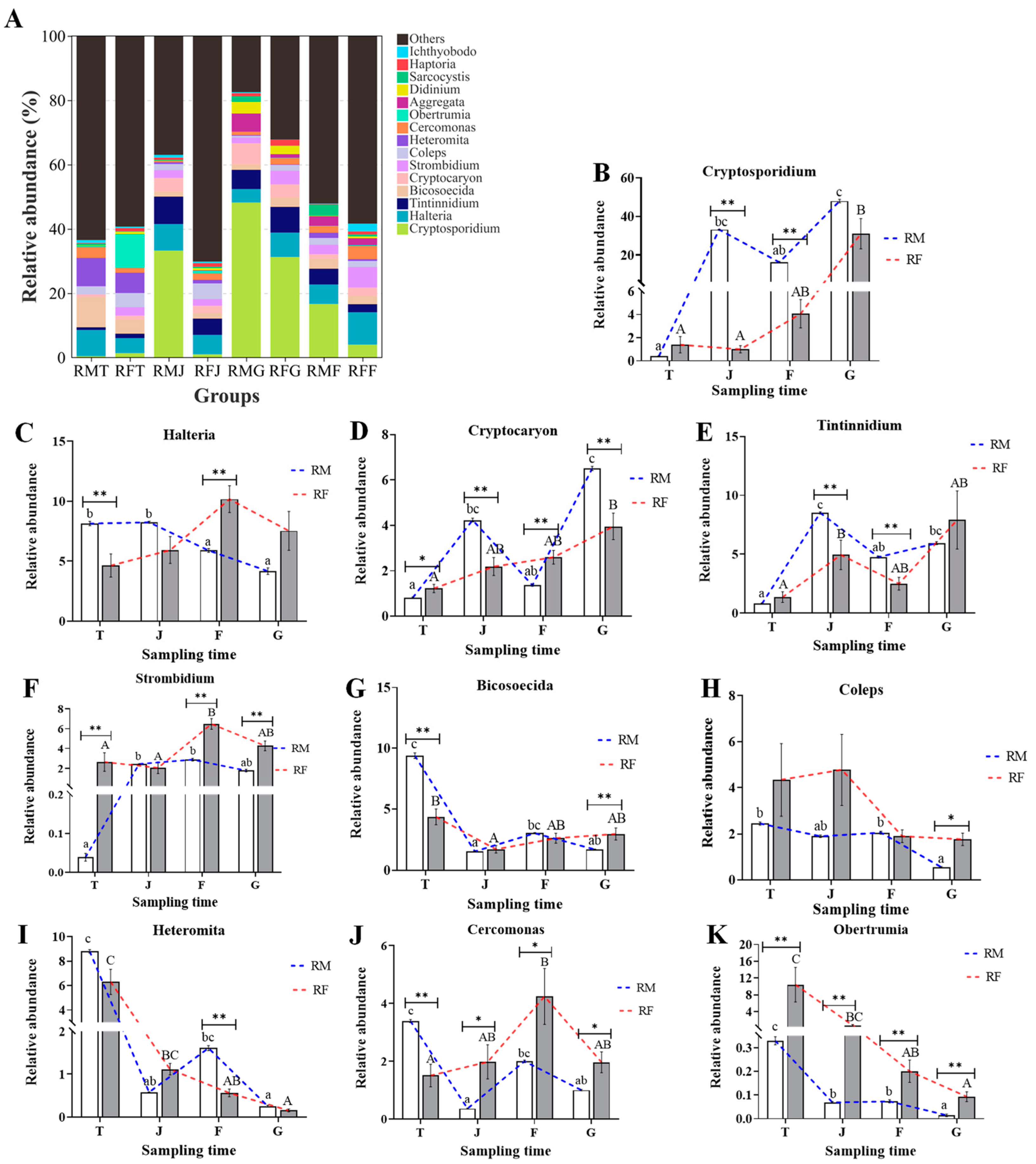
3.5. Differences in Compositions of Microzooplankton
3.6. Correlations of Environmental Factors with Water Plankton Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, N.; Hornbuckle, J.; Turchini, G.M. Blue-green water utilization in rice-fish cultivation towards sustainable food production. Ambio 2022, 51, 1933–1948. [Google Scholar] [CrossRef] [PubMed]
- National Aquatic Technology Extension Station. Report on the Development of China’s Rice-Fishery Comprehensive Breeding Industry (2024); National Aquatic Technology Extension Station: Beijing, China, 2024. [Google Scholar]
- Xie, J.; Hu, L.; Tang, J.; Wu, X.; Li, N.; Yuan, Y.; Yang, H.; Zhang, J.; Luo, S.; Chen, X. Ecological mechanisms underlying the sustainability of the agricultural heritage rice-fish coculture system. Proc. Natl. Acad. Sci. USA 2011, 108, E1381–E1387. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ren, W.; Tang, J.; Li, N.; Zhang, J.; Chen, X. The productivity of traditional rice-fish co-culture can be increased without increasing nitrogen loss to the environment. Agric. Ecosyst. Environ. 2013, 177, 28–34. [Google Scholar] [CrossRef]
- Li, Y.; Phonexay, M.; Zhang, Z.; Li, C.; Li, J.; Zhang, W. Status of rice-fish farming and rice field fisheries in Northern Laos. Front. Sustain. Food Syst. 2023, 7, 1174172. [Google Scholar] [CrossRef]
- Wan, N.-F.; Li, S.-X.; Li, T.; Cavalieri, A.; Weiner, J.; Zheng, X.-Q.; Ji, X.-Y.; Zhang, J.-Q.; Zhang, H.-L.; Zhang, H. Ecological intensification of rice production through rice-fish co-culture. J. Clean. Prod. 2019, 234, 1002–1012. [Google Scholar] [CrossRef]
- Ahmed, N.; Turchini, G.M. The evolution of the blue-green revolution of rice-fish cultivation for sustainable food production. Sustain. Sci. 2021, 16, 1375–1390. [Google Scholar] [CrossRef]
- Rahman, M.A. Integration of aquaculture with rice farming: A way to increase farm productivity, food security, livelihood improvement and better environment. Agric. Syst. 2016, 8, 10. [Google Scholar]
- Liu, D.; Tang, R.; Xie, J.; Tian, J.; Shi, R.; Zhang, K. Valuation of ecosystem services of rice–fish coculture systems in Ruyuan County, China. Ecosyst. Serv. 2020, 41, 101054. [Google Scholar] [CrossRef]
- Bakhtiyar, Y.; Arafat, M.Y.; Andrabi, S.; Tak, H.I. Zooplankton: The significant ecosystem service provider in aquatic environment. In Bioremediation and Biotechnology, Vol 3: Persistent and Recalcitrant Toxic Substances; Springer: Berlin/Heidelberg, Germany, 2020; pp. 227–244. [Google Scholar]
- Lobus, N.V.; Kulikovskiy, M.S. The co-evolution aspects of the biogeochemical role of phytoplankton in aquatic ecosystems: A review. Biology 2023, 12, 92. [Google Scholar] [CrossRef]
- Pace, M.L.; Lovett, G.M.; Carey, C.C.; Thomas, R.Q. Primary production: The foundation of ecosystems. In Fundamentals of Ecosystem Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 29–53. [Google Scholar]
- Brierley, A.S. Plankton. Curr. Biol. 2017, 27, R478–R483. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdiscip. Rev. Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Steinberg, D.K.; Landry, M.R. Zooplankton and the ocean carbon cycle. Annu. Rev. Mar. Sci. 2017, 9, 413–444. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Xiong, W.; Li, S.; Zhan, A. Conventional net tow versus environmental DNA for metabarcoding-based analysis of plankton-environment interactions in polluted aquatic ecosystems. Ecol. Indic. 2024, 158, 111356. [Google Scholar] [CrossRef]
- Xiong, W.; Ni, P.; Chen, Y.; Gao, Y.; Li, S.; Zhan, A. Biological consequences of environmental pollution in running water ecosystems: A case study in zooplankton. Environ. Pollut. 2019, 252, 1483–1490. [Google Scholar] [CrossRef]
- Vanni, M.J. Effects of food availability and fish predation on a zooplankton community. Ecol. Monogr. 1987, 57, 61–88. [Google Scholar] [CrossRef]
- Palupi, M.; Wijaya, R.; Chanda, R.A.; Azhari, R.F.; Fitriadi, R. Biodiversity and abundance of phytoplankton in rice-fish farming system. Iraqi J. Agric. Sci. 2023, 54, 1084–1093. [Google Scholar] [CrossRef]
- Vromant, N.; Chau, N.T.H.; Ollevier, F. The effect of rice seeding rate and fish stocking on the floodwater ecology of the rice field in direct-seeded, concurrent rice-fish systems. Hydrobiologia 2001, 445, 151–164. [Google Scholar] [CrossRef]
- Frei, M.; Razzak, M.A.; Hossain, M.M.; Oehme, M.; Dewan, S.; Becker, K. Performance of common carp, Cyprinus carpio L. and Nile tilapia, Oreochromis niloticus (L.) in integrated rice-fish culture in Bangladesh. Aquaculture 2007, 262, 250–259. [Google Scholar] [CrossRef]
- Mohanty, R.K.; Thakur, A.K.; Ghosh, S.; Patil, D.U. Impact of rice-fish-prawn culture on rice field ecology and productivity. Indian J. Agric. Sci. 2010, 80, 597–602. [Google Scholar]
- Nayak, P.K.; Nayak, A.K.; Panda, B.B.; Lal, B.; Gautam, P.; Poonam, A.; Shahid, M.; Tripathi, R.; Kumar, U.; Mohapatra, S.D.; et al. Ecological mechanism and diversity in rice based integrated farming system. Ecol. Indic. 2018, 91, 359–375. [Google Scholar] [CrossRef]
- Nørgaard, L.; Olesen, C.R.; Trøjelsgaard, K.; Pertoldi, C.; Nielsen, J.L.; Taberlet, P.; Ruiz-González, A.; De Barba, M.; Iacolina, L. eDNA metabarcoding for biodiversity assessment, generalist predators as sampling assistants. Sci. Rep. 2021, 11, 6820. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Guo, N.; Gao, J.; Xiao, N. Using eDNA to survey amphibians: Methods, applications, and challenges. Biotechnol. Bioeng. 2024, 121, 456–471. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Jeffery, N.; Stanley, R.; Hamilton, L.; Rubidge, E.; Abbott, C. eDNA metabarcoding enriches traditional trawl survey data for monitoring biodiversity in the marine environment. ICES J. Mar. Sci. 2023, 80, 1529–1538. [Google Scholar] [CrossRef]
- Lv, J.; Lin, Y.; Zhao, Z.; Zhou, X. eDNA metabarcoding revealed the seasonal and spatial variation of phytoplankton functional groups in the Chai river and their relationship with environmental factors. J. Freshw. Ecol. 2023, 38, 2176374. [Google Scholar] [CrossRef]
- Šimunović, M.; Kulaš, A.; Žutinić, P.; Gligora Udovič, M. Phytoplankton Diversity of a Natural Karst Lake Combining Morphological and Molecular Approaches. Water 2023, 15, 1379. [Google Scholar] [CrossRef]
- SC/T 1135.1-2017; Technical Specification for Integrated Farming of Rice and Aquaculture Animal Part 1: General Principle. Fisheries Administration of the People’s Republic of China: Beijing, China, 2017.
- Wang, Y.; Wu, F.; Li, X.; Li, C.; Zhao, Y.; Gao, Y.; Liu, J. Effects of plants and soil microorganisms on organic carbon and the relationship between carbon and nitrogen in constructed wetlands. Environ. Sci. Pollut. Res. 2023, 30, 62249–62261. [Google Scholar] [CrossRef]
- Zhu, S.; Feng, W. Improvement of the Ammonium Molybdate Spectrophotometry Method for Determining Total Phosphorus in Water. J. Henan Sci. Technol. 2013, 15, 212–213. [Google Scholar]
- Dai, X.; Zeng, Q. Alkaline potassium persulfate digestion UV spectrophotometric method forthe determination of total nitrogen. Environ. Dev. 2018, 30, 139–140. [Google Scholar] [CrossRef]
- Li-qiong, C.; Yong, H. Influencing factors on determination of ammonia nitrogen in water by nessler’s reagent spectrophotometry. In Proceedings of the 2011 International Symposium on Water Resource and Environmental Protection, Xi’an, China, 20–22 May 2011; pp. 1173–1176. [Google Scholar]
- Aydın, A.; Ercan, Ö.; Taşcıoğlu, S. A novel method for the spectrophotometric determination of nitrite in water. Talanta 2005, 66, 1181–1186. [Google Scholar] [CrossRef]
- Armstrong, F. Determination of Nitrate in Water Ultraviolet Spectrophotometry. Anal. Chem. 1963, 35, 1292–1294. [Google Scholar] [CrossRef]
- Guo, P.; Li, C.; Liu, J.; Chai, B. Predation has a significant impact on the complexity and stability of microbial food webs in subalpine lakes. Microbiol. Spectr. 2023, 11, e02411–e02423. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wang, S.; Li, H.; Yan, Z.; Zhang, Y.; Zheng, X.; Wang, P. Modeling the ecological status response of rivers to multiple stressors using machine learning: A comparison of environmental DNA metabarcoding and morphological data. Water Res. 2020, 183, 116004. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “all-species living tree project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef]
- Chavhan, A. Community structure and monthly dynamics of zooplankton in high altitude rice fish system in Eastern Himalayan region of India. Int. J. Life Sci. 2017, 5, 362–378. [Google Scholar]
- Sun, Y.; Li, H.; Wang, X.; Jin, Y.; Nagai, S.; Lin, S. Phytoplankton and Microzooplankton Community Structure and Assembly Mechanisms in Northwestern Pacific Ocean Estuaries with Environmental Heterogeneity and Geographic Segregation. Microbiol. Spectr. 2023, 11, e04926–e04922. [Google Scholar] [CrossRef]
- Stirling, G.; Wilsey, B. Empirical relationships between species richness, evenness, and proportional diversity. Am. Nat. 2001, 158, 286–299. [Google Scholar] [CrossRef]
- Faith, D.P. Phylogenetic diversity and conservation evaluation: Perspectives on multiple values, indices, and scales of application. In Phylogenetic Diversity: Applications and Challenges in Biodiversity Science; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–26. [Google Scholar]
- Xia, Y.; Sun, J. Alpha diversity. In Bioinformatic and Statistical Analysis of Microbiome Data: From Raw Sequences to Advanced Modeling with QIIME 2 and R; Springer: Berlin/Heidelberg, Germany, 2023; pp. 289–333. [Google Scholar]
- Zhai, J.; Sun, J. Diversity and community structure of microzooplankton in the eastern Indian Ocean during the inter-monsoon period. Front. Mar. Sci. 2023, 10, 1249281. [Google Scholar] [CrossRef]
- Fang, J.; Xu, Y.; Nie, Z.; Xu, G.; Jiang, Z.; Shao, N.; Xiao, Y.; Fang, J. Food sources of common carp in a Hani Terrace integrated rice-fish system (Yunnan Province, China). Aquac. Rep. 2022, 22, 100937. [Google Scholar] [CrossRef]
- Guan, W.; Li, K.; Li, K. Bacterial communities in co-cultured fish intestines and rice field soil irrigated with aquaculture wastewater. AMB Express 2022, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Arendt, K.E.; Nielsen, T.G.; Rysgaard, S.; Tönnesson, K. Differences in plankton community structure along the Godthåbsfjord, from the Greenland Ice Sheet to offshore waters. Mar. Ecol. Prog. Ser. 2010, 401, 49–62. [Google Scholar] [CrossRef]
- Chen, B.; Laws, E.A. Is there a difference of temperature sensitivity between marine phytoplankton and heterotrophs? Limnol. Oceanogr. 2017, 62, 806–817. [Google Scholar] [CrossRef]
- Rodrigues, L.H.R.; Canterle, E.B.; Becker, V.; Gazulha, V.; Hamester, A.; Motta Marques, D. Dynamics of plankton and fish in a subtropical temporary wetland: Rice fields. Sci. Res. Essays 2011, 6, 2069–2077. [Google Scholar]
- Liu, Y.; Zou, G.; Yuan, Q.; Huang, W.; Zhou, W. Phytoplankton community characteristics in rice paddy fields under different nitrogen fertiliser applications. Acta Physiol. Plant. 2020, 42, 33. [Google Scholar] [CrossRef]
- Iwai, N.; Koyama, N.; Tsuji, S.; Maruyama, A. Functions of indigenous animals in paddy fields: An in situ experiment on their effects on water quality, phytoplankton, weeds, soil structure, and rice growth. Paddy Water Environ. 2018, 16, 89–98. [Google Scholar] [CrossRef]
- Nam, N.D.G.; Giao, N.T.; Nguyen, M.N.; Downes, N.K.; Ngan, N.V.C.; Anh, L.H.H.; Trung, N.H. The Diversity of Phytoplankton in a Combined Rice-Shrimp Farming System in the Coastal Area of the Vietnamese Mekong Delta. Water 2022, 14, 487. [Google Scholar] [CrossRef]
- Das, D.; Haque, M.; Choudury, B.; Haque, M.; Alam, M. Study on monthly variations of plankton in relation to the physico-chemical condition of rice-fish fields in boro season. Int. J. Sustain. Crop Prod. 2011, 6, 43–49. [Google Scholar]
- Fan, T.; Amzil, H.; Fang, W.; Xu, L.; Lu, A.; Wang, S.; Wang, X.; Chen, Y.; Pan, J.; Wei, X. Phytoplankton-Zooplankton Community Structure in Coal Mining Subsidence Lake. Int. J. Environ. Res. Public Health 2022, 20, 484. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, J.; Gao, Y.; Liu, Y.; Zhou, C.; Meng, X.; Li, X.; Li, M.; Wang, Y.; Dai, D.; et al. Patterns of phytoplankton community structure and diversity in aquaculture ponds, Henan, China. Aquaculture 2021, 544, 737078. [Google Scholar] [CrossRef]
- Kopp, R.; Řezníčková, P.; Hadašová, L.; Petrek, R.; Brabec, T. Water quality and phytoplankton communities in newly created fishponds. Acta Univ. Agric. Et Silvic. Mendel. Brun. 2016, 64, 71–80. [Google Scholar] [CrossRef]
- Gao, W.; Xiong, F.; Lu, Y.; Xin, W.; Wang, H.; Feng, G.; Kong, C.; Fang, L.; Gao, X.; Chen, Y. Water quality and habitat drive phytoplankton taxonomic and functional group patterns in the Yangtze River. Ecol. Process. 2024, 13, 11. [Google Scholar] [CrossRef]
- Jakhar, P. Role of phytoplankton and zooplankton as health indicators of aquatic ecosystem: A review. Int. J. Innov. Res. Study 2013, 2, 489–500. [Google Scholar]
- Randall, S.; Cartozzo, C.; Simmons, T.; Swall, J.L.; Singh, B. Prediction of minimum postmortem submersion interval (PMSImin) based on eukaryotic community succession on skeletal remains recovered from a lentic environment. Forensic Sci. Int. 2021, 323, 110784. [Google Scholar] [CrossRef]
- Venter, P.C.; Nitsche, F.; Arndt, H. The Hidden Diversity of Flagellated Protists in Soil. Protist 2018, 169, 432–449. [Google Scholar] [CrossRef]
- Fayer, R.; Xiao, L. Cryptosporidium and Cryptosporidiosis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welde, G.T.; Li, B.; Hou, Y.; Ayana, G.U.; Zhou, L.; Jia, R.; Zhu, J. Effect of Rice–Carp Coculture on Phytoplankton and Microzooplankton Community Composition in Paddy Water during Different Rice Growth Stages. Water 2024, 16, 2775. https://doi.org/10.3390/w16192775
Welde GT, Li B, Hou Y, Ayana GU, Zhou L, Jia R, Zhu J. Effect of Rice–Carp Coculture on Phytoplankton and Microzooplankton Community Composition in Paddy Water during Different Rice Growth Stages. Water. 2024; 16(19):2775. https://doi.org/10.3390/w16192775
Chicago/Turabian StyleWelde, Geleta Tiko, Bing Li, Yiran Hou, Gelana Urgesa Ayana, Linjun Zhou, Rui Jia, and Jian Zhu. 2024. "Effect of Rice–Carp Coculture on Phytoplankton and Microzooplankton Community Composition in Paddy Water during Different Rice Growth Stages" Water 16, no. 19: 2775. https://doi.org/10.3390/w16192775
APA StyleWelde, G. T., Li, B., Hou, Y., Ayana, G. U., Zhou, L., Jia, R., & Zhu, J. (2024). Effect of Rice–Carp Coculture on Phytoplankton and Microzooplankton Community Composition in Paddy Water during Different Rice Growth Stages. Water, 16(19), 2775. https://doi.org/10.3390/w16192775








