Source and Origin of Subsurface Brine of the Kongquehe Sag Area in Western Lop Nur, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Analysis
3. Results
3.1. Brine Water Chemical Characteristics
3.1.1. Types of Water Chemistry
3.1.2. Ion Content
3.1.3. Hydro-Geochemical Coefficients
3.2. Characteristics of Isotopic Composition of Brine
3.2.1. H–O Isotope Composition Characteristics
3.2.2. Characteristics of S Isotope Composition
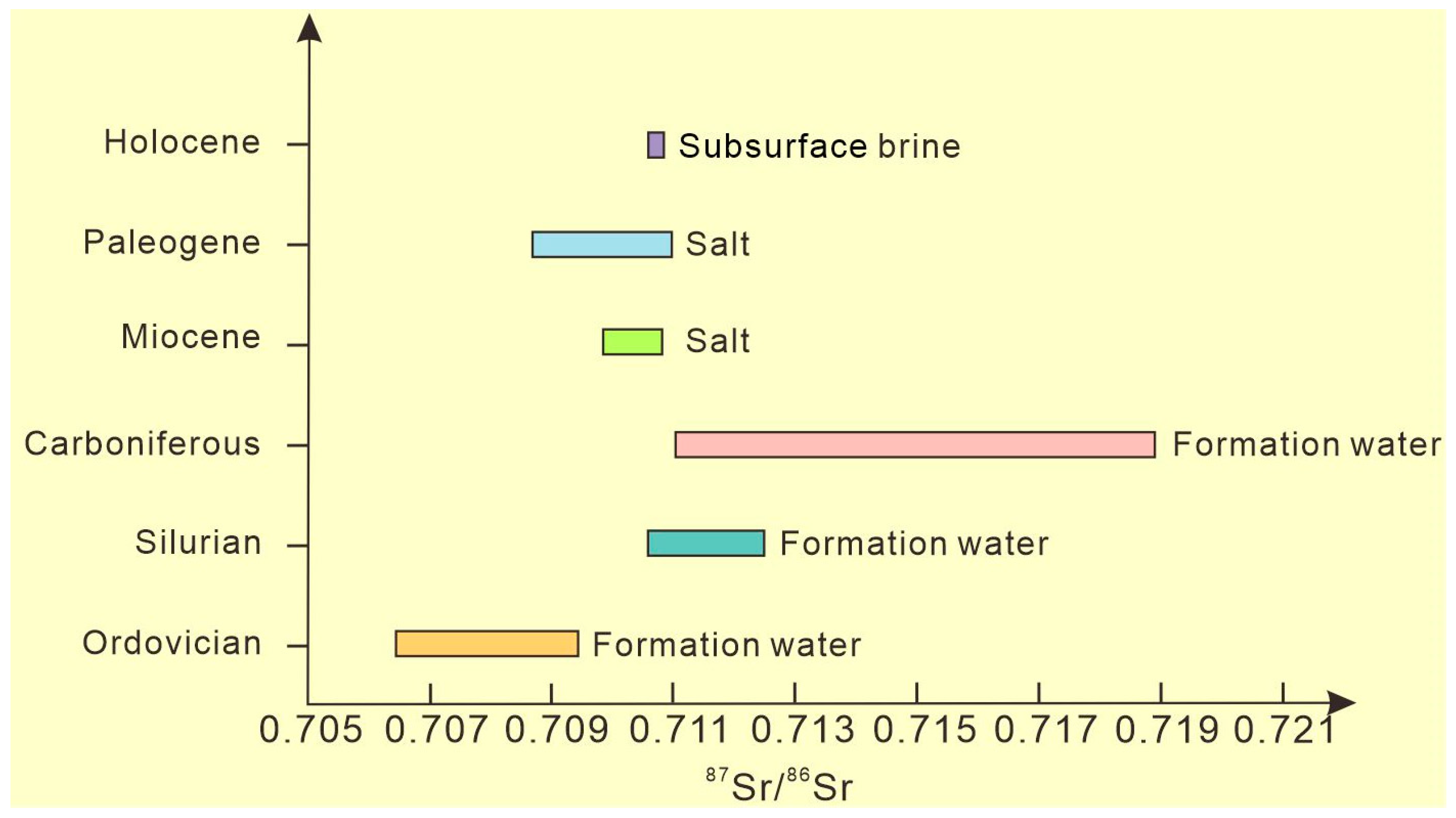
3.2.3. Characteristics of Sr Isotope Composition
4. Discussions
4.1. Source of Brine Supply
4.2. Cause Analysis
4.3. Genetic Analysis of Brine
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munk, L.A.; Hynek, S.A.; Bradley, D.C.; Boutt, D.; Labay, K.; Jochens, H. Lithium brines: A global perspective. Rev. Econ. Geol. 2016, 18, 339–365. [Google Scholar]
- Ihsanullah, I.; Mustafa, J.; Zafar, A.M.; Obaid, M.; Atieh, M.A.; Ghaffour, N. Waste to wealth: A critical analysis of resource recovery from desalination brine. Desalination 2022, 543, 116093. [Google Scholar] [CrossRef]
- Samantray, J.; Anand, A.; Dash, B.; Ghosh, M.K.; Behera, A.K. Silicate minerals-Potential source of potash—A review. Miner. Eng. 2022, 179, 107463. [Google Scholar] [CrossRef]
- Liu, C.L.; Lowenstein, T.K.; Wang, A.; Zheng, C.; Yu, J. Brine: Genesis and Sustainable Resource Recovery Worldwide. Annu. Rev. Environ. Resour. 2023, 48, 371–394. [Google Scholar] [CrossRef]
- Khalil, A.; Mohammed, S.; Hashaikeh, R.; Hilal, N. Lithium recovery from brine: Recent developments and challenges. Desalination 2022, 528, 115611. [Google Scholar] [CrossRef]
- Mojid, M.R.; Lee, K.J.; You, J. A review on advances in direct lithium extraction from continental brines: Ion-sieve adsorption and electrochemical methods for varied Mg/Li ratios. Sustain. Mater. Technol. 2024, 40, e00923. [Google Scholar] [CrossRef]
- Mischke, S.; Liu, C.; Zhang, J.; Zhang, C.; Zhang, H.; Jiao, P.; Plessen, B. The world’s earliest Aral-Sea type disaster: The decline of the Loulan Kingdom in the Tarim Basin. Sci. Rep. 2017, 7, 43102. [Google Scholar] [CrossRef]
- Liu, C.L.; Jiao, P.C.; Lü, F.L.; Wang, Y.Z.; Sun, X.H.; Zhang, H.; Wang, L.C.; Yao, F.J. The impact of the linked factors of provenance, tectonics and climate on potash formation: An example from the potash Deposits of Lop Nur depression in Tarim Basin, Xinjiang, Western China. Acta Geol. Sin.-Engl. Ed. 2015, 89, 2030–2047. [Google Scholar]
- Warren, J.K.; Warren, J.K. Potash resources: Occurrences and controls. In Evaporites: A Geological Compendium; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1081–1185. [Google Scholar]
- Álvarez-Amado, F.; Tardani, D.; Poblete-González, C.; Godfrey, L.; Matte-Estrada, D. Hydrogeochemical processes controlling the water composition in a hyperarid environment: New insights from Li, B, and Sr isotopes in the Salar de Atacama. Sci. Total Environ. 2022, 835, 155470. [Google Scholar] [CrossRef]
- Miao, W.; Zhang, X.; Li, Y.; Li, W.; Yuan, X.; Li, C. Lithium and strontium isotopic systematics in the Nalenggele River catchment of Qaidam basin, China: Quantifying contributions to lithium brines and deciphering lithium behavior in hydrological processes. J. Hydrol. 2022, 614, 128630. [Google Scholar] [CrossRef]
- Yu, X.; Yuan, X.; Guo, H.; Zhang, Y.; Cao, H.; Luo, T.; Gong, Z.; Huang, H. Coupling Hydrochemistry and Stable Isotopes (δ2H, δ18O and 87Sr/86Sr) to Identify the Major Factors Affecting the Hydrochemical Process of Groundwater and Surface Water in the Lower Reaches of the Yarlung-Zangbo River, Southern Tibet, Southwestern China. Water 2022, 14, 3906. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, N.; Shen, L.; Li, R.; Liu, C.; Jiao, P.; Hu, Y.; Zhao, X. Contribution of deep material sources to shallow potash formation of the Quaternary Mahai Salt Lake in the Qaidam Basin: Evidence from isotopes and trace elements. Ore Geol. Rev. 2024, 171, 106166. [Google Scholar] [CrossRef]
- Yang, F.; Fan, Q.; Han, G.; Wang, W.; Liu, J.; Bai, H. Origin and Evolution of Deep K-Rich Confined Brine in Mahai Basin, Qinghai–Tibet Plateau. Aquat. Geochem. 2024. [Google Scholar] [CrossRef]
- Yang, H.; Wei, J.; Shi, K. Hydrochemical and Isotopic Characteristics and the Spatiotemporal Differences of Surface Water and Groundwater in the Qaidam Basin, China. Water 2023, 16, 169. [Google Scholar] [CrossRef]
- Wang, S.; Zuo, Q.; Zhou, K.; Wang, J.; Wang, W. Predictions of Land Use/Land Cover Change and Landscape Pattern Analysis in the Lower Reaches of the Tarim River, China. Land 2023, 12, 1093. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, F.; Krijgsman, W.; Zhang, C.; Wei, W.; Chen, L.; Yang, S.; Liu, X.; Lu, Y. Palaeogeographic reconstructions of the Eocene-Oligocene Tarim Basin (NW China): Sedimentary response to late Eocene sea retreat. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 587, 110796. [Google Scholar] [CrossRef]
- Shvarts, A.A.; Kaplan, E.M.; Rumynin, V.G.; Borovitskaya, E.Y.; Erzova, V.A. Natural radioactivity of groundwater in Vendian deposits in St. Petersburg Region. J. Environ. Radioact. 2023, 264, 107189. [Google Scholar] [CrossRef]
- Mirzavand, M.; Walter, J. Delineating the mechanisms controlling groundwater salinization using chemo-isotopic data and meta-heuristic clustering algorithms (case study: Saguenay-Lac-Saint-Jean region in the Canadian Shield, Quebec, Canada). Environ. Sci. Pollut. Res. 2024, 31, 42406–42427. [Google Scholar] [CrossRef]
- Jiang, L.; Duan, X.; Yang, Z.; He, L.; Dong, Y.; Wang, Y. Fluid inclusion and stable isotope (H–O–S–Pb) constraints on the genesis of the Haxi gold deposit, west Junggar, China. J. Asian Earth Sci. X 2023, 9, 100131. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q. Research advances in identifying sulfate contamination sources of water environment by using stable isotopes. Int. J. Environ. Res. Public Health 2019, 16, 1914. [Google Scholar] [CrossRef]
- Christensen, J.N.; Dafflon, B.; Shiel, A.E.; Tokunaga, T.K.; Wan, J.; Faybishenko, B.; Dong, W.; Williams, K.H.; Hobson, C.; Brown, S.T.; et al. Using strontium isotopes to evaluate the spatial variation of groundwater recharge. Sci. Total Environ. 2018, 637, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Boussaada, N.; Bouselsal, B.; Benhamida, S.A.; Hammad, N.; Kharroubi, M. Geochemistry and water quality assessment of continental intercalary aquifer in Ouargla region (Sahara, Algeria). J. Ecol. Eng. 2023, 24, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.; Samiei-Fard, R. Geochemical characteristics of saline soils formed during the recent retreat of the Caspian Sea. Catena 2024, 243, 108208. [Google Scholar] [CrossRef]
- Adams, J.J.; Bachu, S. Equations of state for basin geofluids: Algorithm review and intercomparison for brines. Geofluids 2002, 2, 257–271. [Google Scholar] [CrossRef]
- Bazamad, M.; Tangestani, M.H.; Asadi, S.; Staubwasser, M. Investigating the geochemical behavior and exploration potential of lithium in brines; a case study of Bam salt plug, Zagros Zone, southern Iran. Sci. Rep. 2023, 13, 21567. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, K.; Pan, S.; Ren, Y.; Zhang, W. Effect of Moisture Sources on the Isotopic Composition of Precipitation in Northwest China. Water 2023, 15, 1584. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, J.; Wang, P.; Wang, T.; Li, Y. Groundwater-fed oasis in arid Northwest China: Insights into hydrological and hydrochemical processes. J. Hydrol. 2021, 597, 126154. [Google Scholar] [CrossRef]
- Li, Q.; Fan, Q.; Wei, H.; Qin, Z.; Zhang, X.; Du, Y.; Shan, F. Sulfur isotope constraints on the formation of MgSO4-deficient evaporites in the Qarhan salt Lake, western China. J. Asian Earth Sci. 2020, 189, 104160. [Google Scholar] [CrossRef]
- Makhnach, A.; Mikhajlov, N.; Kolosov, I.; Gulis, L.; Shimanovich, V.; Demeneva, O. Comparative analysis of sulfur isotope behavior in the basins with evaporites of chloride and sulfate types. Sediment. Geol. 2020, 134, 343–360. [Google Scholar] [CrossRef]
- Tan, H.; Ma, H.; Wei, H.; Xu, J.; Li, T. Chlorine, sulfur and oxygen isotopic constraints on ancient evaporite deposit in the Western Tarim Basin, China. Geochem. J. 2006, 40, 569–577. [Google Scholar] [CrossRef][Green Version]
- Liu, C.L.; Wang, L.; Yan, M.; Zhao, Y.; Cao, Y.; Fang, X.; Shen, L.; Wu, C.; Lü, F.; Ding, T. The Mesozoic-Cenozoic tectonic settings, paleogeography and evaporitic sedimentation of Tethyan blocks within China: Implications for potash formation. Ore Geol. Rev. 2018, 102, 406–425. [Google Scholar] [CrossRef]
- Veizer, J.; Demovic, R. Strontium as a tool in facies analysis. J. Sediment. Res. 1974, 44, 93–115. [Google Scholar]
- Liu, C.; Wang, M.; Jiao, P.; Li, S.; Chen, Y. Features and formation mechanism of faults and potash-forming effect in the Lop Nur Salt Lake, Xinjiang, China. Acta Geol. Sin. (Engl. Ed.) 2006, 80, 936–943. [Google Scholar]
- Xu, Y.; Cao, Y.; Liu, C.; Zhang, H.; Nie, X. The history of transgressions during the Late Paleocene-Early Eocene in the Kuqa Depression, Tarim Basin: Constraints from COS-Sr isotopic geochemistry. Minerals 2020, 10, 834. [Google Scholar] [CrossRef]
- Liu, C.L.; Zhang, J.F.; Jiao, P.; Mischke, S. The Holocene history of Lop Nur and its palaeoclimate implications. Quat. Sci. Rev. 2016, 148, 163–175. [Google Scholar] [CrossRef]
- Jiang, L.; Cai, C.; Worden, R.H.; Crowley, S.F.; Jia, L.; Zhang, K.; Duncan, I.J. Multiphase dolomitization of deeply buried Cambrian petroleum reservoirs, Tarim Basin, north-west China. Sedimentology 2016, 63, 2130–2157. [Google Scholar] [CrossRef]
- Setiati, R.; Akbar, F.; Prasucipto Karisma, G.; Ramadhani, A.; Setiawan, S.; Aditya, R.; Taufiq Fathaddin, M.; Giat Sukaryo, S.; Bharoto, B.; Sumirat, I. Application of Neutron Computed Tomography in Enhanced Oil Recovery for Analysing Oil Distribution in Berea Sandstone using Bagasse Surfactant. Rud.-Geološko-Naft. Zb. 2023, 38, 87–95. [Google Scholar] [CrossRef]
- Irfan, M.; Hamza, S.; Waqar Azeem, M.; Mahmud, S.; Nawaz-ul-Huda, S.; Qadir, A. Groundwater Exploration and Salinity Intrusion Studies using Electrical Resistivity Survey (ERS)-Winder, Balochistan, Pakistan. Rud.-Geološko-Naft. Zb. 2022, 37, 31–44. [Google Scholar] [CrossRef]
- Shang, K.; Tian, J.; Lv, H.; Zhang, X.; Li, J.; Zhang, Y. Geochemical Characteristics and the Sedimentary Environment of Lower Cambrian Argillaceous Rocks on the Kongquehe Slope, Tarim Basin, China. Energies 2022, 15, 5400. [Google Scholar] [CrossRef]
- Li, L.; Ni, W.; Cheng, Y.; Wang, H.; Yuan, K.; Zhou, B. Evaluation of the eco-geo-environment in the Qaidam Basin, China. Environ. Earth Sci. 2021, 80, 27. [Google Scholar] [CrossRef]
- Zachara, J.M.; Moran, J.J.; Resch, C.T.; Lindemann, S.R.; Felmy, A.R.; Bowden, M.E.; Cory, A.B.; Fredrickson, J.K. Geo-and biogeochemical processes in a heliothermal hypersaline lake. Geochim. Cosmochim. Acta 2016, 181, 144–163. [Google Scholar] [CrossRef]
- Zheng, M.P.; Hou, X.H.; Zhang, Y.S.; Xing, E.Y.; Li, H.P.; Yin, H.W.; Yu, C.Q.; Wang, N.J.; Deng, X.L.; Wei, Z.; et al. Progress in the investigation of potash resources in western China. China Geol. 2018, 1, 392–401. [Google Scholar] [CrossRef]
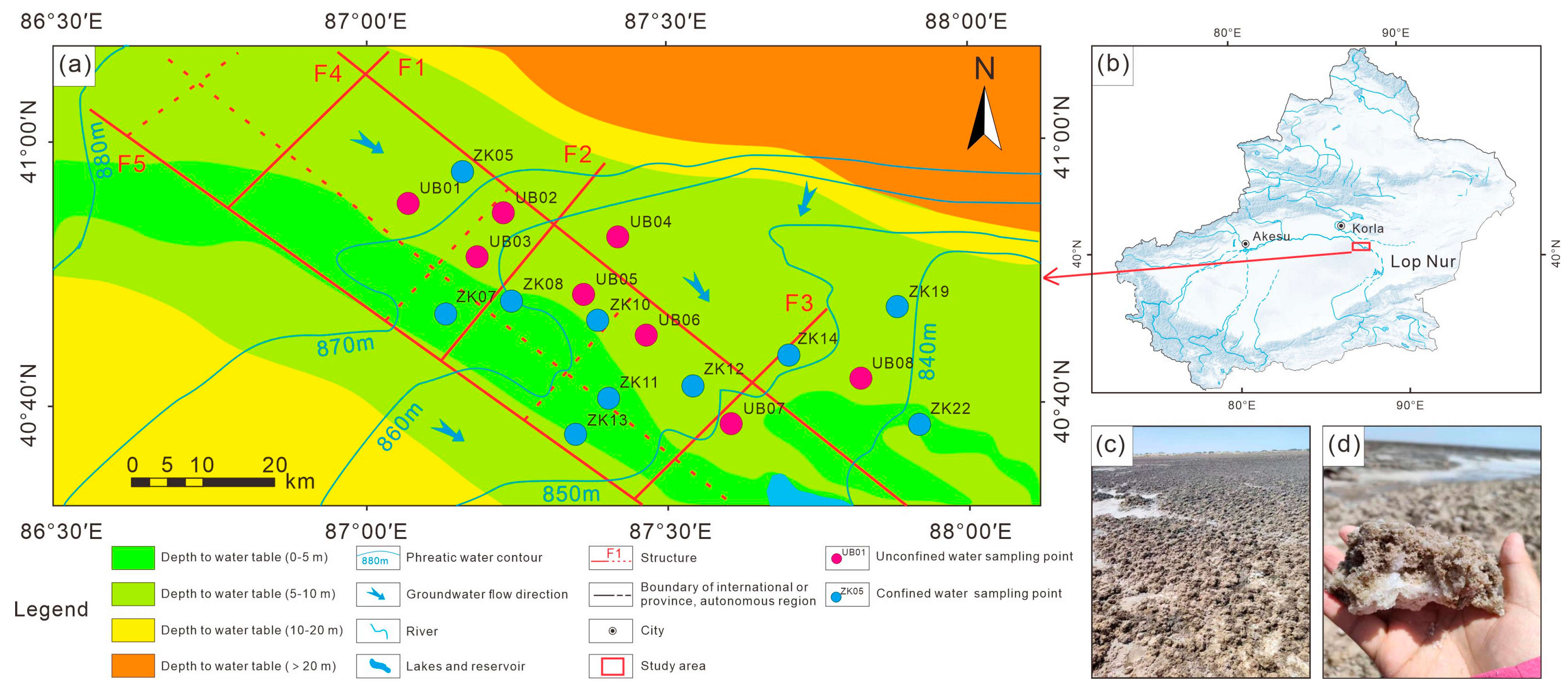

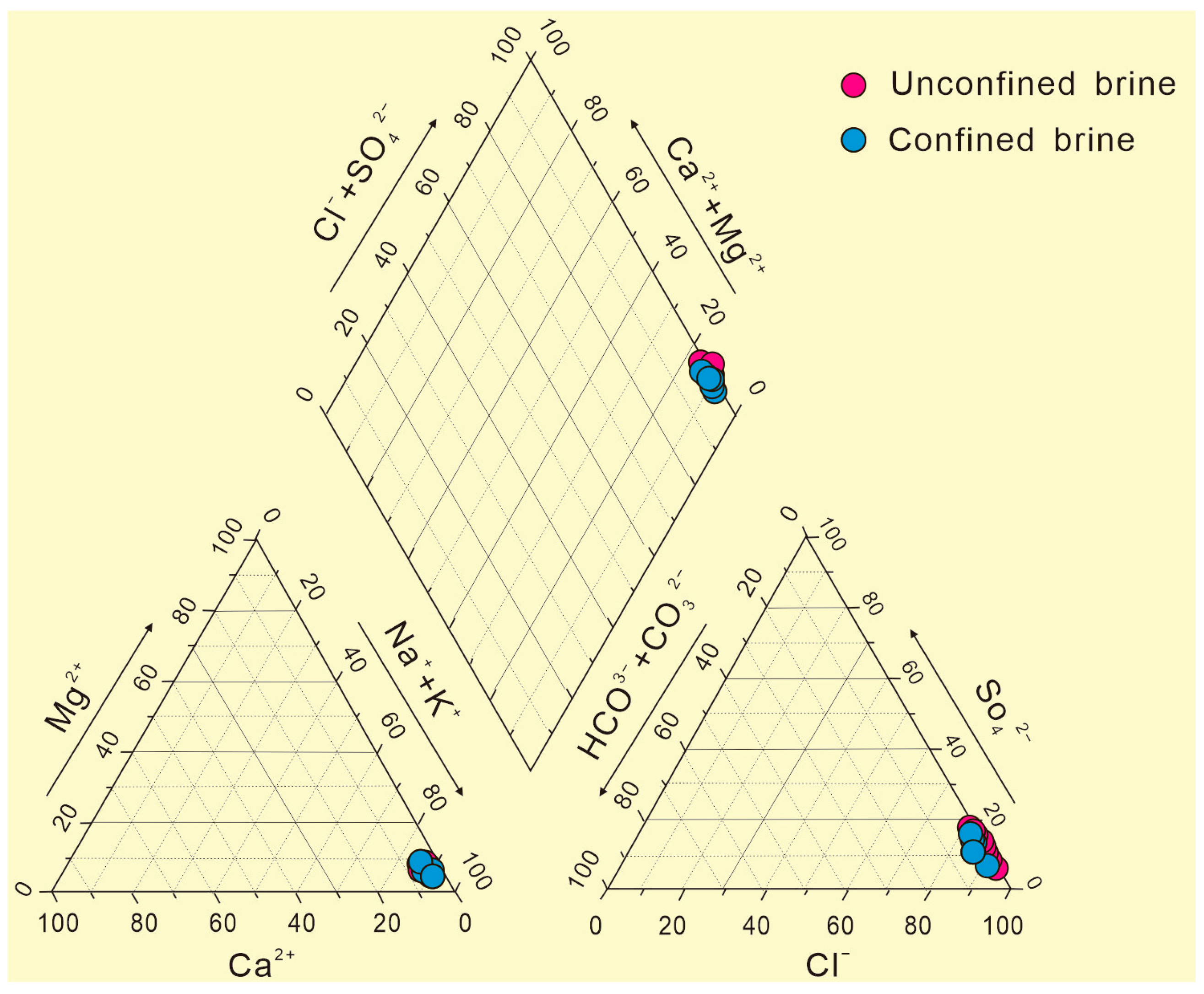
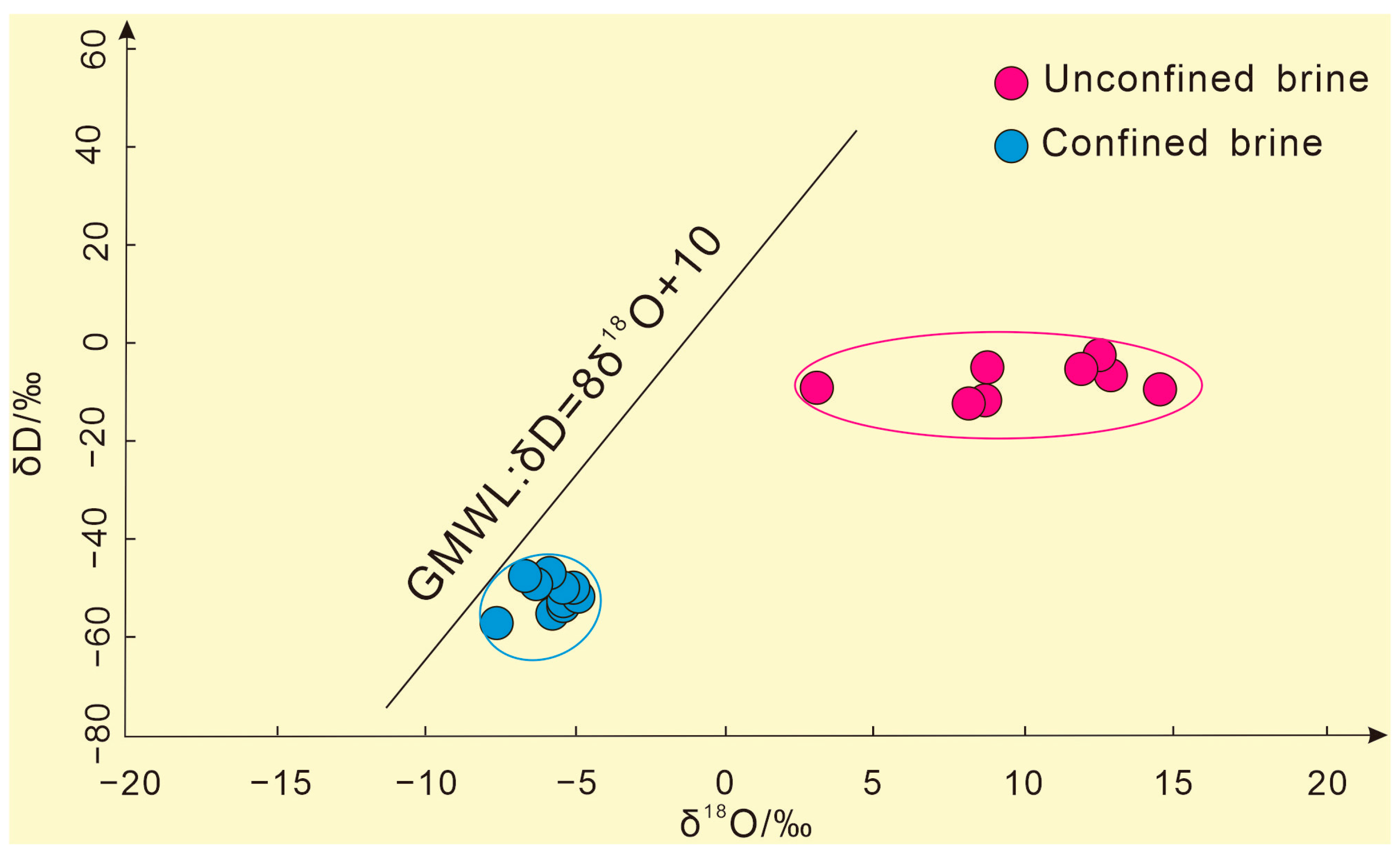
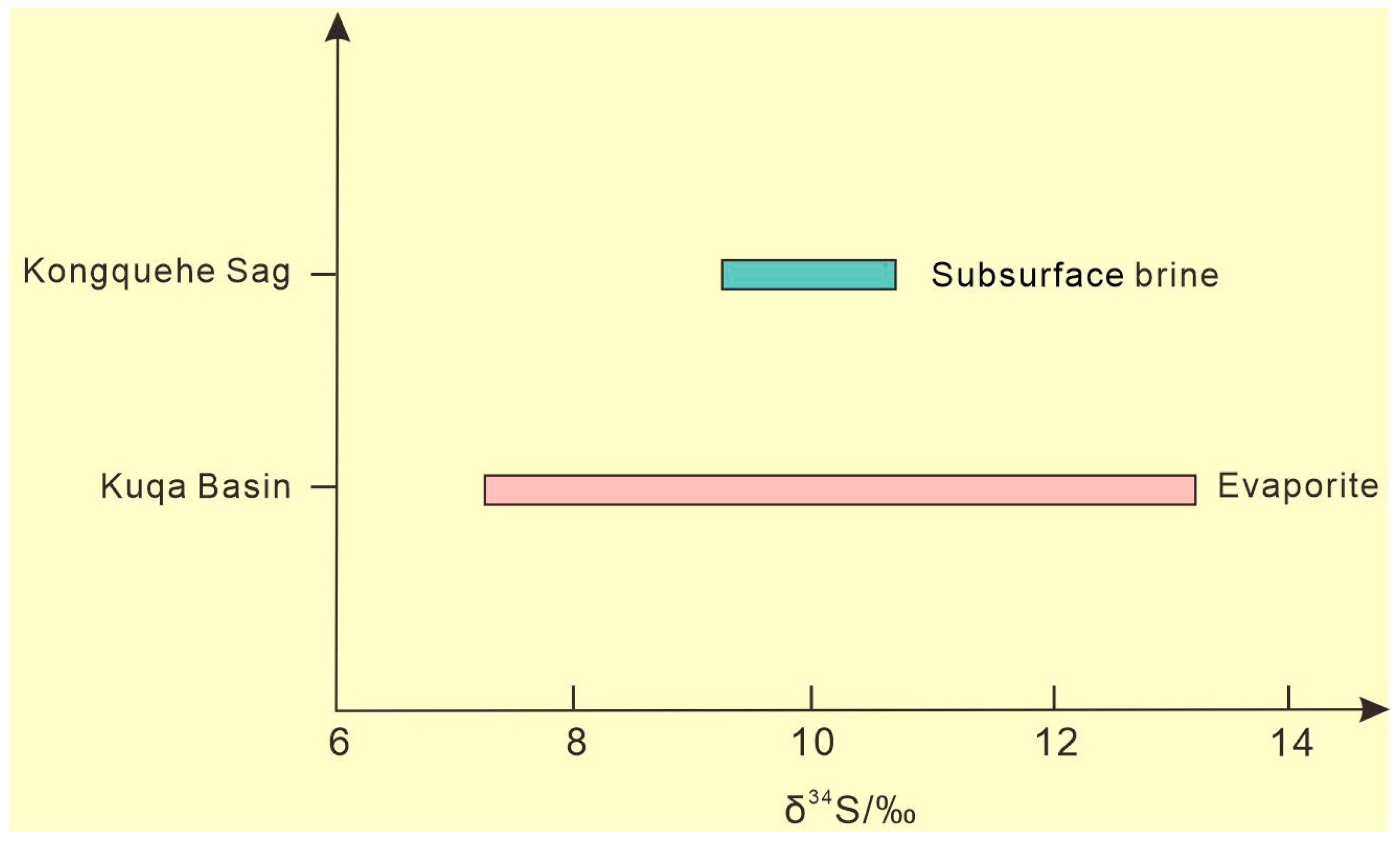

| Types | Number | Mineralization | K+ | Ca2+ | Na+ | Mg2+ | Cl− | SO42− | HCO3− | Br− | Sr2+ | Li+ | pH | Density |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g/L | mg/L | g/cm3 | ||||||||||||
| Confined brines | ZK05 | 75.83 | 0.17 | 1.21 | 24.95 | 1.74 | 40.45 | 7.19 | 0.11 | 10.31 | 37.68 | 0.38 | 8.15 | 1.13 |
| ZK07 | 50.50 | 0.11 | 1.09 | 15.05 | 1.36 | 30.31 | 2.45 | 0.12 | 8.26 | 45.13 | 0.42 | 8.06 | 1.05 | |
| ZK08 | 58.41 | 0.18 | 1.22 | 18.78 | 1.38 | 30.72 | 6.01 | 0.12 | 5.57 | 34.56 | 0.27 | 8.12 | 1.08 | |
| ZK10 | 60.48 | 0.09 | 1.27 | 19.30 | 1.51 | 31.47 | 6.67 | 0.16 | 7.89 | 28.98 | 0.33 | 8.21 | 1.09 | |
| ZK11 | 50.98 | 0.06 | 0.58 | 17.03 | 0.75 | 26.63 | 3.81 | 0.10 | 9.98 | 44.57 | 0.51 | 8.17 | 1.06 | |
| ZK12 | 57.86 | 0.10 | 1.18 | 18.79 | 1.30 | 30.67 | 5.60 | 0.21 | 13.24 | 39.68 | 0.45 | 8.16 | 1.08 | |
| ZK13 | 91.14 | 0.02 | 2.11 | 29.79 | 1.30 | 53.62 | 4.23 | 0.06 | 5.88 | 33.59 | 0.25 | 7.31 | 1.15 | |
| ZK14 | 52.75 | 0.11 | 1.28 | 16.58 | 1.36 | 27.78 | 5.45 | 0.17 | 11.98 | 31.89 | 0.47 | 8.17 | 1.07 | |
| ZK19 | 71.25 | 0.18 | 1.02 | 23.75 | 1.52 | 38.23 | 6.28 | 0.25 | 14.87 | 39.21 | 0.36 | 8.27 | 1.11 | |
| ZK22 | 72.15 | 0.16 | 1.18 | 22.12 | 1.79 | 38.29 | 6.74 | 0.19 | 13.65 | 29.23 | 0.49 | 8.14 | 1.08 | |
| Unconfined brines | UB01 | 68.87 | 0.04 | 1.67 | 24.02 | 0.84 | 39.37 | 2.75 | 0.11 | 5.57 | 33.90 | 0.16 | 8.22 | 1.13 |
| UB02 | 63.38 | 0.13 | 1.33 | 20.63 | 1.84 | 33.46 | 6.48 | 0.16 | 2.86 | 29.20 | 0.49 | 8.15 | 1.09 | |
| UB03 | 67.59 | 0.27 | 1.02 | 21.35 | 2.02 | 34.60 | 8.13 | 0.41 | 6.32 | 27.89 | 0.32 | 8.31 | 1.12 | |
| UB04 | 70.23 | 0.12 | 1.21 | 22.78 | 1.46 | 38.98 | 5.76 | 0.18 | 6.88 | 32.45 | 0.41 | 8.22 | 1.13 | |
| UB05 | 65.89 | 0.03 | 1.76 | 21.34 | 1.49 | 36.24 | 3.45 | 0.14 | 6.45 | 25.67 | 0.37 | 8.28 | 1.09 | |
| UB06 | 66.91 | 0.16 | 1.45 | 20.28 | 1.92 | 37.72 | 4.56 | 0.32 | 5.34 | 28.98 | 0.29 | 8.15 | 1.09 | |
| UB07 | 79.87 | 0.15 | 1.41 | 24.78 | 1.59 | 45.12 | 6.45 | 0.18 | 6.09 | 26.71 | 0.32 | 8.21 | 1.14 | |
| UB08 | 71.56 | 0.14 | 1.22 | 22.31 | 1.69 | 39.08 | 5.79 | 0.22 | 4.57 | 27.98 | 0.35 | 8.19 | 1.13 | |
| Types | Number | nNa+/nCl− | ρBr−*103/ρCl− | nSO42−*50/nCl− | nCa2+/nMg2+ | ρK+*103/ρCl− |
|---|---|---|---|---|---|---|
| Confined brines | ZK05 | 0.95 | 0.25 | 6.56 | 0.42 | 4.16 |
| ZK07 | 0.85 | 0.3 | 3.31 | 0.49 | 3.94 | |
| ZK08 | 0.94 | 0.18 | 7.22 | 0.54 | 3.41 | |
| ZK10 | 0.95 | 0.25 | 7.82 | 0.51 | 2.9 | |
| ZK11 | 0.99 | 0.37 | 5.28 | 0.47 | 2.32 | |
| ZK12 | 0.94 | 0.43 | 6.74 | 0.55 | 3.22 | |
| ZK13 | 0.88 | 0.47 | 2.6 | 1.28 | 0.68 | |
| ZK14 | 0.92 | 0.43 | 7.24 | 0.57 | 4.11 | |
| ZK19 | 0.96 | 0.39 | 6.07 | 0.41 | 4.81 | |
| ZK22 | 0.92 | 0.45 | 8.21 | 0.4 | 5.17 | |
| Unconfined brines | UB01 | 0.94 | 0.36 | 5.87 | 0.31 | 4.12 |
| UB02 | 0.96 | 0.28 | 7.93 | 0.65 | 3.14 | |
| UB03 | 0.83 | 0.31 | 7.91 | 0.38 | 4.98 | |
| UB04 | 0.95 | 0.35 | 5.98 | 0.39 | 4.78 | |
| UB05 | 0.98 | 0.34 | 7.98 | 0.48 | 3.09 | |
| UB06 | 0.93 | 0.32 | 5.66 | 0.29 | 3.97 | |
| UB07 | 0.96 | 0.33 | 7.45 | 0.48 | 4.32 | |
| UB08 | 0.91 | 0.21 | 6.43 | 0.39 | 4.01 |
| Types | Number | δD | δ18O | δ34S | 87Sr/86Sr |
|---|---|---|---|---|---|
| ‰ | |||||
| Confined brines | ZK05 | −54.13 | −5.52 | 10.40 | 0.710750 |
| ZK07 | −55.67 | −5.80 | 10.70 | 0.710642 | |
| ZK08 | −51.01 | −4.99 | 10.10 | 0.710768 | |
| ZK10 | −47.94 | −6.70 | 10.20 | 0.710773 | |
| ZK11 | −47.39 | −5.85 | 9.60 | 0.710791 | |
| ZK12 | −57.60 | −7.64 | 10.30 | 0.710757 | |
| ZK13 | −54.89 | −5.78 | 9.70 | 0.710732 | |
| ZK14 | −48.64 | −6.25 | 9.20 | 0.710820 | |
| ZK19 | −49.55 | −6.32 | 9.80 | 0.710805 | |
| ZK22 | −50.73 | −5.28 | 10.30 | 0.710837 | |
| Unconfined brines | UB01 | −5.06 | 8.78 | 10.20 | 0.710796 |
| UB02 | −9.41 | 3.08 | 9.90 | 0.710812 | |
| UB03 | −12.48 | 8.15 | 10.20 | 0.710793 | |
| UB04 | −5.54 | 11.91 | 10.40 | 0.710821 | |
| UB05 | −11.94 | 8.71 | 9.80 | 0.710779 | |
| UB06 | −6.70 | 12.89 | 10.40 | 0.710783 | |
| UB07 | −3.34 | 12.56 | 10.50 | 0.710809 | |
| UB08 | −9.58 | 14.56 | 10.30 | 0.710818 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Wang, Y.; Guo, S.; He, L.; Zeng, X.; Han, F.; Yang, Z.; Zu, B. Source and Origin of Subsurface Brine of the Kongquehe Sag Area in Western Lop Nur, China. Water 2024, 16, 2709. https://doi.org/10.3390/w16192709
Jiang L, Wang Y, Guo S, He L, Zeng X, Han F, Yang Z, Zu B. Source and Origin of Subsurface Brine of the Kongquehe Sag Area in Western Lop Nur, China. Water. 2024; 16(19):2709. https://doi.org/10.3390/w16192709
Chicago/Turabian StyleJiang, Lei, Ying Wang, Shuai Guo, Liang He, Xize Zeng, Feng Han, Zhen Yang, and Bo Zu. 2024. "Source and Origin of Subsurface Brine of the Kongquehe Sag Area in Western Lop Nur, China" Water 16, no. 19: 2709. https://doi.org/10.3390/w16192709
APA StyleJiang, L., Wang, Y., Guo, S., He, L., Zeng, X., Han, F., Yang, Z., & Zu, B. (2024). Source and Origin of Subsurface Brine of the Kongquehe Sag Area in Western Lop Nur, China. Water, 16(19), 2709. https://doi.org/10.3390/w16192709






