Feasibility Research on Surface Water Reinjection into the Sandstone Geothermal Reservoir of the Guantao Formation in Tianjin Based on Laboratory Experiments
Abstract
1. Introduction
2. Materials and Methods
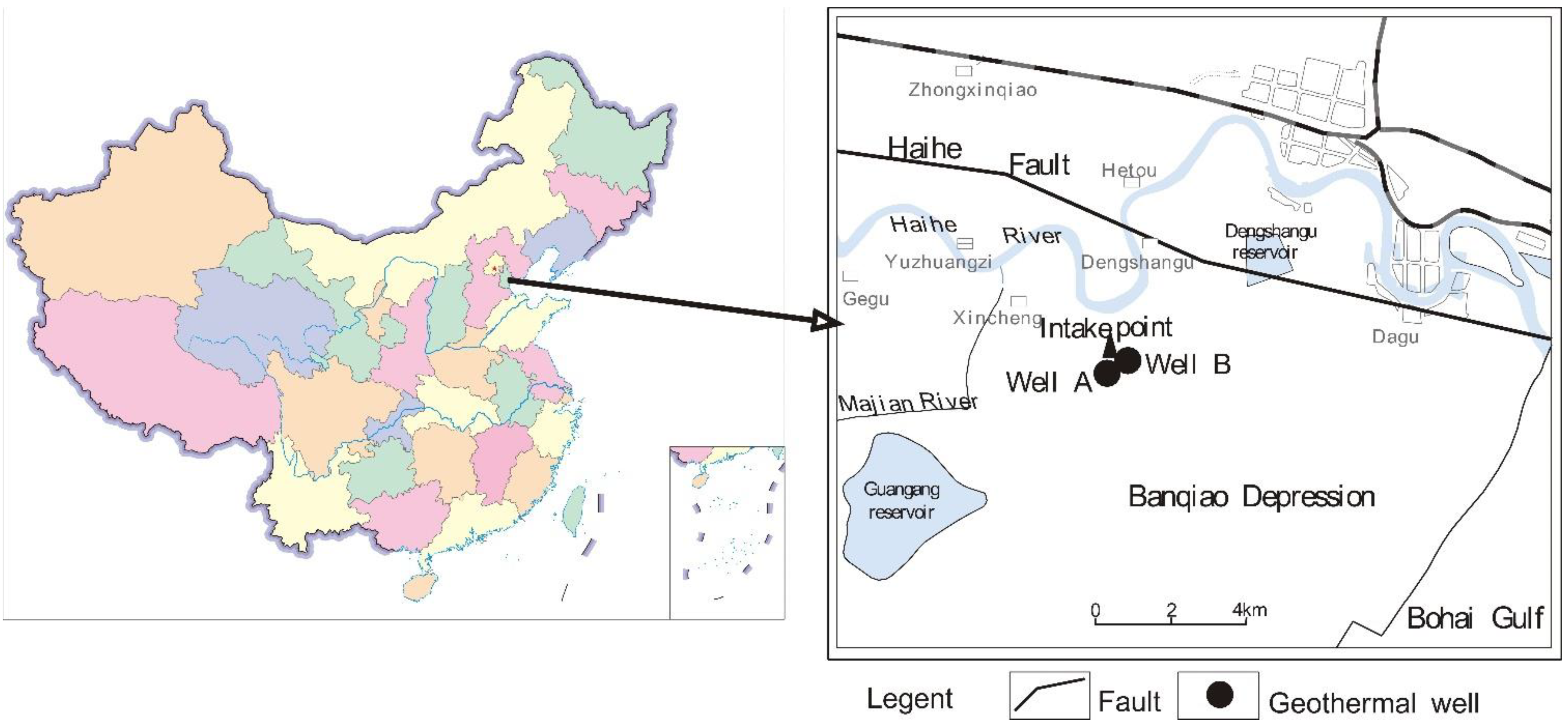
2.1. Project Overview
2.1.1. Geothermal Wells
2.1.2. Surface Water
2.2. Surface Water Reinjection Experiment
2.2.1. Experimental Platform
2.2.2. Simulation Experiment
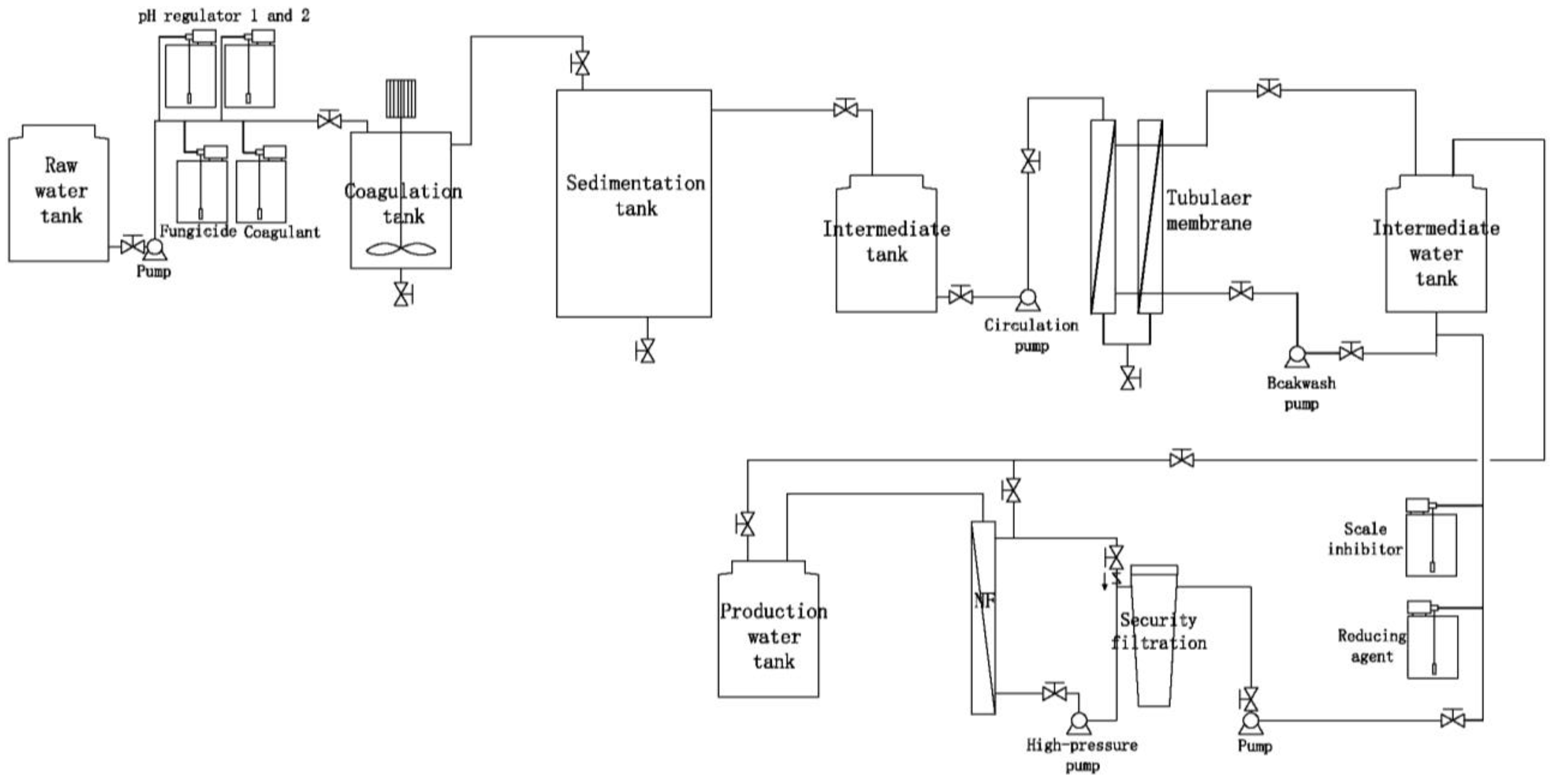
2.2.3. Determination of Water Treatment Process
- (1)
- The inclusion of a nanofiltration module is essential in the water treatment process of the experiment to effectively reduce the total dissolved solids (TDS).
- (2)
- Sand filtration and precision filtration processes demonstrate a relatively low filtration accuracy for suspended solids (SS) and other impurities in water, necessitating higher raw water quality standards. Additionally, the sand filter tank occupies a significant area, making it less feasible to prioritize when spatial constraints at the station are inadequate.
- (3)
- During actual operations, the sponge iron in the deaeration tank is susceptible to contamination by microorganisms present in the water, which leads to a diminished deaeration capacity and ineffective backwashing. Consequently, the deaerator tanks frequently fail to fulfill their designed functions. Furthermore, the deaerator tank poses a challenge due to its large footprint, which limits its prioritization.
- (4)
- The biological aerated filter (BAF) requires the pretreatment of the influent water; otherwise, the presence of numerous impurities and suspended solids (SS) can obstruct the aeration and water distribution system, adversely affecting system operation. As BAF is inherently a biochemical system, its effluent contains microorganisms and their metabolites, necessitating the addition of fungicides and deoxidants in subsequent processes. This introduces uncertainty in water quality and increases the operational load.
- (5)
- As a membrane separation technology, tubular membranes offer several advantages, including a large diameter, low flow resistance, high surface flow velocity, reduced retention of pollutants on the membrane surface, ease of cleaning, enhanced pollution resistance, and lower requirements for water inflow. Consequently, tubular membranes are well-suited for treating lake water with fluctuating quality and demonstrate stability during laboratory experiments.
- (6)
- In designing the reinjection process, it is essential to establish independent dosing tanks for the fungicide, coagulant, and pH regulator, while also considering the sequence of delivery.
2.3. Water–Rock Reaction Experiment
3. Results and Discussion
3.1. Reservoir Fluid Response
3.2. Reservoir Lithology Response
3.3. Geochemical Analysis
4. Conclusions
- (1)
- The surface water treatment process for reinjection into sandstone geothermal reservoirs was established through water treatment simulation experiments. It is feasible to utilize the mixed water (HHS) produced from nanofiltration and intermediate water as a source for reinjection.
- (2)
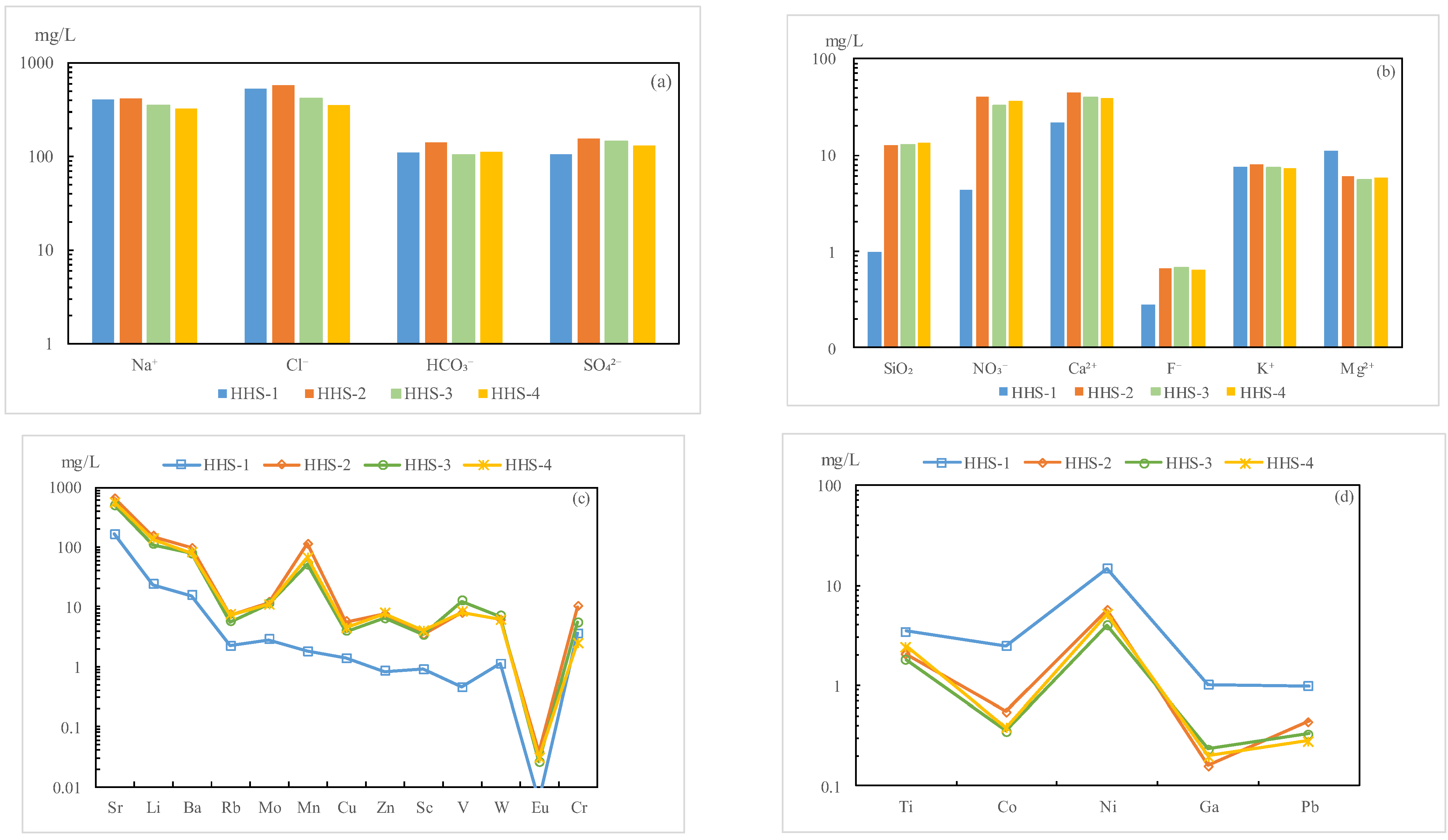
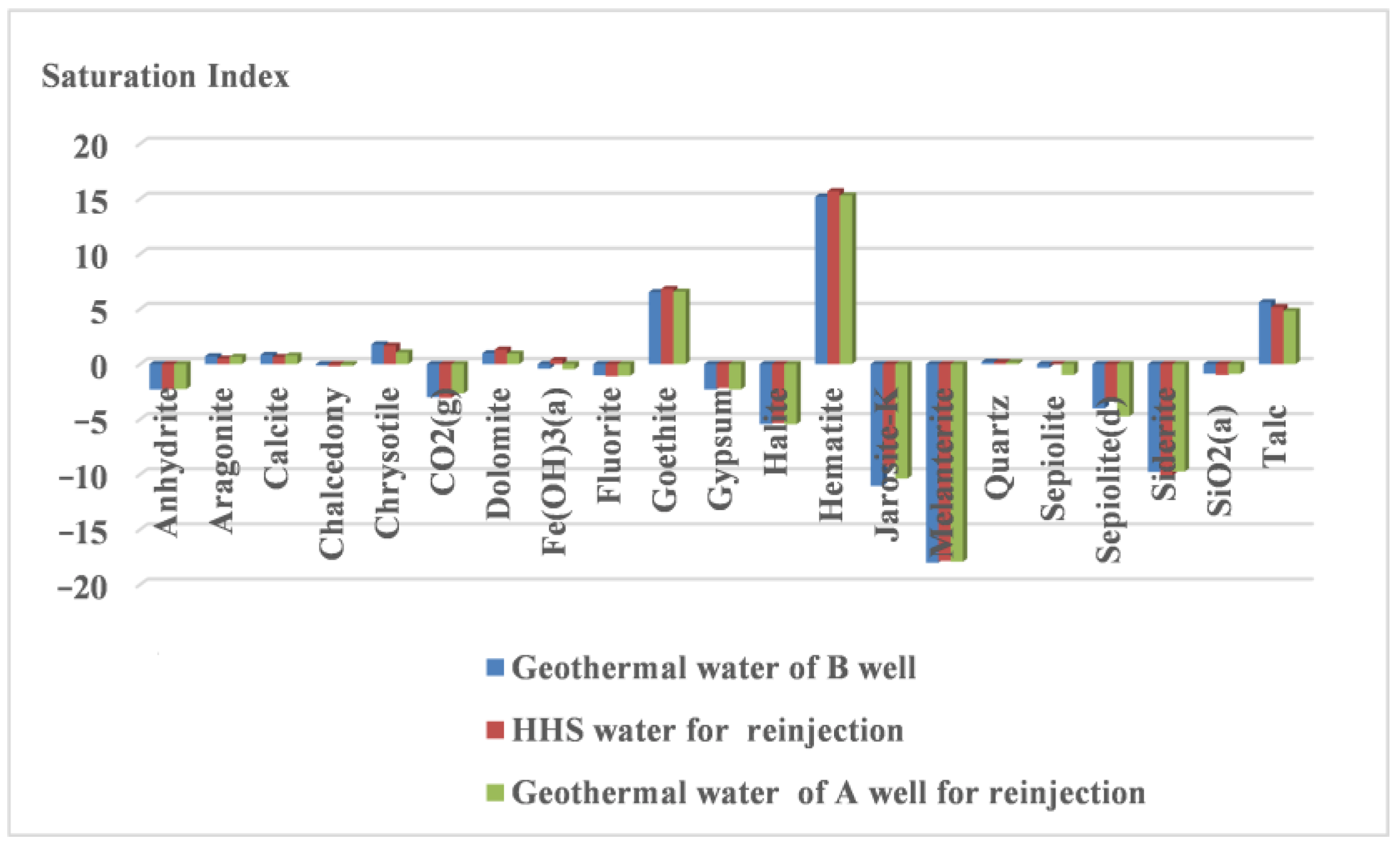
- Following the reinjection of mixed water (HHS) into the sandstone reservoir, the pH of the water exhibited a downward trend, while the total dissolved solids (TDS) content decreased by 94.2 mg/L, indicating the production of acidic substances and precipitates. The hydrochemical type consistently remained Cl-Na. The variation in trace elements paralleled that of conventional hydrochemical components, suggesting that they are influenced by related water–rock reactions. The rock minerals enriched in oxygen isotopes precipitated from the solution, with the amount of precipitation being lower than that of dissolution.
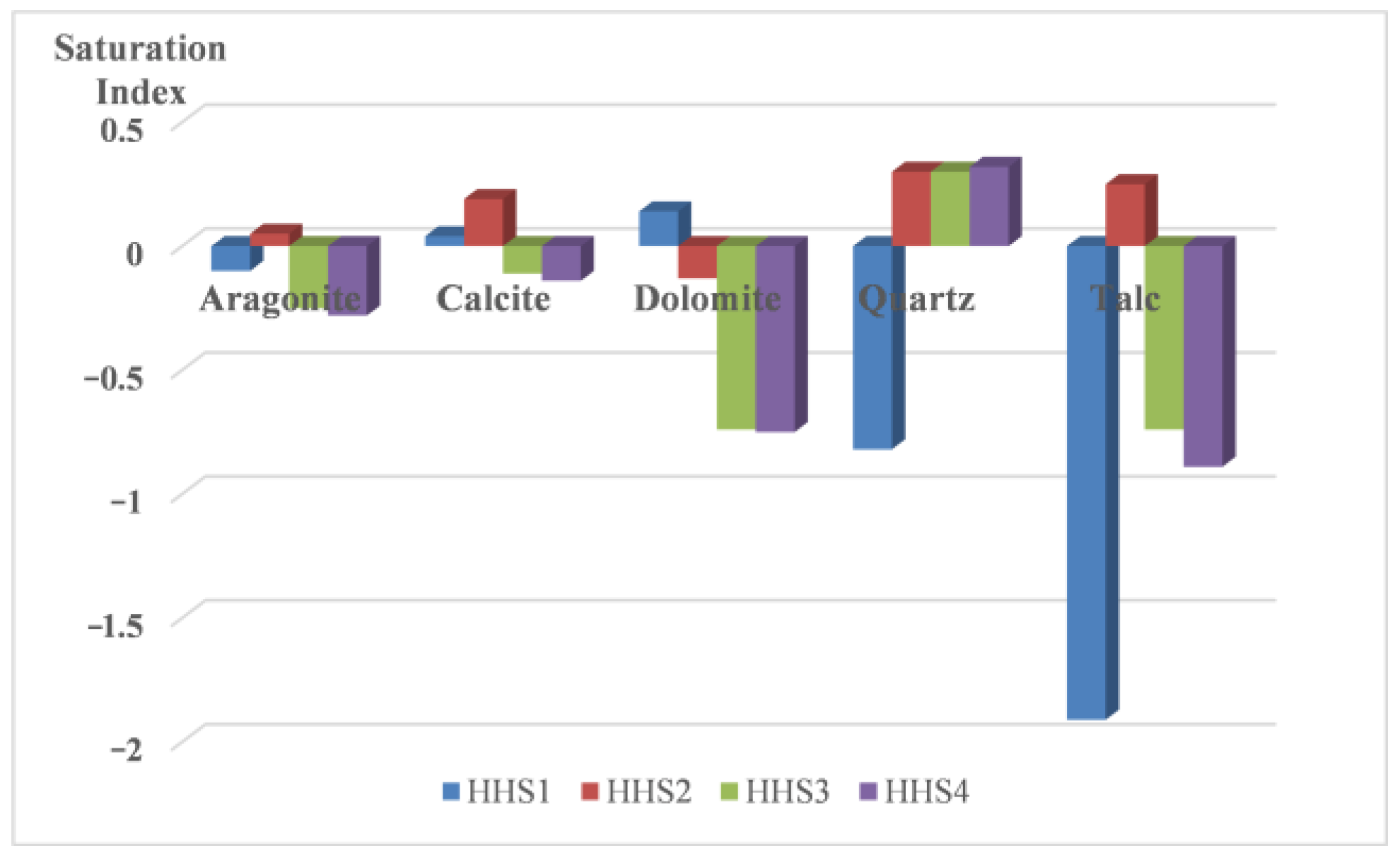
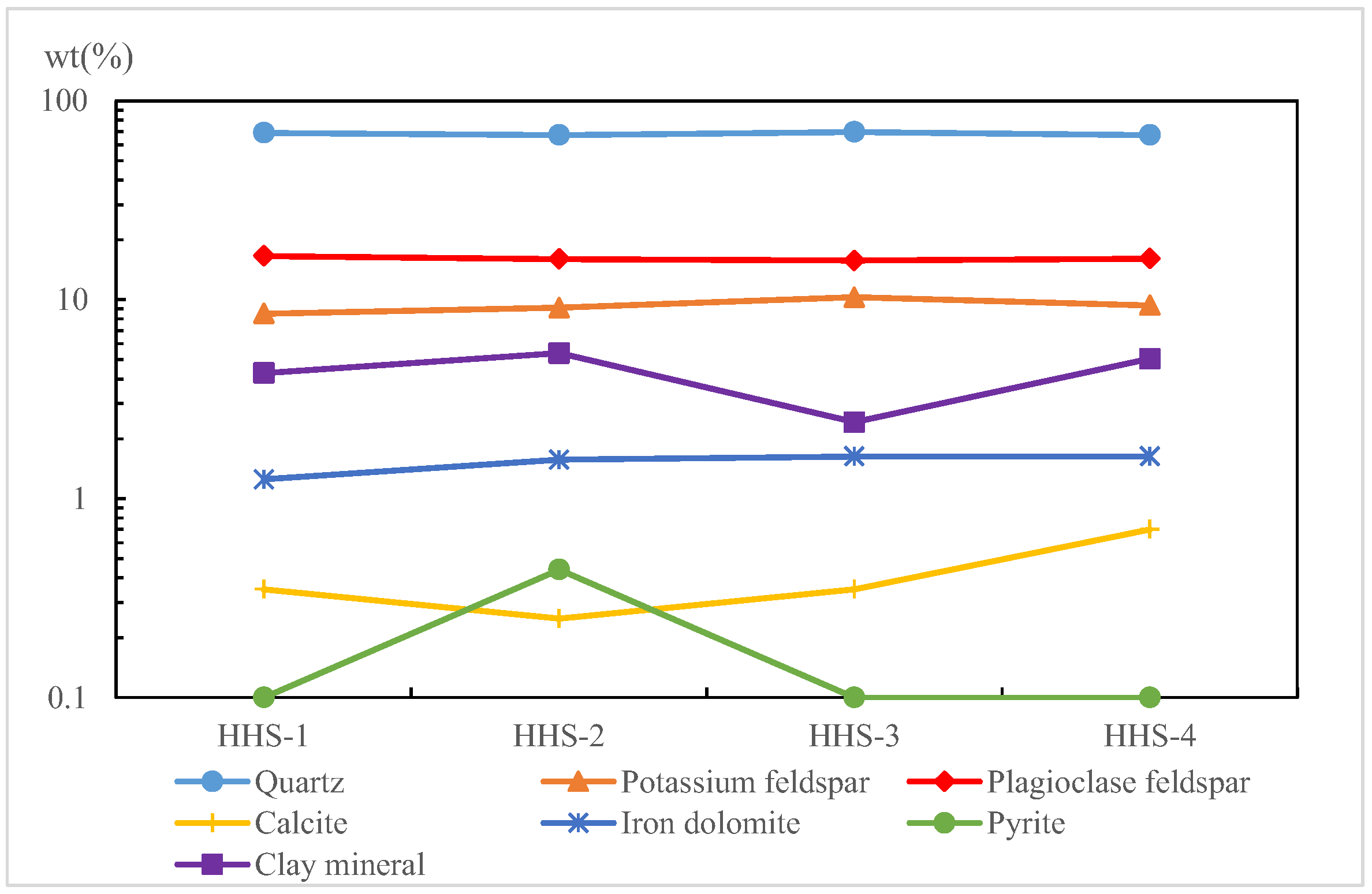
- (3)
- Following the reinjection of mixed water (HHS) into the sandstone reservoir, the minerals within the reservoir exhibited minimal precipitation, primarily consisting of potassium feldspar and iron dolomite. During the experiment, a total of 28.3 mg of rock minerals was produced, accounting for only 0.08% of the debris involved in the reaction. This precipitation had a negligible impact on the reservoir.
- (4)
- Similar hydrogeochemical changes were observed in the geothermal fluid after the reinjection of mixed water (HHS) and geothermal water. From the perspective of laboratory experiments, the reinjection of treated surface water into the sandstone geothermal reservoir is feasible. Future efforts should concentrate on conducting field tests to validate these findings, establishing a long-term geothermal fluid dynamic monitoring network, and utilizing numerical simulations to evaluate the application effects.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, G.L.; Liu, Y.G.; Zhu, X.; Zhang, W. The status and development trend of geothermal resources in China. Earth Sci. Front. 2020, 27, 1–9. [Google Scholar]
- Liu, J.; Song, M.Y.; Tian, G.H. Development situation of the geothermal resources and suggestion on sustainable development utilization in Tianjin. Geol. Surv. Res. 2012, 35, 67–73. [Google Scholar]
- Cao, Q.; Fang, C.H.; Li, Y.; Wang, H.X.; Fang, Q.; Shi, X.Y. Development status of geothermal reinjection at home and abroad and its enlightenment. Oil Drill. Prod. Technol. 2021, 43, 203–211. [Google Scholar]
- Yang, J.L.; Wang, D.M.; Niu, W.C.; Xiang, Z.Q.; Liu, Y.; Zhao, Z.L.; Cheng, X.Y. Prospects and problems of geothermal resources exploitation and utilization in Tianjin. North China Geol. 2022, 45, 1–6. [Google Scholar]
- Yin, X.X.; Zao, S.M.; Cai, Y.; Yan, J.X.; Xu, L. Dynamic evolution of geothermal reservoir characteristics in Tianjin in the last three decades of large—Scale development. Acia Geothermal Sin. 2024, 98, 297–313. [Google Scholar]
- Zhao, S.M.; Cheng, W.Q.; Zhang, W. Analysis of the geothermal reinjection blockage problem in Neogene sandstone thermal reservoir. Geothermal Energy 2013, 7–12. [Google Scholar]
- Axelsson, G. Importance of Geothermal Reinjection. In Proceedings of the Workshop for Decision Makers on Direct Heating Use of Geothermal Resources in Asia, Tinjin, China, 11–18 May 2008. [Google Scholar]
- Capetti, G.; Parisi, L.; Ridolfi, A.; Stefani, G. Fifteen years of Reinjection in the Larderello-Valle Secolo Area: Analysis of the Production Data. In Proceedings of the World Geothermal Congress 1995, Florence, Italy, 18–31 May 1995. [Google Scholar]
- Stark, M.A.; Tom, W.; Beall, J.J.; Goyal, K.P.; Pingol, A.S. The Santa Rosa—Geysers Recharge Project, Geysers Geothermal Field, California, USA. In Proceedings of the Transactions Geothermal Resources Council 2005, Antalya, Turkey, 14–29 April 2005. [Google Scholar]
- Ungemach, P.; Antics, M. Sustainable Geothermal Reservoir Management Practice. In Proceedings of the International Course and EGEC Business Seminar on Organization of Successful Development of a Geothermal Project, Casta Papiernicka, Slovakia, 26–29 May 2009. [Google Scholar]
- Allen, A.; Milenic, D. Low-enthalpy geothermal energy resources from groundwater in fluvioglacial gravels of buried valleys. Appl. Energy 2003, 74, 9–19. [Google Scholar] [CrossRef]
- Bodvarsson, G.S.S.; Tefansson, V. Some theoretical and field aspects of reinjection in geothermal reservoirs. Water Resour. Res. 1989, 25, 1235–1248. [Google Scholar] [CrossRef]
- Darwis, R.S.; Tampubolon, T.; Simatupang, R.; Asdassah, D. Study of Water Reinjection on the Kamojang Geothermal Reservoir Performance, Indonesia. In Proceedings of the 17th NZ Geothermal Workshop 1995, Auckland, New Zealand, 1 January 1995. [Google Scholar]
- Diazr, A.R.; Kaya, E.; Zarrouk, S.J. Reinjection in geothermal fields—A worldwide review update. Renew. Sustain. Energy Rev. 2016, 53, 105–162. [Google Scholar]
- Kamila, Z.; Kaya, E.; Zarrouk, S.J. Reinjection in geothermal fields: An updated worldwide review 2020. Geothermics 2021, 89, 101970. [Google Scholar] [CrossRef]
- Zhao, S.M. Development and Utilization of Geothermal Field in Sedimentary Basin; Geology Press: Beijing, China, 2013. [Google Scholar]
- Wang, L.C. A Study of Geothermal Reinjection in the Guantao Reservoir in Tianjin; China University of Geosciences: Beijing, China, 2015. [Google Scholar]
- Wang, X.P.; Liu, H.; Jiang, S.J.; Deng, R.Q. Reinjection experimental research on sedimentary basin sandstone reservoir—A case study of Yucheng City’ Shandong. Geol. Rev. 2020, 66, 485–492. [Google Scholar]
- Zhou, X. Reinjection Plugging Mechanism Researching of Sedimentary Basin Type Porous Geothermal Water—As the Reinjection Well in Sanqiao of Xi’an for an Example; Chang’an University: Xi’an, China, 2013. [Google Scholar]
- Dai, Q.; Wang, C.; Luo, Y.; Xu, B.B.; Yin, W.T.; Wang, P.F. Research on sandstone geothermal reservoir reinjection plugging mechanism and measures against it. Adv. Fine Petrochem. 2017, 18, 10–13. [Google Scholar]
- Wu, D.D. Discussion on the countermeasures for blockage control of sandstone geothermal reinjection wells. Explor. Dev. 2022, 18, 166–167. [Google Scholar]
- Jiang, G.S.; Wang, G.H.; Zhao, N.; Huang, X.L.; Shen, J. Application of gravel-packed completion technology of Guantao geothermal reinjection well in the Western Binhai New Area. Geol. Surv. Res. 2014, 37, 149–154. [Google Scholar]
- Zhao, Y.T.; Shen, J.; Zhao, S.M.; Wen, S.; Zhang, S. Well completion technology optimization and application effect analysis of medium –deep sandstone reinjection wells: A case study of Minghuazhen Formation in Tianjin. Pet. Reserv. Eval. Dev. 2023, 13, 765–772. [Google Scholar]
- Feng, S.T.; Wang, C.M.; Yang, Y.B.; Song, W.H.; Liu, S.; Zhao, J.C. Evaluation of the impact of sandstone thermal reservoir recharge on reservoirs: Taking the geothermal region of the northwest Shandong Depression as an example. J. Geol. 2019, 93, 158–167. [Google Scholar]
- Wang, H.J.; Yu, M.; Zhao, S.M.; Gao, X.Z.; Liu, F. Microscopic analysis of pore characteristics and comparison of permeability of sandstone in geothermal wells of Neogene. Acta Energiae Solaris Sin. 2019, 40, 1790–1796. [Google Scholar]
- Li, S.; Sun, X.L.; Yang, B.M.; Gao, X.Z. Evaluation and analysis of heat reservoir reinjection ability of Neogene Guantao Formation in Tianjin. North China Geol. 2023, 46, 31–44. [Google Scholar]
- Chen, Z.Y. Modeling water-rock interaction of geothermal reinjection in the Tanggu low-temperature field, Tianjin. Earth Sci. J. China Univ. Geosci. 1998, 23, 513–518. [Google Scholar]
- Shi, X.F.; Zhang, W.J.; Wang, H.M.; Jiao, X.; He, H.Y. Modeling of water-rock interaction during the artificial recharge. J. Jilin Univ. Earth Sci. Ed. 2013, 43, 220–227. [Google Scholar]
- Wu, K.Y.; Xiong, Y.; Tan, X.C.; Liu, X.J.; Zhang, Y.F.; Chen, X.D.; Li, Y.F. Study of the crystallization kinetics for “water-rock” interactions in the reservoir pore-system: An overview. Acta Sedimentol. Sin. 2022, 40, 996–1009. [Google Scholar]
- Su, Y.J.; Yang, F.T.; Wang, B.; Jia, Z.; Duan, Z.F. Reinjection of cooled water into sandstone geothermal reservoirs in China: A review. Geosci. J. 2018, 22, 199–207. [Google Scholar] [CrossRef]
- Wang, B.; Xia, Y.B.; Liu, D.L.; Tian, G.H.; Cai, Y.; Zong, Z.H. Analysis on PHREEQC simulation surface water reinjection of Jxw geothermal wells in Baodi District, Tianjin. Geol. Prospect. 2015, 38, 317–320. [Google Scholar]
- Liu, D.L.; Li, Y.M.; Pang, Z.H.; Zhao, S.M.; Fan, Y.F. Geochemical responses of carbonate reservoir to untreated lake water reinjection. J. Eng. Geol. 2019, 27, 178–183. [Google Scholar]
- Li, Y.M.; Shen, J.; Zhao, S.M.; Pang, Z.H.; Liu, D.L. Feasibility study on high-effective co-reinjection of waste geothermal water and water from other sources into carbonate reservoir. Sci. Technol. Dev. 2020, 16, 332–337. [Google Scholar]
- Xia, Y.B.; Wang, B.; Zhang, F.N.; Jia, Z. Optimization and transformation of water treatment technology for surface water reinjection into geothermal reservoir in Dongli Lake area of Tianjin. Energy Rep. 2023, 9, 25–29. [Google Scholar] [CrossRef]
- Standardization Administration of China. GB/T 19772-2005. Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT19772-2005 (accessed on 25 June 2024).
- National Energy Administration of China. SY/T 5329-2012. Available online: https://www.chinesestandard.net/PDF/English.aspx/SYT5329-2012 (accessed on 25 June 2024).
- Ministry of Natural Resources of the People’s Republic of China. DZ/T 0330-2019. Available online: https://www.chinesestandard.net/PDF/English.aspx/DZT0330-2019 (accessed on 25 June 2024).
- Guo, Y.L.; Xu, Y.H.; Wang, H.J.; Shen, J.; Zhao, S.M. Experimental investigation of water-rock reaction for the reinjection of sandstone geothermal reservoirs: A case from Neogene Guantao Formation in Tianjin. Renew. Energy 2023, 210, 203–214. [Google Scholar] [CrossRef]
- Jiang, W.J.; Wang, G.C.; Sheng, Y.Z.; Shi, Z.M.; Zhang, H. Isotopes in groundwater (2H, 18O, 14C) revealed the climate and groundwater recharge in the Northern China. Sci. Total Environ. 2019, 666, 298–307. [Google Scholar] [CrossRef]
- Jiang, W.J.; Meng, L.S.; Liu, F.T.; Sheng, Y.Z.; Chen, S.M.; Yang, J.L.; Mao, H.R.; Zhang, J.; Zhang, Z.; Ning, H. Distribution, source investigation, and risk assessment of topsoil heavy metals in areas with intensive anthropogenic activities using the positive matrix factorization (PMF) model coupled with self-organizing map (SOM). Environ. Geochem. Health 2023, 45, 6353–6370. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Jiang, W.J.; Sheng, Y.Z.; Wang, K.L.; Chen, S.M.; Zhang, Z.; Liu, F.T. Comprehensive evaluation of nitrogen contamination in water ecosystems of the Miyun reservoir watershed, northern China: Distribution, source apportionment and risk assessment. Environ. Geochem. Health 2024, 46, 278. [Google Scholar] [CrossRef]
- Garrels, R.M.; Christ, C.L. Solutions, Minerals, and Equilibria; Jones and Bartlett Publishiers: Boston, MA, USA, 1965. [Google Scholar]
- Feng, G.; Wang, X.C.; Kang, Y.; Zhang, Z.T. Effect of thermal cycling-dependent cracks on physical and mechanical properties of granite for enhanced geothermal system. Int. J. Rock Mech. Min. Sci. 2020, 134, 104476. [Google Scholar] [CrossRef]
- Feng, G.; Zhu, C.; Wang, X.; Tang, S. Thermal effects on prediction accuracy of dense granite mechanical behaviors using modified maximum tangential stress criterion. J. Rock Mech. Geotech. Eng. 2023, 15, 1734–1748. [Google Scholar] [CrossRef]
- Zhu, D.F.; Yu, B.B.; Wang, D.Y.; Zhang, Y.J. Fusion of finite element and machine learning methods to predict rock shear strength parameters. J. Geophys. Eng. 2024, 21, 1183–1193. [Google Scholar] [CrossRef]


| Name | Na+ | K+ | Ca2+ | Mg2+ | Cl− | NO3− | SO42− | HCO3− | CO32− | SiO2 | F− | TDS | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 490 | 3.6 | 10.5 | 0.9 | 363 | 22.4 | 231 | 510 | 6 | 27.7 | 4.7 | 1408.4 | 8.25 |
| B | 503.6 | 3.4 | 9.5 | 0.6 | 361.6 | 0.25 | 266 | 393.6 | 6 | 33.6 | 5.46 | 1577.9 | 8.44 |
| Lake | 787.07 | 25.69 | 31.64 | 51.69 | 1072.4 | 2657.3 | 306.03 | 361.2 | 19.8 | 1.78 | - | 2657.3 | 8.67 |
| Name | Process Flow | TDS | SS | DO | PO43− | pH |
|---|---|---|---|---|---|---|
| Process 1 | raw water–coagulation–sedimentation–intermediate tank–sand filtration–deoxygenation–precision filter–water production | 575 | 2.54 | 6 | <0.02 | 7.83 |
| Process 2 | raw water–coagulation–sedimentation–intermediate tank–tubular microfiltration membrane–nanofiltration–produced water | 620 | 1 | 8 | <0.02 | 7.8 |
| Process 3 | Raw water–coagulation–sedimentation–BAF–intermediate tank–tubular microfiltration membrane–produced water | 3050 | 0.98 | 8.25 | <0.02 | 8.1 |
| Name | Na+ | K+ | Ca2+ | Mg2+ | Cl− | NH4+ | SO42− | HCO3− | SiO2 | F− | CO2 | TDS | COD | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RW | 787.07 | 25.69 | 31.64 | 51.69 | 1072.4 | 0.28 | 306.03 | 361.2 | 1.78 | 2.358 | 0 | 2657.3 | 20.69 | 8.67 |
| NF | 201.49 | 4.18 | 25.09 | 3.1 | 264.1 | 0.05 | 45.48 | 137.3 | 1.12 | 0.155 | 16.3 | 681.9 | 4 | 7.77 |
| HHS | 406 | 7.5 | 22.1 | 11.2 | 529.6 | 0.23 | 106.1 | 110 | 1 | 0.3 | 11.9 | 1142 | 4 | 8.25 |
| Time/d | Na+ | K+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO3− | SiO2 | F− | TDS | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 406 | 7.5 | 22.1 | 11.2 | 529.6 | 106.1 | 110.0 | 4.3 | 1 | 0.3 | 1142 | 8.25 |
| 5 | 418 | 7.9 | 44.6 | 6.1 | 577.7 | 156.1 | 142.0 | 40 | 12.7 | 0.7 | 1322 | 8.01 |
| 10 | 351 | 7.4 | 40 | 5.4 | 416.3 | 144.7 | 104.1 | 32.7 | 12.7 | 0.7 | 1050 | 7.86 |
| 15 | 325 | 7.2 | 38.3 | 5.7 | 355.2 | 130.8 | 112.4 | 36.4 | 12.7 | 0.6 | 956 | 7.81 |
| Time/d | Sr | Li | Ba | Rb | Mo | Mn | Cu | Zn | Sc | V | W | Eu | Cr | Ti | Co | Ni | Ga | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 161.0 | 23.3 | 15.3 | 2.2 | 2.8 | 1.8 | 1.4 | 0.8 | 0.9 | 0.5 | 1.1 | 0.006 | 3.5 | 3.5 | 2.5 | 14.9 | 1.0 | 1.0 |
| 5 | 650.0 | 151.0 | 95.1 | 7.4 | 11.7 | 113.0 | 5.7 | 7.4 | 3.4 | 7.9 | 6.2 | 0.037 | 10.2 | 2.1 | 0.5 | 5.7 | 0.2 | 0.4 |
| 10 | 496.0 | 110.0 | 78.0 | 5.7 | 11.1 | 51.2 | 3.8 | 6.4 | 3.4 | 12.3 | 6.9 | 0.026 | 5.4 | 1.8 | 0.4 | 4.0 | 0.2 | 0.3 |
| 15 | 551.0 | 137.0 | 80.0 | 7.4 | 10.8 | 66.0 | 4.5 | 7.8 | 4.0 | 8.2 | 6.0 | 0.030 | 2.5 | 2.5 | 0.4 | 5.2 | 0.2 | 0.3 |
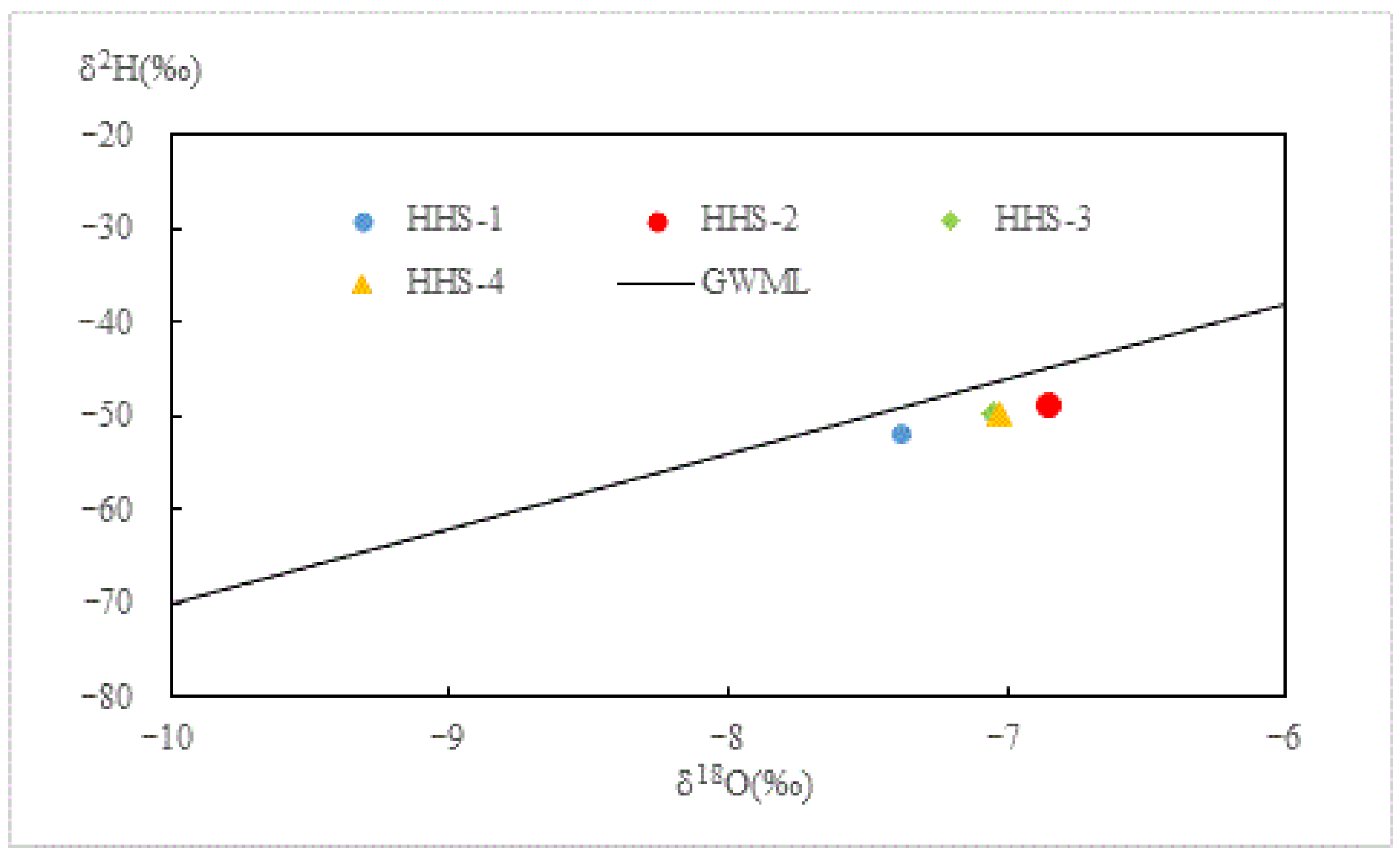
| Time/d | δ18O (‰) | δ2H (‰) |
|---|---|---|
| 0 | −7.4 | −52.0 |
| 5 | −6.8 | −49.2 |
| 10 | −7.1 | −49.8 |
| 15 | −7.0 | −49.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhao, Y.; Cai, Y.; Zhang, S.; Yang, B.; Liu, F. Feasibility Research on Surface Water Reinjection into the Sandstone Geothermal Reservoir of the Guantao Formation in Tianjin Based on Laboratory Experiments. Water 2024, 16, 2475. https://doi.org/10.3390/w16172475
Wang B, Zhao Y, Cai Y, Zhang S, Yang B, Liu F. Feasibility Research on Surface Water Reinjection into the Sandstone Geothermal Reservoir of the Guantao Formation in Tianjin Based on Laboratory Experiments. Water. 2024; 16(17):2475. https://doi.org/10.3390/w16172475
Chicago/Turabian StyleWang, Bing, Yanting Zhao, Yun Cai, Sen Zhang, Baomei Yang, and Fei Liu. 2024. "Feasibility Research on Surface Water Reinjection into the Sandstone Geothermal Reservoir of the Guantao Formation in Tianjin Based on Laboratory Experiments" Water 16, no. 17: 2475. https://doi.org/10.3390/w16172475
APA StyleWang, B., Zhao, Y., Cai, Y., Zhang, S., Yang, B., & Liu, F. (2024). Feasibility Research on Surface Water Reinjection into the Sandstone Geothermal Reservoir of the Guantao Formation in Tianjin Based on Laboratory Experiments. Water, 16(17), 2475. https://doi.org/10.3390/w16172475






