The Isolation and Identification of Novel Arsenic-Resistant Bacteria from an Arsenic-Contaminated Region—A Study to Understand the Efficiency of Bacteria for Arsenic Removal from Aqueous Media
Abstract
1. Introduction
2. Materials and Methods
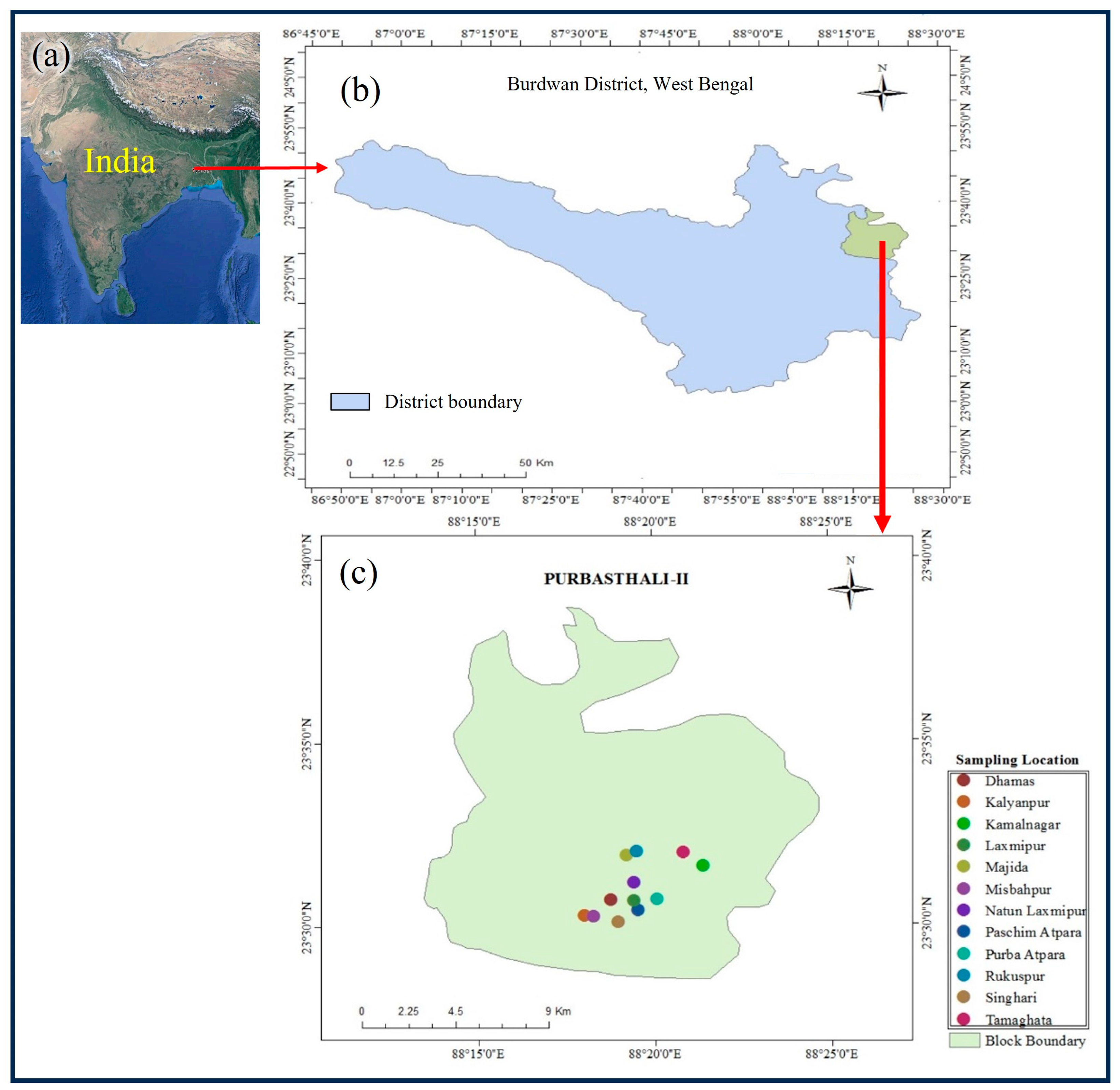
2.1. Study Area
2.2. Collection of Soil Samples
2.3. Chemicals and Reagents
2.4. Habitat Characterization
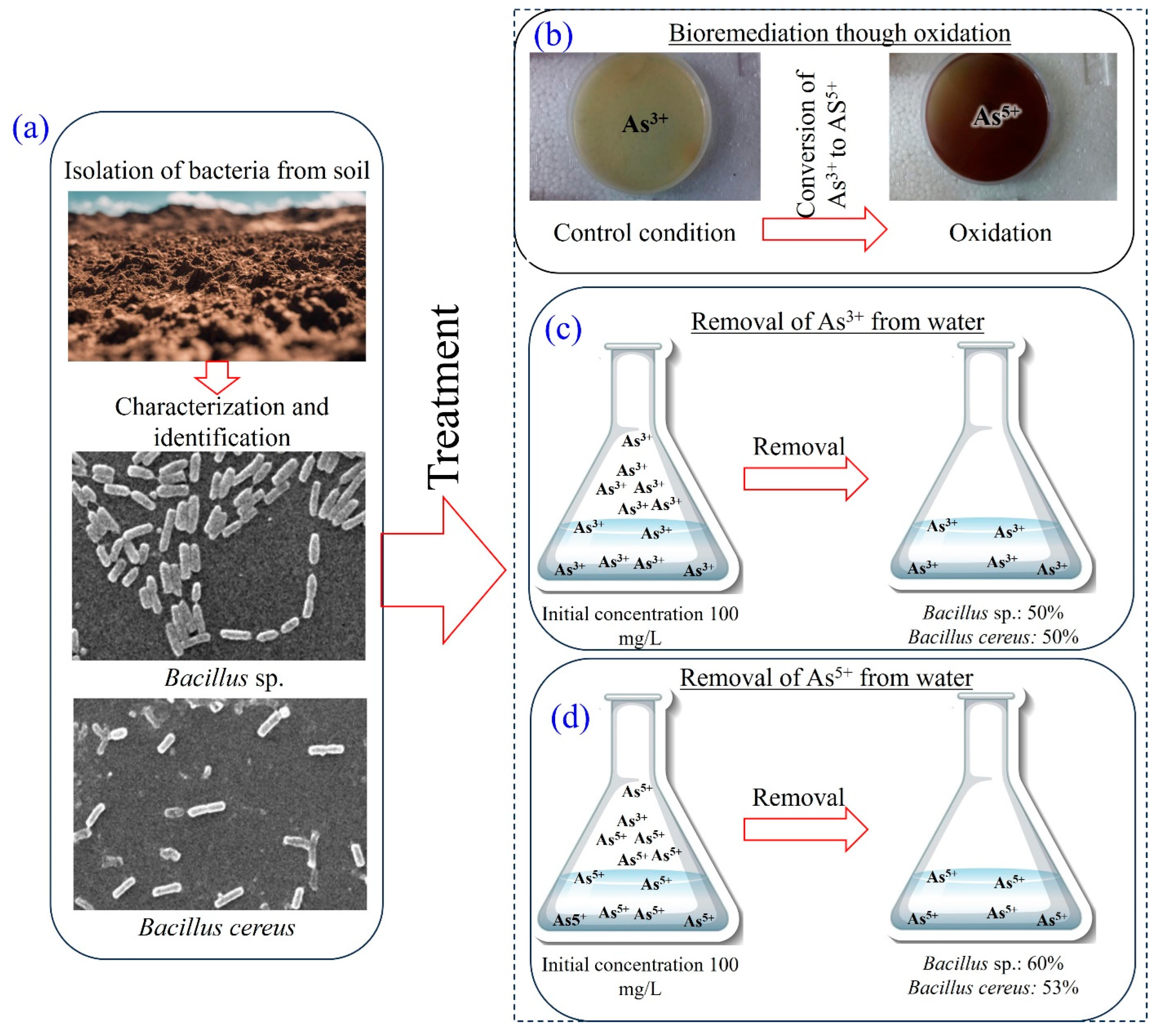
2.5. Isolation of Bacteria
2.6. Viable Cell Count
2.7. Establishment of Minimum Inhibitory Concentration
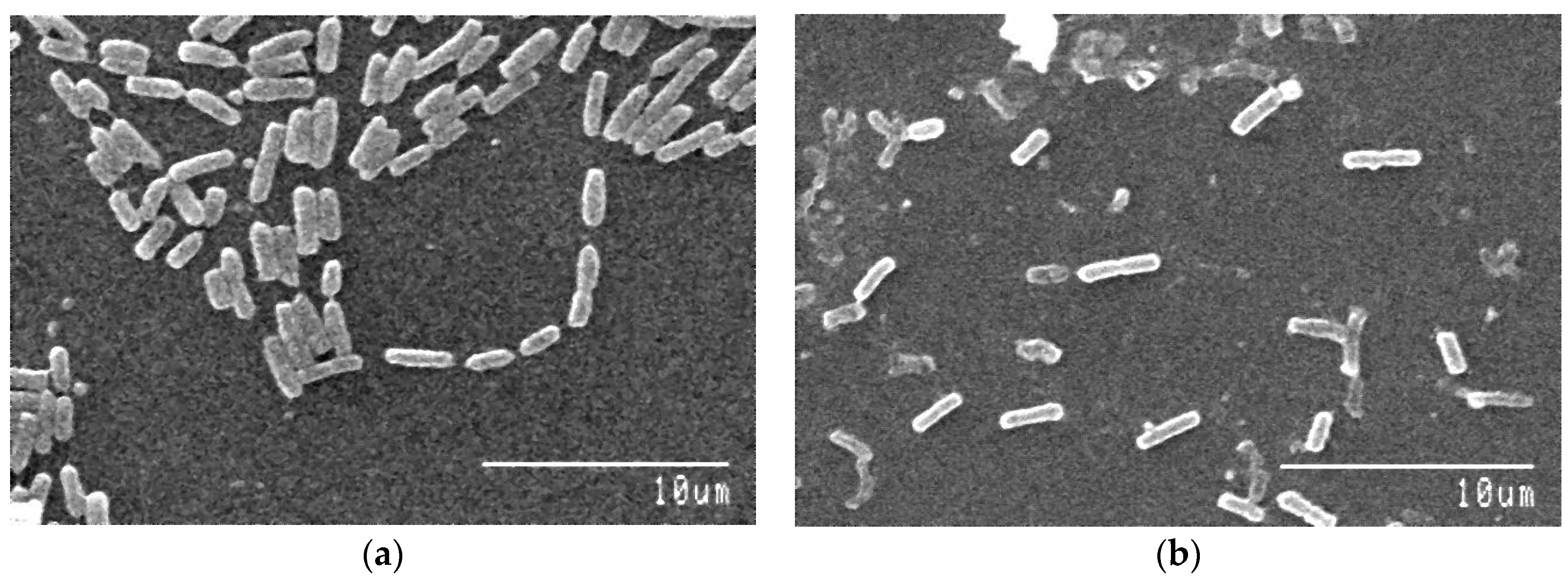
2.8. Morphological and Biochemical Characterization and Scanning Electron Microscopy of the Isolated Arsenic-Resistant Bacteria
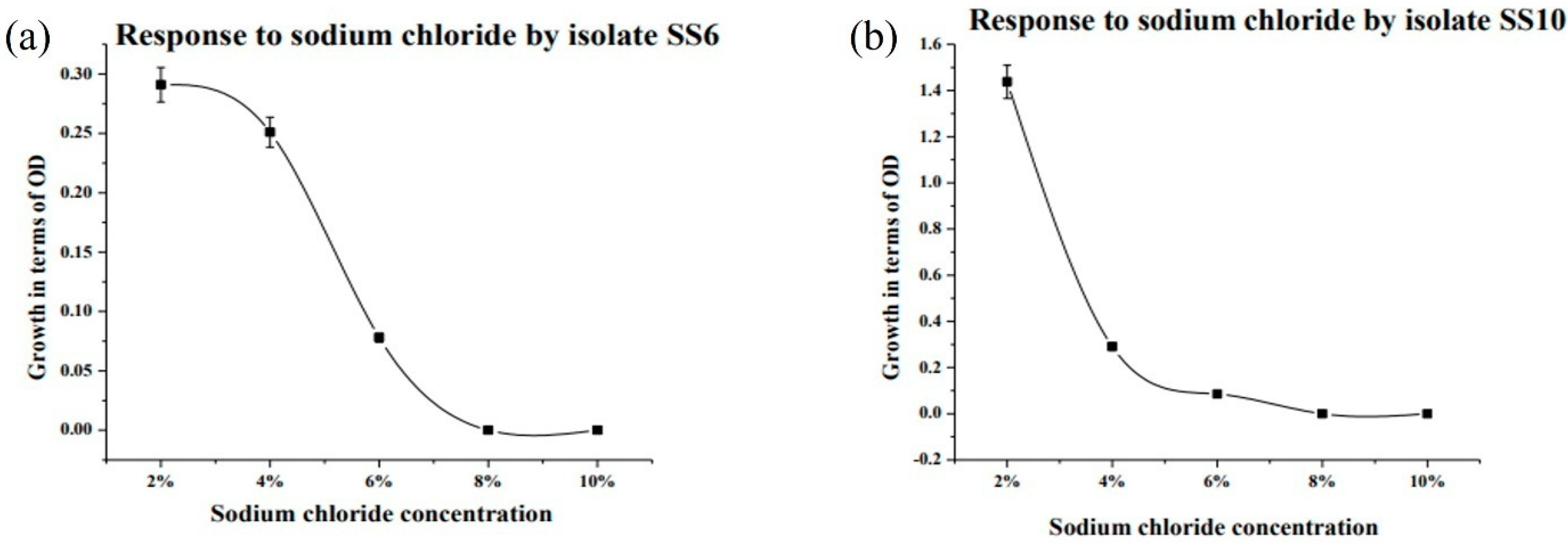
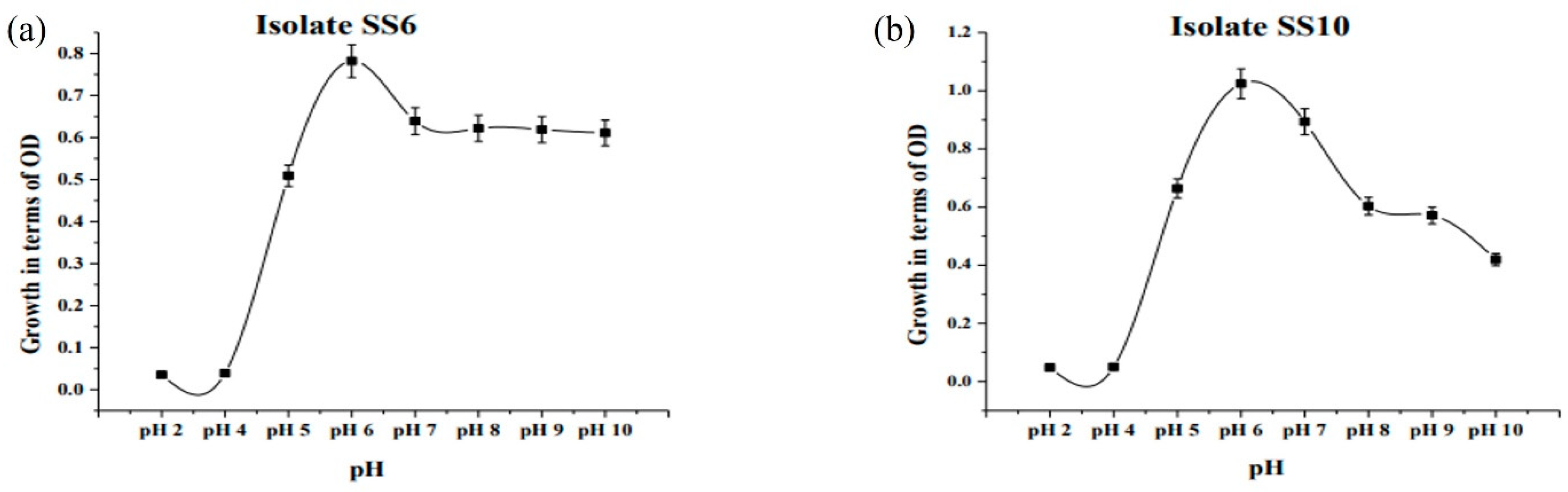
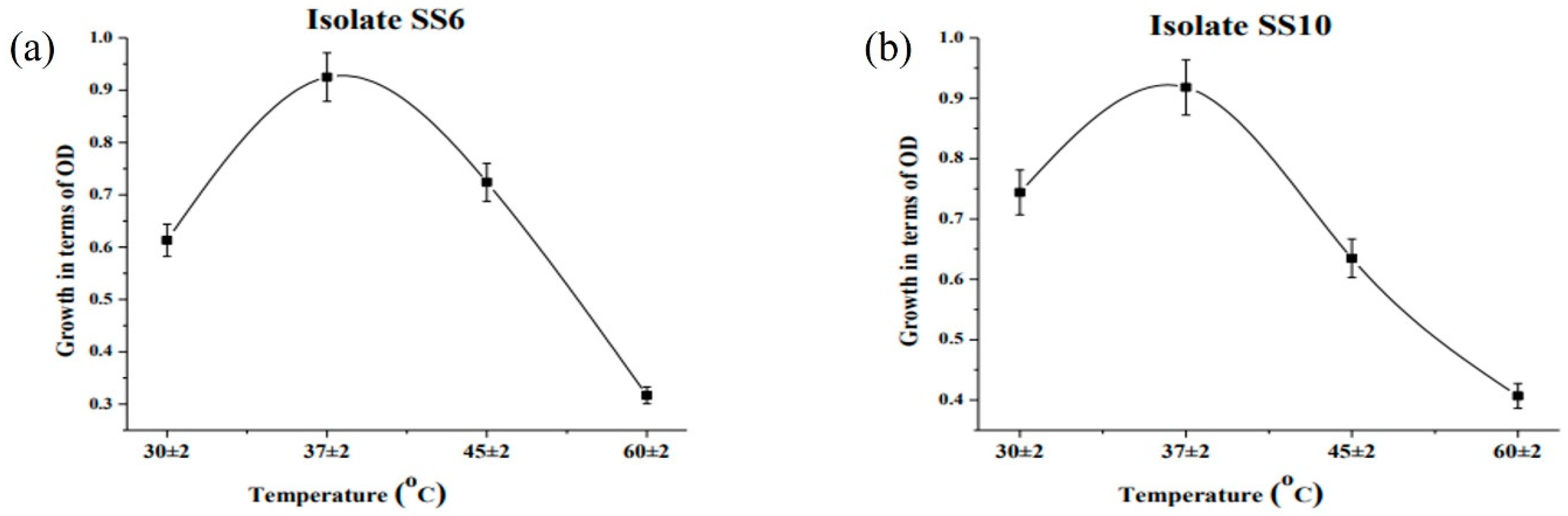
2.9. Determination of Optimum pH and Temperature
2.10. Arsenic Bioremediation Test by the Isolated Bacteria
2.11. Determination of Arsenic Oxidation and Reduction Potentiality of the Isolated Bacteria
2.12. Genomic DNA Sequencing
3. Results
3.1. Soil Physicochemical Properties
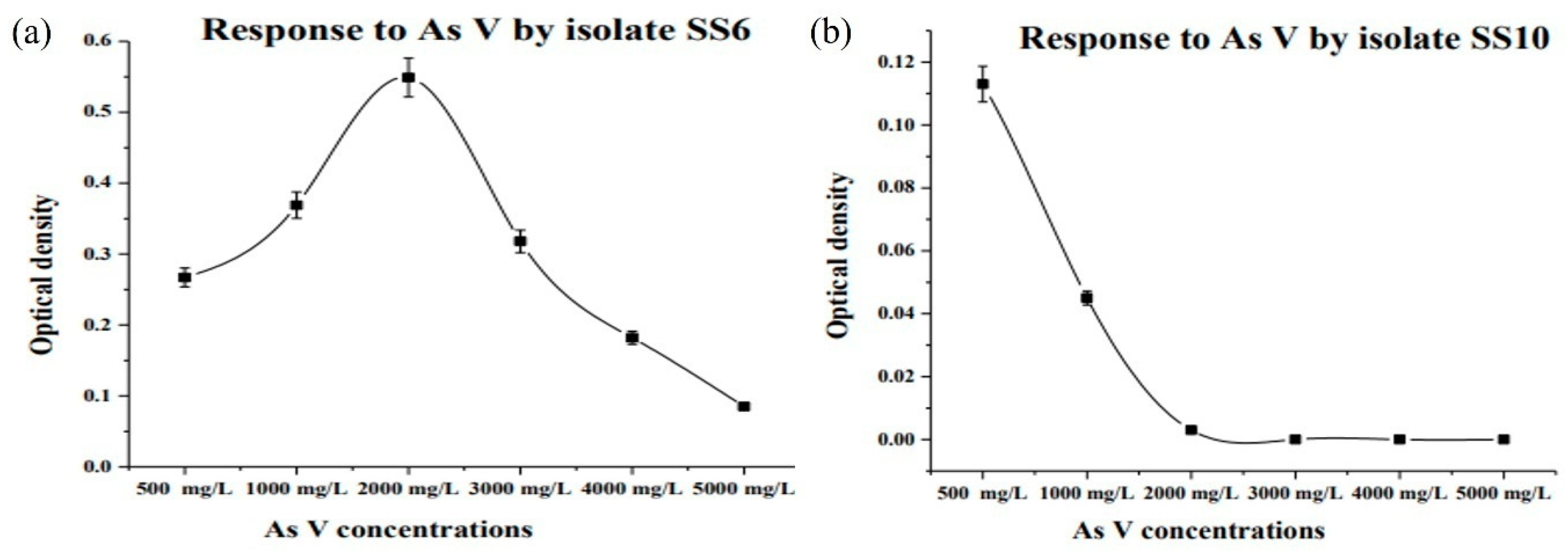
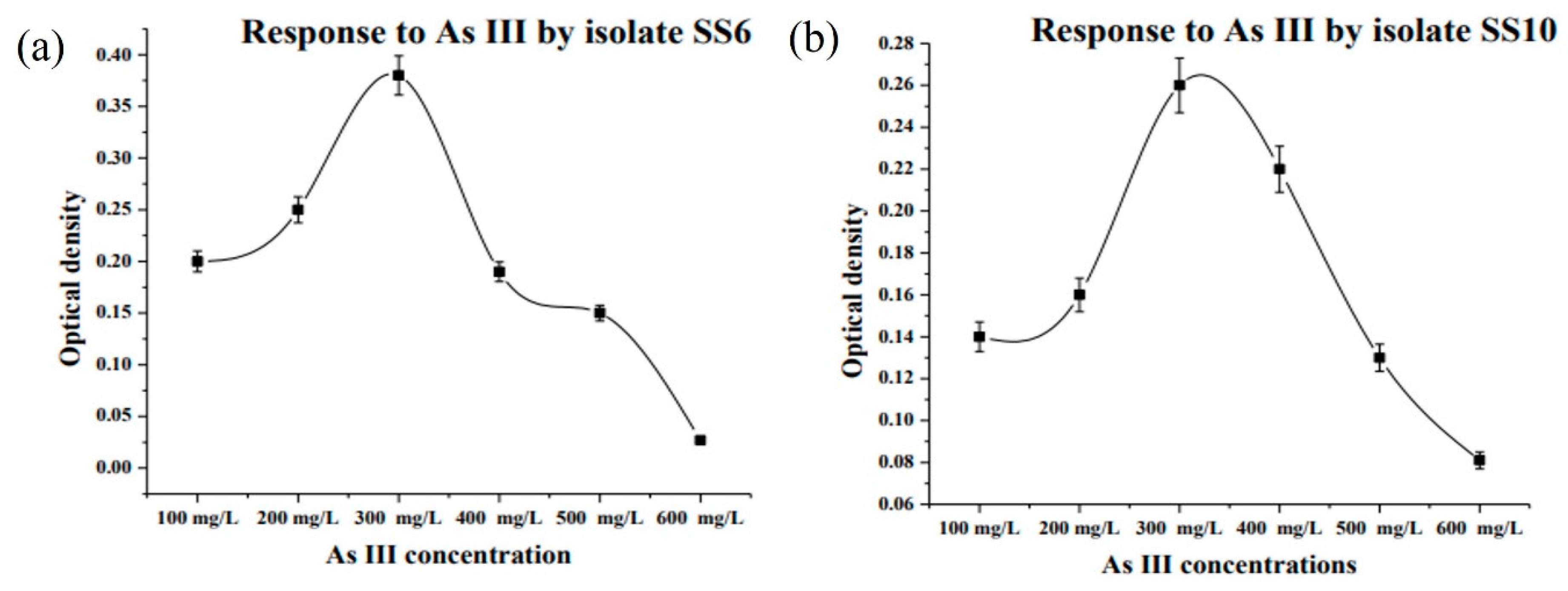
3.2. Isolation and MIC of Arsenic Resistant Bacteria
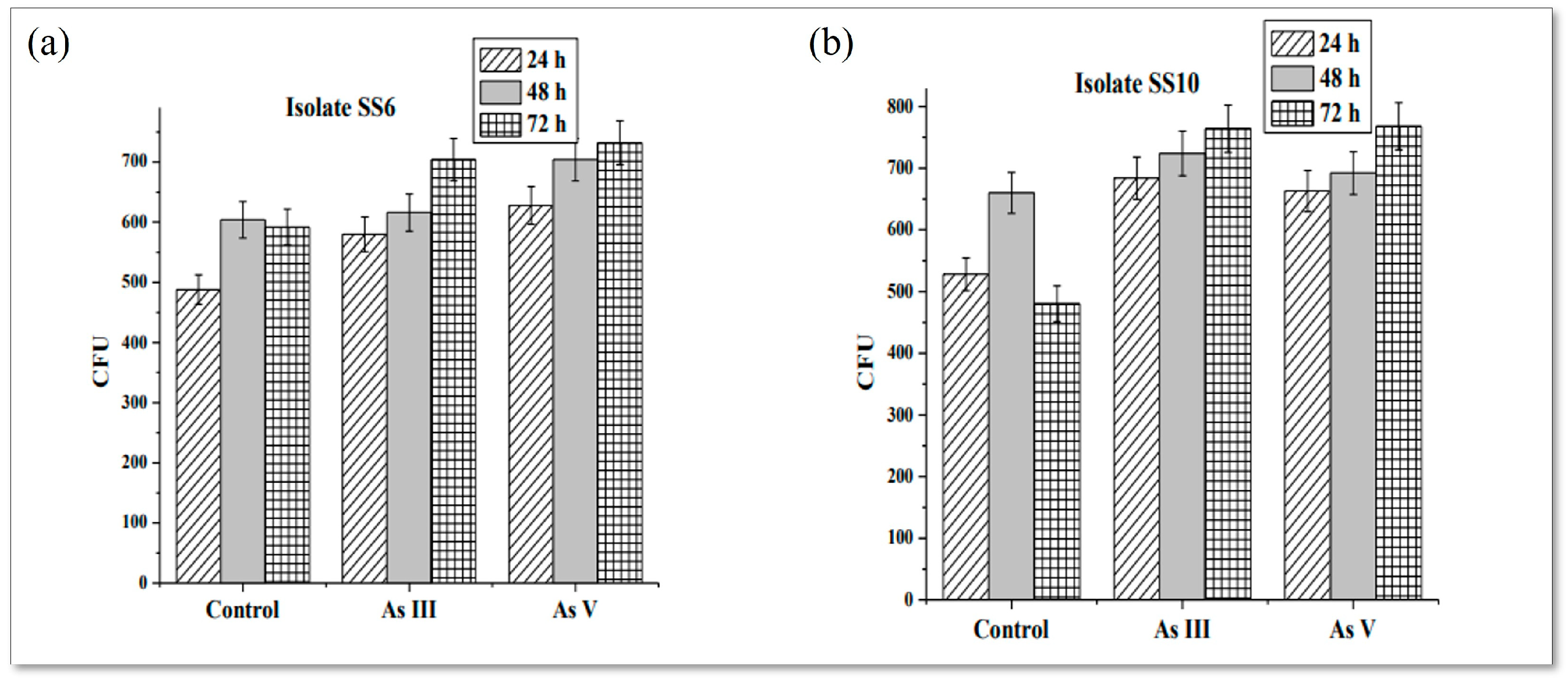
3.3. Presence of Viable Cells
3.4. Morphology and Biochemistry of the Isolates
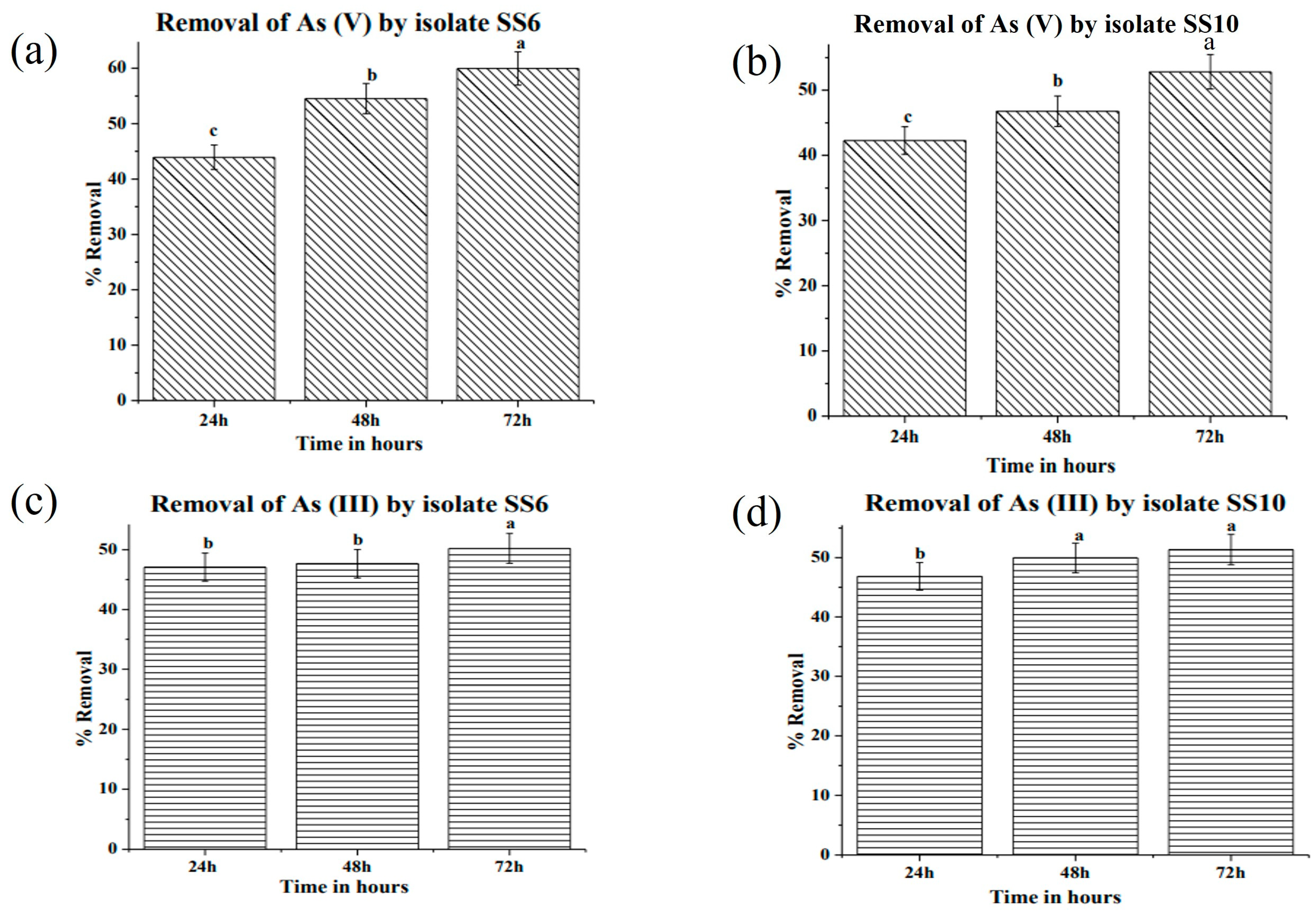
3.5. Bioremediation of Arsenic
3.6. Microbial Oxidation of Arsenic
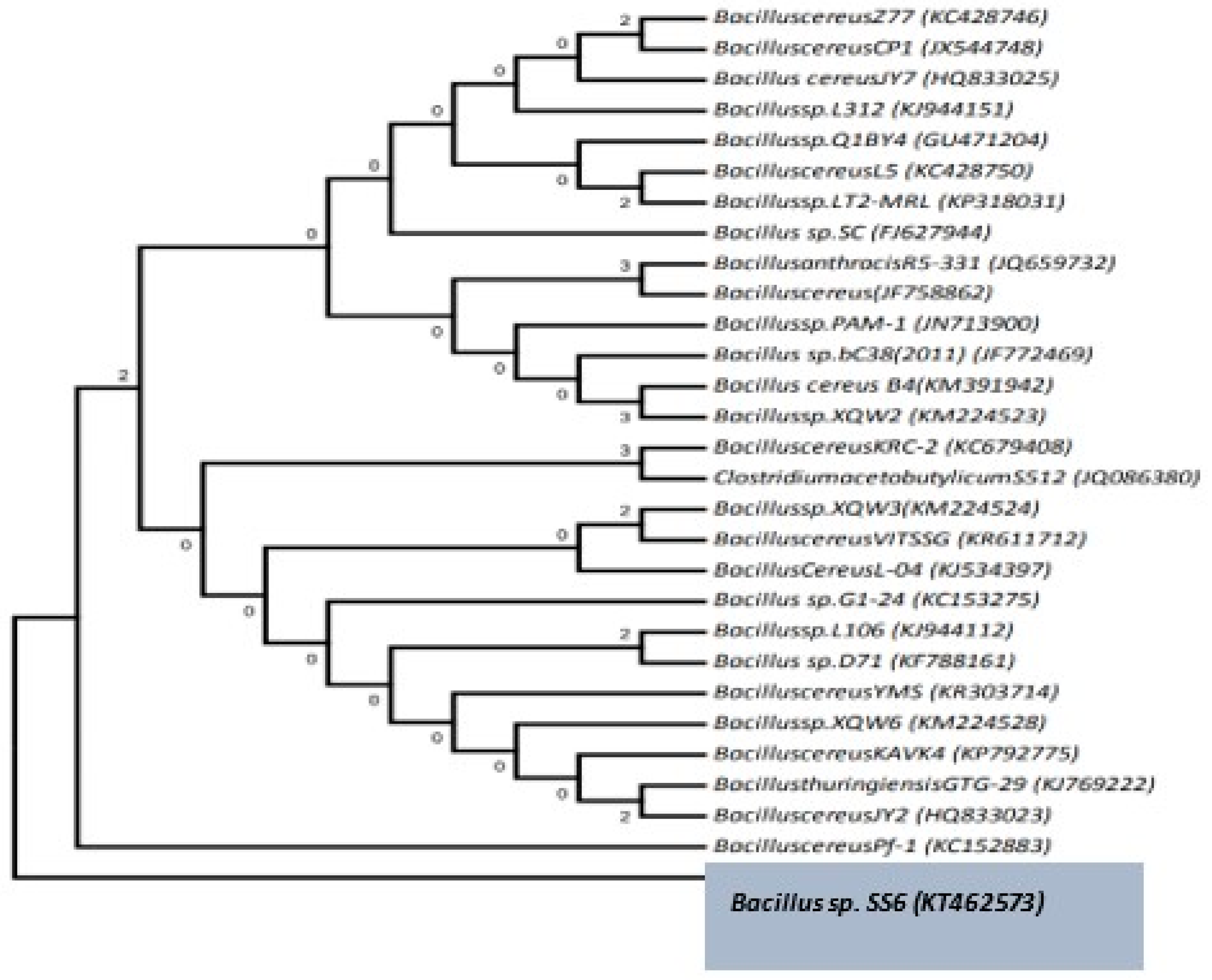
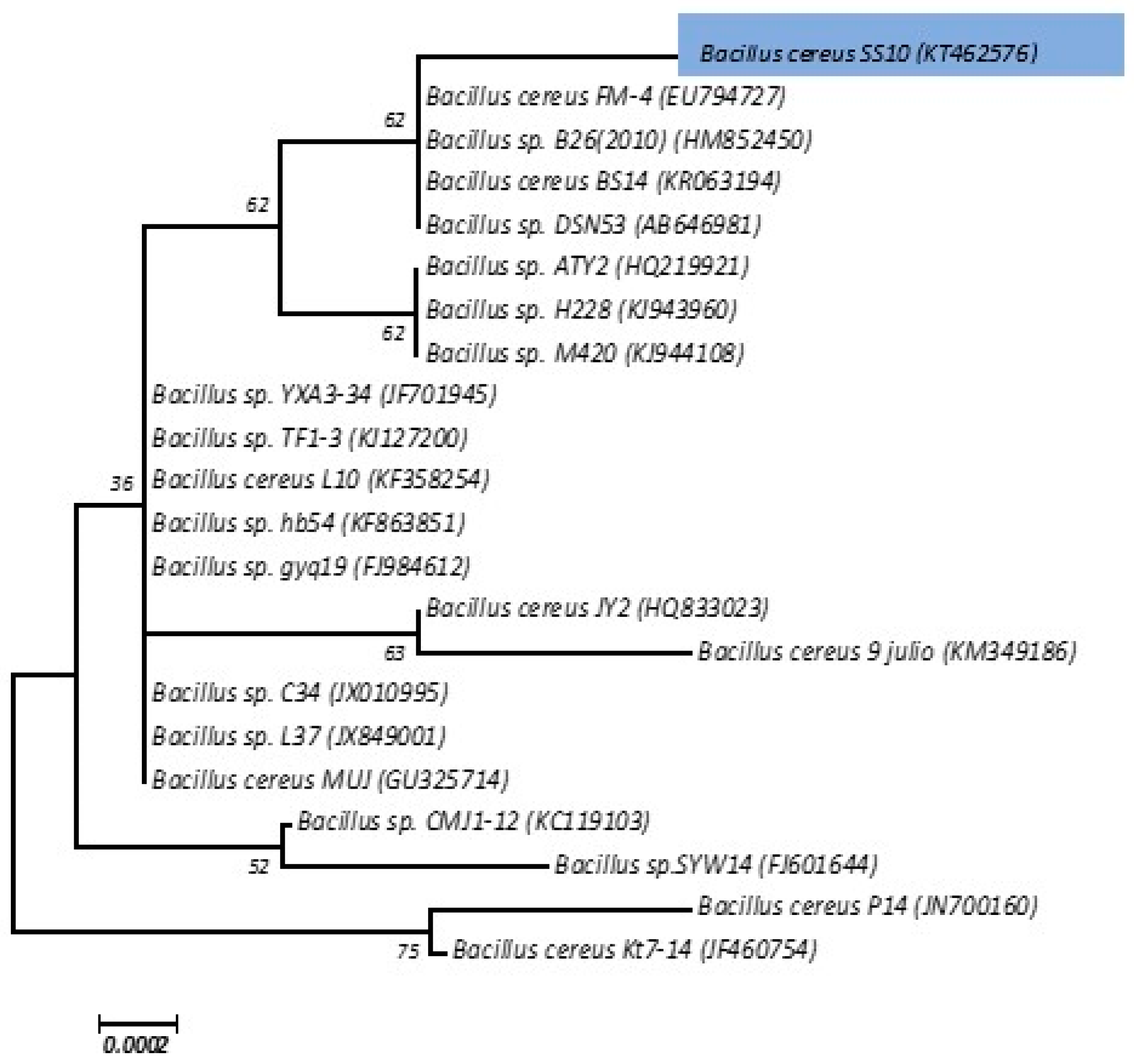
3.7. Identification of the Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability, and Risks of Metals; Springer: New York, NY, USA, 2001; Volume 860. [Google Scholar]
- Abernathy, C.O.; Thomas, D.J.; Calderon, R.L. Health effects and risk assessment of arsenic. J. Nutr. 2003, 133, 1536S–1538S. [Google Scholar] [CrossRef] [PubMed]
- Ravenscroft, P.; Brammer, H.; Richards, K. Arsenic Pollution: A Global Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 28. [Google Scholar]
- Tashan, H.; Harighi, B.; Rostamzadeh, J.; Azizi, A. Characterization of arsenic-resistant endophytic bacteria from alfalfa and chickpea plants. Front. Plant Sci. 2021, 12, 696750. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, M.K.; Yadav, P.; Shukla, A.; Srivastava, S. Utilizing the potential of microorganisms for managing arsenic contamination: A feasible and sustainable approach. Front. Environ. Sci. 2018, 6, 24. [Google Scholar] [CrossRef]
- Datta, D.V.; Kaul, M.K. Arsenic content of drinking water in villages in northern India. A concept of arsenicosis. J. Assoc. Physicians India 1976, 24, 599–604. [Google Scholar] [PubMed]
- Garai, R.; Chakraborty, A.K.; Dey, S.B.; Saha, K.C. Chronic arsenic poisoning from tube-well water. J. Indian Med. Assoc. 1984, 82, 34–35. [Google Scholar]
- Mukherjee, A.B.; Bhattacharya, P.; Jacks, G.; Banerjee, D.M.; Ramanathan, A.L.; Mahanta, M.; Chandrashekharam, D.; Chatterjee, D.C.D.; Naidu, R. Groundwater Arsenic Contamination in India: Extent and Severity; CSIRO Publishing: Clayton, Australia, 2006; pp. 553–593. [Google Scholar]
- Chakraborti, D.; Singh, S.K.; Rahman, M.M.; Dutta, R.N.; Mukherjee, S.C.; Pati, S.; Kar, P.B. Groundwater arsenic contamination in the Ganga River Basin: A future health danger. Int. J. Environ. Res. Public Health 2018, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Mukherjee, A.; Fryar, A.E.; Scanlon, B.R.; Bhattacharya, P.; Bhattacharya, A. Elevated arsenic in deeper groundwater of the western Bengal basin, India: Extent and controls from regional to local scale. Appl. Geochem. 2011, 26, 600–613. [Google Scholar] [CrossRef]
- Chakraborty, M.; Mukherjee, A.; Ahmed, K.M. A review of groundwater arsenic in the Bengal Basin, Bangladesh and India: From source to sink. Curr. Pollut. Rep. 2015, 1, 220–247. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Riaz, M.; Ramzan, N.; Zahid, M.T.; Shakoori, F.R.; Rafatullah, M. Isolation and characterization of arsenic resistant bacteria from wastewater. Braz. J. Microbiol. 2014, 45, 1309–1315. [Google Scholar] [CrossRef]
- Aksu, A.; Balkıs, N.; Erşan, M.S.; Müftüoğlu, A.E.; Apak, R. Biogeochemical cycle of arsenic and calculating the enrichment factor by using Li element. Environ. Geochem. Health 2010, 32, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol. Appl. Pharmacol. 2004, 197, 67–83. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kawahata, H.; Gupta, L.P.; Kita, N.; Morishita, Y.; Ono, Y.; Komai, T. Arsenic resistance and removal by marine and non-marine bacteria. J. Biotechnol. 2007, 127, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.L.; Singh, S.; Chen, W. Arsenic metabolism by microbes in nature and the impact on arsenic remediation. Curr. Opin. Biotechnol. 2009, 20, 659–667. [Google Scholar] [CrossRef]
- Titah, H.S.; Abdullah, S.R.S.; Idris, M.; Anuar, N.; Basri, H.; Mukhlisin, M.; Tangahu, B.V.; Purwanti, I.F.; Kurniawan, S.B. Arsenic Resistance and Biosorption by Isolated Rhizobacteria from the Roots of Ludwigia octovalvis. Hindawi Int. J. Microbiol. 2018, 2018, 3101498. [Google Scholar] [CrossRef]
- Dey, U.; Das, K.; Roy, P.; Chatterjee, S.N.; Mondal, N.K. Searching of microbial agent for bioremediation of arsenic. Int. J. Extensive Res. 2016, 5, 60–64. [Google Scholar]
- Dey, U.; Chatterjee, S.; Mondal, N.K. Isolation and characterization of arsenic-resistant bacteria and possible application in bioremediation. Biotechnol. Rep. 2016, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nwuche, C.O.; Ugoji, E.O. Effects of heavy metal pollution on the soil microbial activity. Int. J. Environ. Sci. Technol. 2008, 5, 409–414. [Google Scholar] [CrossRef]
- Ghodsi, H.; Hoodaji, M.; Tahmourespour, A.; Gheisari, M.M. Investigation of bioremediation of arsenic by bacteria isolated from contaminated soil. Afr. J. Microbiol. Res. 2011, 5, 5889–5895. [Google Scholar]
- Dhuldhaj, U.P.; Yadav, I.C.; Singh, S.; Sharma, N.K. Microbial interactions in the arsenic cycle: Adoptive strategies and applications in environmental management. Rev. Environ. Contam. Toxicol. 2012, 224, 1–38. [Google Scholar]
- Vidali, M. Bioremediation. An overview *. Pure Appl. Chem. 2001, 73, 1163–1172. [Google Scholar] [CrossRef]
- Oremland, R.S.; Stolz, J.F.; Hollibaugh, J.T. The microbial arsenic cycle in Mono Lake, California. FEMS Microbiol. Ecol. 2004, 48, 15–27. [Google Scholar] [CrossRef]
- Suresh, K.; Prabagaran, S.R.; Sengupta, S.; Shivaji, S. Bacillus indicus sp. nov., an arsenic-resistant bacterium isolated from an aquifer in West Bengal, India. Int. J. Syst. Evol. Microbiol. 2004, 54, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Dey, U.; Chatterjee, S.; Mondal, N.K. Investigation of bioremediation of arsenic by bacteria isolated from an arsenic contaminated area. Environ. Process. 2017, 4, 183–199. [Google Scholar] [CrossRef]
- Gupta, D.; Gantayet, L.M.; Chatterjee, D. Groundwater arsenic contamination in West Bengal, India: A review. Environ. Geochem. Health 2009, 31, 325–336. [Google Scholar]
- Roy, P.; Mondal, N.K.; Das, B.; Das, K. Arsenic contamination in groundwater: A statistical modeling. J. Urban Environ. Eng. 2013, 7, 24–29. [Google Scholar] [CrossRef]
- Mondal, N.K.; Dey, U.; Ghosh, S.; Datta, J.K. Soil enzyme activity under arsenic-stressed area of Purbasthali, West Bengal, India. Arch. Agron. Soil Sci. 2015, 61, 73–87. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total nitrogen analysis of soil and plant tissues. J. Assoc. Off. Anal. Chem. 1980, 63, 770–778. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate (No. 939); US Department of Agriculture: Washington, DC, USA, 1984. [Google Scholar]
- Sarkar, D.; Haldar, A. Physical and Chemical Methods in Soil Analysis, 2nd ed.; New Age International: Delhi, India, 2010; ISBN 978-81-224-2725-7. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Kamsonlian, S.; Suresh, S.; Majumder, C.B.; Chand, S. Biosorption of arsenic from contaminated water onto solid Psidium guajava leaf surface: Equilibrium, kinetics, thermodynamics, and desorption study. Bioremediat. J. 2012, 16, 97–112. [Google Scholar] [CrossRef]
- Kamsonlian, S.; Suresh, S.; Majumder, C.B.; Chand, S. Biosorption of As(III) from contaminated water onto low cost palm bark biomass. Int. J. Curr. Eng. Technol. 2012, 2, 153–158. [Google Scholar]
- Bhakat, P.B.; Gupta, A.K.; Ayoob, S. Feasibility analysis of As (III) removal in a continuous flow fixed bed system by modified calcined bauxite (MCB). J. Hazard. Mater. 2007, 139, 286–292. [Google Scholar] [CrossRef]
- Anyanwu, C.U.; Ugwu, C.E. Incidence of arsenic resistant bacteria isolated from a sewage treatment plant. Int. J. Basic Appl. Sci. 2010, 10, 64–78. [Google Scholar]
- Rahman, S.; Kim, K.H.; Saha, S.K.; Swaraz, A.M.; Paul, D.K. Review of remediation techniques for arsenic (As) contamination: A novel approach utilizing bio-organisms. J. Environ. Manag. 2014, 134, 175–185. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Svenstrup, H.F.; Glupczynski, Y. Defining and investigating the epidemiology of bacterial resistance to antimicrobial agents: A clinical and public health priority. Clin. Microbiol. Infect. 2012, 18, 821–830. [Google Scholar]
- Brown, A.E. Benson’s Microsbiological Applications: Laboratory Manual in General Microbiology, 10th ed.; Short Version; The McGraw Hill Companies: New York, NY, USA, 2010. [Google Scholar]
- Holt, J.G.; Noel, K.R.; Peter, S.H.A.; James, S.T.; Stanley, W.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1994; p. 11. [Google Scholar]
- Pelczar, M.J.; Bard, R.C.; Burnett, G.W.; Conn, H.J.; Demoss, R.D.; Euans, E.E.; Weiss, F.A.; Jennison, M.W.; Meckee, A.P.; Riker, A.J.; et al. Manual of Microbiological Methods. Society of American Bacteriology; McGraw Hill Book Company: New York, NY, USA, 1957; p. 315. [Google Scholar]
- Lacey, L.A. (Ed.) Manual of Techniques in Insect Pathology; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Banerjee, S.; Datta, S.; Chattyopadhyay, D.; Sarkar, P. Arsenic accumulating and transforming bacteria isolated from contaminated soil for potential use in bioremediation. J. Environ. Sci. Health Part A 2011, 46, 1736–1747. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Vincze, T.; Posfai, J.; Roberts, R.J. NEBcutter: A program to cleave DNA with restriction enzymes. Nucleic Acids Res. 2003, 31, 3688–3691. [Google Scholar] [CrossRef] [PubMed]
- Uguen, P.; Hamelin, J.; Le Pennec, J.P.; Blanco, C. Influence of osmolarity and the presence of an osmoprotectant on Lactococcus lactis growth and bacteriocin production. Appl. Environ. Microbiol. 1999, 65, 291–293. [Google Scholar] [CrossRef]
- Cai, L.; Liu, G.; Rensing, C.; Wang, G. Genes involved in arsenic transformation and resistance associated with different levels of arsenic-contaminated soils. BMC Microbiol. 2009, 9, 4. [Google Scholar] [CrossRef]
- Das, S.; Jean, J.S.; Kar, S.; Chou, M.L.; Chen, C.Y. Screening of plant growth-promoting traits in arsenic-resistant bacteria isolated from agricultural soil and their potential implication for arsenic bioremediation. J. Hazard. Mater. 2014, 272, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Maizel, D.; Blum, J.S.; Ferrero, M.A.; Utturkar, S.M.; Brown, S.D.; Rosen, B.P.; Oremland, R.S. Characterization of the extremely arsenic-resistant Brevibacterium linens strain AE038-8 isolated from contaminated groundwater in Tucumán, Argentina. Int. Biodeterior. Biodegrad. 2016, 107, 147–153. [Google Scholar] [CrossRef]
- Satyapal, G.K.; Mishra, S.K.; Srivastava, A.; Ranjan, R.K.; Prakash, K.; Haque, R.; Kumar, N. Possible bioremediation of arsenic toxicity by isolating indigenous bacteria from the middle Gangetic plain of Bihar, India. Biotechnol. Rep. 2018, 17, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Kepel, B.; Bodhi, W.; Tallei, T.E. Isolation and Identification of Arsenic-resistant Bacteria for Possible Application in Arsenic Bioremediation. Pak. J. Biol. Sci. PJBS 2020, 23, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.; Varma, A.; Chaudhary, D.; Aggarwal, H. Novel Arsenic resistant bacterium Sporosarcinaluteola M10 having potential bioremediation properties. J. Microbiol. Exp. 2010, 8, 213–218. [Google Scholar]
- Silver, S.; Phung, L.T. A bacterial view of the periodic table: Genes and proteins for toxic inorganic ions. J. Ind. Microbiol. Biotechnol. 2005, 32, 587–605. [Google Scholar] [CrossRef]
- Qin, J.; Rosen, B.P.; Zhang, Y.; Wang, G.; Franke, S.; Rensing, C. Arsenic detoxification and evolution of trimethylarsine gas by a microbial arsenite-S-adenosylmethionine methyltransferase. Proc. Natl. Acad. Sci. USA 2006, 103, 2075–2080. [Google Scholar] [CrossRef]
- Huang, A.; Teplitski, M.; Rathinasabapathi, B.; Ma, L. Characterization of arsenic-resistant bacteria from the rhizosphere of arsenic hyperaccumulator Pteris vittata. Can. J. Microbiol. 2010, 56, 236–246. [Google Scholar] [CrossRef]
- Berlanga, G.A.; Persans, M.W.; Eubanks, T.; Lowe, K.L. Characterization of arsenic-tolerant bacterial cultures from the Lower Laguna Madre of south Texas. Tex. J. Sci. 2010, 61, 259–279. [Google Scholar]
- Chang, J.S.; Kim, I.S. Arsenite oxidation by Bacillus sp. strain SeaH-As22w isolated from coastal seawater in Yeosu Bay. Environ. Eng. Res. 2010, 15, 15–21. [Google Scholar] [CrossRef]
- Davolos, D.; Pietrangeli, B. A molecular study on bacterial resistance to arsenic-toxicity in surface and underground waters of Latium (Italy). Ecotoxicol. Environ. Saf. 2013, 96, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Bhattacharyya, K.; Bhattacharyya, S.; Kole, S.C. Arsenic-tolerant, arsenite-oxidising bacterial strains in the contaminated soils of West Bengal, India. Sci. Total Environ. 2013, 463, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Rosen, B.P. New mechanisms of bacterial arsenic resistance. Biomed. J. 2016, 39, 5–13. [Google Scholar] [CrossRef]
- Andreoni, V.; Zanchi, R.; Cavalca, L.; Corsini, A.; Romagnoli, C.; Canzi, E. Arsenite oxidation in ancylobacter dichloromethanicus As3-1b strain: Detection of genes involved in arsenite oxidation and CO2 fixation. Curr. Microbiol. 2012, 65, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Cook, G.M. Isolation and characterization of arsenate-reducing bacteria from arsenic-contaminated sites in New Zealand. Curr. Microbiol. 2004, 48, 341–347. [Google Scholar] [CrossRef] [PubMed]

| Isolate No. | Fermenter | CO2 or Other Gas Production | Butt Color | Slant Color | H2S Production |
|---|---|---|---|---|---|
| SS6 | Glucose fermentation only | +ve | Yellow | Pink | −ve |
| SS10 | Glucose, lactose, and sucrose non-fermenter | −ve | Pink | Pink | −ve |
| Carbon Source | SS6 | SS10 |
|---|---|---|
| Glucose | +/gas | + |
| Sucrose | + | + |
| Lactose | +/gas | − |
| Mannitol | +/gas | − |
| Arabinose | + | + |
| Fructose | + | + |
| Maltose | + | + |
| Salicin | + | + |
| Inositol | + | + |
| Mannose | + | + |
| Aesculin | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, U.; Mondal, N.K.; Chatterjee, S.; Das, K.; Raj, D.; Kumar, P.; Meraj, G. The Isolation and Identification of Novel Arsenic-Resistant Bacteria from an Arsenic-Contaminated Region—A Study to Understand the Efficiency of Bacteria for Arsenic Removal from Aqueous Media. Water 2024, 16, 2401. https://doi.org/10.3390/w16172401
Dey U, Mondal NK, Chatterjee S, Das K, Raj D, Kumar P, Meraj G. The Isolation and Identification of Novel Arsenic-Resistant Bacteria from an Arsenic-Contaminated Region—A Study to Understand the Efficiency of Bacteria for Arsenic Removal from Aqueous Media. Water. 2024; 16(17):2401. https://doi.org/10.3390/w16172401
Chicago/Turabian StyleDey, Uttiya, Naba Kumar Mondal, Soumendranath Chatterjee, Kousik Das, Deep Raj, Pankaj Kumar, and Gowhar Meraj. 2024. "The Isolation and Identification of Novel Arsenic-Resistant Bacteria from an Arsenic-Contaminated Region—A Study to Understand the Efficiency of Bacteria for Arsenic Removal from Aqueous Media" Water 16, no. 17: 2401. https://doi.org/10.3390/w16172401
APA StyleDey, U., Mondal, N. K., Chatterjee, S., Das, K., Raj, D., Kumar, P., & Meraj, G. (2024). The Isolation and Identification of Novel Arsenic-Resistant Bacteria from an Arsenic-Contaminated Region—A Study to Understand the Efficiency of Bacteria for Arsenic Removal from Aqueous Media. Water, 16(17), 2401. https://doi.org/10.3390/w16172401








