Bacteria and Cyanobacteria Inactivation Using UV-C, UV-C/H2O2, and Solar/H2O2 Processes: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bacteria Solution
2.2. Preparation of Cyanobacteria Solution
2.3. UV-C, Solar, UV-C/H2O2, and Solar/H2O2 Processes
2.4. Enumeration of Bacteria and Cyanobacteria
3. Results and Discussion
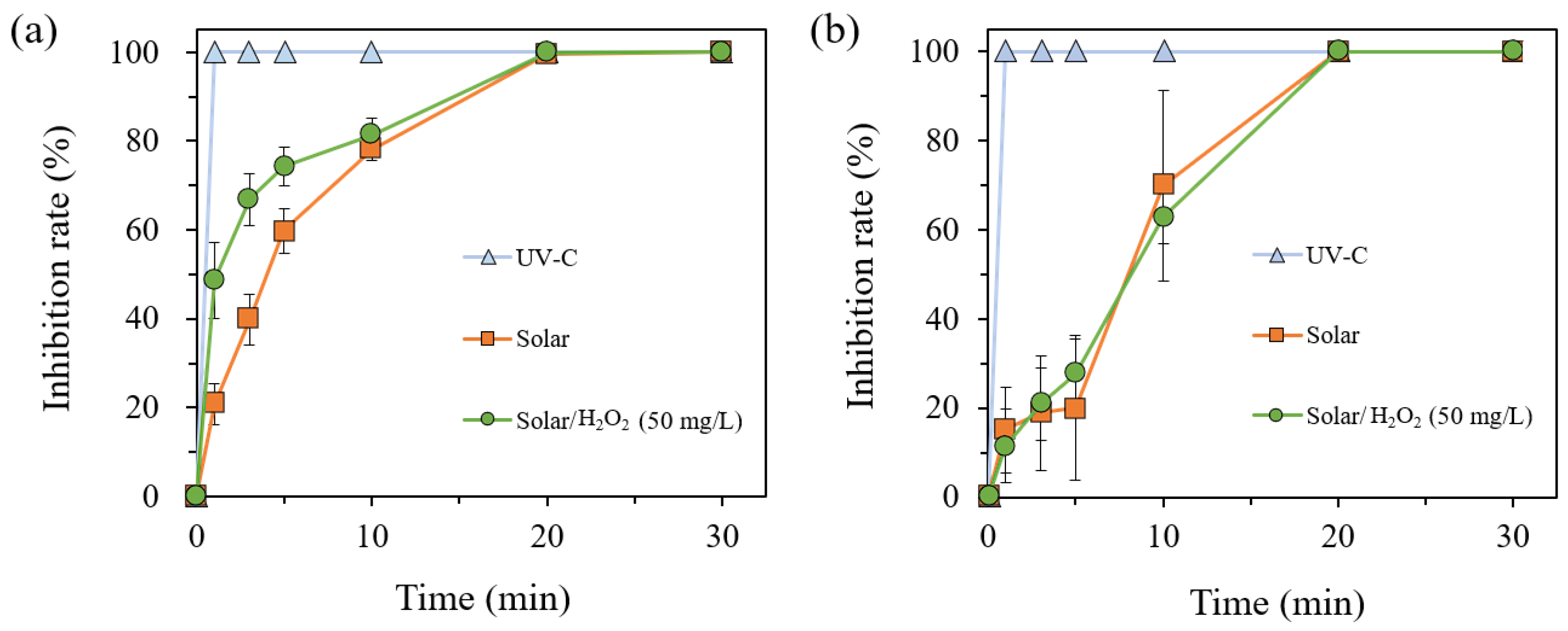
3.1. Inhibition of E. coli and B. Subtilis under UV-C, Solar, and Solar/H2O2 Processes
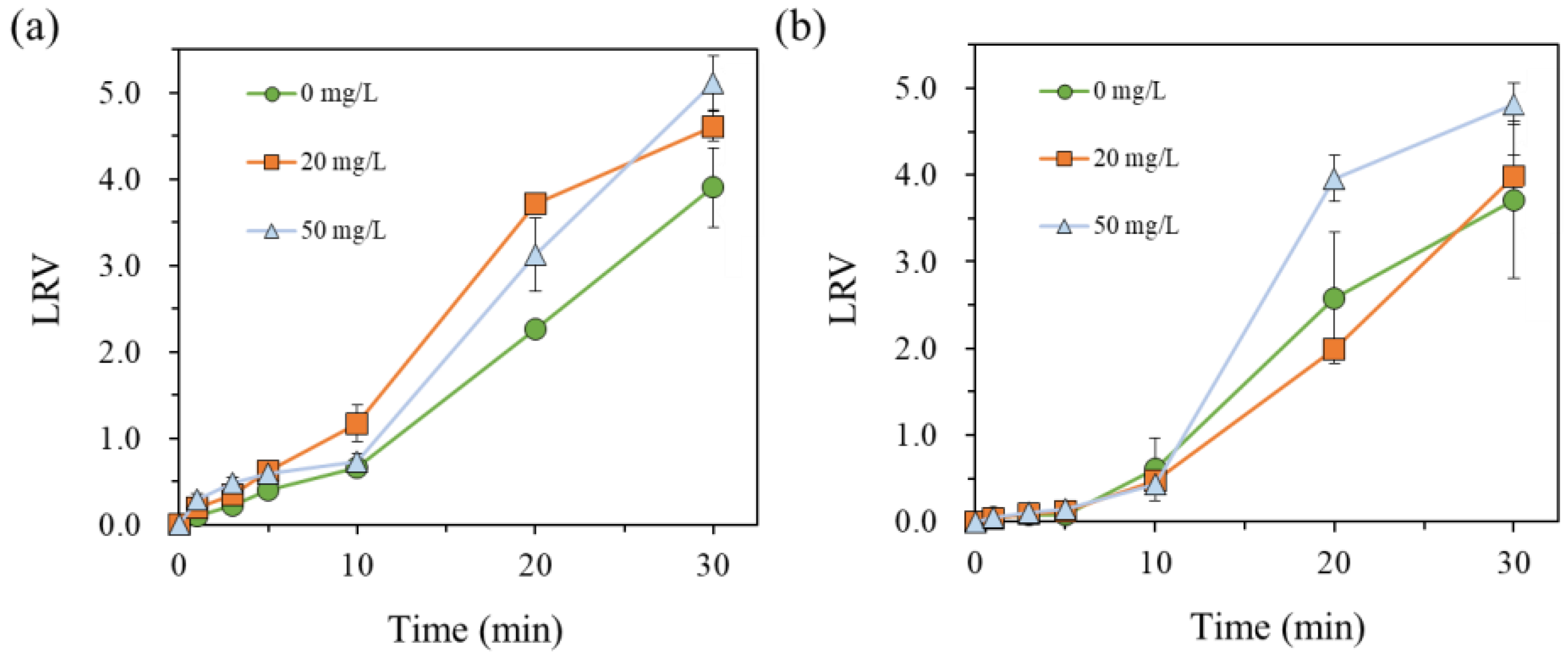
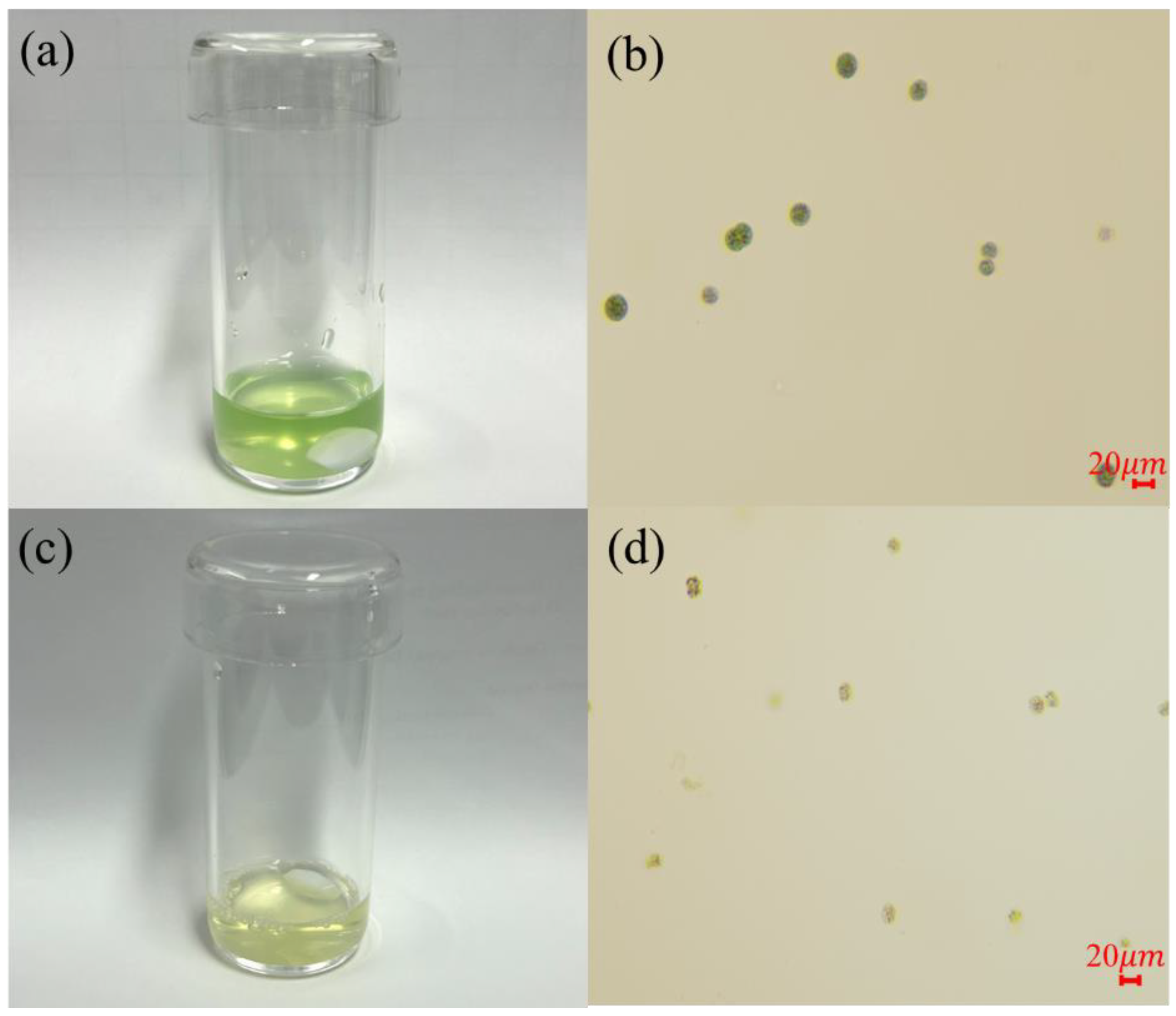
3.2. Inhibition of M. aeruginosa Using UV-C, UV-C/H2O2, Solar, and Solar/H2O2 Processes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chamberlain, K.; Lyons, A.C. Routledge International Handbook of Critical Issues in Health and Illness; Routledge, Taylor & Francis Group: Abingdon, UK, 2022. [Google Scholar]
- Okafor, C.O.; Ude, U.I.; Okoh, F.N.; Eromonsele, B.O. Safe Drinking Water: The Need and Challenges in Developing Countries. In Water Quality-New Perspectives; IntechOpen: London, UK, 2024. [Google Scholar]
- Cho, K.H.; Wolny, J.; Kase, J.A.; Unno, T.; Pachepsky, Y. Interactions of E. coli with algae and aquatic vegetation in natural waters. Water Res. 2022, 209, 117952. [Google Scholar] [CrossRef] [PubMed]
- Prüss-Ustün, A.; Wolf, J.; Bartram, J.; Clasen, T.; Cumming, O.; Freeman, M.C.; Gordon, B.; Hunter, P.R.; Medlicott, K.; Johnston, R. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: An updated analysis with a focus on low-and middle-income countries. Int. J. Hyg. Environ. Health 2019, 222, 765–777. [Google Scholar] [CrossRef]
- Girmay, A.M.; Mengesha, S.D.; Dinssa, D.A.; Alemu, Z.A.; Wagari, B.; Weldegebriel, M.G.; Serte, M.G.; Alemayehu, T.A.; Kenea, M.A.; Weldetinsae, A. Access to water, sanitation and hygiene (WASH) services and drinking water contamination risk levels in households of Bishoftu Town, Ethiopia: A cross-sectional study. Health Sci. Rep. 2023, 6, e1662. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhang, C.; Struewing, I.; Li, X.; Allen, J.; Lu, J. Cyanotoxin-encoding genes as powerful predictors of cyanotoxin production during harmful cyanobacterial blooms in an inland freshwarer lake: Evaluating a novel early-warning system. Sci. Total Environ. 2022, 830, 154568. [Google Scholar] [CrossRef]
- Ahn, J.M.; Kim, J.; Park, L.J.; Jeon, J.; Jong, J.; Min, J.-H.; Kang, T. Predicting cyanobacterial harmful algal blooms (CyanoHABs) in a regulated river using a revised EFDC model. Water 2021, 13, 439. [Google Scholar] [CrossRef]
- Wilson, A.E.; Sarnelle, O.; Neilan, B.A.; Salmon, T.P.; Gehringer, M.M.; Hay, M.E. Genetic variation of the bloom-forming cyanobacterium Microcystis aeruginosa within and among lakes: Implications for harmful algal blooms. Appl. Environ. Microbiol. 2005, 71, 6126–6133. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; He, Y.; Lu, S.; He, M.; Liu, Z.; Deng, Y.; Liu, Z.; Xu, T.; Zhang, H. Activation of peroxymonosulfate by La2CuO4 perovskite for synergistic removal of Microcystis aeruginosa and microcystin-LR in harmful algal bloom impacted water. Appl. Catal. B Environ. 2023, 328, 122511. [Google Scholar] [CrossRef]
- Elala, D.; Labhasetwar, P.; Tyrrel, S.F. Deterioration in water quality from supply chain to household and appropriate storage in the context of intermittent water supplies. Water Sci. Technol. Water Supply 2011, 11, 400–408. [Google Scholar] [CrossRef]
- Remucal, C.; Manley, D. Emerging investigators series: The efficacy of chlorine photolysis as an advanced oxidation process for drinking water treatment. Environ. Sci. Water Res. Technol. 2016, 2, 565–579. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Ahn, K.; Lee, Y.; Choi, J.; Ahn, H.; Seo, K.; Oh, J.; Jung, W.; Park, S. A Study on Occurance and Control of Disinfection by-Products (DBPs) in Drinking Water (I)-Monitoring of DBPs and Its Precursors; NEIR No. 2010-46-1221; National Institute of Environmental Research: Incheon, Republic of Korea, 2010. [Google Scholar]
- Li, X.; Cai, M.; Wang, L.; Niu, F.; Yang, D.; Zhang, G. Evaluation survey of microbial disinfection methods in UV-LED water treatment systems. Sci. Total Environ. 2019, 659, 1415–1427. [Google Scholar] [CrossRef]
- Gelover, S.; Gómez, L.A.; Reyes, K.; Leal, M.T. A practical demonstration of water disinfection using TiO2 films and sunlight. Water Res. 2006, 40, 3274–3280. [Google Scholar] [CrossRef]
- Tavares, R.D.; Tacão, M.; Figueiredo, A.S.; Duarte, A.S.; Esposito, F.; Lincopan, N.; Manaia, C.M.; Henriques, I. Genotypic and phenotypic traits of blaCTX-M-carrying Escherichia coli strains from an UV-C-treated wastewater effluent. Water Res. 2020, 184, 116079. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.I.; Ho, L.; Brookes, J.D. Effect of chlorination on Microcystis aeruginosa cell integrity and subsequent microcystin release and degradation. Environ. Sci. Technol. 2007, 41, 4447–4453. [Google Scholar] [CrossRef]
- Lürling, M.; Kang, L.; Mucci, M.; van Oosterhout, F.; Noyma, N.P.; Miranda, M.; Huszar, V.L.; Waajen, G.; Marinho, M.M. Coagulation and precipitation of cyanobacterial blooms. Ecol. Eng. 2020, 158, 106032. [Google Scholar] [CrossRef]
- Wu, X.; Joyce, E.M.; Mason, T.J. The effects of ultrasound on cyanobacteria. Harmful Algae 2011, 10, 738–743. [Google Scholar] [CrossRef]
- Tao, Y.; Mao, X.; Hu, J.; Mok, H.; Wang, L.; Au, D.; Zhu, J.; Zhang, X. Mechanisms of photosynthetic inactivation on growth suppression of Microcystis aeruginosa under UV-C stress. Chemosphere 2013, 93, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Gárcia-Fernández, I.; Polo-López, M.I.; Oller, I.; Fernandez-Ibanez, P. Bacteria and fungi inactivation using Fe3+/sunlight, H2O2/sunlight and near neutral photo-Fenton: A comparative study. Appl. Catal. B Environ. 2012, 121, 20–29. [Google Scholar] [CrossRef]
- Boyle, M.; Sichel, C.; Fernández-Ibáñez, P.; Arias-Quiroz, G.; Iriarte-Puná, M.; Mercado, A.; Ubomba-Jaswa, E.; McGuigan, K. Bactericidal effect of solar water disinfection under real sunlight conditions. Appl. Environ. Microbiol. 2008, 74, 2997–3001. [Google Scholar] [CrossRef] [PubMed]
- Eleren, S.C.; Alkan, U.; Teksoy, A. Inactivation of E. Coli and B. subtilis by solar and solar/H2O2 processes in humic surface waters. Fresen. Environ. Bull. 2014, 23, 1397–1406. [Google Scholar]
- Huang, J.; Zhang, Y.; Arhonditsis, G.B.; Gao, J.; Chen, Q.; Peng, J. The magnitude and drivers of harmful algal blooms in China’s lakes and reservoirs: A national-scale characterization. Water Res. 2020, 181, 115902. [Google Scholar] [CrossRef]
- Rahn, R.O. Potassium Iodide as a Chemical Actinometer for 254 nm Radiation: Use of lodate as an Electron Scavenger. Photochem. Photobiol. 1997, 66, 450–455. [Google Scholar] [CrossRef]
- Liu, W.; Andrews, S.A.; Stefan, M.I.; Bolton, J.R. Optimal methods for quenching H2O2 residuals prior to UFC testing. Water Res. 2003, 37, 3697–3703. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environment (MOE) of South Korea. Water Pollution Standard Method; Korean Literature: Hanja, Republic of Korea, 2022. [Google Scholar]
- Nyangaresi, P.O.; Rathnayake, T.; Beck, S.E. Evaluation of disinfection efficacy of single UV-C, and UV-A followed by UV-C LED irradiation on Escherichia coli, B. spizizenii and MS2 bacteriophage, in water. Sci. Total Environ. 2023, 859, 160256. [Google Scholar] [CrossRef] [PubMed]
- Lui, G.Y.; Roser, D.; Corkish, R.; Ashbolt, N.; Jagals, P.; Stuetz, R. Photovoltaic powered ultraviolet and visible light-emitting diodes for sustainable point-of-use disinfection of drinking waters. Sci. Total Environ. 2014, 493, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Kaidzu, S.; Sugihara, K.; Sasaki, M.; Nishiaki, A.; Igarashi, T.; Tanito, M. Evaluation of acute corneal damage induced by 222-nm and 254-nm ultraviolet light in Sprague–Dawley rats. Free Radic. Res. 2019, 53, 611–617. [Google Scholar] [CrossRef]
- Castro-Alférez, M.; Polo-López, M.I.; Marugán, J.; Fernandez-Ibanez, P. Mechanistic model of the Escherichia coli inactivation by solar disinfection based on the photo-generation of internal ROS and the photo-inactivation of enzymes: CAT and SOD. Chem. Eng. J. 2017, 318, 214–223. [Google Scholar] [CrossRef]
- Feng, L.; Peillex-Delphe, C.; Lü, C.; Wang, D.; Giannakis, S.; Pulgarin, C. Employing bacterial mutations for the elucidation of photo-Fenton disinfection: Focus on the intracellular and extracellular inactivation mechanisms induced by UVA and H2O2. Water Res. 2020, 182, 116049. [Google Scholar] [CrossRef]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef]
- Zhang, J.K.; Su, P.D.; Chen, H.H.; Qiao, M.; Yang, B.; Zhao, X. Impact of reactive oxygen species on cell activity and structural integrity of Gram-positive and Gram-negative bacteria in electrochemical disinfection system. Chem. Eng. J. 2023, 451, 138879. [Google Scholar] [CrossRef]
- Chen, L.; Keramati, L.; Helmann, J.D. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. USA 1995, 92, 8190–8194. [Google Scholar] [CrossRef]
- Ou, H.S.; Gao, N.Y.; Deng, Y.; Wang, H.; Zhang, H.X. Inactivation and degradation of by UV-C irradiation. Chemosphere 2011, 85, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-Y.; Häder, D.-P. UV-B-induced formation of reactive oxygen species and oxidative damage of the cyanobacterium Anabaena sp.: Protective effects of ascorbic acid and N-acetyl-L-cysteine. J. Photochem. Photobiol. B Biol 2002, 66, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Farnood, R. Effects of hydrogen peroxide concentration and ultraviolet light intensity on methyl tert-butyl ether degradation kinetics. Chem. Eng. Sci. 2005, 60, 1641–1648. [Google Scholar] [CrossRef]


| Process | M. aeruginosa | Chl-a | ||
|---|---|---|---|---|
| k (1/min) | R2 | k (1/min) | R2 | |
| UV-C/H2O2 (50 mg/L) | 0.053 | 0.959 | 0.022 | 0.855 |
| UV-C/H2O2 (20 mg/L) | 0.046 | 0.989 | 0.027 | 0.908 |
| UV-C | 0.041 | 0.973 | 0.031 | 0.958 |
| Solar/H2O2 (20 mg/L) | 0.011 | 0.937 | 0.017 | 0.783 |
| Solar | 0.005 | 0.989 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-H.; Shin, J.; Yoon, S.; Jang, T.; Lee, J.; Kim, H.-K.; Park, J.-A. Bacteria and Cyanobacteria Inactivation Using UV-C, UV-C/H2O2, and Solar/H2O2 Processes: A Comparative Study. Water 2024, 16, 2392. https://doi.org/10.3390/w16172392
Choi J-H, Shin J, Yoon S, Jang T, Lee J, Kim H-K, Park J-A. Bacteria and Cyanobacteria Inactivation Using UV-C, UV-C/H2O2, and Solar/H2O2 Processes: A Comparative Study. Water. 2024; 16(17):2392. https://doi.org/10.3390/w16172392
Chicago/Turabian StyleChoi, Jin-Hyuk, Jeongmin Shin, Soyeong Yoon, Taesoon Jang, Jooyoung Lee, Hyun-Kyung Kim, and Jeong-Ann Park. 2024. "Bacteria and Cyanobacteria Inactivation Using UV-C, UV-C/H2O2, and Solar/H2O2 Processes: A Comparative Study" Water 16, no. 17: 2392. https://doi.org/10.3390/w16172392
APA StyleChoi, J.-H., Shin, J., Yoon, S., Jang, T., Lee, J., Kim, H.-K., & Park, J.-A. (2024). Bacteria and Cyanobacteria Inactivation Using UV-C, UV-C/H2O2, and Solar/H2O2 Processes: A Comparative Study. Water, 16(17), 2392. https://doi.org/10.3390/w16172392






