Reflections on How to Reach the “30 by 30” Target: Identification of and Suggestions on Global Priority Marine Areas for Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Description and Sources
2.1.1. Surveyed Nearshore, Offshore, and Deep-Sea Habitats
2.1.2. Endangered, Rare, and Commercially Valuable Marine Species
2.1.3. Abiotic Elements
2.2. Methodology
2.2.1. Research Area and Data Pre-Processing
2.2.2. Priority for Protection Calculation
2.2.3. Visualization and Comparison
3. Results
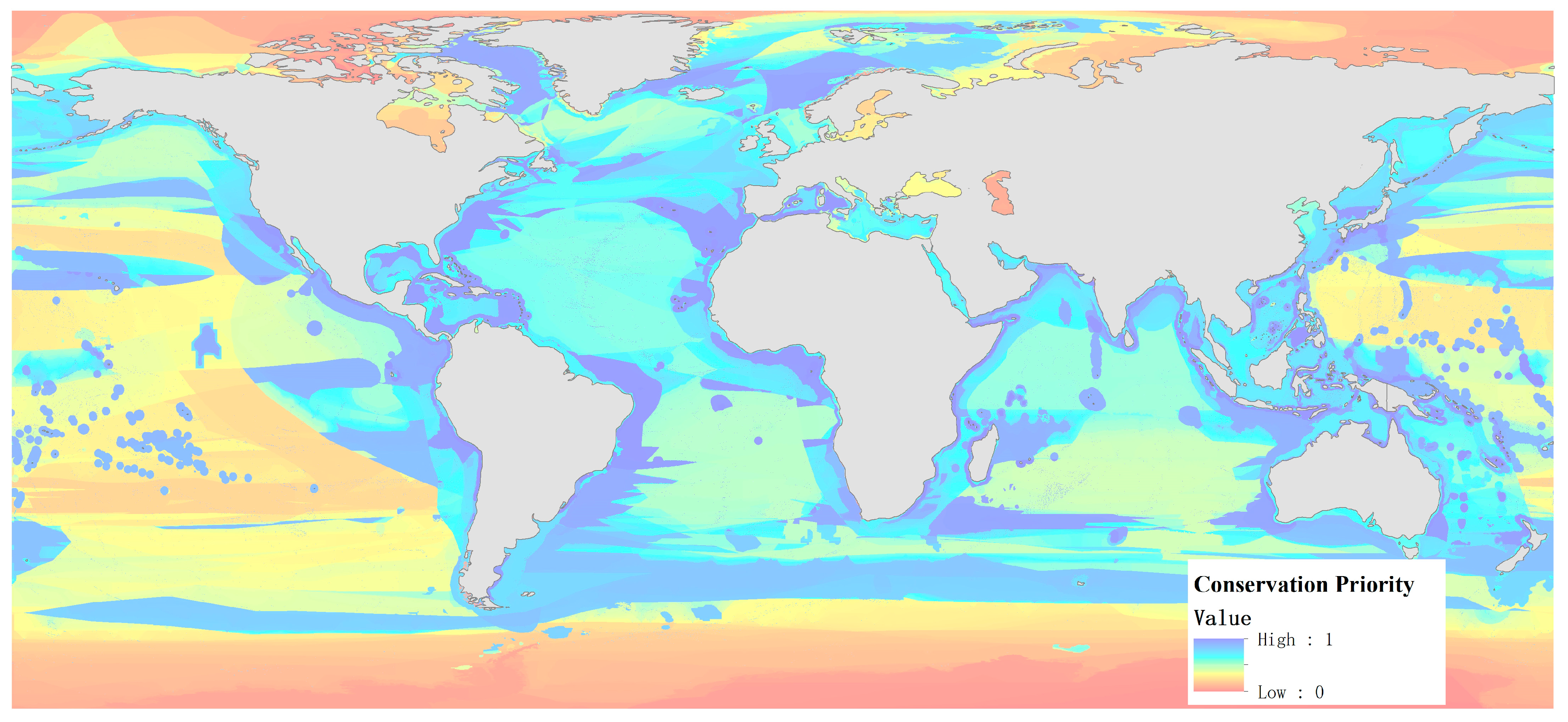
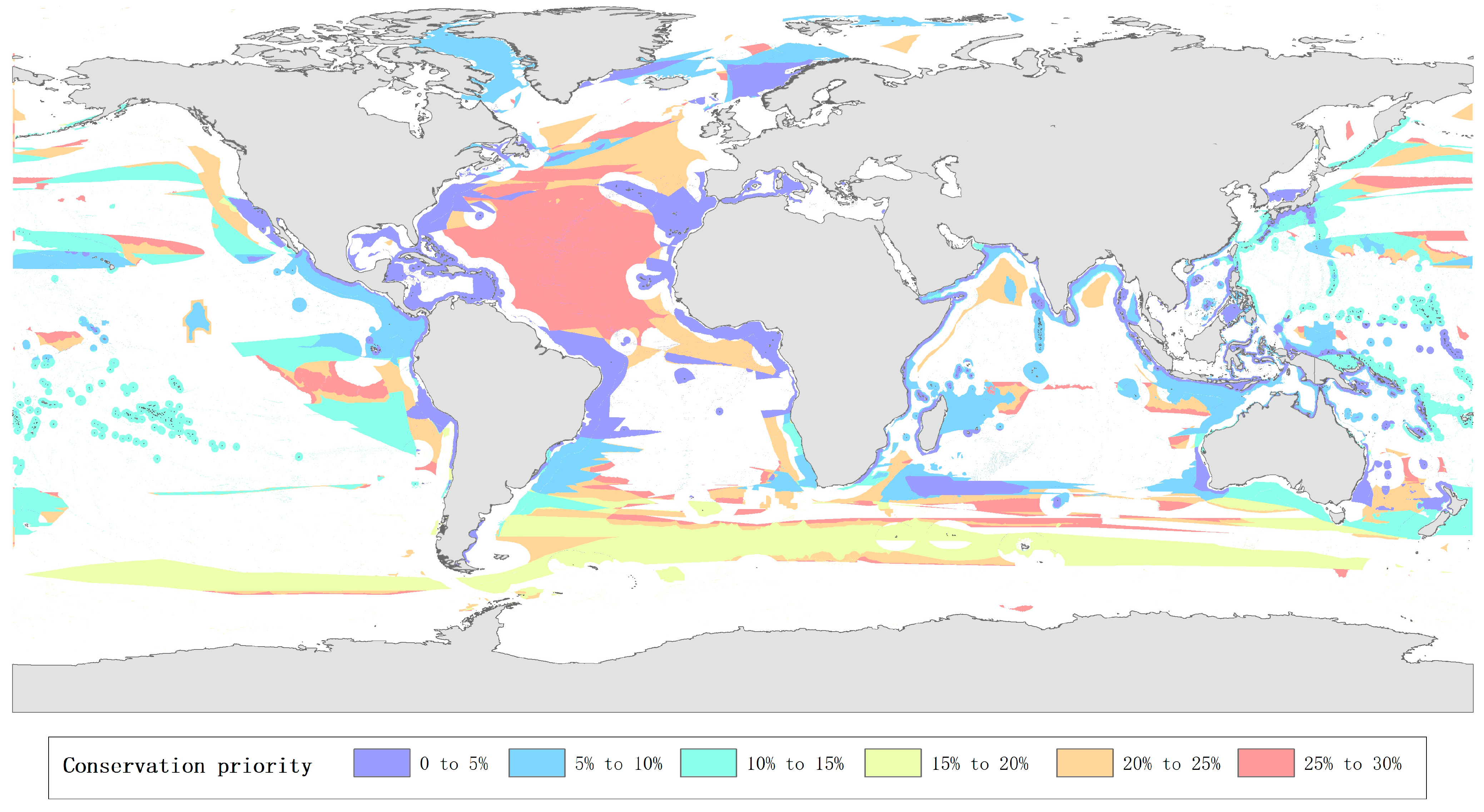
3.1. Description of Global Priority Marine Areas for Protection Layout
3.2. Distribution of Priority Marine Areas for Protection from the Coastline to Deep Ocean Trenches
4. Discussion
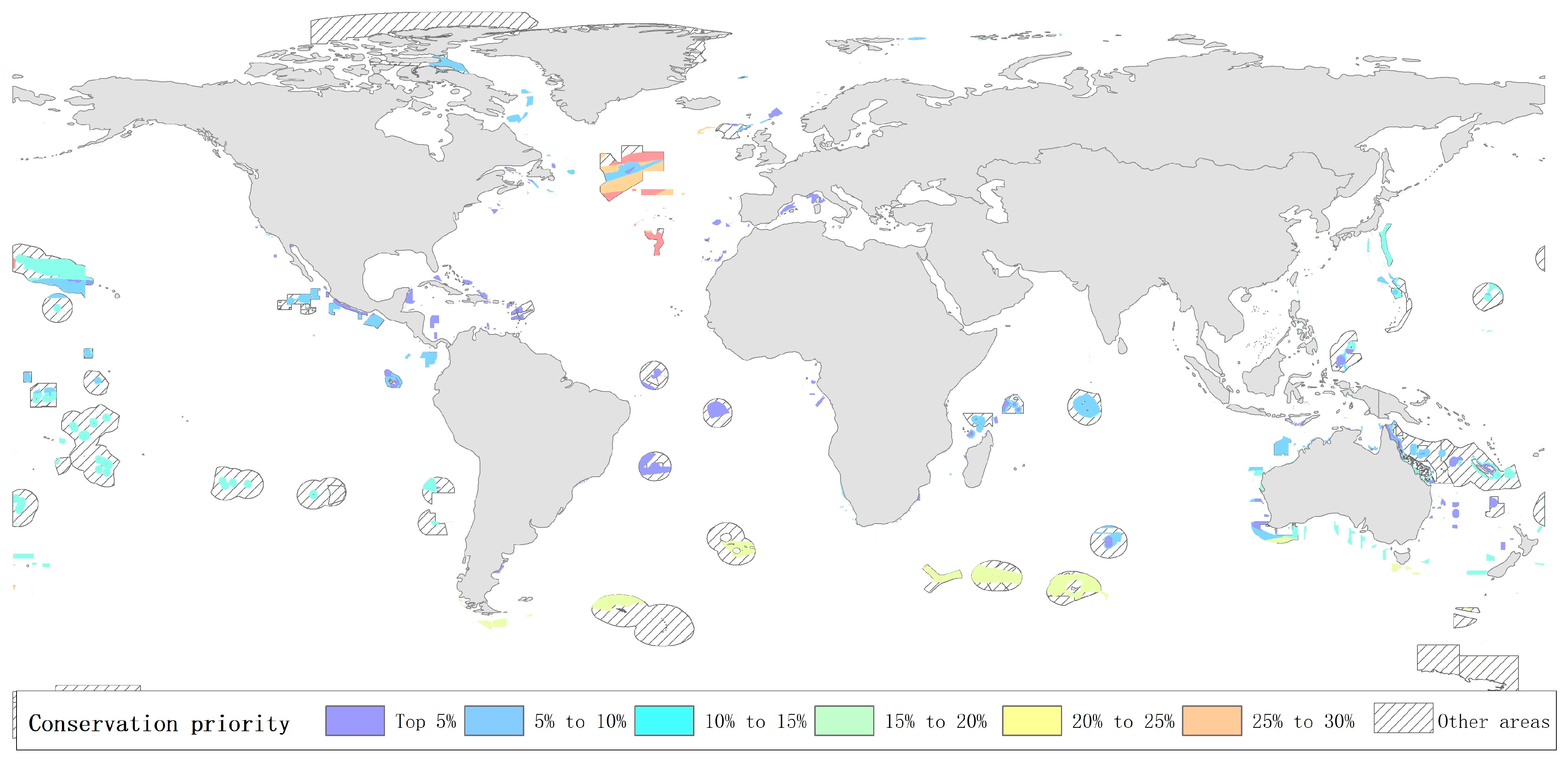
4.1. Comparison with Marine Protected Areas and Ecologically or Biologically Significant Marine Areas
4.2. Discussion on Management of Priority Marine Areas for Protection
4.3. Limitations
4.4. Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- NOAA. ‘Why Should We Care about the Ocean?’, National Ocean Service National Oceanic and Atmospheric Administration. Available online: https://oceanservice.noaa.gov/facts/why-care-about-ocean.html (accessed on 17 August 2023).
- Fischer, M.; Maxwell, K.; Nuunoq; Pedersen, H.; Greeno, D.; Jingwas, N.; Graham Blair, J.; Hugu, S.; Mustonen, T.; Murtomäki, E.; et al. Empowering her guardians to nurture our Ocean’s future. Rev. Fish Biol. Fish. 2022, 32, 271–296. [Google Scholar] [CrossRef]
- Tigchelaar, M.; Leape, J.; Micheli, F.; Allison, E.H.; Basurto, X.; Bennett, A.; Bush, S.R.; Cao, L.; Cheung, W.W.L.; Crona, B.; et al. The vital roles of blue foods in the global food system. Glob. Food Secur. 2022, 33, 100637. [Google Scholar] [CrossRef]
- Newman, D.; Cragg, G. Drugs and Drug Candidates from Marine Sources: An Assessment of the Current “State of Play”. Planta Med. 2016, 82, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.; Harrould-Kolieb, E.; Jones, E.; Taylor, M.L. The current status of deep-sea mining governance at the International Seabed Authority. Mar. Policy 2023, 147, 105396. [Google Scholar] [CrossRef]
- Sala, E.; Mayorga, J.; Bradley, D.; Cabral, R.B.; Atwood, T.B.; Auber, A.; Cheung, W.; Costello, C.; Ferretti, F.; Friedlander, A.M.; et al. Protecting the global ocean for biodiversity, food and climate. Nature 2021, 592, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Quéré, C.L.; Moriarty, R.; Andrew, R.M.; Peters, G.P.; Ciais, P.; Friedlingstein, P.; Jones, S.D.; Sitch, S.; Tans, P.; Arneth, A.; et al. Global Carbon Budget 2014. Earth Syst. Sci. Data 2015, 7, 47–85. [Google Scholar] [CrossRef]
- Vikas, M.; Dwarakish, G.S. Coastal Pollution: A Review. Aquat. Procedia 2015, 4, 381–388. [Google Scholar] [CrossRef]
- Estes, M.; Anderson, C.; Appeltans, W.; Bax, N.; Bednaršek, N.; Canonico, G.; Djavidnia, S.; Escobar, E.; Fietzek, P.; Gregoire, M.; et al. Enhanced monitoring of life in the sea is a critical component of conservation management and sustainable economic growth. Mar. Policy 2021, 132, 104699. [Google Scholar] [CrossRef]
- Lodge, M. The International Seabed Authority and Deep Seabed Mining, United Nations. Available online: https://www.un.org/en/chronicle/article/international-seabed-authority-and-deep-seabed-mining#:~:text=Terrestrial%20mineral%20deposits%20are%20coming%20under%20increasing%20pressure,Easily%20mined%2C%20high-grade%20ore%20deposits%20are%20quickly%20declining (accessed on 17 August 2023).
- Butchart, S.; Scharlemann, J.; Evans, M.; Quader, S.; Aricò, S.; Arinaitwe, J.; Balman, M.; Bennun, L.; Bertzky, B.; Besançon, C.; et al. Protecting Important Sites for Biodiversity Contributes to Meeting Global Conservation Targets. PLoS ONE 2012, 7, e32529. [Google Scholar] [CrossRef]
- Lester, S.; Halpern, B. Biological responses in marine no-take reserves versus partially protected areas. Mar. Ecol. Prog. Ser. 2008, 367, 49–56. [Google Scholar] [CrossRef]
- CBD 15/4. Kunming-Montreal Global Biodiversity Framework. Available online: https://www.cbd.int/doc/decisions/cop-15/cop-15-dec-04-en.pdf (accessed on 12 March 2023).
- CBD. Decision Adopted by the Conference of the Parties to the Convention on Biological Diversity at Its Tenth Meeting, October 2010. Available online: https://www.cbd.int/doc/decisions/cop-10/cop-10-dec-02-en.pdf (accessed on 17 August 2023).
- Protected Planet. Protected Planet Report 2018. Available online: https://livereport.protectedplanet.net/ (accessed on 12 March 2023).
- Protected Planet. Marine Protected Areas. Available online: https://www.protectedplanet.net/en/thematic-areas/marine-protected-areas (accessed on 12 March 2023).
- Protected Planet. Discover the World’s Protected and Conserved Areas. Available online: https://www.protectedplanet.net/en (accessed on 10 March 2024).
- Dinerstein, E.; Vynne, C.; Sala, E.; Joshi, A.R.; Fernando, S.; Lovejoy, T.E.; Mayorga, J.; Olson, D.; Asner, G.P.; Baillie, J.E.M.; et al. A Global Deal For Nature: Guiding principles, milestones, and targets. Sci. Adv. 2019, 5, eaaw2869. [Google Scholar] [CrossRef] [PubMed]
- Un Environment (Ed.) Global Environment Outlook–GEO-6: Healthy Planet, Healthy People, 1st ed.; Cambridge University Press: Cambridge, UK, 2019; ISBN 978-1-108-62714-6. [Google Scholar]
- United Nations. UNCITRAL Expedited Arbitration Rules 2021: UNCITRAL Rules on Transparency in Treaty-Based Investor-State Arbitration; United Nations: New York, NY, USA, 2022; Available online: https://uncitral.un.org/sites/uncitral.un.org/files/media-documents/uncitral/en/rules-on-transparency-e.pdf (accessed on 17 February 2023). [CrossRef]
- United Nations. UN Delegates Reach Historic Agreement on Protecting Marine Biodiversity in International Waters. UN News Global Perspective Human Stories. Available online: https://news.un.org/en/story/2023/03/1134157 (accessed on 15 March 2023).
- Gownaris, N.J.; Santora, C.M.; Davis, J.B.; Pikitch, E.K. Gaps in Protection of Important Ocean Areas: A Spatial Meta-Analysis of Ten Global Mapping Initiatives. Front. Mar. Sci. 2019, 6, 650. [Google Scholar] [CrossRef]
- Johnson, D.; Gunn, V.; Bax, N.; Dunn, D. Special Places in the Ocean: A Decade of Describing Ecologically or Biologically Significant Marine Areas (EBSAs); Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2021; Available online: https://www.cbd.int/marine/ebsa/booklet-ebsa-impact-en.pdf (accessed on 17 August 2023).
- Greenpeace. 30 × 30: A Blueprint for Ocean Protection. Available online: https://www.greenpeace.org/international/publication/21604/30x30-a-blueprint-for-ocean-protection/ (accessed on 12 March 2023).
- Pew. A Path to Creating the First Generation of High Seas Protected Areas Science-Based Method Highlights 10 Sites That Would Help Safeguard Biodiversity beyond National Waters. Available online: https://www.pewtrusts.org/en/research-and-analysis/reports/2020/03/a-path-to-creating-the-first-generation-of-high-seas-protected-areas (accessed on 12 March 2023).
- Werner, S.R.; Spurgeon, J.P.G.; Isaksen, G.H.; Smith, J.P.; Springer, N.K.; Gettleson, D.A.; N’Guessan, L.; Dupont, J.M. Rapid prioritization of marine ecosystem services and ecosystem indicators. Mar. Policy 2014, 50, 178–189. [Google Scholar] [CrossRef]
- Ortiz Cajica, A.K.; Hinojosa-Arango, G.; Garza-Pérez, J.R.; Rioja-Nieto, R. Seascape metrics, spatio-temporal change, and intensity of use for the spatial conservation prioritization of a Caribbean marine protected area. Ocean Coast. Manag. 2020, 194, 105265. [Google Scholar] [CrossRef]
- Winiarski, K.J.; Miller, D.L.; Paton, P.W.C.; McWilliams, S.R. A spatial conservation prioritization approach for protecting marine birds given proposed offshore wind energy development. Biol. Conserv. 2014, 169, 79–88. [Google Scholar] [CrossRef]
- Holness, S.D.; Harris, L.R.; Chalmers, R.; De Vos, D.; Goodall, V.; Truter, H.; Oosthuizen, A.; Bernard, A.T.F.; Cowley, P.D.; Da Silva, C.; et al. Using systematic conservation planning to align priority areas for biodiversity and nature-based activities in marine spatial planning: A real-world application in contested marine space. Biol. Conserv. 2022, 271, 109574. [Google Scholar] [CrossRef]
- Pruckner, S.; Bedford, J.; Murphy, L.; Turner, J.A.; Mills, J. Adapting to heatwave-induced seagrass loss: Prioritizing management areas through environmental sensitivity mapping. Estuar. Coast. Shelf Sci. 2022, 272, 107857. [Google Scholar] [CrossRef] [PubMed]
- Hogg, O.; Huvenne, V.; Griffiths, H.; Linse, K. On the ecological relevance of landscape mapping and its application in the spatial planning of very large marine protected areas. Sci. Total Environ. 2018, 626, 384–398. [Google Scholar] [CrossRef]
- Jenkins, C.N.; Houtan, K.S.V.; Pimm, S.L.; Sexton, J.O. US protected lands mismatch biodiversity priorities. Proc. Natl. Acad. Sci. USA 2015, 112, 5081–5086. [Google Scholar] [CrossRef]
- Davidson, L.N.K.; Dulvy, N.K. Global marine protected areas to prevent extinctions. Nat. Ecol. Evol. 2017, 1, 0040. [Google Scholar] [CrossRef]
- Visalli, M.E.; Best, B.D.; Cabral, R.B.; Cheung, W.W.L.; Clark, N.A.; Garilao, C.; Kaschner, K.; Kesner-Reyes, K.; Lam, V.W.Y.; Maxwell, S.M.; et al. Data-driven approach for highlighting priority areas for protection in marine areas beyond national jurisdiction. Mar. Policy 2020, 122, 103927. [Google Scholar] [CrossRef]
- Belote, R.T.; Barnett, K.; Dietz, M.S.; Burkle, L.; Jenkins, C.N.; Dreiss, L.; Aycrigg, J.L.; Aplet, G.H. Options for prioritizing sites for biodiversity conservation with implications for “30 by 30”. Biol. Conserv. 2021, 264, 109378. [Google Scholar] [CrossRef]
- Lehtomäki, J.; Moilanen, A. Methods and workflow for spatial conservation prioritization using Zonation. Environ. Model. Softw. 2013, 47, 128–137. [Google Scholar] [CrossRef]
- Wallace, B.; DiMatteo, A.; Hurley, B.; Finkbeiner, E.; Bolten, A.B.; Chaloupka, M.Y.; Hutchinson, B.J.; Abreu-Grobois, F.A.; Amorocho, D.; Bjorndal, K.A.; et al. Regional Management Units for Marine Turtles: A Novel Framework for Prioritizing Conservation and Research across Multiple Scales. PLoS ONE 2010, 5, e15465. [Google Scholar] [CrossRef]
- Wagner, D.; Friedlander, A.M.; Pyle, R.L.; Brooks, C.M.; Gjerde, K.M.; Wilhelm, T. ‘Aulani Coral Reefs of the High Seas: Hidden Biodiversity Hotspots in Need of Protection. Front. Mar. Sci. 2020, 7, 567428. [Google Scholar] [CrossRef]
- Lascelles, B.G.; Langham, G.M.; Ronconi, R.A.; Reid, J.B. From hotspots to site protection: Identifying Marine Protected Areas for seabirds around the globe. Biol. Conserv. 2012, 156, 5–14. [Google Scholar] [CrossRef]
- Walsh, J.; Connors, K.; Hertz, E.; Kehoe, L.; Martin, T.; Connors, B.; Bradford, M.; Freshwater, C.; Frid, A.; Halverson, J.; et al. Prioritizing conservation actions for Pacific salmon in Canada. J. Appl. Ecol. 2020, 57, 1688–1699. [Google Scholar] [CrossRef]
- Williams, R.; Grand, J.; Hooker, S.; Buckland, S.; Reeves, R.R.; Rojas-Bracho, L.; Sandilands, D.; Kaschner, K. Prioritizing global marine mammal habitats using density maps in place of range maps. Ecography 2014, 37, 212–220. [Google Scholar] [CrossRef]
- Tardin, R.; Maciel, I.; Espécie, M.; Melo-Santos, G.; Simão, S.; Alves, M. Modelling habitat use by the Guiana dolphin, Sotalia guianensis, in south-eastern Brazil: Effects of environmental and anthropogenic variables, and the adequacy of current management measures. Aquat. Conserv Mar. Freshw. Ecosyst. 2020, 30, 775–786. [Google Scholar] [CrossRef]
- Villa, F.; Tunesi, L.; Agardy, T. Zoning Marine Protected Areas through Spatial Multiple-Criteria Analysis: The Case of the Asinara Island National Marine Reserve of Italy. Conserv. Biol. 2002, 16, 515–526. [Google Scholar] [CrossRef]
- Dunn, D.C.; Van Dover, C.L.; Etter, R.J.; Smith, C.R.; Levin, L.A.; Morato, T.; Colaço, A.; Dale, A.C.; Gebruk, A.V.; Gjerde, K.M.; et al. A strategy for the conservation of biodiversity on mid-ocean ridges from deep-sea mining. Sci. Adv. 2018, 4, eaar4313. [Google Scholar] [CrossRef] [PubMed]
- Yamakita, T.; Yamamoto, H.; Nakaoka, M.; Yamano, H.; Fujikura, K.; Hidaka, K.; Hirota, Y.; Ichikawa, T.; Kakehi, S.; Kameda, T.; et al. Identification of important marine areas around the Japanese Archipelago: Establishment of a protocol for evaluating a broad area using ecologically and biologically significant areas selection criteria. Mar. Policy 2015, 51, 136–147. [Google Scholar] [CrossRef]
- Klein, C.J.; Brown, C.J.; Halpern, B.S.; Segan, D.B.; McGowan, J.; Beger, M.; Watson, J.E.M. Shortfalls in the global protected area network at representing marine biodiversity. Sci. Rep. 2015, 5, 17539. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Van Houtan, K. Global and regional priorities for marine biodiversity protection. Biol. Conserv. 2016, 204, 333–339. [Google Scholar] [CrossRef]
- Fan, H.; Huang, M.; Chen, Y.; Zhou, W.; Hu, Y.; Wei, F. Conservation priorities for global marine biodiversity across multiple dimensions. Natl. Sci. Rev. 2023, 10, nwac241. [Google Scholar] [CrossRef] [PubMed]
- Dodge, R.; Birkeland, C.; Hatziolos, M.; Kleypas, J.; Palumbi, S.; Hoegh-Guldberg, O.; Van Woesik, R.; Ogden, J.; Aronson, R.; Causey, B.; et al. A Call to Action for Coral Reefs. Science 2008, 322, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Hong, H.S.; Zhang, L.P.; Chen, W.Q. Emergy Value of Mangrove Ecosystem Services in China. Resour. Sci. 2007, 29, 147–154. [Google Scholar]
- Moilanen, A.; Franco, A.; Early, R.; Fox, R.; Wintle, B.; Thomas, C. Prioritizing multiple-use landscapes for conservation: Methods for large multi-species planning problems. Proc. R. Soc. B 2005, 272, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Takao, K.; Uehara, T.; Mineo, K.; Obata, N.; Nakagami, K.; Yoshioka, T.; Sakurai, R.; Hidaka, T.; Seino, S. Chapter 5-Environmental Economics, Culture, and Negotiation in the Coastal Sea. In Integrated Coastal Management in the Japanese Satoumi-Restoring Estuaries and Bays; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–193. [Google Scholar]
- Job, S.; Sekadende, B.; Yona, G.; George, R.; Lugendo, B.R.; Kimirei, I.A. Effect of seagrass cover loss on seawater carbonate chemistry: Implications for the potential of seagrass meadows to mitigate ocean acidification. Reg. Stud. Mar. Sci. 2023, 60, 102816. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Costa, M.D.P.; Atwood, T.B.; Friess, D.A.; Kelleway, J.J.; Kennedy, H.; Lovelock, C.E.; Serrano, O.; Duarte, C.M. Blue carbon as a natural climate solution. Nat. Rev. Earth Environ. 2021, 2, 826–839. [Google Scholar] [CrossRef]
- Garmendia, J.M.; Rodríguez, J.G.; Borja, Á.; Pouso, S.; Del Campo, A.; Galparsoro, I.; Fernandes-Salvador, J.A. Restoring seagrass meadows in Basque estuaries: Nature-based solution for successful management. Nat.-Based Solut. 2023, 4, 100084. [Google Scholar] [CrossRef]
- Joseph, A. Chapter 6-Seafloor Hot Chimneys and Cold Seeps: Mysterious Life around Them. In Investigating Seafloors and Oceans-from Mud Volcanoes to Giant Squid; Elsevier: Amsterdam, The Netherlands, 2017; pp. 307–375. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780128093573000060 (accessed on 12 December 2023).
- Rogers, A.D. The Biology of Seamounts. Adv. Mar. Biol. 1994, 30, 305–350. [Google Scholar] [CrossRef]
- Stone, K.; Fenner, D.; LeBlanc, D.; Vaisey, B.; Purcell, I.; Eliason, B. Chapter 30-Tonga. In World Seas: An Environmental Evaluation (Second Edition)-Volume II: The Indian Ocean to the Pacific; Academic Press: Cambridge, MA, USA, 2019; pp. 661–678. [Google Scholar]
- Gage, J.; Tyler, P. Deep-Sea Biology: A Natural History of Organisms at the Deep-Sea Floor; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Clark, M.R.; Bennun, L.; Rowden, A.; Guinotte, J.; Smith, C. A global seamount classification to aid the scientific design of marine protected area networks. Ocean Coast. Manag. 2011, 54, 19–36. [Google Scholar] [CrossRef]
- CBD. Global Open Oceans and Deep Seabed (Goods)-Biogeographic Classification, April 2010. Available online: https://www.cbd.int/doc/meetings/sbstta/sbstta-14/information/sbstta-14-inf-10-en.pdf (accessed on 17 March 2023).
- Charles, A.; Westlund, L.; Bartley, D.; Fletcher, W.; Garcia, S.; Govan, H.; Sanders, J. Fishing livelihoods as key to marine protected areas: Insights from the World Parks Congress: Fishing Livelihoods and Marine Protected Areas. Aquat. Conserv Mar. Freshw. Ecosyst. 2016, 26, 165–184. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Siddique, M.R.H.; Hossain, M.; Rashid, A.Z.M.M. The dilemma of prioritizing conservation over livelihoods: Assessing the impact of fishing restriction to the fishermen of the Sundarbans. Trees For. People 2023, 11, 100366. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2. Available online: https://www.iucnredlist.org (accessed on 12 March 2023).
- Birdlife International. Data Zone. Available online: http://datazone.birdlife.org/site/ibasindanger (accessed on 12 January 2023).
- Donald, P.; Fishpool, L.; Ajagbe, A.; Bennun, L.; Bunting, G.; Burfield, I.; Butchart, S.; Capellan, S.; Crosby, M.; Dias, M.; et al. Important Bird and Biodiversity Areas (IBAs): The development and characteristics of a global inventory of key sites for biodiversity. Bird. Conserv. Int. 2019, 29, 177–198. [Google Scholar] [CrossRef]
- Sutton, T.; Clark, M.; Dunn, D.; Halpin, P.; Rogers, A.D.; Guinotte, J.; Bograd, S.; Angel, M.; Perez, J.; Wishner, K.; et al. A global biogeographic classification of the mesopelagic zone. Deep Sea Res. Part I 2017, 126, 85–102. [Google Scholar] [CrossRef]
- Mikkonen, N.; Moilanen, A. Identification of top priority areas and management landscapes from a national Natura 2000 network. Environ. Sci. Policy 2013, 27, 11–20. [Google Scholar] [CrossRef]
- Leathwick, J.; Moilanen, A.; Francis, M.; Elith, J.; Taylor, P.; Julian, K.; Hastie, T.; Duffy, C. Novel methods for the design and evaluation of marine protected areas in offshore waters: Designing offshore MPAs. Conserv. Lett. 2008, 1, 91–102. [Google Scholar] [CrossRef]
- Virtanen, E.; Viitasalo, M.; Lappalainen, J.; Moilanen, A. Evaluation, Gap Analysis, and Potential Expansion of the Finnish Marine Protected Area Network. Front. Mar. Sci. 2018, 5, 402. [Google Scholar] [CrossRef]
- Dunn, D.C.; Ardron, J.; Bax, N.; Bernal, P.; Cleary, J.; Cresswell, I.; Donnelly, B.; Dunstan, P.; Gjerde, K.; Johnson, D.; et al. The Convention on Biological Diversity’s Ecologically or Biologically Significant Areas: Origins, Development, and Current Status. Mar. Policy 2014, 49, 137–145. [Google Scholar] [CrossRef]
- CBD. Report of the Southern Indian Ocean Regional Workshop to Facilitate the Description of Ecologically or Biologically Significant Marine Areas. Mauritius, June 2013. Available online: https://www.cbd.int/doc/meetings/mar/ebsa-sio-01/official/ebsa-sio-01-04-en.pdf (accessed on 17 March 2023).
- Nur, N.; Jahncke, J.; Herzog, M.P.; Howar, J.; Hyrenbach, K.D.; Zamon, J.E.; Ainley, D.G.; Wiens, J.A.; Morgan, K.; Ballance, L.T.; et al. Where the wild things are: Predicting hotspots of seabird aggregations in the California Current System. Ecol. Appl. 2011, 21, 2241–2257. [Google Scholar] [CrossRef]
- González-Solís, J.; Croxall, J.P.; Afanasyev, V. Offshore spatial segregation in giant petrelsMacronectes spp.: Differences between species, sexes and seasons. Aquat. Conserv. Mar. Freshw. Ecosyst. 2007, 17, S22–S36. [Google Scholar] [CrossRef]
- Johnson, D.E.; Barrio Froján, C.; Turner, P.J.; Weaver, P.; Gunn, V.; Dunn, D.C.; Halpin, P.; Bax, N.J.; Dunstan, P.K. Reviewing the EBSA process: Improving on success. Mar. Policy 2018, 88, 75–85. [Google Scholar] [CrossRef]
- Katikiro, R.E.; Kweka, O.L.; Minja, R.; Namkesa, F.; Ponte, S. Stakeholder engagement and conservation outcomes in marine protected areas: Lessons from the Mnazi Bay-Ruvuma Estuary Marine Park (MBREMP) in Tanzania. Ocean Coast. Manag. 2021, 202, 105502. [Google Scholar] [CrossRef]
- Agardy, T.; Di Sciara, G.N.; Christie, P. Mind the gap: Addressing the shortcomings of marine protected areas through large scale marine spatial planning. Mar. Policy 2011, 35, 226–232. [Google Scholar] [CrossRef]
- Horta, E.; Costa, B.; Claudet, J.; Franco, G.; Erzini, K.; Caro, A.; Gonçalves, E.J. A regulation-based classification system for Marine Protected Areas (MPAs). Mar. Policy 2016, 72, 192–198. [Google Scholar] [CrossRef]
- Rife, A.N.; Erisman, B.; Sanchez, A.; Aburto-Oropeza, O. When good intentions are not enough … Insights on networks of “paper park” marine protected areas: Concerns regarding marine “paper parks”. Conserv. Lett. 2013, 6, 200–212. [Google Scholar] [CrossRef]
- Failler, P.; Touron-Gardic, G.; Drakeford, B.; Sadio, O.; Traoré, M.-S. Perception of threats and related management measures: The case of 32 marine protected areas in West Africa. Mar. Policy 2020, 117, 103936. [Google Scholar] [CrossRef]
- Day, J.; Dudley, N.; Hockings, M.; Holmes, G.; Laffoley, D.; Stolton, S.; Wells, S. Guidelines for Applying the IUCN Protected Area Management Categories to Marine Protected Areas; IUCN: Gland, Switzerland, 2012; Available online: https://portals.iucn.org/library/node/10201 (accessed on 15 February 2024).
- Vimal, R.; Navarro, L.M.; Jones, Y.; Wolf, F.; Le Moguédec, G.; Réjou-Méchain, M. The global distribution of protected areas management strategies and their complementarity for biodiversity conservation. Biol. Conserv. 2021, 256, 109014. [Google Scholar] [CrossRef]
- Marsac, F.; Galletti, F.; Ternon, J.-F.; Romanov, E.V.; Demarcq, H.; Corbari, L.; Bouchet, P.; Roest, W.R.; Jorry, S.J.; Olu, K.; et al. Seamounts, plateaus and governance issues in the southwestern Indian Ocean, with emphasis on fisheries management and marine conservation, using the Walters Shoal as a case study for implementing a protection framework. Deep Sea Res. Part II 2020, 176, 104715. [Google Scholar] [CrossRef]
- Heerah, K.; Dias, M.P.; Delord, K.; Oppel, S.; Barbraud, C.; Weimerskirch, H.; Bost, C.A. Important areas and conservation sites for a community of globally threatened marine predators of the Southern Indian Ocean. Biol. Conserv. 2019, 234, 192–201. [Google Scholar] [CrossRef]
- Clark, M.R.; Rowden, A.A.; Schlacher, T.; Williams, A.; Consalvey, M.; Stocks, K.I.; Rogers, A.D.; O’Hara, T.D.; White, M.; Shank, T.M.; et al. The Ecology of Seamounts: Structure, Function, and Human Impacts. Annu. Rev. Mar. Sci. 2010, 2, 253–278. [Google Scholar] [CrossRef]
- FAO. International Guidelines for the Management of Deep-Sea Fisheries in the High Seas; FAO: Roma, Italy, 2009. [Google Scholar]
- Brown, R.M.; Jordan, W.C. Characterization of polymorphic microsatellite loci from Round Island Petrels (Pterodroma arminjoniana) and their utility in other seabird species. J. Ornithol. 2009, 150, 925–929. [Google Scholar] [CrossRef]
- CBD. Report of the North-West Indian Ocean and Adjacent Gulf Areas Regional Workshop to Facilitate the Description of Ecologically or Biologically Significant Marine Areas. 2016. Available online: https://www.cbd.int/doc/meetings/sbstta/sbstta-20/information/sbstta-20-inf-23-en.pdf (accessed on 17 March 2023).
- Jones, P.J.S.; De Santo, E.M. Viewpoint–Is the race for remote, very large marine protected areas (VLMPAs) taking us down the wrong track? Mar. Policy 2016, 73, 231–234. [Google Scholar] [CrossRef]
- International Cable Protection Committee. International Cable Protection Committee Welcomes New Marine Biodiversity Treaty, Calls on All Parties to Promote Regulatory Certainty and Network Resilience. Available online: https://www.iscpc.org/news/ (accessed on 17 August 2023).
- Dutka-Gianelli, J.; Crandall, C.; Garlock, T.; Camp, E.; Lorenzen, K. Effects of short educational workshops on stakeholder knowledge and attitudes on coastal fish stocking programmes. Fish. Manag. Ecol. 2019, 26, 306–309. [Google Scholar] [CrossRef]
- Gilman, E.; Dunn, D.; Read, A.; Hyrenbach, K.D.; Warner, R. Designing criteria suites to identify discrete and networked sites of high value across manifestations of biodiversity. Biodivers. Conserv. 2011, 20, 3363–3383. [Google Scholar] [CrossRef][Green Version]
- Bridges, A.E.H.; Barnes, D.K.A.; Bell, J.B.; Ross, R.E.; Voges, L.; Howell, K.L. Filling the data gaps: Transferring models from data-rich to data-poor deep-sea areas to support spatial management. J. Environ. Manag. 2023, 345, 118325. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.L.; Hilário, A.; Allcock, A.L.; Bailey, D.M.; Baker, M.; Clark, M.R.; Colaço, A.; Copley, J.; Cordes, E.E.; Danovaro, R.; et al. A Blueprint for an Inclusive, Global Deep-Sea Ocean Decade Field Program. Front. Mar. Sci. 2020, 7, 584861. [Google Scholar] [CrossRef]
- Stat, M.; Huggett, M.J.; Bernasconi, R.; DiBattista, J.D.; Berry, T.E.; Newman, S.J.; Harvey, E.S.; Bunce, M. Ecosystem biomonitoring with eDNA: Metabarcoding across the tree of life in a tropical marine environment. Sci. Rep. 2017, 7, 12240. [Google Scholar] [CrossRef]
- Deasy, K. What we know about the new High Seas Treaty. NPJ Ocean Sustain. 2023, 2, 7. [Google Scholar] [CrossRef]
- Zhao, C. The Development History and Prospects of Marine Protected Areas in China. Green Technol. 2022, 24, 207–211. [Google Scholar] [CrossRef]
- BirdLife International. Important Bird and Biodiversity Area (IBA) Digital Boundaries: September 2020 Version; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- Bunting, P.; Rosenqvist, A.; Lucas, R.M.; Rebelo, L.M.; Hilarides, L.; Thomas, N.; Hardy, A.; Itoh, T.; Shimada, M.; Finlayson, C.M. The Global Mangrove Watch—A New 2010 Global Baseline of Mangrove Extent. Remote Sens. 2018, 10, 1669. [Google Scholar] [CrossRef]
- EBSAs. Ecologically or Biologically Significant Marine Areas. 2023. Available online: https://www.cbd.int/ebsa/ (accessed on 15 February 2024).
- Flanders Marine Institute. Union of the ESRI Country Shapefile and the Exclusive Economic Zones (version 3). Available online: https://www.marineregions.org/ (accessed on 15 February 2024).
- GBSCO. The GEBCO_2021 Grid. 2023. Available online: https://www.gebco.net/data_and_products/gridded_bathymetry_data/gebco_2021/ (accessed on 15 February 2024).
- German, C.R.; Ramirez-Llodra, E.; Baker, M.C.; Tyler, P.A.; ChEss Scientific Steering Committee. Deep-Water Chemosynthetic Ecosystem Research during the Census of Marine Life Decade and Beyond: A Proposed Deep-Ocean Road Map. PLoS ONE 2011, 6, e23259. [Google Scholar] [CrossRef] [PubMed]
- Jayathilake, D.R.M.; Costello, M.J. A modelled global distribution of the kelp biome. Biol. Conserv. 2020, 252, 108815. [Google Scholar] [CrossRef]
- Mcowen, C.; Weatherdon, L.V.; Bochove, J.; Sullivan, E.; Blyth, S.; Zockler, C.; Stanwell-Smith, D.; Kingston, N.; Martin, C.S.; Spalding, M.; et al. A global map of saltmarshes (v6.1). Biodivers. Data J. 2017, 5, e11764. [Google Scholar] [CrossRef]
- OBIS. OBIS The OBIS Web Portal Search Interface (2015). 2022. Available online: http://iobis.org/mapper/ (accessed on 15 February 2024).
- Sayre, R.; Noble, S.; Hamann, S.; Smith, R.; Wright, D.; Breyer, S.; Butler, K.; Van Graafeiland, K.; Frye, C.; Karagulle, D.; et al. A new 30 meter resolution global shoreline vector and associated global islands database for the development of standardized ecological coastal units. J. Oper. Oceanogr. 2019, 12, S47–S56. [Google Scholar] [CrossRef]
- UNEP-WCMC; IUCN. Protected Planet: The World Database on Protected Areas (WDPA). 2023. Available online: www.protectedplanet.net (accessed on 15 February 2024).
- UNEP-WCMC. Short FT. 2021. Global Distribution of Seagrasses (Version 7.1). Seventh Update to the Data Layer Used in Green and Short; UN Environment World Conservation Monitoring Centre: Cambridge, UK, 2003. [Google Scholar]
- UNEP-WCMC; WorldFish Centre; WRI; TNC. Global Distribution of Warm-Water Coral Reefs, Compiled from Multiple Sources Including the Millennium Coral Reef Mapping Project. Version 4.1. Includes contributions from IMaRS-USF and IRD (2005), IMaRS-USF (2005) and Spalding et al. (2001); UN Environment World Conservation Monitoring Centre: Cambridge, UK, 2021. [Google Scholar]

| Order of Priority Marine Areas for Protection | Percentage of Territorial Sea and Exclusive Economic Zone | Percentage of High Seas and International Seabed Areas |
|---|---|---|
| Top 5% | 82.42% | 17.58% |
| From 5% to 10% | 71.27% | 28.73% |
| From 10% to 15% | 53.16% | 46.85% |
| From 15% to 20% | 15.09% | 84.91% |
| From 20% to 25% | 0.63% | 98.47% |
| From 25% to 30% | 0.16% | 99.84% |
| Order of Priority Marine Areas for Protection | Proportion of Existing Marine Protected Areas | Proportion of Ecologically or Biologically Significant Marine Areas |
|---|---|---|
| Top 5% | 5.26% | 6.59% |
| From 5% to 10% | 7.16% | 7.12% |
| From 10% to 15% | 7.29% | 7.27% |
| From 15% to 20% | 5.28% | 2.29% |
| From 20% to 25% | 1.75% | 10.54% |
| From 25% to 30% | 1.22% | 8.31% |
| Sum | 27.96% | 42.11% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Ge, Y.; Zheng, M. Reflections on How to Reach the “30 by 30” Target: Identification of and Suggestions on Global Priority Marine Areas for Protection. Water 2024, 16, 2293. https://doi.org/10.3390/w16162293
Zhao C, Ge Y, Zheng M. Reflections on How to Reach the “30 by 30” Target: Identification of and Suggestions on Global Priority Marine Areas for Protection. Water. 2024; 16(16):2293. https://doi.org/10.3390/w16162293
Chicago/Turabian StyleZhao, Chang, Yuejing Ge, and Miaozhuang Zheng. 2024. "Reflections on How to Reach the “30 by 30” Target: Identification of and Suggestions on Global Priority Marine Areas for Protection" Water 16, no. 16: 2293. https://doi.org/10.3390/w16162293
APA StyleZhao, C., Ge, Y., & Zheng, M. (2024). Reflections on How to Reach the “30 by 30” Target: Identification of and Suggestions on Global Priority Marine Areas for Protection. Water, 16(16), 2293. https://doi.org/10.3390/w16162293





