Efficient Phosphate Adsorption from Groundwater by Mn-FeOOHs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Synthesis of Mn-FeOOH
2.3. Characterization
2.4. Batch Experiments
3. Results
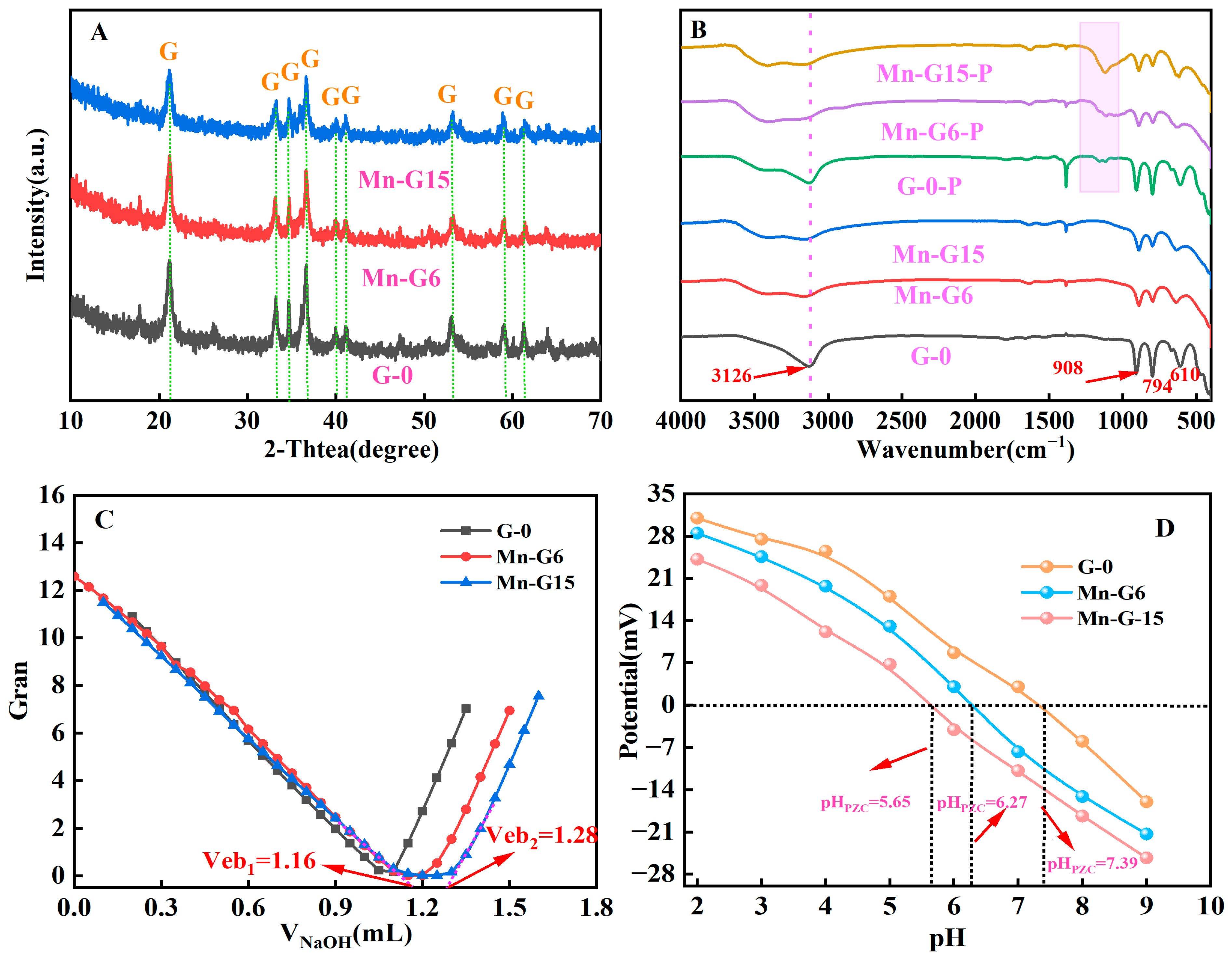
3.1. Characterization
3.2. Adsorption Kinetics
3.3. Effect of pH and Ionic Strength
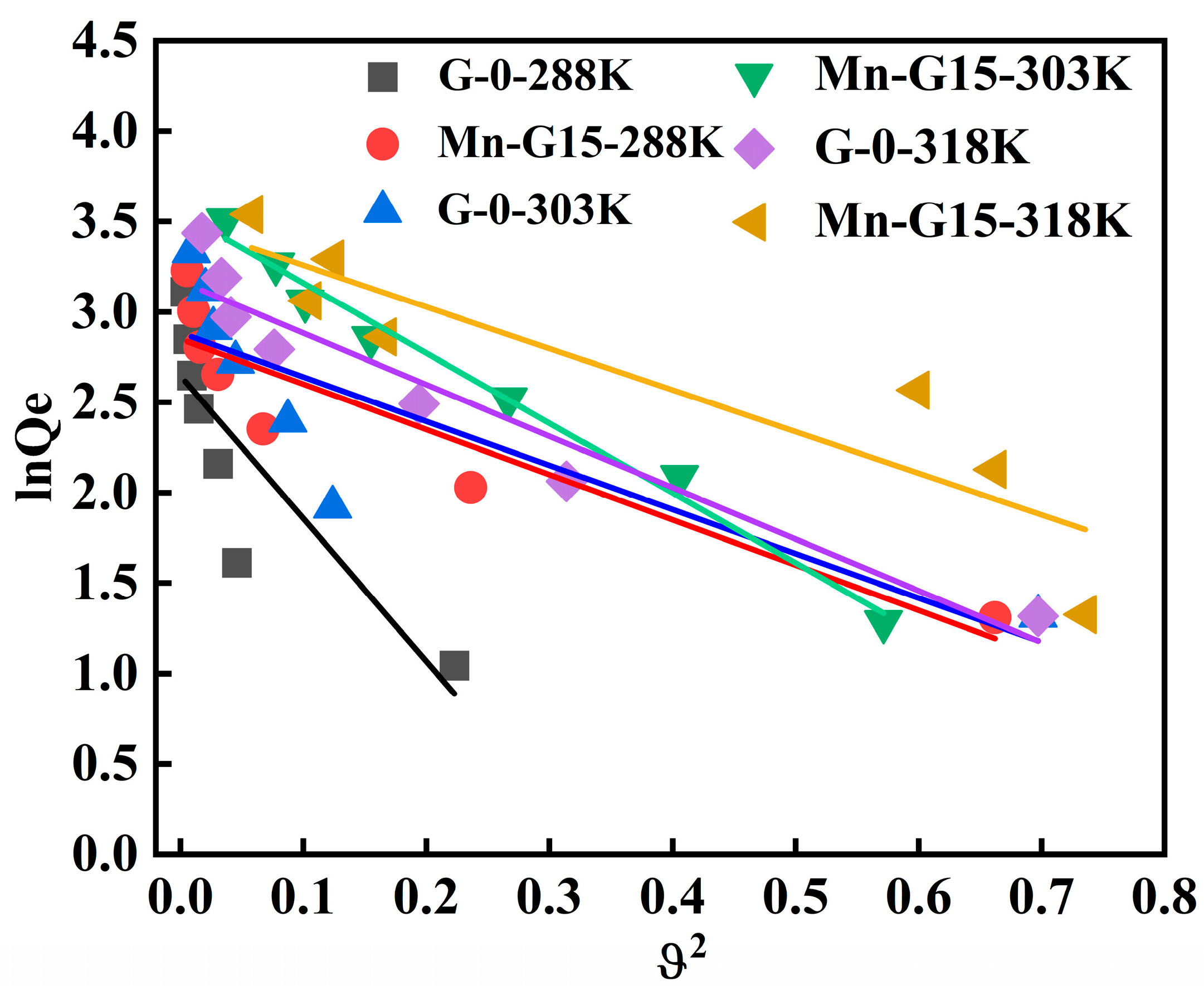
3.4. Adsorption Isotherms
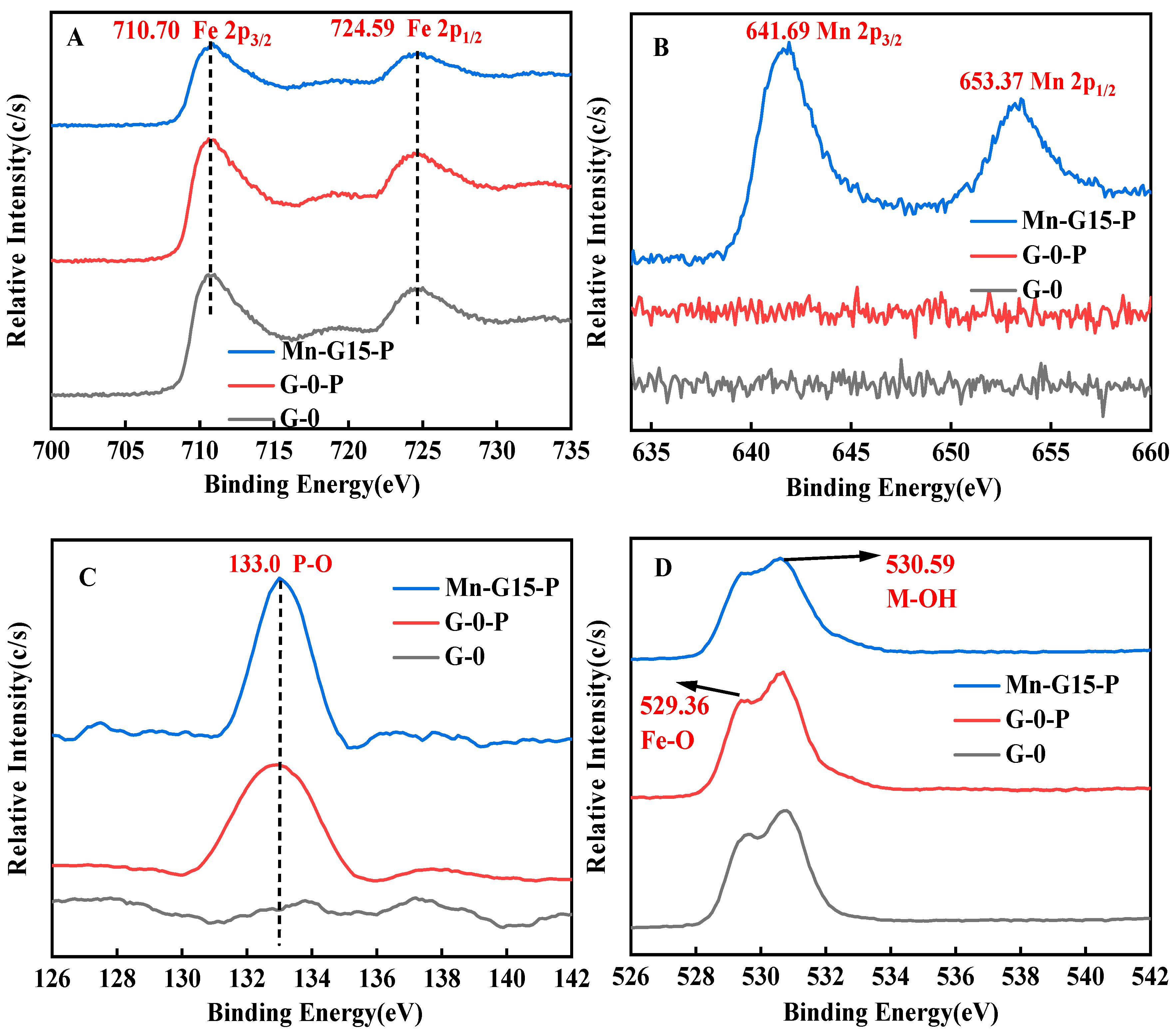
3.5. XPS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cui, Q.; Xu, J.; Wang, W.; Tan, L.; Cui, Y.; Wang, T. Phosphorus recovery by core-shell gamma-Al2O3/Fe3O4 biochar composite from aqueous phosphate solutions. Sci. Total Environ. 2020, 729, 138892. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, K.; Yang, J.; Yu, Z.; Yu, W.; Xu, Q. Phosphorus recovery from the liquid phase of anaerobic digestate using biochar derived from iron-rich sludge: A potential phosphorus fertilizer. Water Res. 2020, 174, 115629. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, P.; Liu, Z.; Zhang, J.; Huang, X. Simultaneous recovery of phosphorus with nickel purification in nickel-plating wastewater via Fe/C activated H2O2 oxidation. Chem. Eng. J. 2020, 381, 122702. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Shen, N.; Yan, W.; Wang, J.; Wang, G. Phosphorus release and recovery from Fe-enhanced primary sedimentation sludge via alkaline fermentation. Bioresour. Technol. 2019, 278, 266–271. [Google Scholar] [CrossRef]
- Jack, J.; Huggins, T.; Huang, Y.; Fang, Y.; Ren, Z. Production of magnetic biochar from waste-derived fungal biomass for phosphorus removal and recovery. J. Clean. Prod. 2019, 224, 100–106. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Liu, H.; Zou, X.; Zhu, L.; Ma, L. Spectroscopic and surface complexation modeling of phosphate enrichment on porous iron-carbon composites. Water Air Soil Pollut. 2023, 234, 392. [Google Scholar] [CrossRef]
- Zhu, L.; Tong, L.; Zhao, N.; Li, J.; Lv, Y. Coupling interaction between porous biochar and nano zero valent iron/nano α-hydroxyl iron oxide improves the remediation efficiency of cadmium in aqueous solution. Chemosphere 2019, 219, 493–503. [Google Scholar] [CrossRef]
- Shuai, W.; Gu, C.; Fang, G.; Zhou, D.; Gao, J. Effects of iron (hydr)oxides on the degradation of diethyl phthalate ester in heterogeneous (photo)-Fenton reactions. J. Environ. Sci. 2019, 80, 5–13. [Google Scholar] [CrossRef]
- Hong, H.; Yang, J.; Yang, J.; Jeong, H. Highly enhanced heavy metal adsorption performance of iron oxide (Fe-Oxide). Mater. Transact. 2017, 58, 71–75. [Google Scholar] [CrossRef]
- Jin, J.; Liang, Y.; Wang, M.; Fang, L.; Xiong, J.; Hou, J. Generic CD-MUSIC-eSGC model parameters to predict the surface reactivity of iron (hydr)oxides. Water Res. 2023, 230, 119534. [Google Scholar] [CrossRef]
- Alvarez-Cruz, J.; Garrido-Hoyos, S. Effect of the mole ratio of Mn/Fe composites on arsenic (V) adsorption. Sci. Total Environ. 2019, 668, 47–55. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F. Kinetic study on removal of copper(II) using goethite and hematite nano-photocatalysts. J. Colloid. Interf. Sci. 2010, 347, 277–281. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Li, P.; Qin, H.; Fan, Q. The adsorption of U(VI) on magnetite, ferrihydrite and goethite. Environ. Technol. Innov. 2021, 23, 101615. [Google Scholar] [CrossRef]
- Wang, C.; Han, Z.; Zou, X.; Liu, H.; Wang, H.; Shu, D. Ultrathin MnO2-coated FeOOH catalyst for indoor formaldehyde oxidation at ambient temperature: New insight into surface reactive oxygen species and in-field testing in an air cleaner. Environ. Sci. Technol. 2022, 56, 10963–10976. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Chen, T.; Chen, D.; Wang, C.; Wei, L. Efficient U(VI) adsorption on iron/carbon composites derived from the coupling of cellulose with iron oxides: Performance and mechanism. Sci. Total Environ. 2020, 703, 135604. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Zou, X.; Xie, Q.; Qing, C.; Chen, D. Removal of phosphorus using NZVI derived from reducing natural goethite. Chem. Eng. J. 2013, 234, 80–87. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, J.; Zhao, N.; Yang, X.; Chen, C.; Shang, J. Goethite modified biochar as a multifunctional amendment for cationic Cd(II), anionic As(III), roxarsone, and phosphorus in soil and water. J. Clean. Prod. 2020, 247, 119579. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Chen, T.; Hayat, T.; Alharbi, N.S.; Chen, C. Adsorption of Europium on Al-substituted goethite. J. Mol. Liq. 2017, 236, 445–451. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Zou, X.; Qing, C.; Frost, R.L. Effect of Al content on the structure of Al-substituted goethite: A micro-Raman spectroscopic study. J. Raman Spectrosc. 2013, 44, 1609–1614. [Google Scholar] [CrossRef]
- Ma, M.; Gao, H.; Sun, Y.; Huang, M. The adsorption and desorption of Ni(II) on Al substituted goethite. J. Mol. Liq. 2015, 201, 30–35. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Liu, L.; Song, W.; Sun, Y. Effect of Staphylococcus epidermidis on U(VI) sequestration by Al-goethite. J. Hazard. Mater. 2019, 368, 52–62. [Google Scholar] [CrossRef]
- Campo, B.; Rosseler, O.; Alvarez, M.; Rueda, E.; Volpe, M. On the nature of goethite, Mn-goethite and Co-goethite as supports for gold nanoparticles. Mater. Chem. Physics. 2008, 109, 448–454. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Chen, T.; Wei, L.; Wang, C.; Hu, W. The transformation of α-(Al, Fe)OOH in natural fire: Effect of Al substitution amount on fixation of phosphate. Chem. Geol. 2019, 524, 368–382. [Google Scholar] [CrossRef]
- Alvarez, M.; Tufo, A.E.; Zenobi, C.; Ramos, C.P.; Sileo, E.E. Chemical, structural and hyperfine characterization of goethites with simultaneous incorporation of manganese, cobalt and aluminum ions. Chem. Geol. 2015, 414, 16–27. [Google Scholar] [CrossRef]
- Liu, H.; Shu, D.; Sun, F.; Li, Q.; Chen, T.; Xing, B. Effect of manganese substitution on the crystal structure and decomposition kinetics of siderite. J. Therm. Anal. Calorim. 2018, 136, 1315–1322. [Google Scholar] [CrossRef]
- Wang, C.; Zou, X.; Liu, H.; Chen, T.; Suib, S.; Chen, D. A highly efficient catalyst of palygorskite-supported manganese oxide for formaldehyde oxidation at ambient and low temperature: Performance, mechanism and reaction kinetics. Appl. Surf. Sci. 2019, 486, 420–430. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, X.; Chen, B. Magnetic biochar supported alpha-MnO2 nanorod for adsorption enhanced degradation of 4-chlorophenol via activation of peroxydisulfate. Sci. Total Environ. 2020, 724, 138278. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y.; Liu, W. Effects of incorporating Mn into goethite on adsorption of dissolved organic matter and potentially toxic elements in soil: Isotherms, kinetics, and mechanisms. Environ. Res. 2023, 231, 116260. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Wang, J.; Ma, L.; Pan, Z.; Ji, W. Structural evolution of Mn-substituted FeOOH and its adsorption mechanism for U(VI): Effect of the mole ratio of Mn/(Fe + Mn). Water 2024, 16, 1795. [Google Scholar] [CrossRef]
- Li, M.; Kang, Y.; Ma, H.; Dong, J.; Wang, Y.; Kuang, S. Efficient removal of heavy metals from aqueous solutions using Mn-doped FeOOH: Performance and mechanisms. Environ. Res. 2023, 231, 116161. [Google Scholar] [CrossRef]
- Liu, H.; Lu, X.; Flynn, E.; Catalano, J. Fate of arsenic during the interactions between Mn-substituted goethite and dissolved Fe(II). Geochim. Cosmochim. Acta 2023, 356, 1–13. [Google Scholar] [CrossRef]
- Li, L.; Stanforth, R. Distinguishing adsorption and surface precipitation of phosphate on Ggoethite (alpha-FeOOH). J. Colloid. Interf. Sci. 2000, 230, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Schwertmann, U.; Cambier, P.; Murad, E. Properties of goethites of varying crystallinity. Clays Clay Miner. 1985, 33, 369. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, S.; Wang, Q.; Alsaedi, A.; Wang, X. Sequestration of uranium on fabricated aluminum co-precipitated with goethite (Al-FeOOH). Radiochim. Acta 2014, 102, 797–804. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Zhu, H.; Gao, H.; Zhang, S.; Chen, T. Kinetics and mechanism of Sr(II) adsorption by Al-Fe2O3: Evidence from XPS analysis. J. Mol. Liq. 2017, 233, 364–369. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Luo, W.; Sun, J.; Xu, Q.; Chen, F. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge. Bioresour. Technol. 2018, 247, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, S.; Tzou, Y.; Chen, J.; Wang, M. The adsorption and catalytic transformations of chromium on Mn substituted goethite. Appl. Catal. B Environ. 2007, 75, 272–280. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Xiang, Z.; Yang, J.; Yin, J.; Guo, X. Mn doping accelerates regeneration of Fe2+ in FeOOH and promotes efficient H2O2 activation for degradation of As(III). Colloids Surf. A 2022, 655, 130166. [Google Scholar] [CrossRef]
- Ren, X.; Li, J.; Tan, X.; Wang, X. Comparative study of graphene oxide, activated carbon and carbon nanotubes as adsorbents for copper decontamination. Dalton Trans. 2013, 42, 5266–5274. [Google Scholar] [CrossRef]
- Chen, C.; Hu, J.; Xu, D.; Tan, X.; Meng, Y.; Wang, X. Surface complexation modeling of Sr(II) and Eu(III) adsorption onto oxidized multiwall carbon nanotubes. J. Colloid Interf. Sci. 2008, 323, 33–41. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, D.; Liu, H.; Zou, X.; Wu, Z.; Xie, J.; Qing, C.; Kong, D.; Chen, T. Synergetic effects of anhydrite and brucite-periclase materials on phosphate removal from aqueous solution. J. Mol. Liq. 2018, 254, 145–153. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Zhou, B.; Awasthi, M.; Ali, A.; Zhang, Z. Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate-based fertilizer substitute. Bioresour. Technol. 2016, 215, 209–214. [Google Scholar] [CrossRef]
- Jung, K.; Ahn, K. Fabrication of porosity-enhanced MgO/biochar for removal of phosphate from aqueous solution: Application of a novel combined electrochemical modification method. Bioresour. Technol. 2016, 200, 1029–1032. [Google Scholar] [CrossRef]
- Goscianska, J.; Ptaszkowska-Koniarz, M.; Frankowski, M.; Franus, M.; Panek, R.; Franus, W. Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash. J. Colloid Interf. Sci. 2018, 513, 72–81. [Google Scholar] [CrossRef]
- Hu, W.; Li, M.; Chen, T.; Zhang, Z.; Chen, D.; Liu, H. Enrichment of U(VI) on Bacillus subtilis/Fe3O4 nanocomposite. J. Mol. Liq. 2018, 258, 244–252. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Z.; Li, M.; Liu, H.; Zhang, C.; Chen, T. Enhanced uptake capacity for uranium(VI) in aqueous solutions by activated natural siderite: Performance and mechanism. Appl. Geochem. 2019, 100, 96–103. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Duan, S.; Wang, Y.; Liu, X.; Shao, D.; Hayat, T.; Alsaedi, A.; Li, J. Removal of U(VI) from Aqueous Solution by Amino Functionalized Flake Graphite Prepared by Plasma Treatment. ACS Sustainable Chem. Eng. 2017, 5, 4073–4085. [Google Scholar] [CrossRef]
- Hutson, N.; Yang, R. Theoretical basis for the Dubinin-Radushkevitch (D-R) adsorption isotherm equation. Adsorption 1997, 3, 189–195. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, D.; Liu, H.; Zou, X.; Zhang, Y.; Xie, J. Enhanced adsorption capacity for phosphate in wastewater from thermally activated flue gas desulfurization gypsum. J. Chem. Technol. Biotechnol. 2018, 93, 1733–1741. [Google Scholar] [CrossRef]
- Cui, X.; Dai, X.; Khan, K.; Li, T.; Yang, X.; He, Z. Removal of phosphate from aqueous solution using magnesium-alginate/chitosan modified biochar microspheres derived from Thalia dealbata. Bioresour. Technol. 2016, 218, 1123–1132. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Dong, Y.; Wang, L. Preferable adsorption of phosphate using lanthanum-incorporated porous zeolite: Characteristics and mechanism. Appl. Surf. Sci. 2017, 426, 995–1004. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Chen, T.; Lin, W. Nano-hematite prepared by activation of natural siderite and its performance on immobilization of Eu(III). Appl. Geochem. 2017, 84, 154–161. [Google Scholar] [CrossRef]




| Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|
| Qe (mg∙g−1) | K1 (g∙(mg·min)−1) | R2 | Qe (mg∙g−1) | K1 (g∙(mg·min)−1) | R2 | |
| G-0 | 2.8 | 0.27 | 0.9848 | 6.63 | 0.32 | 0.9985 |
| Mn-G6 | 0.77 | 0.18 | 0.9440 | 6.56 | 0.30 | 0.9999 |
| Mn-G15 | 0.34 | 0.20 | 0.6381 | 7.00 | 1.36 | 0.9998 |
| Intraparticle Diffusion Model | ||||
|---|---|---|---|---|
| K1 (g∙(mg·min)−1) | R2 | C | ||
| First stage | 0.57 | 0.9754 | 1.63 | |
| G-0 | Second stage | 0.88 | 0.9722 | 2.76 |
| Third stage | 0.46 | 1.0000 | 3.60 | |
| First stage | 0.28 | 0.8770 | 1.09 | |
| Mn-G6 | Second stage | 5.18 | 0.9111 | 31.30 |
| Third stage | 0.44 | 1.0000 | 3.65 | |
| First stage | 0.42 | 0.9787 | 2.01 | |
| Mn-G15 | Second stage | 5.48 | 0.8262 | 34.53 |
| Third stage | 0.53 | 1.0000 | 3.39 |
| Langmuir | Freundlich | D-R | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T(K) | Sample | Qm (mg·g−1) | KL (L·mg−1) | R2 | KF ((mg·g−1)/(mg·L)−n) | 1/n | R2 | β (mol2·J−2) | Qm (mg·g−1) | R2 |
| 288 | G-0 | 78.86 | 0.09 | 0.9982 | 2.17 | 0.79 | 0.9532 | 7.89 | 14.11 | 0.6768 |
| Mn-G15 | 82.56 | 0.16 | 0.9955 | 4.98 | 0.62 | 0.9846 | 2.50 | 17.30 | 0.8237 | |
| 303 | G-0 | 82.78 | 0.06 | 0.9972 | 5.38 | 0.65 | 0.9325 | 2.44 | 17.90 | 0.6658 |
| Mn-G15 | 85.54 | 0.12 | 0.9965 | 9.31 | 0.60 | 0.7841 | 3.87 | 34.81 | 0.9853 | |
| 318 | G-0 | 83.06 | 0.05 | 0.9722 | 7.46 | 0.60 | 0.8869 | 2.85 | 23.81 | 0.9153 |
| Mn-G15 | 87.18 | 0.02 | 0.9743 | 11.38 | 0.57 | 0.7685 | 2.30 | 32.79 | 0.7932 | |
| Adsorbents | Adsorption Conditions | Qmax (mg/g) | Refs. |
|---|---|---|---|
| flue gas desulfurization gypsum | T = 298 K, pH = 7.0 | 22.54 | [50] |
| MgCl2-alginate-modified biochar | T = 301 K, pH = 3.0 | 46.56 | [51] |
| lanthanum-modified zeolites | T = 298 K, pH = 5.0 | 58.20 | [44] |
| lanthanum-incorporated porous zeolite | T = 313 K, pH = 6.0 | 17.20 | [52] |
| α-(Al, Fe)OOH | T = 298 K, pH = 5.5 | 5.96 | [23] |
| magnesium oxide/biochar | T = 296 K, pH = 4.0 | 121.25 | [42] |
| Mn-G15 | T = 288 K, pH = 5.5 | 82.56 | This work |
| Adsorbents | SBET (m2/g) | Pore Volume (cm3/g) | C(SOH) (mmol/g) | pHPZC |
|---|---|---|---|---|
| G-0 | 7.32 | 0.04 | 0.22 | 7.39 |
| Mn-G6 | 43.86 | 0.34 | 0.41 | 6.27 |
| Mn-G15 | 77.18 | 0.40 | 0.73 | 5.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Sun, G.; Chu, Z.; Wang, J.; Qiu, Y. Efficient Phosphate Adsorption from Groundwater by Mn-FeOOHs. Water 2024, 16, 2294. https://doi.org/10.3390/w16162294
Li M, Sun G, Chu Z, Wang J, Qiu Y. Efficient Phosphate Adsorption from Groundwater by Mn-FeOOHs. Water. 2024; 16(16):2294. https://doi.org/10.3390/w16162294
Chicago/Turabian StyleLi, Mengxue, Guanghui Sun, Ziyang Chu, Jing Wang, and Yu Qiu. 2024. "Efficient Phosphate Adsorption from Groundwater by Mn-FeOOHs" Water 16, no. 16: 2294. https://doi.org/10.3390/w16162294
APA StyleLi, M., Sun, G., Chu, Z., Wang, J., & Qiu, Y. (2024). Efficient Phosphate Adsorption from Groundwater by Mn-FeOOHs. Water, 16(16), 2294. https://doi.org/10.3390/w16162294






