Groundwater Quality Assessment at East El Minia Middle Eocene Carbonate Aquifer: Water Quality Index (WQI) and Health Risk Assessment (HRA)
Abstract
1. Introduction
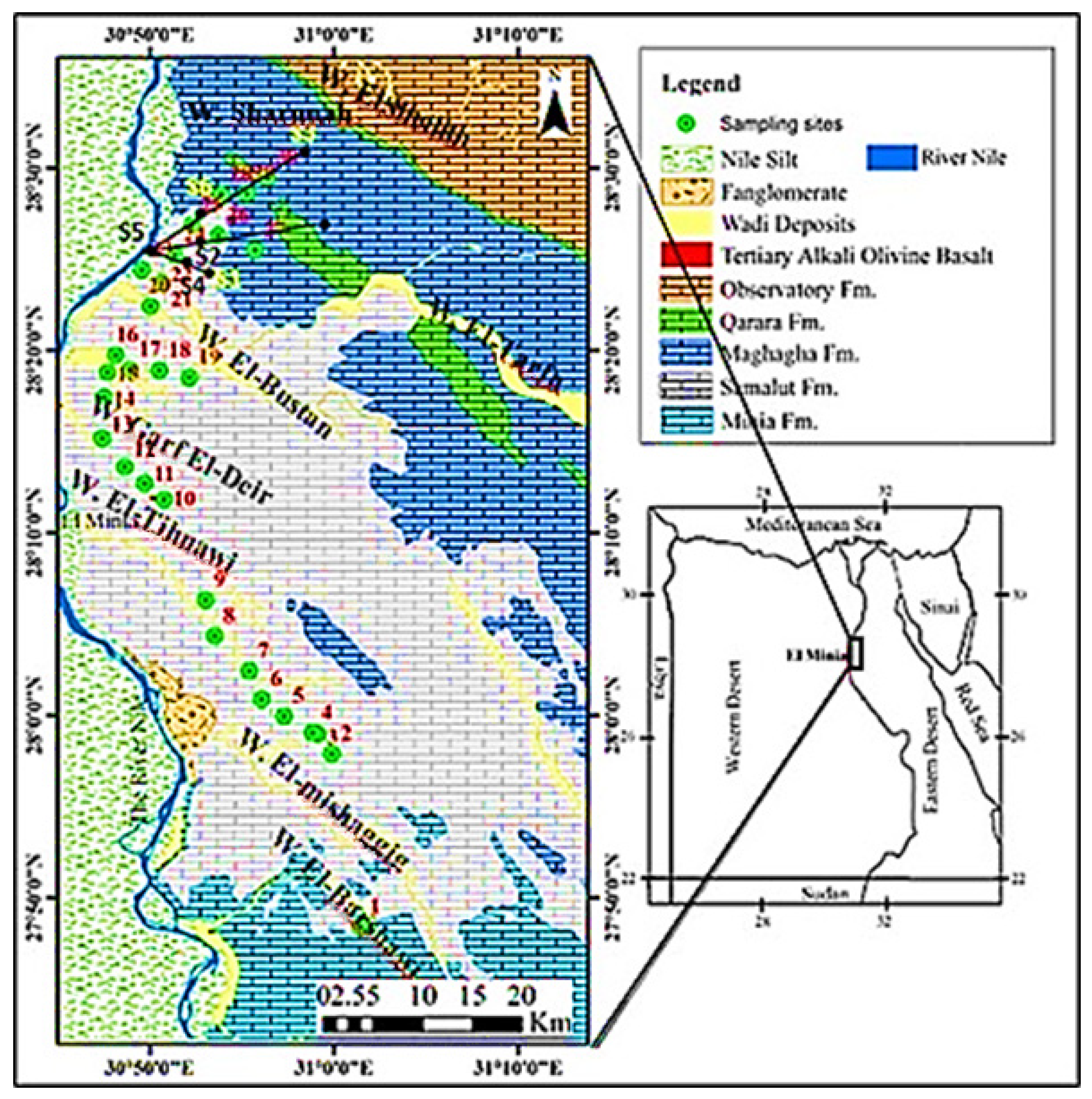
Location and Climate
2. Materials and Methods
2.1. Sampling and Analyses
2.2. Water Quality Evaluation for Irrigation
2.3. Water Quality Index (WQI)
- The number of times through which a separate concentration is more than (or less than, when the objective is a minimum) the objective is termed an “excursion” and is expressed as follows:
- 2.
- The collective amount by which specific tests are out of compliance is found by summing the excursions of separate tests from their objectives and dividing by the total number of tests (both those meeting objectives and those not meeting objectives). This variable, referred to as the normalized sum of excursions, or nse, is calculated as follows:
- 3.
- F3 is then found by an asymptotic function that scales the normalized sum of the excursions from objectives (nse) to produce a range between 0 and 100.
2.4. Health Risk Assessment (HRA)
3. Results and Discussion
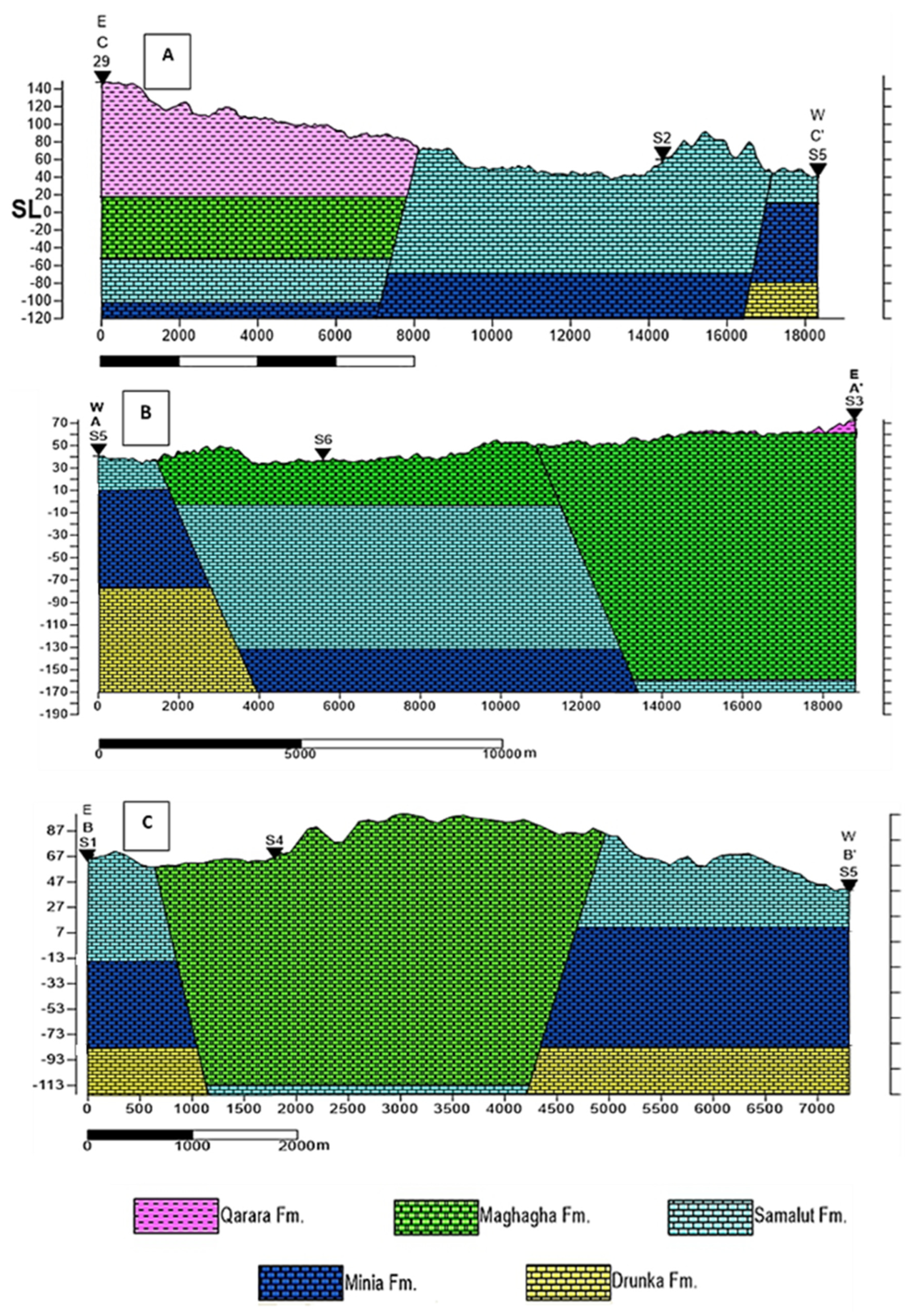
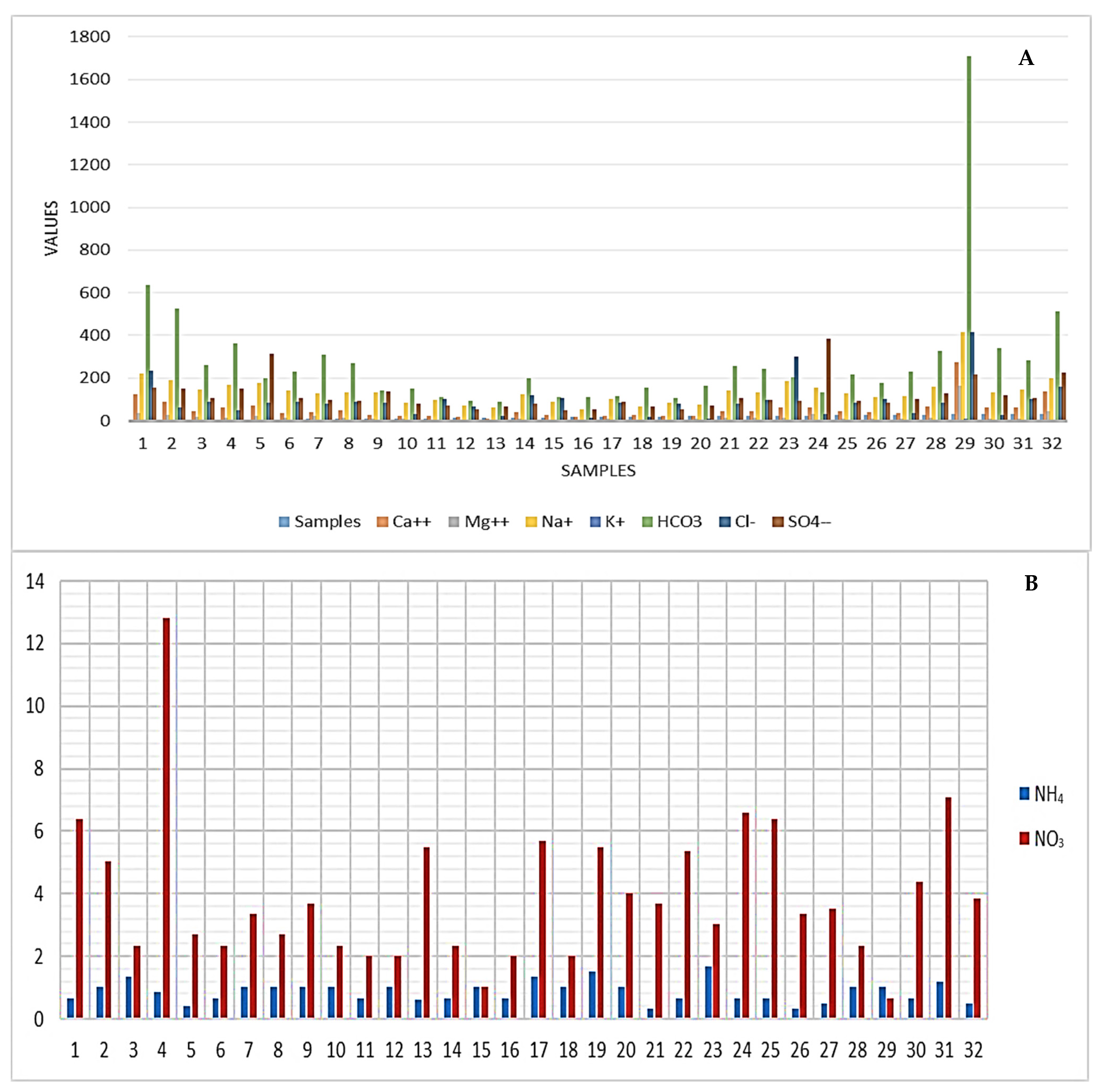
3.1. Hydrogeochemistry
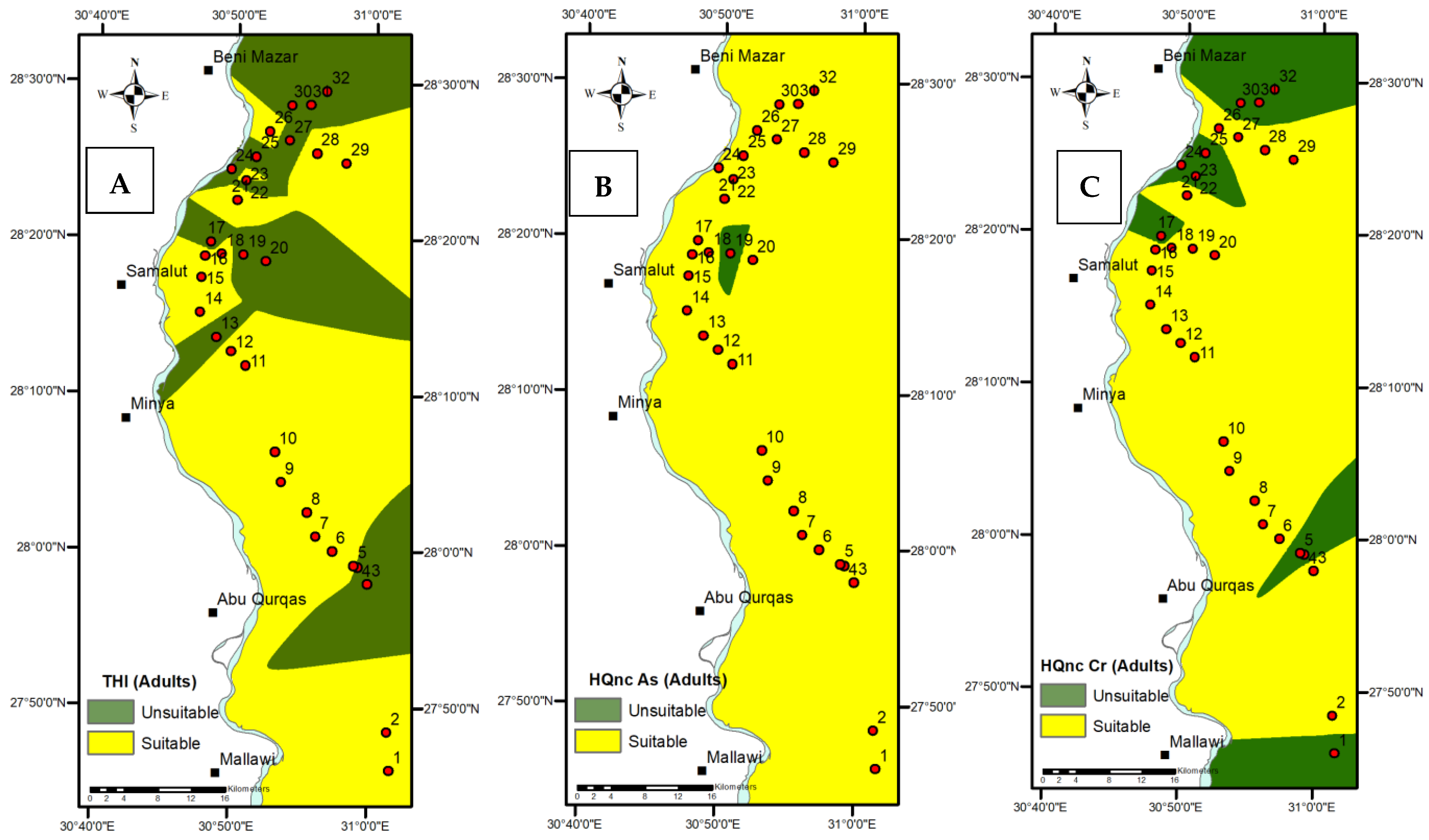
3.2. Appraisal of WQI and HRA
4. Conclusions
- Most examined water samples are categorized as fresh water and acceptable for drinking use; also, the majority of studied water samples, with total hardness (TH) values ranging from 32.19 to 1035.44 mg/L, are suitable for household use;
- The increase in ammonia concentrations in the research area’s northern direction is regarded as the use of N-fertilizers. Numerous serious illnesses, including liver damage, kidney failure, and genetic problems, could be brought on by these high quantities. Additionally, it might cause harm and irritate the eyes, lips, throat, and lungs;
- Most groundwater samples (68.8%) are classified as alkaline water with prevailing SO42− and Cl−, indicating the excess gypsum and halite dissolved in the water samples;
- Of the samples collected, 46.87% and 100% had THI values unsuitable for adults (more than 1) and children. As a result, the combined effect of Fe, Mn, As, Cr, Cd, Cu, and Pb in drinking water tends to harm human health;
- According to the THI, most of the research area’s northeastern and southeastern regions are extremely sensitive.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Shafy, H.I.; Kamel, A.H. Groundwater in Egypt issue: Resources, location, amount, contamination, protection, renewal, future overview. Egyp. J. Chem. 2016, 59, 321–362. [Google Scholar] [CrossRef]
- Abdel-Magged, G.M. Hydrogeological and Hydrogeochemical Studies of the Nile Valley Area, Beni-Suef Governorate, Egypt. Master’s Thesis, Cairo University, Giza, Egypt, 1998; p. 210. [Google Scholar]
- Korany, E.; Sakr, S.; Darwish, M.; Morsy, S. Hydrogeologic modelling for the assessment of continuous rise of groundwater levels in the quaternary aquifer, Nile valley, Egypt: Case study. In Proceedings of the International Conference of the Geology of the Arab World, Cairo University, Giza, Egypt, 24–27 March 2008; pp. 703–711. [Google Scholar]
- Korany, E.A.; Tempel, R.N.; Gomaa, M.A.; Mohamed, R.G. Detecting the roles of the physiochemical processes on groundwater evolution, Assiut area, Egypt—applications of hydrogeochemical and isotopic approaches. Egypt. J. Geol. 2013, 57, 63–83. [Google Scholar]
- El Kashouty, M.; El Sayed, E.; Kamel, A.A. The hydrochemical characteristics and evolution of groundwater and surface water in the western part of the River Nile, El Minia district, Upper Egypt. J. Geosci. 2010, 5, 637–652. [Google Scholar] [CrossRef]
- Ibrahim, R.G.; Lyons, W.B. Assessment of the hydrogeochemical processes affecting groundwater quality in the Eocene limestone aquifer at the desert fringes of El minia governorate, Egypt. Aquat. Geochem. 2016, 23, 33–52. [Google Scholar] [CrossRef]
- Ammar, A.I.; Kamal, K.A. Resistivity method contribution in determining of fault zone and hydro-geophysical characteristics of carbonate aquifer, eastern desert, Egypt. Appl. Water Sci. 2018, 8, 1. [Google Scholar] [CrossRef]
- Ramadan, E.M.; Badr, A.M.; Abdelradi, F.; Negm, A.; Nosair, A.M. Detection of Groundwater Quality Changes in Minia Governorate, West Nile River. Sustainability 2023, 15, 4076. [Google Scholar] [CrossRef]
- Asmoay, A. Evaluating groundwater quality and salinity dynamics in the Western-west area of El Minya Governorate, Egypt, based on geochemical modelling and multivariate analysis. J. Umm Al-Qura Univ. Appl. Sci. 2024, 10, 91–101. [Google Scholar] [CrossRef]
- Makhlouf, A.; Sharaan, M.; El-Rawy, M.; Kanae, S.; Ibrahim, M.G. Investigating the effects of surface water recharge on groundwater quality using hydrochemistry and ANFIS model: A case study Minia Governorate, Egypt. J. Environ. Manag. 2024, 362, 121269. [Google Scholar] [CrossRef]
- Ismail, E.; Alexakis, D.E.; Heleika, M.A.; Hashem, M.; Ahmed, M.S.; Hamdy, D.; Ali, A. Applying Geophysical and Hydrogeochemical Methods to Evaluate Groundwater Potential and Quality in Middle Egypt. Hydrology 2023, 10, 173. [Google Scholar] [CrossRef]
- Ismail, E.; Snousy, M.G.; Alexakis, D.E.; Gamvroula, D.E.; Howard, G.; El Sayed, E.; Ahmed, M.S.; Ali, A.; Abdelhalim, A. Multivariate Statistical Analysis and Geospatial Mapping for Assessing Groundwater Quality in West El Minia District, Egypt. Water 2023, 15, 2909. [Google Scholar] [CrossRef]
- Ismail, E.; Abdelhalim, A.; Ali, A.; Ahmed, M.S.; Scholger, R.; Khalil, M.M. Isotopic, Geophysical, and Hydrogeochemical Investigations of Groundwater in West Middle Upper Egypt. ACS Omega 2022, 7, 44000–44011. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, Y.; Xu, Z.; Qiao, X. Hydrochemical and isotopic characteristics of groundwater in the north-eastern Tennger Desert, northern China. Hydrogeol. J. 2017, 25, 2363–2375. [Google Scholar] [CrossRef]
- El Tahlawi, M.R.; Farrag, A.A.; Ahmed, S.S. Groundwater of Egypt: “An environmental overview”. Environ. Geol. 2008, 55, 639–652. [Google Scholar] [CrossRef]
- Said, R. The Geological Evolution of the River Nile; Springer: New York, NY, USA, 1981; p. 151. [Google Scholar]
- Adimalla, N.; Sudarshan, V. Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in the semi-arid region of Basara, Telangana State, South India. Appl. Water Sci. 2018, 8, 44. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Azad, C.; Mukherjee, S.; Rina, K. An assessment of groundwater salinization in Haryana state in India using hydrochemical tools in association with GIS. Environ. Earth Sci. 2017, 76, 465. [Google Scholar] [CrossRef]
- Melegy, A.; El-Kammar, A.; Yehia, M.M.; Miro, G. Hydrogeochemical characteristics and assessment of water resources in Beni Suef Governorate, Egypt. Open J. Geol. 2014, 4, 14. [Google Scholar] [CrossRef]
- Salman, S.A.; Asmoay, A.A.; El-Gohary, A.; Sabet, H. Evaluation of human risks of surface water and groundwater contaminated with Cd and Pb in the southern El-Minya Governorate, Egypt. Drink. Water Eng. Sci. 2019, 12, 23–30. [Google Scholar] [CrossRef]
- Seleem, E.M.; Abdelhafiz, M.A.; Elnazer, A.A.; Mostafa, A.; Al-Gamal, A.G.; Salman, S.A.; Feng, X. Chemical and bacterial quality monitoring of the Nile River water and associated health risks in Qena–Sohag sector, Egypt. Environ. Geochem. Health 2021, 43, 4089–4104. [Google Scholar]
- Mahmood, A.; Malik, R.N. Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arab. J. Chem. 2014, 7, 91–99. [Google Scholar] [CrossRef]
- Wu, M.; Wu, J.; Liu, J.; Wu, J.; Zheng, C. Effect of groundwater quality on sustainability of groundwater resource: A case study in the North China Plain. J. Contam. Hydrol. 2015, 179, 132–147. [Google Scholar] [CrossRef]
- Mohsen, A.; Elshemy, M.; Zeidan, B. Water quality monitoring of Lake Burullus (Egypt) using Landsat satellite imageries. Environ. Sci. Pollut. Res. 2021, 28, 15687–15700. [Google Scholar] [CrossRef] [PubMed]
- CCME (Canadian Council of Ministers of the Environment). Water Quality, Index 1.0, User’s Manual; CCME (Canadian Council of Ministers of the Environment): Winnipeg, MB, Canada, 2001. [Google Scholar]
- USEPA. Risk Assessment Guidance for Superfund; Volume I, Human Health Evaluation Manual (Part A); USEPA: Washington, DC, USA, 1989. [Google Scholar]
- CONOCO. Geological Maps of Egypt Scale 1:500,000. Sheet Nos. NG 36 NW Cairo; The Egyptian General Petroleum Corporation: Cairo, Egypt, 1987. [Google Scholar]
- Ahmed, A.; El Ammawy, M.; Hewaidy, A.G.; Moussa, B.; Abdel Hafz, N.; El Abd, E.S. Mapping of lineaments for groundwater assessment in the Desert Fringes East El-Minia, Eastern Desert, Egypt. Environ. Monit. Assess. 2019, 191, 556. [Google Scholar] [CrossRef]
- Oceanic and Atmospheric Administration (NOAA). Mean Values of Climate Data in El-Minia National; Oceanic and Atmospheric Administration (NOAA): Washington, DC, USA, 2023. [Google Scholar]
- World Meteorological Organization (WMO). Mean Values of Climate Data in El-Minia; World Meteorological Organization (WMO): Geneva, Switzerland, 2023. [Google Scholar]
- Bishay, Y. Biostratigraphic Study of the Eocene in the Eastern Desert between Samalut and Assiut by the Large Foraminifera; S N Publisher: Delhi, India, 1961; 7p. [Google Scholar]
- Said, R. The Geology of Egypt; El-Sevier Publ. Co.: Amsterdam, NY, USA, 1962; p. 377. [Google Scholar]
- Yousef, A.F.; Salem, A.A.; Baraka, A.M.; Aglan, O.S. The impact of geological setting on the groundwater occurrences in some Wadis in Shalatein–Abu Ramad Area, South Eastern Desert, Egypt. Eur. Water Publ. 2009, 25, 53–68. [Google Scholar]
- Salem, A. Hydrogeological studies on the shallow aquifers in the area West Samalot, El-Minia Governorate, Egypt. Egyp. J. Pure Appl. Sci. 2015, 53, 49–60. [Google Scholar]
- Abo Habibah, A.; Dahab, K.; Shabana, A.; Kamh, S.; Ibrahim, H. Assessment of Hydrogeochemical Characteristics of Groundwater Resources at Some Wadis, east El Minia Governorate, Eastern Desert, Egypt. Acta Geol. Sin. 2022, 96, 1082–1097. [Google Scholar] [CrossRef]
- Mosaad, S.; El Abd, E.; Kehew, A.E. Integration of geochemical data to assess the groundwater quality in a carbonate aquifer in the southeast of Beni-Suef city, Egypt. J. Afr. Earth Sci. 2019, 158, 103558. [Google Scholar] [CrossRef]
- El Ammawy, M.A.; Hewaidy, A.A.; Moussa, B.M.; Abdel Hafz, N.A.; El Sayed, A.; El Abd, E.A.; Abu Risha, U.A. Hydrogeologicinvestigationsto determine the sources of groundwater recharge of Samalut carbonate aquifer in some wadis, East of El Minia, Egypt. NRIAG J. Astron. Geophys. 2020, 9, 206–219. [Google Scholar] [CrossRef]
- Ibrahim, R.G.M. Geochemical modeling of the hydrogeochemical processes affecting groundwater quality of the Eocene aquifer east of El Minia Governorate-Eastern desert–Egypt. J. Afr. Earth Sci. 2021, 174, 104097. [Google Scholar] [CrossRef]
- Abu Heleika, M.; Toney, S.; Ismail, E. Mapping of groundwater opportunities for multi-purposes use in Beni-Suef province, Egypt. Arab. J. Geosci. 2021, 14, 784. [Google Scholar] [CrossRef]
- Sakram, G.; Adimalla, N. Hydrogeochemical characterization and assessment of water suitability for drinking and irrigation in crystalline rocks of Mothkur region, Telangana State, South India. Appl. Water Sci. 2018, 8, 1–21. [Google Scholar] [CrossRef]
- Adimalla, N.; Li, P.; Venkatayogi, S. Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environ. Proc. 2018, 5, 363–383. [Google Scholar] [CrossRef]
- Eaton, F.M. Significance of Carbonates in Irrigation Waters. Soil Sci. 1950, 69, 123–134. [Google Scholar] [CrossRef]
- Ragunath, H.M. Groundwater; Wiley Eastern Ltd.: New Delhi, India, 1987; p. 563. [Google Scholar]
- Wilcox, L.V. Classification and Use of Irrigation Waters; circular 969; USDA: Washington, DC, USA, 1955; p. 21. [Google Scholar]
- Kelly, W.P. Use of Saline Irrigation Water. Soil Sci. 1963, 95, 355–391. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Exposure Factors Handbook; EPA/600/R-09/052F; United States Environmental Protection Agency (USEPA): Washington, DC, USA, 1989. [Google Scholar]
- Wu, J.; Sun, Z. Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo. Health 2016, 8, 311–329. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Qian, H.; Gao, Y. Assessing nitrate and fluoride contaminants in drinking water and their health risk of rural residents living in a semiarid region of northwest China. Expo. Health 2017, 9, 183–195. [Google Scholar] [CrossRef]
- Adimalla, N. Controlling factors and mechanism of groundwater quality variation in semiarid region of South India: An approach of water quality index (WQI) and health risk assessment (HRA). Environ. Geochem. Health 2020, 42, 1725–1752. [Google Scholar] [CrossRef] [PubMed]
- Kelepertzis, E. Investigating the sources and potential health risks of environmental contaminants in the soils and drinking waters from the rural clusters in Thiva area (Greece), Ecotoxicol. Environ. Saf. 2014, 100, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Furtak, H.; Langguth, H.R. Zurhydrochemischen Kennzeichnung von Grundwassern und Grundwassertypenmittels Kennzahlen. Int. Assoc. Hydrogeol. 1967, 7, 89–96. [Google Scholar]
- US Geological Survey. Classification of Natural Ponds and Lakes; US Geological Survey: Virginia, VA, USA, 2000. [Google Scholar]
- World Health Organization (WHO). Safely Managed Drinking Water: Thematic Report on Drinking Water; World Health Organization (WHO): Brazil, MX, USA, 2017; p. 52. [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH). NIOSH Pocket Guide to Chemical Hazards; DHHS Publication No. (NIOSH) 2005-149; U.S. Department of Health and Human Services: Washington, DC, USA, 2005. [Google Scholar]
- Ahmed, S.I.; Hemada, A.A.; Toney, H.S.H. Response of garlic plants to the application of two bio-fertilizers and four mineral nitrogen levels. Minia J. Agric. Res. Dev. 2012, 32, 593–611. [Google Scholar]
- Ravikumar, P.; Somashekar, R.K. Principal component analysis and hydrochemicalfacies characterization to evaluate groundwater quality in Varahi river basin, Karnataka state, India. Appl. Water Sci. 2017, 7, 745–755. [Google Scholar] [CrossRef]
- Furtak, H.; Langguth, H.R. Zur Hydrochemischenkennzeichnung Von Grundwässern und GrundwassertypenmittelsKennzahlen; Mem. IAH-Congress: Hannover, Germany, 1967; pp. 86–96. [Google Scholar]
- Kaur, L.; Rishi, M.S.; Sharma, S.; Sharma, B.; Lata, R.; Singh, G. Hydrogeochemical characterization of groundwater in alluvial plains of River Yamuna in Northern India: An insight of controlling processes. J. King Saud Univ. Sci. 2019, 31, 1245–1253. [Google Scholar] [CrossRef]
- Ikhlil, A.I. Groundwater quality of springs and dug wells in Dura Area. Master’s Thesis, College of Graduate Studies and Academic Research, Hebron University, Hebron, Palestine, 2009. [Google Scholar]
- Haseen, K.; Amir, A.K.; Sarah, H. The Canadian water quality index: A tool for water resources management. In Proceedings of the Management Term International Conference, Ait, Thailand, 6–10 June 2005. [Google Scholar]
- World Health Organization (WHO). Safe Drinking Water from Desalination; World Health Organization (WHO): Brazil, MX, USA, 2011; p. 28. [Google Scholar]





| Parameters | Concentration in Groundwater Samples | WHO MAL (2017) | Percent of Samples | |||
|---|---|---|---|---|---|---|
| Maximum | Minimum | Mean | Below MAL | Above MAL | ||
| Hydrogen ion concentration (PH) | 9.70 | 7.50 | 7.85 | 9.2 | 96.8% | 3.2% |
| Total dissolved solids (mg/L) | 2327.80 | 271.20 | 668.20 | 1000 | 93.8% | 6.2% |
| Electric conductivity (µS/cm) | 4235.60 | 509.40 | 1218.23 | 1500 | 84.4% | 15.6% |
| Total hardness (mg/L) | 1035.44 | 32.19 | 196.83 | 500 | 93.8% | 6.2% |
| Calcium (mg/L) | 271.97 | 11.02 | 58.71 | 75 | 87.5% | 12.5% |
| Magnesium (mg/L) | 165.03 | 3.34 | 21.90 | 50 | 96.8% | 3.2% |
| Sodium (mg/L) | 415.48 | 60.39 | 145.54 | 200 | 87.5% | 12.5% |
| Potassium (mg/L) | 10.58 | 3.77 | 5.20 | 10 | 96.8% | 3.2% |
| Chloride (mg/L) | 385 | 49.15 | 124.65 | 250 | 93.8% | 6.2% |
| Bicarbonates (mg/L) | 1708.28 | 89.57 | 277.3 | 100 | 9.4% | 90.6% |
| Sulfates(mg/L) | 414.26 | 10.60 | 101.59 | 400 | 96.8% | 3.2% |
| Nitrates(mg/L) | 12.80 | 0.67 | 4.11 | 50 | 100% | - |
| Ammonia (mg/L) | 1.68 | 0.34 | 0.88 | 0.5 | 15.6% | 84.4% |
| Sample No. | WQI Value | WQI Class | Sample No. | WQI Value | WQI Class |
|---|---|---|---|---|---|
| 1 | 36.53 | Poor | 17 | 41.27 | Poor |
| 2 | 38.52 | Poor | 18 | 41.27 | Poor |
| 3 | 41.27 | Poor | 19 | 41.27 | Poor |
| 4 | 41.27 | Poor | 20 | 41.27 | Poor |
| 5 | 41.27 | Poor | 21 | 41.98 | Poor |
| 6 | 41.27 | Poor | 22 | 41.27 | Poor |
| 7 | 41.27 | Poor | 23 | 41.27 | Poor |
| 8 | 41.27 | Poor | 24 | 40.11 | Poor |
| 9 | 41.27 | Poor | 25 | 41.27 | Poor |
| 10 | 41.27 | Poor | 26 | 41.98 | Poor |
| 11 | 41.27 | Poor | 27 | 41.98 | Poor |
| 12 | 41.98 | Poor | 28 | 41.27 | Poor |
| 13 | 41.98 | Poor | 29 | 28.57 | Poor |
| 14 | 41.27 | Poor | 30 | 41.27 | Poor |
| 15 | 41.27 | Poor | 31 | 41.27 | Poor |
| 16 | 41.27 | Poor | 32 | 38.52 | Poor |
| S. No | HQnc (Adults) | THI | HQnc (Children) | THI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Mn | As | Cd | Cr | Cu | Pb | Fe | Mn | As | Cd | Cr | Cu | Pb | |||
| 1 | 0.222034 | 0.008671 | 0.578052 | 0.052458 | 2.355562 | 0.000379 | 0.032206 | 3.249362 | 3.330509 | 0.130062 | 8.670781 | 0.786873 | 35.33343 | 0.00569 | 0.483086 | 48.74044 |
| 2 | 0.173725 | 0.013626 | 0.873581 | 0.049423 | 0.556375 | 0.000488 | 0.031586 | 1.698805 | 2.605879 | 0.204383 | 13.10372 | 0.741352 | 8.345627 | 0.007316 | 0.473796 | 25.48207 |
| 3 | 0.069986 | 0.001239 | 0.4214 | 0.049684 | 0.034683 | 0.000705 | 0.014493 | 0.592188 | 1.049784 | 0.01858 | 6.321 | 0.745254 | 0.520247 | 0.010568 | 0.217389 | 8.882821 |
| 4 | 0.215841 | 0.010529 | 0.823002 | 0.074135 | 2.926389 | 0.000108 | 0.034683 | 4.084686 | 3.237608 | 0.157932 | 12.34502 | 1.112028 | 43.89583 | 0.001626 | 0.520247 | 61.2703 |
| 5 | 0.134087 | 0.004552 | 0.456083 | 0.045088 | 0.12746 | 0.000163 | 0.034188 | 0.801621 | 2.011312 | 0.068282 | 6.841246 | 0.676321 | 1.911907 | 0.002439 | 0.512815 | 12.02432 |
| 6 | 0.247737 | 0.007122 | 0.422701 | 0.048383 | 0.199428 | 0.000217 | 0.014493 | 0.94008 | 3.716049 | 0.106836 | 6.340509 | 0.725744 | 2.99142 | 0.003252 | 0.217389 | 14.1012 |
| 7 | 0.120772 | 0.006348 | 0.358103 | 0.034596 | 0.177751 | 0.000217 | 0.031215 | 0.729002 | 1.811574 | 0.095224 | 5.371549 | 0.518946 | 2.666265 | 0.003252 | 0.468222 | 10.93503 |
| 8 | 0.153597 | 0.013161 | 0.395821 | 0.030434 | 0.368508 | 5.42 × 10−5 | 0.02081 | 0.982386 | 2.30395 | 0.197415 | 5.937317 | 0.456517 | 5.527623 | 0.000813 | 0.312148 | 14.73578 |
| 9 | 0.087946 | 0.001394 | 0.150294 | 0.081939 | 0.101159 | 0.000921 | 0.053883 | 0.477536 | 1.319197 | 0.020903 | 2.254403 | 1.229083 | 1.517387 | 0.013819 | 0.808241 | 7.163033 |
| 10 | 0.043044 | 0.012697 | 0.386717 | 0.029394 | 0.355502 | 0.000271 | 0.014121 | 0.841746 | 0.645664 | 0.190448 | 5.800753 | 0.440909 | 5.33253 | 0.004064 | 0.211815 | 12.62618 |
| 11 | 0.08423 | 0.006503 | 0.09061 | 0.066852 | 0.182086 | 0.001409 | 0.026384 | 0.458074 | 1.263457 | 0.097546 | 1.359145 | 1.002776 | 2.731296 | 0.021135 | 0.395759 | 6.871114 |
| 12 | 0.041496 | 0.0096 | 0.584411 | 0.007283 | 0.268794 | 0.000325 | 0.016722 | 0.928631 | 0.622438 | 0.143997 | 8.76616 | 0.109252 | 4.031913 | 0.004877 | 0.250833 | 13.92947 |
| 13 | 0.071224 | 0.001084 | 0.1633 | 0.114888 | 0.657534 | 0.000325 | 0.014864 | 1.023219 | 1.068364 | 0.016258 | 2.449496 | 1.723318 | 9.863014 | 0.004877 | 0.222963 | 15.34829 |
| 14 | 0.039328 | 0.004645 | 0.208966 | 0.050724 | 0.130062 | 0.000596 | 0.025269 | 0.45959 | 0.589923 | 0.069676 | 3.134487 | 0.760861 | 1.950926 | 0.008942 | 0.379037 | 6.893852 |
| 15 | 0.03747 | 0.006503 | 0.521981 | 0.019249 | 0.182086 | 0.000325 | 0.024898 | 0.792513 | 0.562052 | 0.097546 | 7.829715 | 0.288737 | 2.731296 | 0.004877 | 0.373463 | 11.88769 |
| 16 | 0.017651 | 0.001548 | 0.113587 | 0.066245 | 0.043354 | 0.000325 | 0.024154 | 0.266865 | 0.264768 | 0.023225 | 1.703809 | 0.993672 | 0.650309 | 0.004877 | 0.362315 | 4.002975 |
| 17 | 0.075869 | 0.011148 | 0.885865 | 0.087575 | 2.478398 | 5.42 × 10−5 | 0.012387 | 3.551297 | 1.13804 | 0.167222 | 13.28797 | 1.313623 | 37.17597 | 0.000813 | 0.185802 | 53.26945 |
| 18 | 0.062863 | 0.0096 | 0.357236 | 0.06321 | 0.268794 | 0.000325 | 0.021553 | 0.783582 | 0.942947 | 0.143997 | 5.358543 | 0.94815 | 4.031913 | 0.004877 | 0.323296 | 11.75372 |
| 19 | 0.05605 | 0.007122 | 1.13226 | 0.066331 | 0.354057 | 0.000596 | 0.041496 | 1.657913 | 0.840756 | 0.106836 | 16.98389 | 0.994972 | 5.310854 | 0.008942 | 0.622438 | 24.86869 |
| 20 | 0.172177 | 0.0096 | 0.540479 | 0.043787 | 0.513021 | 0.000596 | 0.035302 | 1.314963 | 2.582654 | 0.143997 | 8.10718 | 0.656812 | 7.695318 | 0.008942 | 0.529537 | 19.72444 |
| 21 | 0.049857 | 0.006193 | 0.132229 | 0.052458 | 0.468222 | 0.000271 | 0.026012 | 0.735244 | 0.747855 | 0.092901 | 1.983441 | 0.786873 | 7.023333 | 0.004064 | 0.390185 | 11.02865 |
| 22 | 0.15081 | 0.017342 | 0.629354 | 0.066765 | 2.89026 | 0.000542 | 0.042735 | 3.797807 | 2.262145 | 0.260123 | 9.440313 | 1.001475 | 43.35391 | 0.008129 | 0.641018 | 56.96711 |
| 23 | 0.129752 | 0.006503 | 0.321686 | 0.052285 | 0.182086 | 0.000379 | 0.016351 | 0.709042 | 1.946281 | 0.097546 | 4.82529 | 0.784272 | 2.731296 | 0.00569 | 0.245259 | 10.63563 |
| 24 | 0.292949 | 0.006101 | 0.976908 | 0.086708 | 3.251543 | 5.42 × 10−5 | 0.041496 | 4.655758 | 4.394228 | 0.091508 | 14.65362 | 1.300617 | 48.77314 | 0.000813 | 0.622438 | 69.83637 |
| 25 | 0.040257 | 0.003097 | 0.585278 | 0.070233 | 3.172061 | 0.000108 | 0.017342 | 3.888376 | 0.603858 | 0.046451 | 8.779166 | 1.0535 | 47.58091 | 0.001626 | 0.260123 | 58.32564 |
| 26 | 0.075869 | 0.011613 | 0.297841 | 0.019249 | 0.325154 | 0.000434 | 0.021058 | 0.751218 | 1.13804 | 0.17419 | 4.46762 | 0.288737 | 4.877314 | 0.006503 | 0.315864 | 11.26827 |
| 27 | 0.02787 | 0.023999 | 0.576173 | 0.036677 | 0.671986 | 0.000542 | 0.012387 | 1.349635 | 0.418056 | 0.359992 | 8.642601 | 0.550161 | 10.07978 | 0.008129 | 0.185802 | 20.24452 |
| 28 | 0.03778 | 0.006348 | 0.2107 | 0.056967 | 0.177751 | 0.000379 | 0.017094 | 0.507019 | 0.566697 | 0.095224 | 3.1605 | 0.854505 | 2.666265 | 0.00569 | 0.256407 | 7.605289 |
| 29 | 0.314625 | 0.010684 | 0.621261 | 0.043007 | 0.299142 | 0.000163 | 0.022916 | 1.311798 | 4.719382 | 0.160255 | 9.318922 | 0.645106 | 4.487129 | 0.002439 | 0.343735 | 19.67697 |
| 30 | 0.054502 | 0.007122 | 0.41692 | 0.061563 | 1.358422 | 5.42 × 10−5 | 0.021058 | 1.919641 | 0.817531 | 0.106836 | 6.253801 | 0.923438 | 20.37634 | 0.000813 | 0.315864 | 28.79462 |
| 31 | 0.122939 | 0.016722 | 0.424146 | 0.067199 | 2.875809 | 0.000163 | 0.029109 | 3.536087 | 1.844089 | 0.250833 | 6.362186 | 1.007978 | 43.13714 | 0.002439 | 0.436636 | 53.0413 |
| 32 | 0.088256 | 0.008206 | 0.38585 | 0.147403 | 1.44513 | 0.000813 | 0.042115 | 2.117774 | 1.323842 | 0.123094 | 5.787747 | 2.211049 | 21.67695 | 0.012193 | 0.631728 | 31.76661 |
| Min. | 0.017651 | 0.001084 | 0.09061 | 0.007283 | 0.034683 | 5.42 × 10−5 | 0.012387 | 0.266865 | 0.264768 | 0.016258 | 1.359145 | 0.109252 | 0.520247 | 0.000813 | 0.185802 | 4.002975 |
| Max. | 0.314625 | 0.023999 | 1.13226 | 0.147403 | 3.251543 | 0.001409 | 0.053883 | 4.655758 | 4.719382 | 0.359992 | 16.98389 | 2.211049 | 48.77314 | 0.021135 | 0.808241 | 69.83637 |
| Mean | 0.109769 | 0.008457 | 0.470087 | 0.057569 | 0.918705 | 0.000384 | 0.026074 | 1.591046 | 1.646529 | 0.126854 | 7.05131 | 0.863529 | 13.78058 | 0.005766 | 0.391114 | 23.86568 |
| S. No. | HQc (Adults) | HQc (Children) | ||||||
|---|---|---|---|---|---|---|---|---|
| As | Cd | Cr | Pb | As | Cd | Cr | Pb | |
| 1 | 0.00026 | 9.96706 × 10−6 | 0.000353334 | 6.19961 × 10−6 | 0.003902 | 0.00015 | 0.0053 | 9.29941 × 10−5 |
| 2 | 0.000393 | 9.39046 × 10−6 | 8.34563 × 10−5 | 6.08039 × 10−6 | 0.005897 | 0.000141 | 0.001252 | 9.12058 × 10−5 |
| 3 | 0.00019 | 9.43988 × 10−6 | 5.20247 × 10−6 | 2.78982 × 10−6 | 0.002844 | 0.000142 | 7.8 × 10−5 | 4.18474 × 10−5 |
| 4 | 0.00037 | 1.40857 × 10−5 | 0.000438958 | 6.6765 × 10−6 | 0.005555 | 0.000211 | 0.006584 | 0.000100148 |
| 5 | 0.000205 | 8.56673 × 10−6 | 1.91191 × 10−5 | 6.58112 × 10−6 | 0.003079 | 0.000129 | 0.000287 | 9.87168 × 10−5 |
| 6 | 0.00019 | 9.19276 × 10−6 | 2.99142 × 10−5 | 2.78982 × 10−6 | 0.002853 | 0.000138 | 0.000449 | 4.18474 × 10−5 |
| 7 | 0.000161 | 6.57332 × 10−6 | 2.66627 × 10−5 | 6.00885 × 10−6 | 0.002417 | 9.86 × 10−5 | 0.0004 | 9.01328 × 10−5 |
| 8 | 0.000178 | 5.78254 × 10−6 | 5.52762 × 10−5 | 4.0059 × 10−6 | 0.002672 | 8.67 × 10−5 | 0.000829 | 6.00885 × 10−5 |
| 9 | 6.76 × 10−5 | 1.55684 × 10−5 | 1.51739 × 10−5 | 1.03724 × 10−5 | 0.001014 | 0.000234 | 0.000228 | 0.000155586 |
| 10 | 0.000174 | 5.58485 × 10−6 | 5.33253 × 10−5 | 2.71829 × 10−6 | 0.00261 | 8.38 × 10−5 | 0.0008 | 4.07743 × 10−5 |
| 11 | 4.08 × 10−5 | 1.27018 × 10−5 | 2.7313 × 10−5 | 5.07891 × 10−6 | 0.000612 | 0.000191 | 0.00041 | 7.61837 × 10−5 |
| 12 | 0.000263 | 1.38386 × 10−6 | 4.03191 × 10−5 | 3.21903 × 10−6 | 0.003945 | 2.08 × 10−5 | 0.000605 | 4.82854 × 10−5 |
| 13 | 7.35 × 10−5 | 2.18287 × 10−5 | 9.86301 × 10−5 | 2.86136 × 10−6 | 0.001102 | 0.000327 | 0.001479 | 4.29204 × 10−5 |
| 14 | 9.4 × 10−5 | 9.63757 × 10−6 | 1.95093 × 10−5 | 4.86431 × 10−6 | 0.001411 | 0.000145 | 0.000293 | 7.29646 × 10−5 |
| 15 | 0.000235 | 3.65734 × 10−6 | 2.7313 × 10−5 | 4.79277 × 10−6 | 0.003523 | 5.49 × 10−5 | 0.00041 | 7.18916 × 10−5 |
| 16 | 5.11 × 10−5 | 1.25865 × 10−5 | 6.50309 × 10−6 | 4.64971 × 10−6 | 0.000767 | 0.000189 | 9.75 × 10−5 | 6.97456 × 10−5 |
| 17 | 0.000399 | 1.66392 × 10−5 | 0.00037176 | 2.38446 × 10−6 | 0.00598 | 0.00025 | 0.005576 | 3.5767 × 10−5 |
| 18 | 0.000161 | 1.20099 × 10−5 | 4.03191 × 10−5 | 4.14897 × 10−6 | 0.002411 | 0.00018 | 0.000605 | 6.22345 × 10−5 |
| 19 | 0.00051 | 1.2603 × 10−5 | 5.31085 × 10−5 | 7.98796 × 10−6 | 0.007643 | 0.000189 | 0.000797 | 0.000119819 |
| 20 | 0.000243 | 8.31961 × 10−6 | 7.69532 × 10−5 | 6.79572 × 10−6 | 0.003648 | 0.000125 | 0.001154 | 0.000101936 |
| 21 | 5.95 × 10−5 | 9.96706 × 10−6 | 7.02333 × 10−5 | 5.00738 × 10−6 | 0.000893 | 0.00015 | 0.001053 | 7.51106 × 10−5 |
| 22 | 0.000283 | 1.26854 × 10−5 | 0.000433539 | 8.2264 × 10−6 | 0.004248 | 0.00019 | 0.006503 | 0.000123396 |
| 23 | 0.000145 | 9.93411 × 10−6 | 2.7313 × 10−5 | 3.14749 × 10−6 | 0.002171 | 0.000149 | 0.00041 | 4.72124 × 10−5 |
| 24 | 0.00044 | 1.64745 × 10−5 | 0.000487731 | 7.98796 × 10−6 | 0.006594 | 0.000247 | 0.007316 | 0.000119819 |
| 25 | 0.000263 | 1.33443 × 10−5 | 0.000475809 | 3.33825 × 10−6 | 0.003951 | 0.0002 | 0.007137 | 5.00738 × 10−5 |
| 26 | 0.000134 | 3.65734 × 10−6 | 4.87731 × 10−5 | 4.05359 × 10−6 | 0.00201 | 5.49 × 10−5 | 0.000732 | 6.08039 × 10−5 |
| 27 | 0.000259 | 6.96871 × 10−6 | 0.000100798 | 2.38446 × 10−6 | 0.003889 | 0.000105 | 0.001512 | 3.5767 × 10−5 |
| 28 | 9.48 × 10−5 | 1.08237 × 10−5 | 2.66627 × 10−5 | 3.29056 × 10−6 | 0.001422 | 0.000162 | 0.0004 | 4.93584 × 10−5 |
| 29 | 0.00028 | 8.17134 × 10−6 | 4.48713 × 10−5 | 4.41126 × 10−6 | 0.004194 | 0.000123 | 0.000673 | 6.61689 × 10−5 |
| 30 | 0.000188 | 1.16969 × 10−5 | 0.000203763 | 4.05359 × 10−6 | 0.002814 | 0.000175 | 0.003056 | 6.08039 × 10−5 |
| 31 | 0.000191 | 1.27677 × 10−5 | 0.000431371 | 5.60349 × 10−6 | 0.002863 | 0.000192 | 0.006471 | 8.40524 × 10−5 |
| 32 | 0.000174 | 2.80066 × 10−5 | 0.00021677 | 8.10718 × 10−6 | 0.002604 | 0.00042 | 0.003252 | 0.000121608 |
| Min. | 4.08 × 10−5 | 1.38386 × 10−6 | 5.20247 × 10−6 | 2.38446 × 10−6 | 0.000612 | 2.08 × 10−5 | 7.8 × 10−5 | 3.5767 × 10−5 |
| Max. | 0.00051 | 2.80066 × 10−5 | 0.000487731 | 1.03724 × 10−5 | 0.007643 | 0.00042 | 0.007316 | 0.000155586 |
| Mean | 0.000212 | 1.0938 × 10−5 | 0.000137806 | 5.0193 × 10−6 | 0.003173 | 0.000164 | 0.002067 | 7.52895 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Aziz, A.-A.A.; Mostafa, A.; Salman, S.A.; Mohamed, R.S.A.; Snousy, M.G.; Ahmed, M.S.; Sambito, M.; Ismail, E. Groundwater Quality Assessment at East El Minia Middle Eocene Carbonate Aquifer: Water Quality Index (WQI) and Health Risk Assessment (HRA). Water 2024, 16, 2288. https://doi.org/10.3390/w16162288
Abdel-Aziz A-AA, Mostafa A, Salman SA, Mohamed RSA, Snousy MG, Ahmed MS, Sambito M, Ismail E. Groundwater Quality Assessment at East El Minia Middle Eocene Carbonate Aquifer: Water Quality Index (WQI) and Health Risk Assessment (HRA). Water. 2024; 16(16):2288. https://doi.org/10.3390/w16162288
Chicago/Turabian StyleAbdel-Aziz, Abdel-Aziz A., Alaa Mostafa, Salman A. Salman, Ramadan S. A. Mohamed, Moustafa Gamal Snousy, Mohamed S. Ahmed, Mariacrocetta Sambito, and Esam Ismail. 2024. "Groundwater Quality Assessment at East El Minia Middle Eocene Carbonate Aquifer: Water Quality Index (WQI) and Health Risk Assessment (HRA)" Water 16, no. 16: 2288. https://doi.org/10.3390/w16162288
APA StyleAbdel-Aziz, A.-A. A., Mostafa, A., Salman, S. A., Mohamed, R. S. A., Snousy, M. G., Ahmed, M. S., Sambito, M., & Ismail, E. (2024). Groundwater Quality Assessment at East El Minia Middle Eocene Carbonate Aquifer: Water Quality Index (WQI) and Health Risk Assessment (HRA). Water, 16(16), 2288. https://doi.org/10.3390/w16162288











