Chromaticity-Based Discrimination of Algal Bloom from Inland and Coastal Waters Using In Situ Hyperspectral Remote Sensing Reflectance
Abstract
1. Introduction
2. Data and Methods
2.1. Data Resources
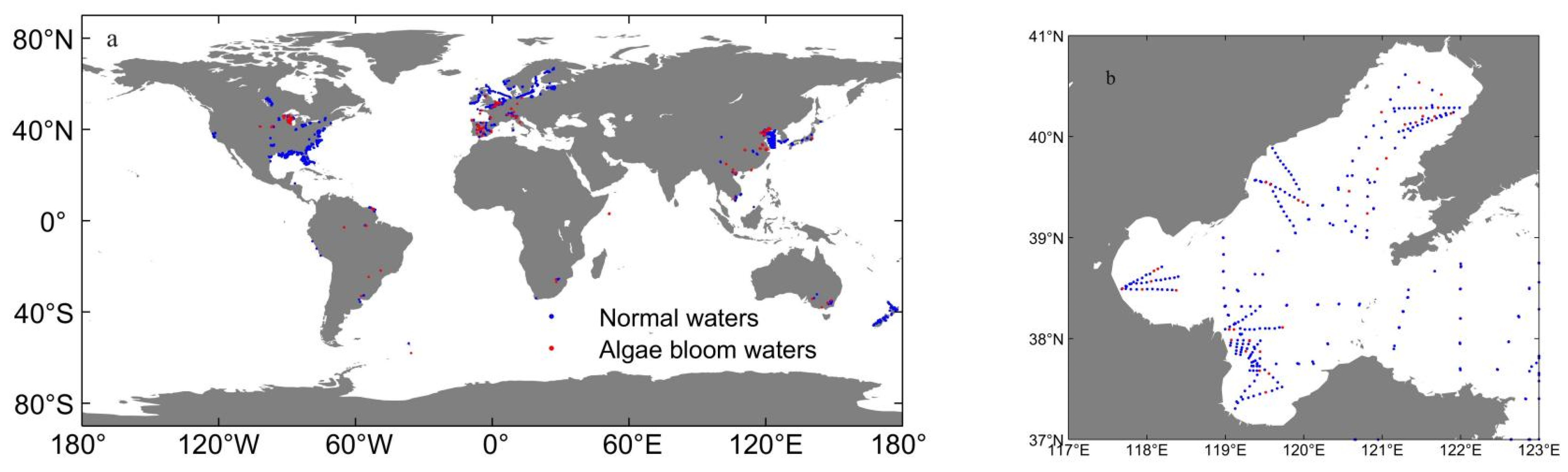
2.2. Case Study Area
2.3. Methods
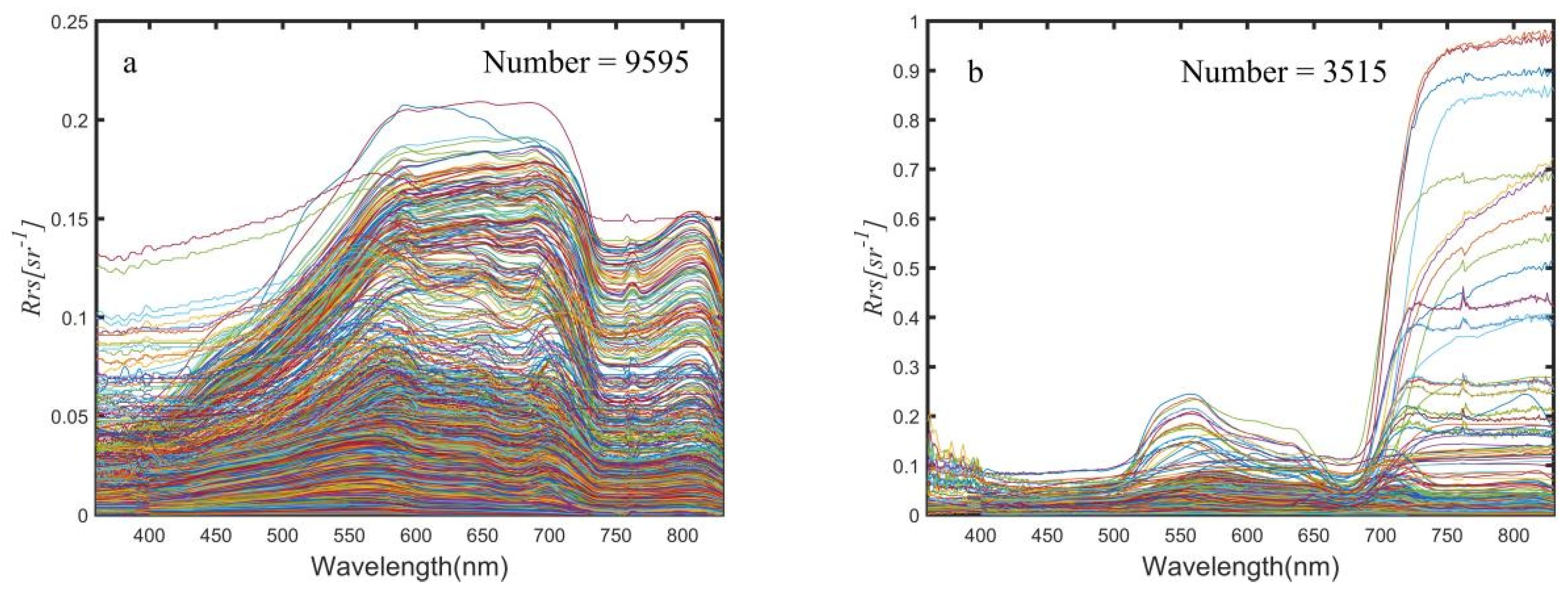
2.3.1. Separation of the Spectra of Algal Bloom Waters from the Inland and Coastal Water
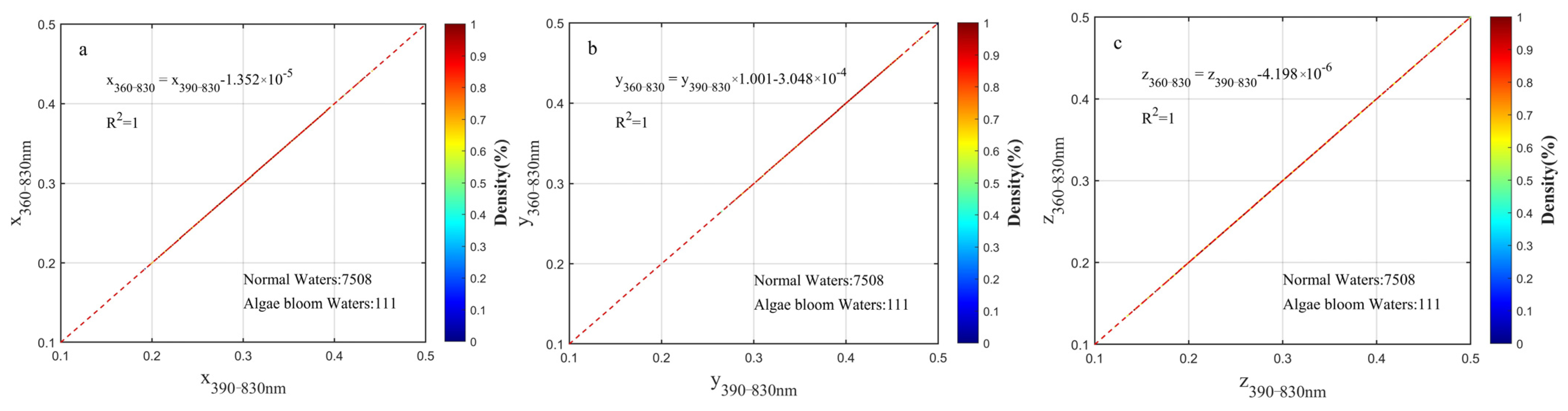
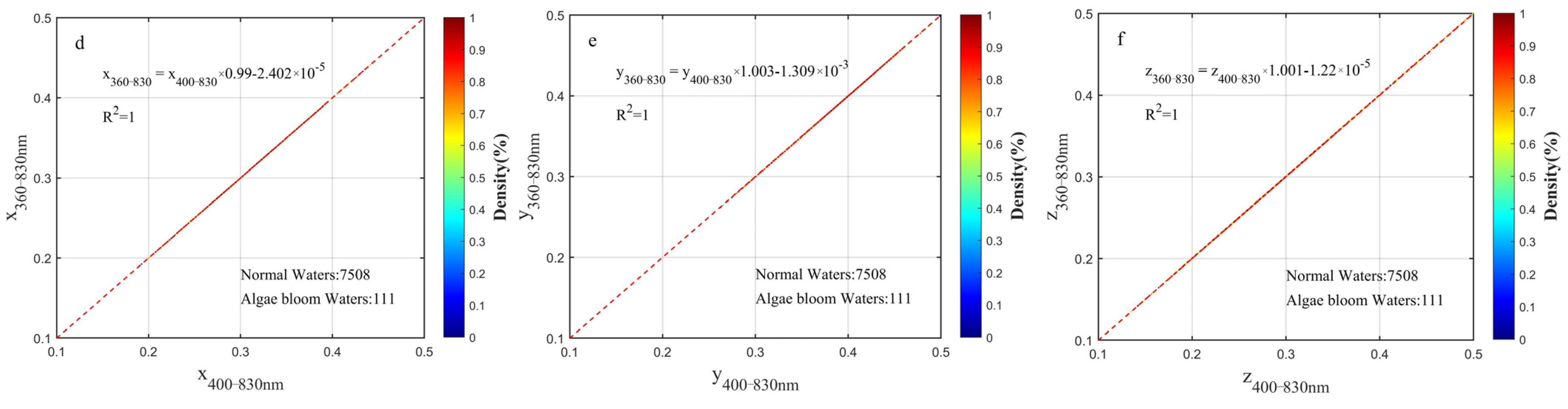
2.3.2. Chromatic Indices Unified to the Wavelength Range of 360–830 nm
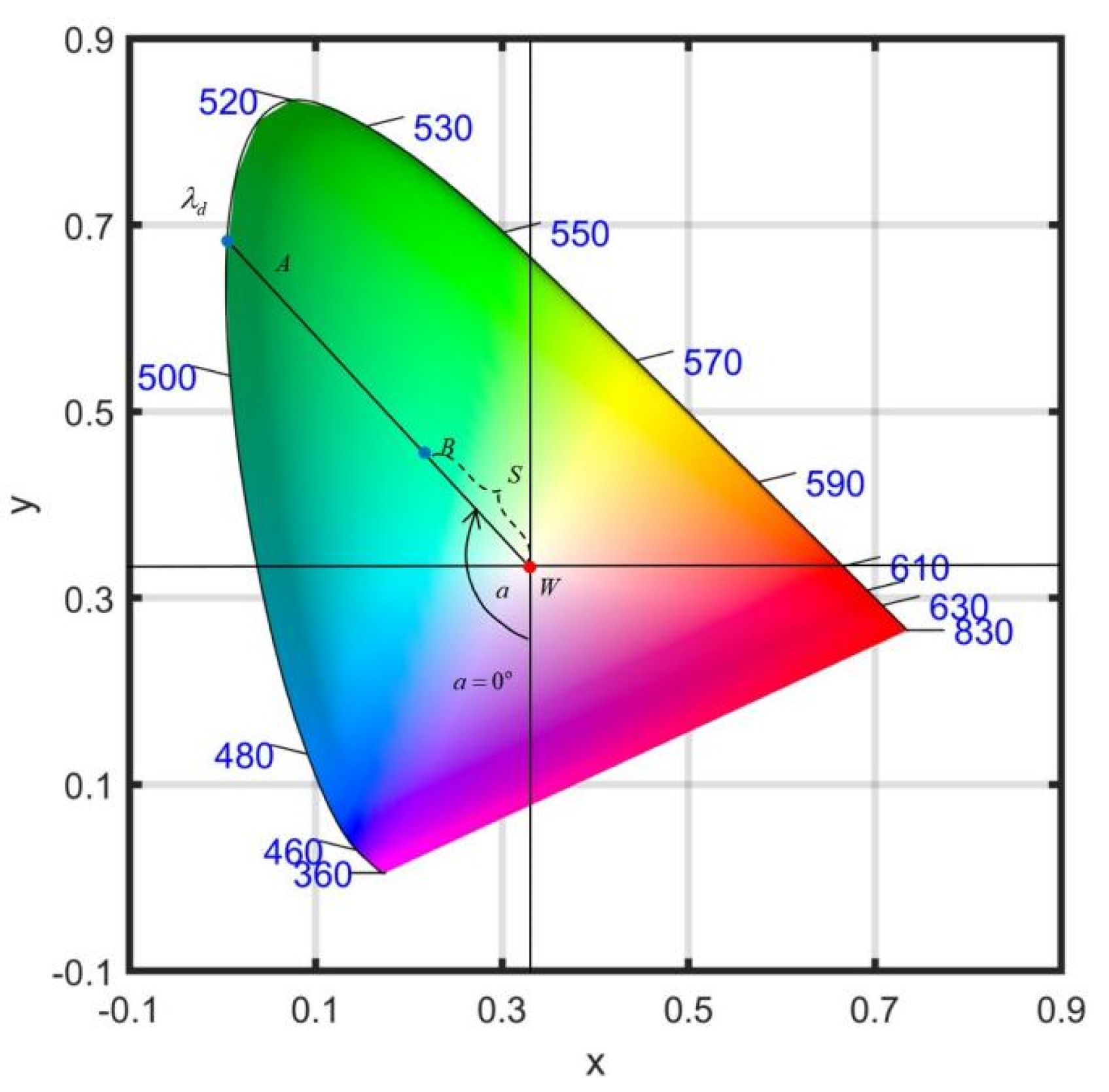
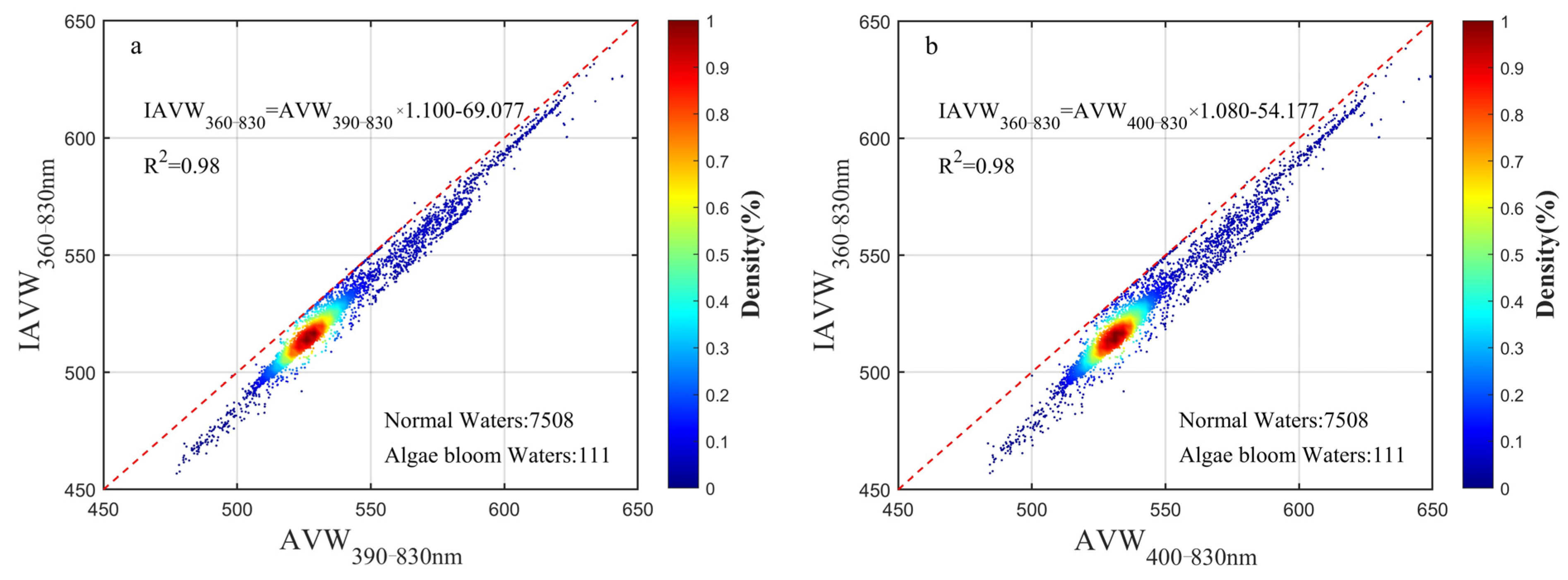
2.3.3. Apparent Visual Wavelength (AVW)
2.3.4. Statistical Analysis Methods
3. Result
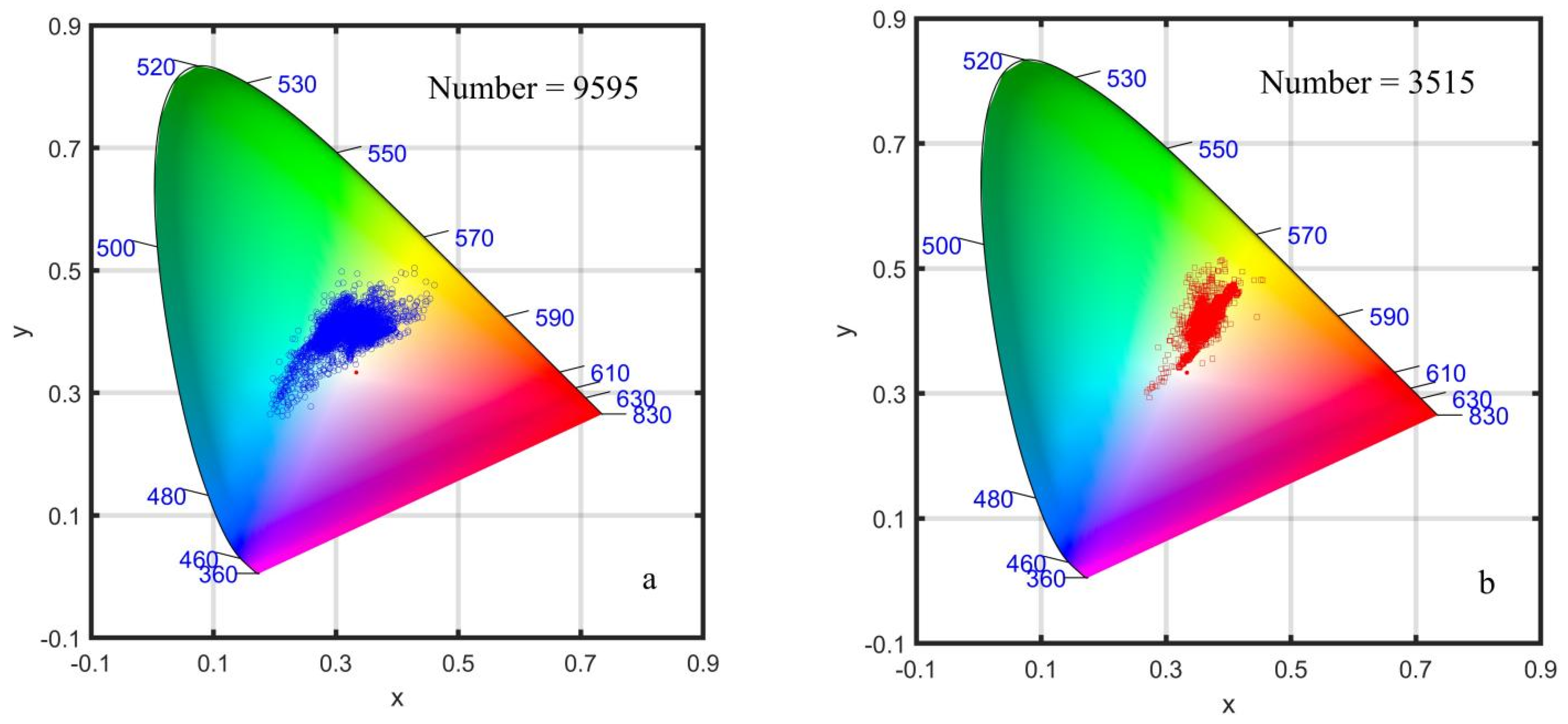
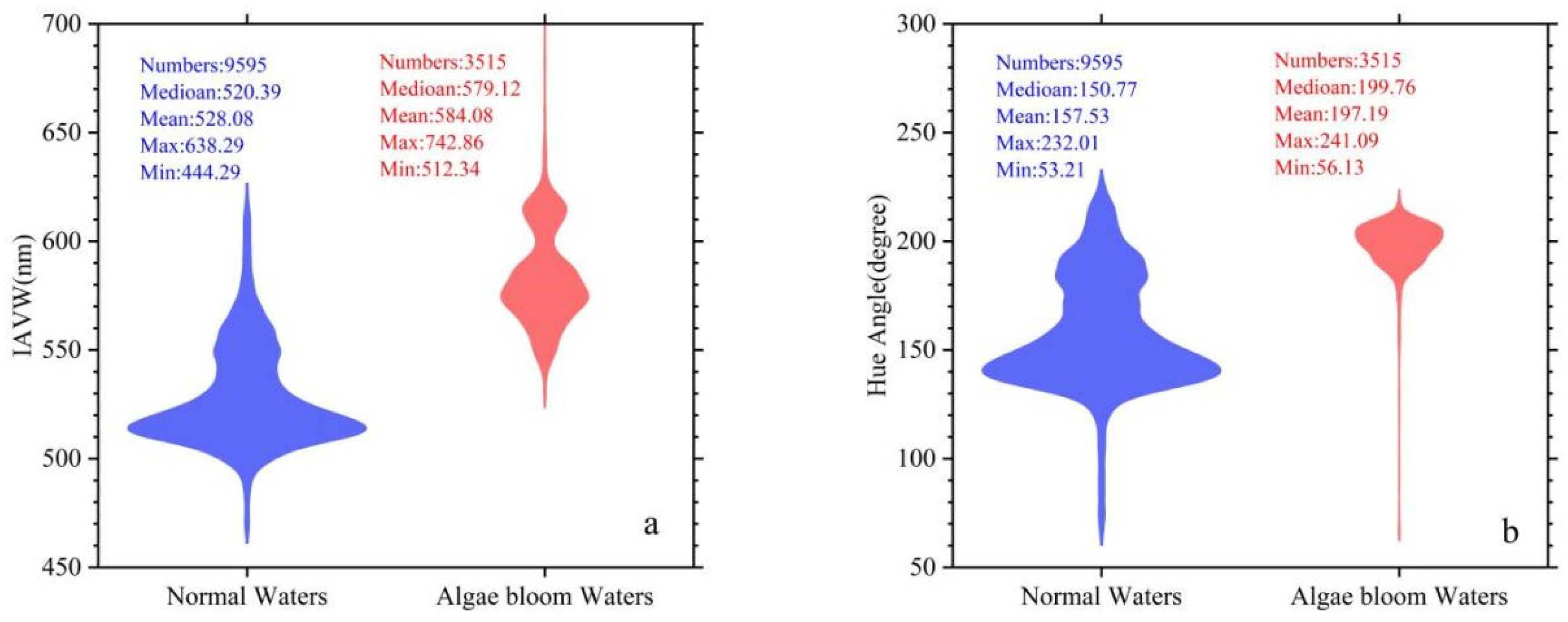
3.1. The Constructed Dataset of Normal Water and Algal Bloom Water
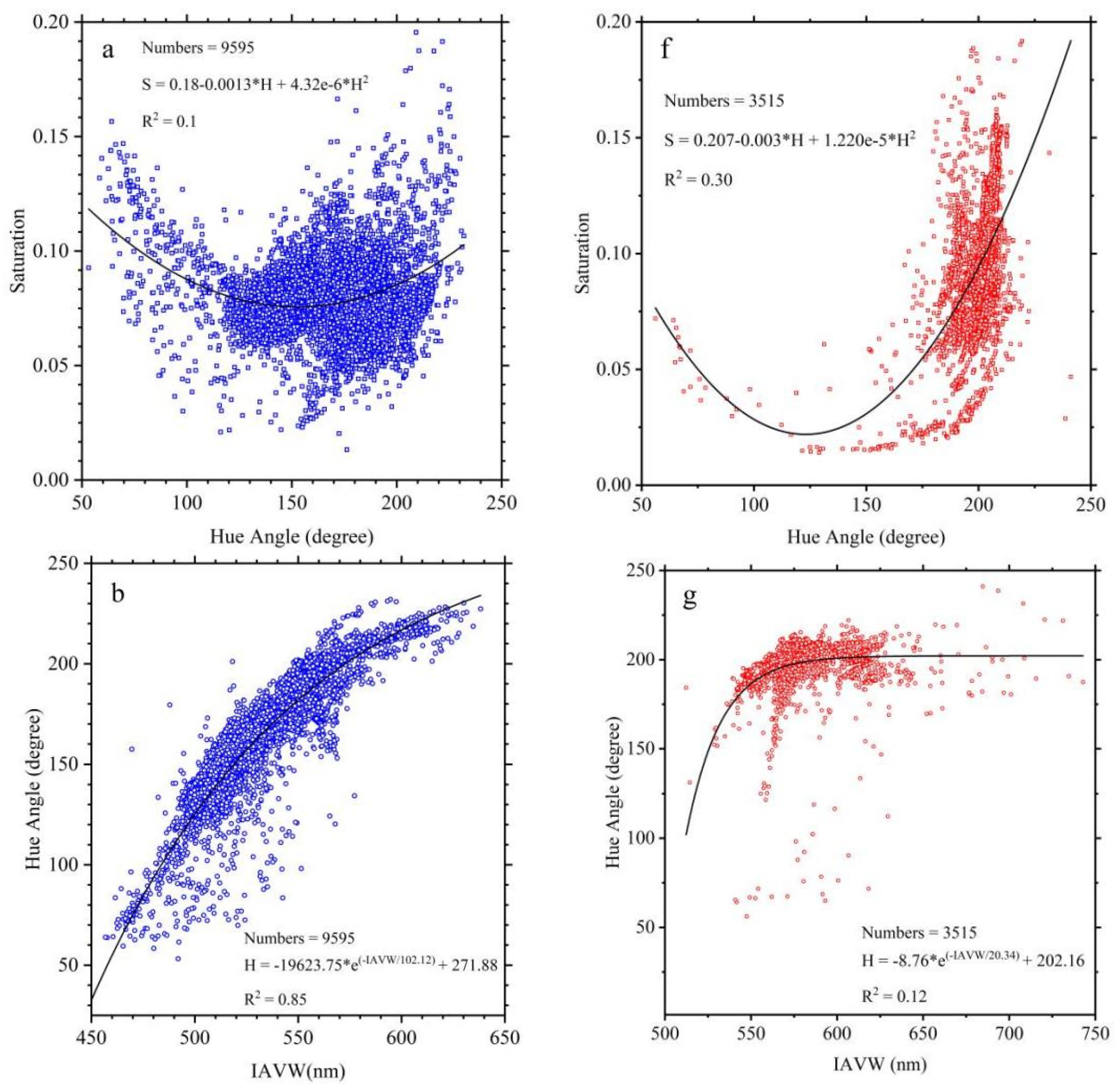
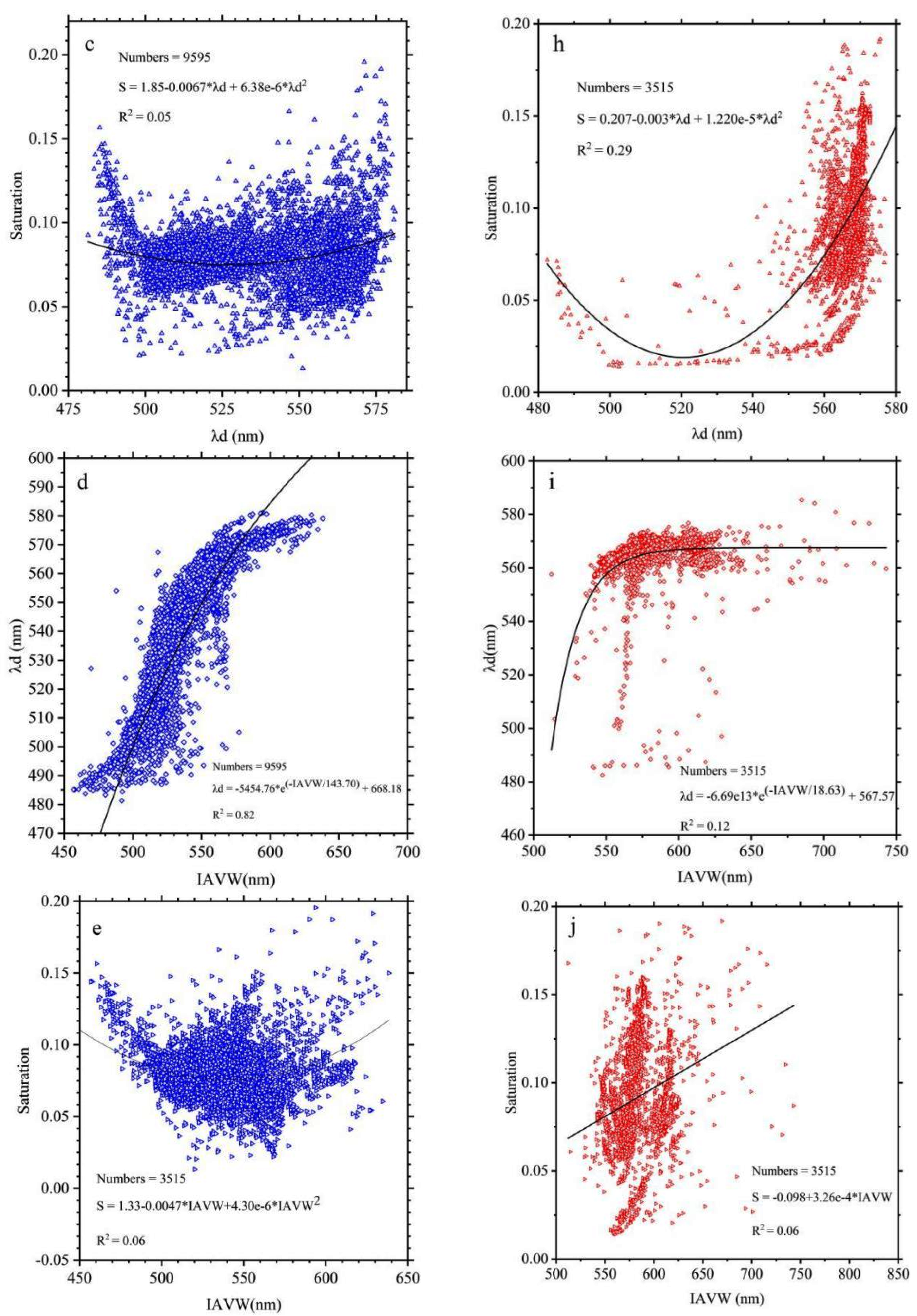
3.2. Wavelength Range Unification Impact to XYZ, Hue Angle, Saturation, λd
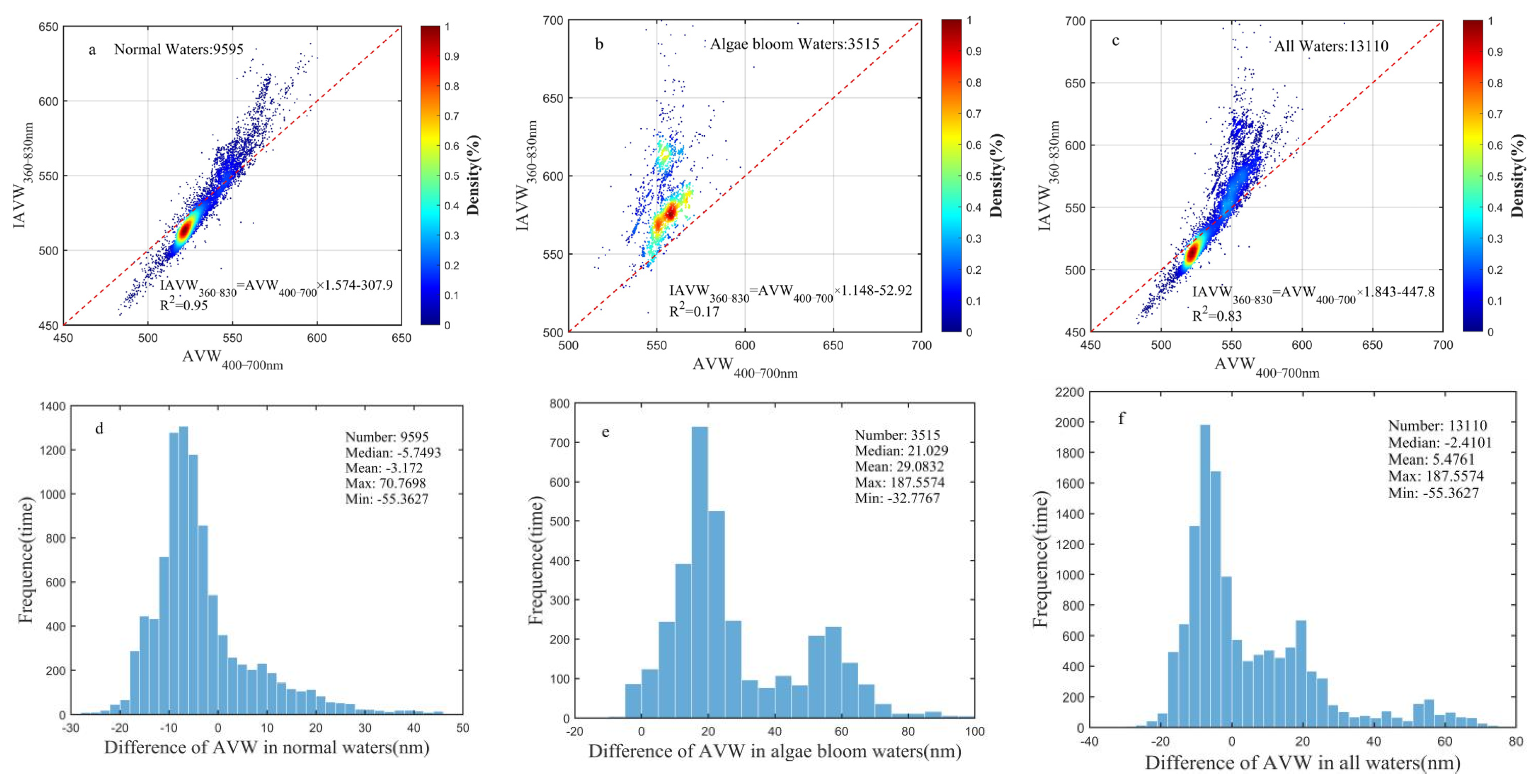
3.3. Wavelength Range Unification Impact on AVW
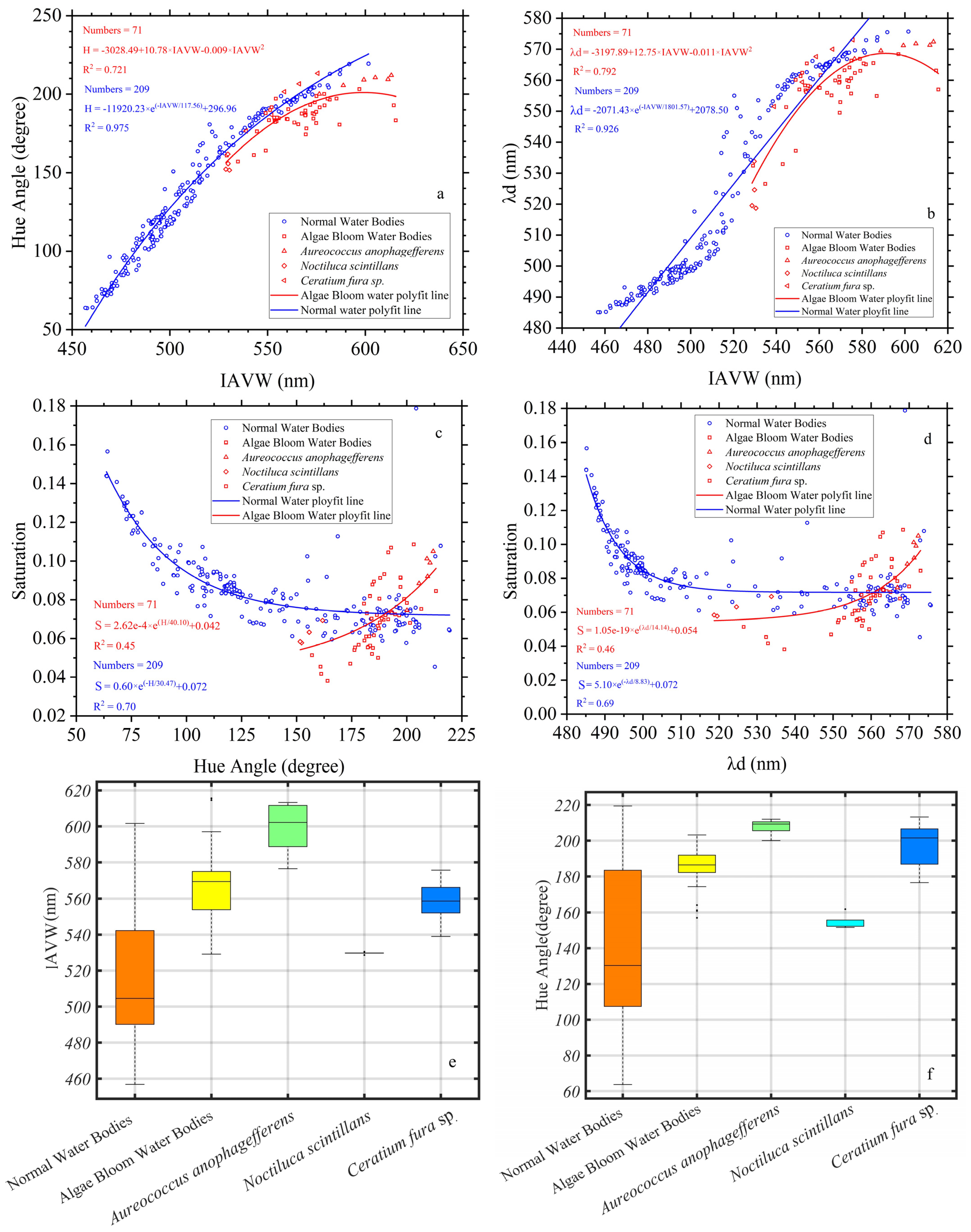
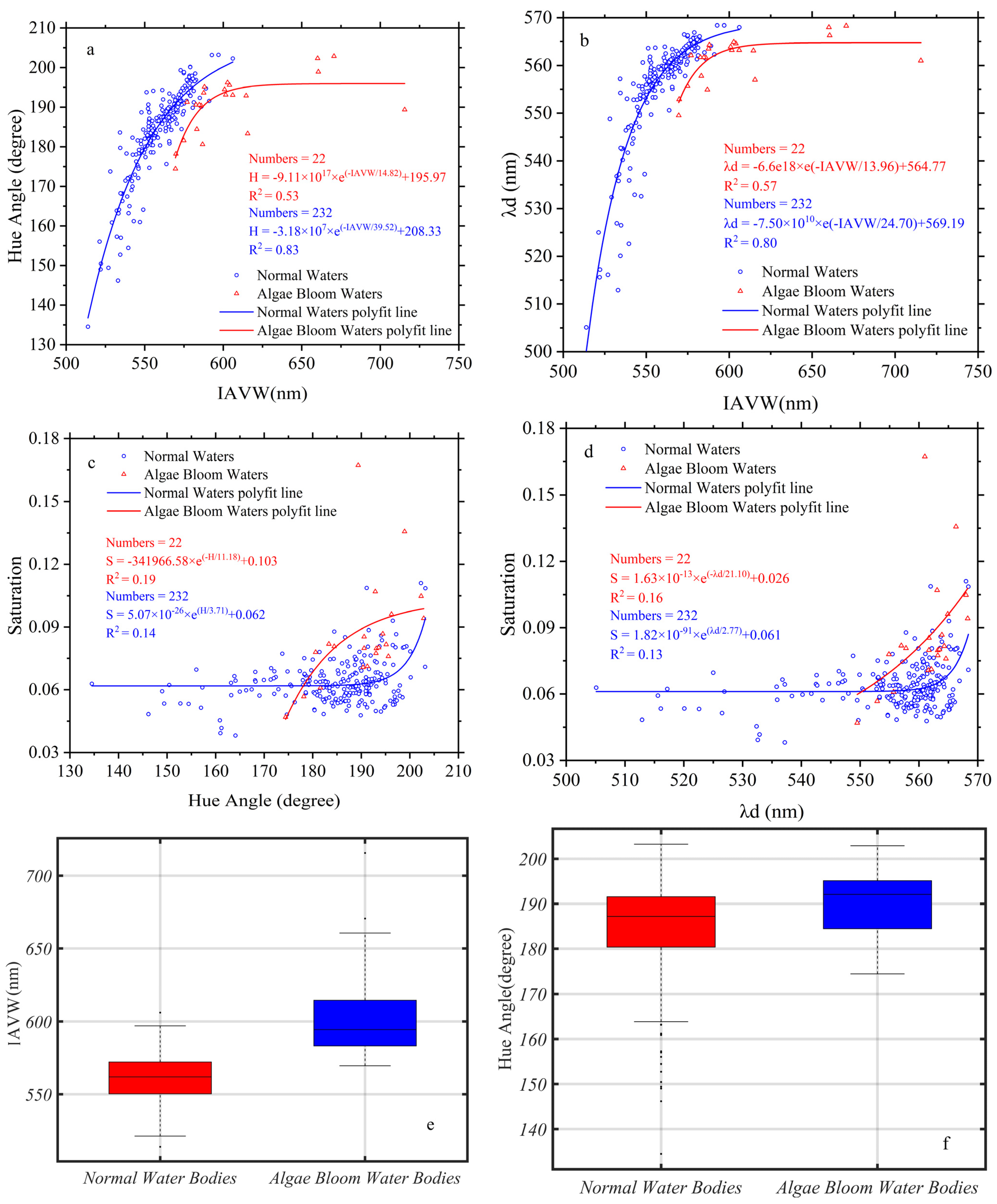
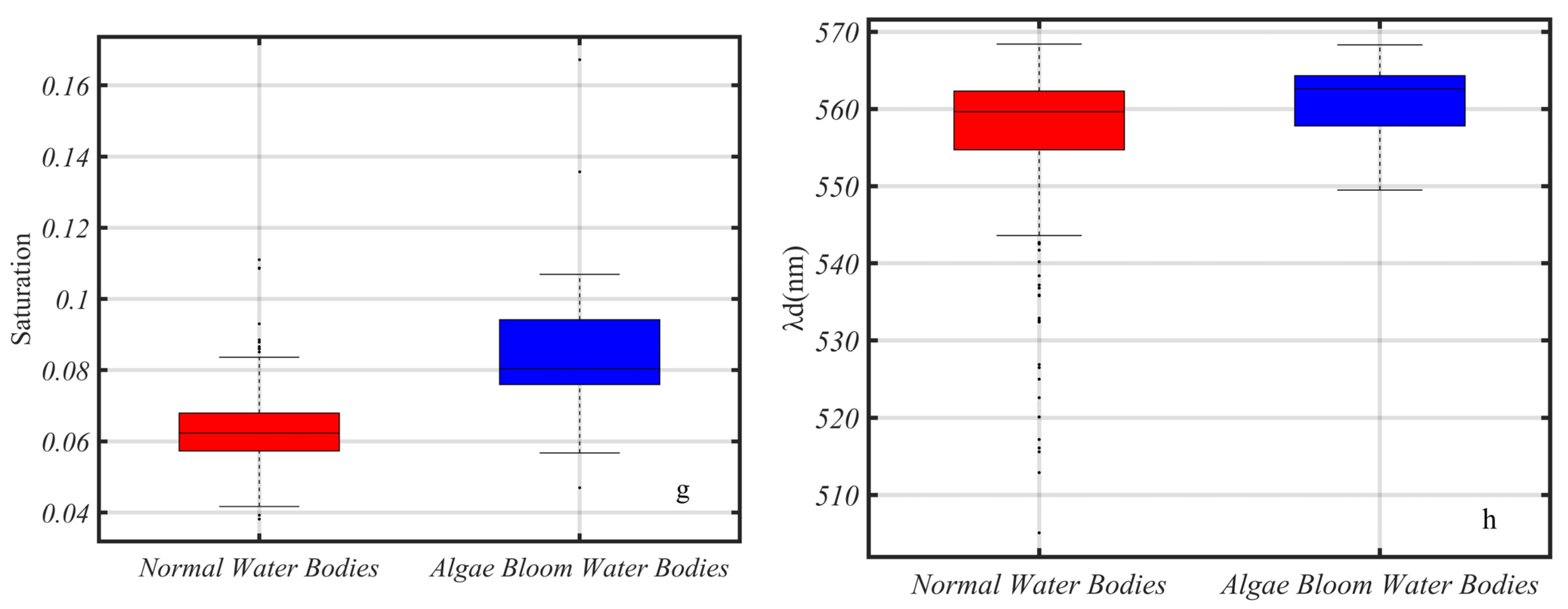
3.4. The Chromatic Indices of Normal Water and Algal Bloom Waters
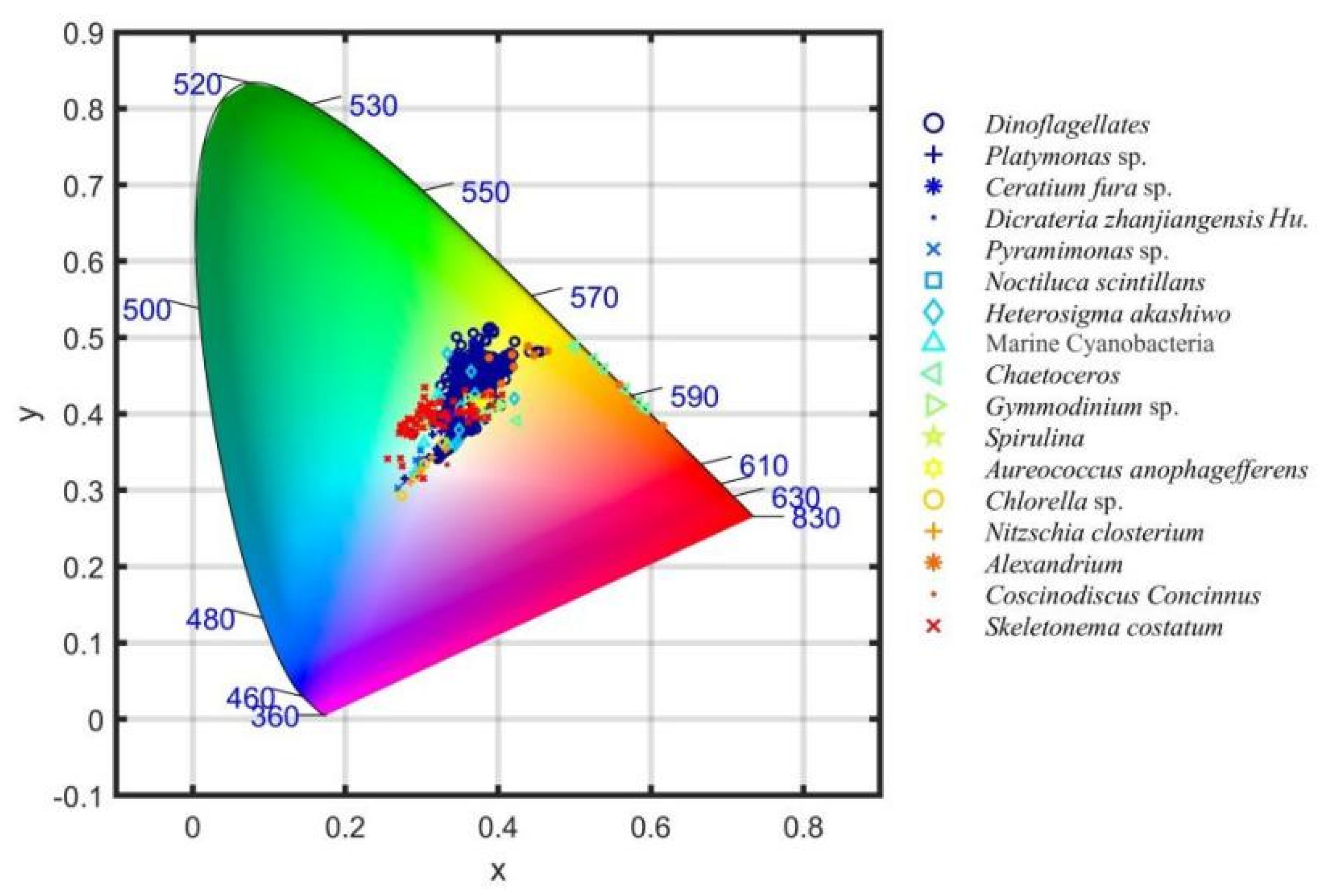
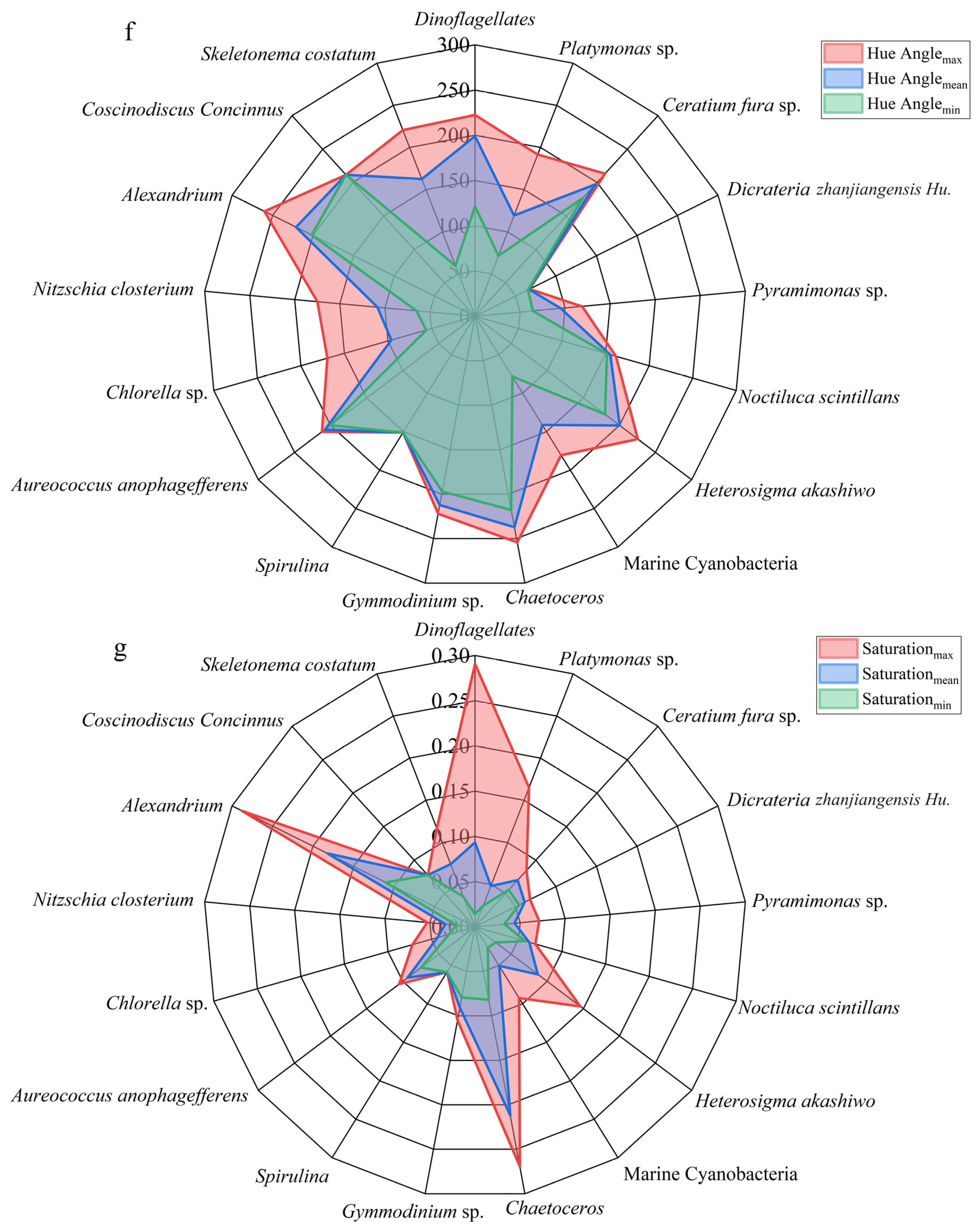
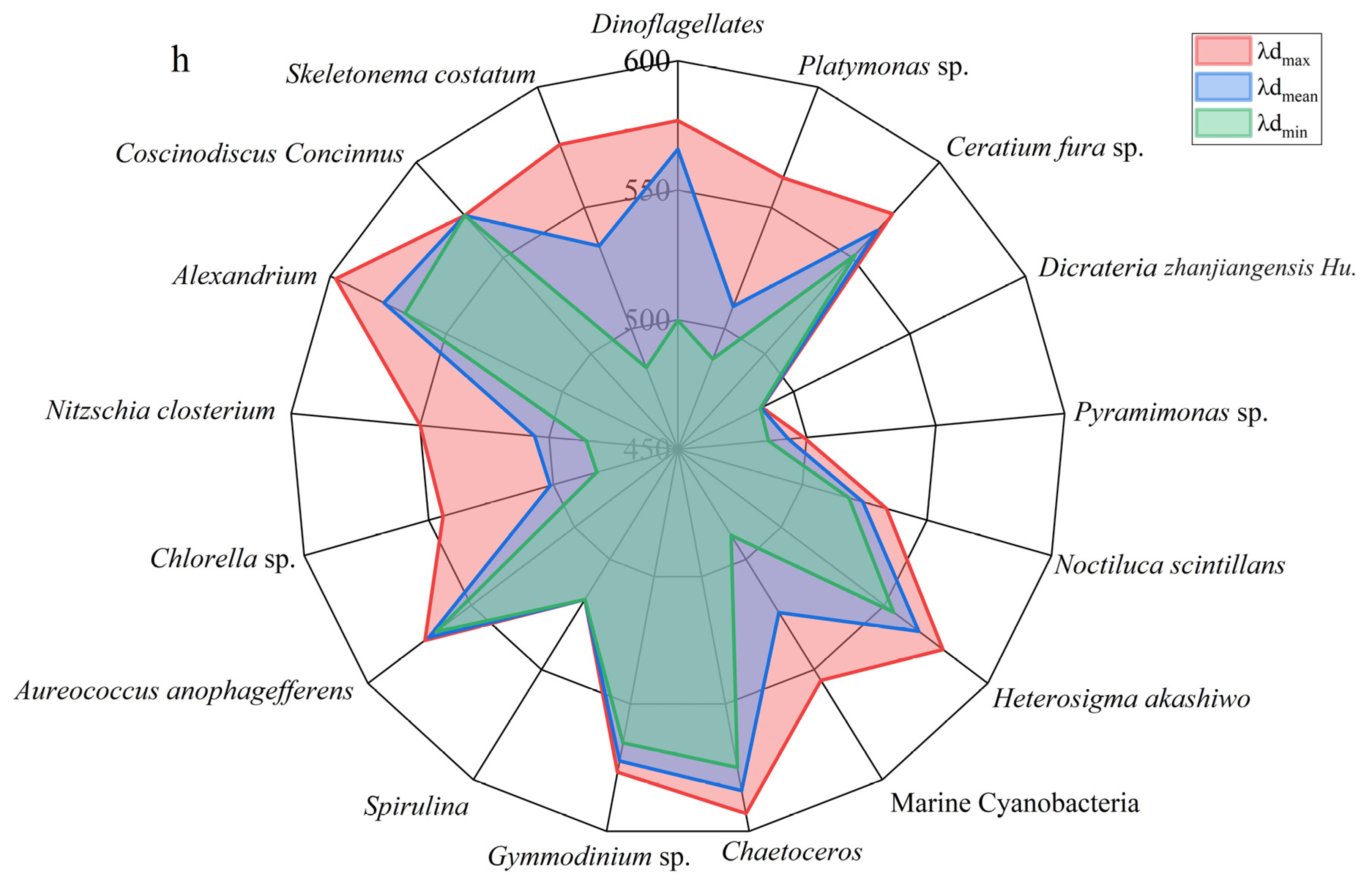
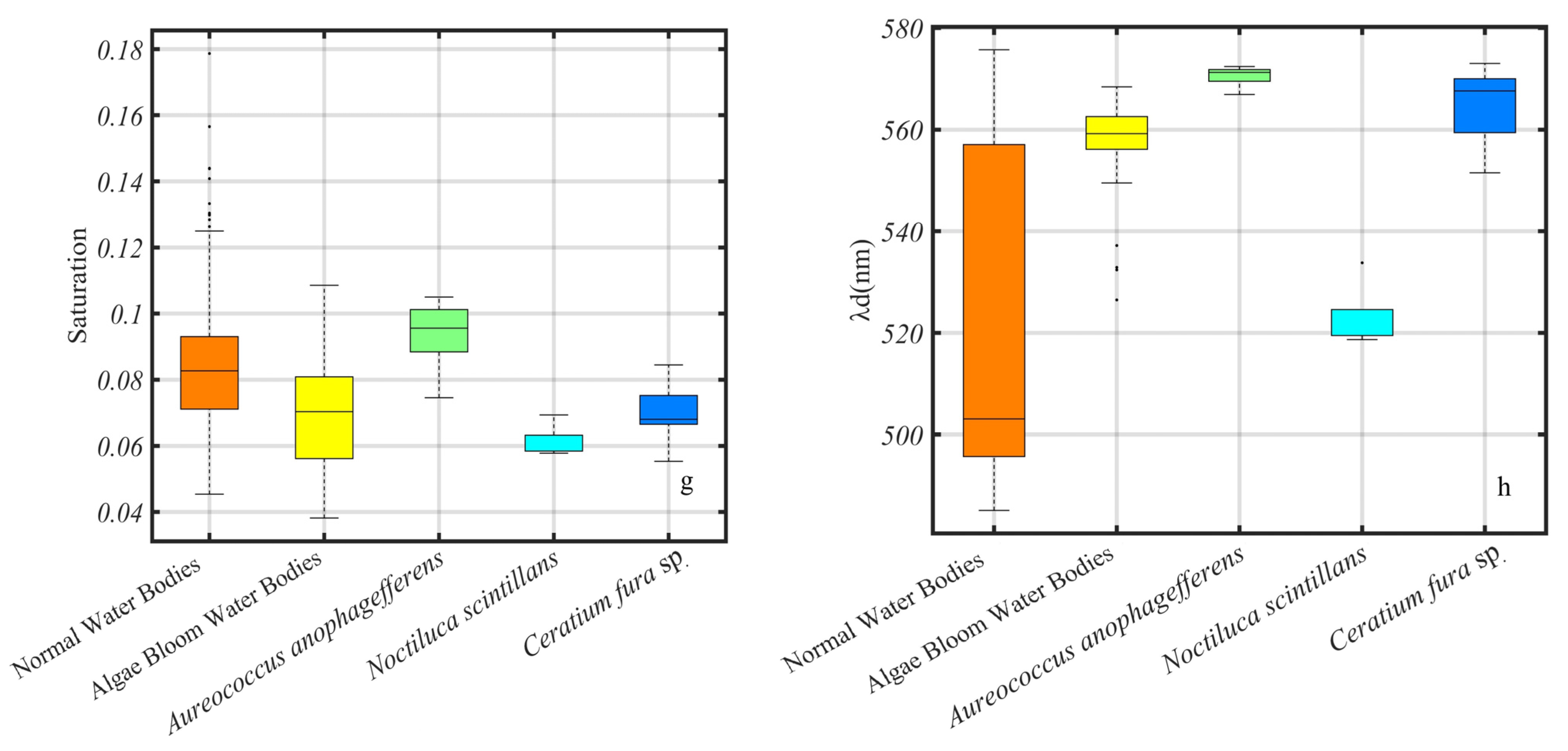
3.5. The Chromatic Indices of the Different Algal Species
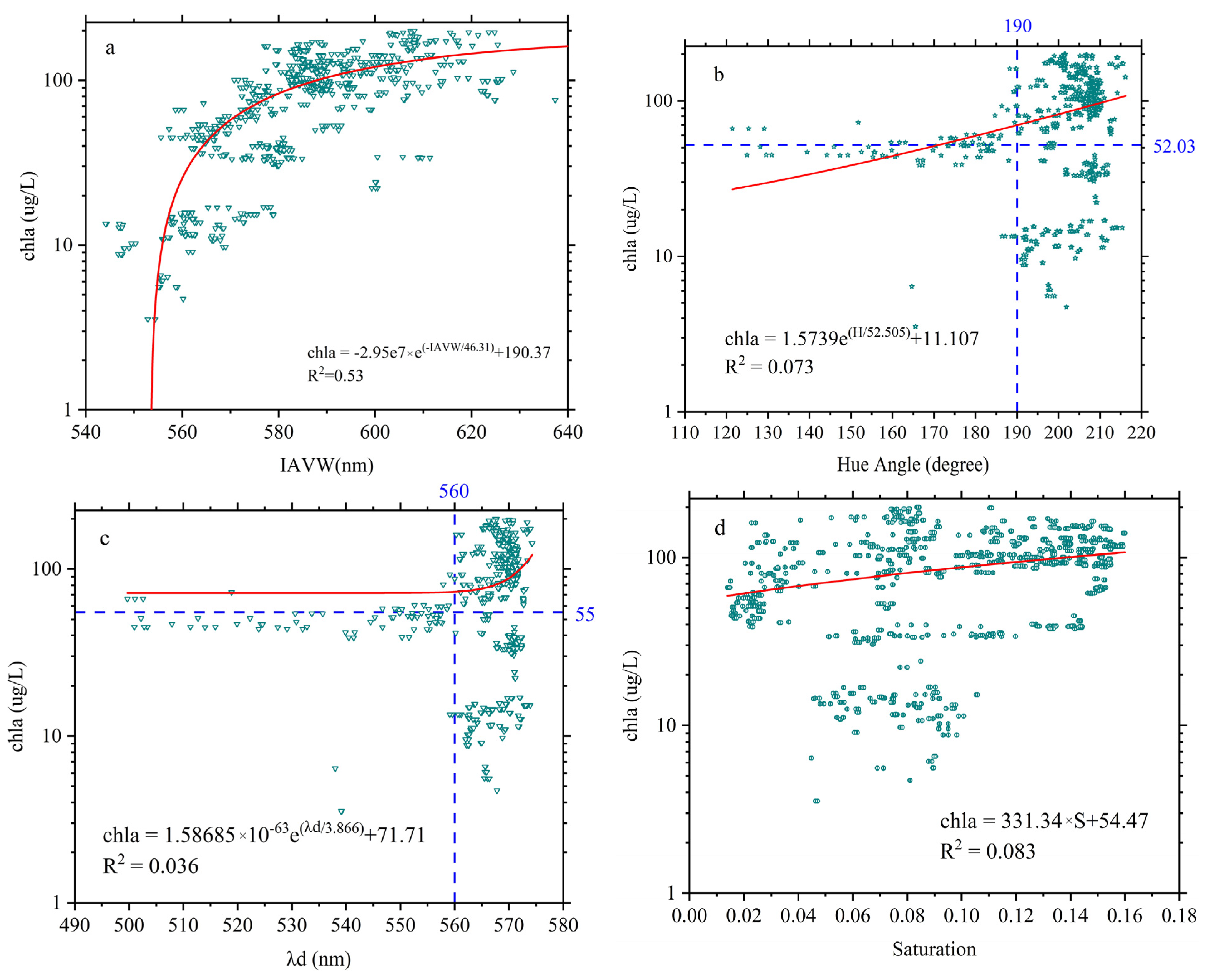
3.6. The Chromatic Indices of the Same Algae with Different Chlorophyll Concentrations
4. Discussion
4.1. IAVW (360–830 nm) and AVW (400–700 nm)
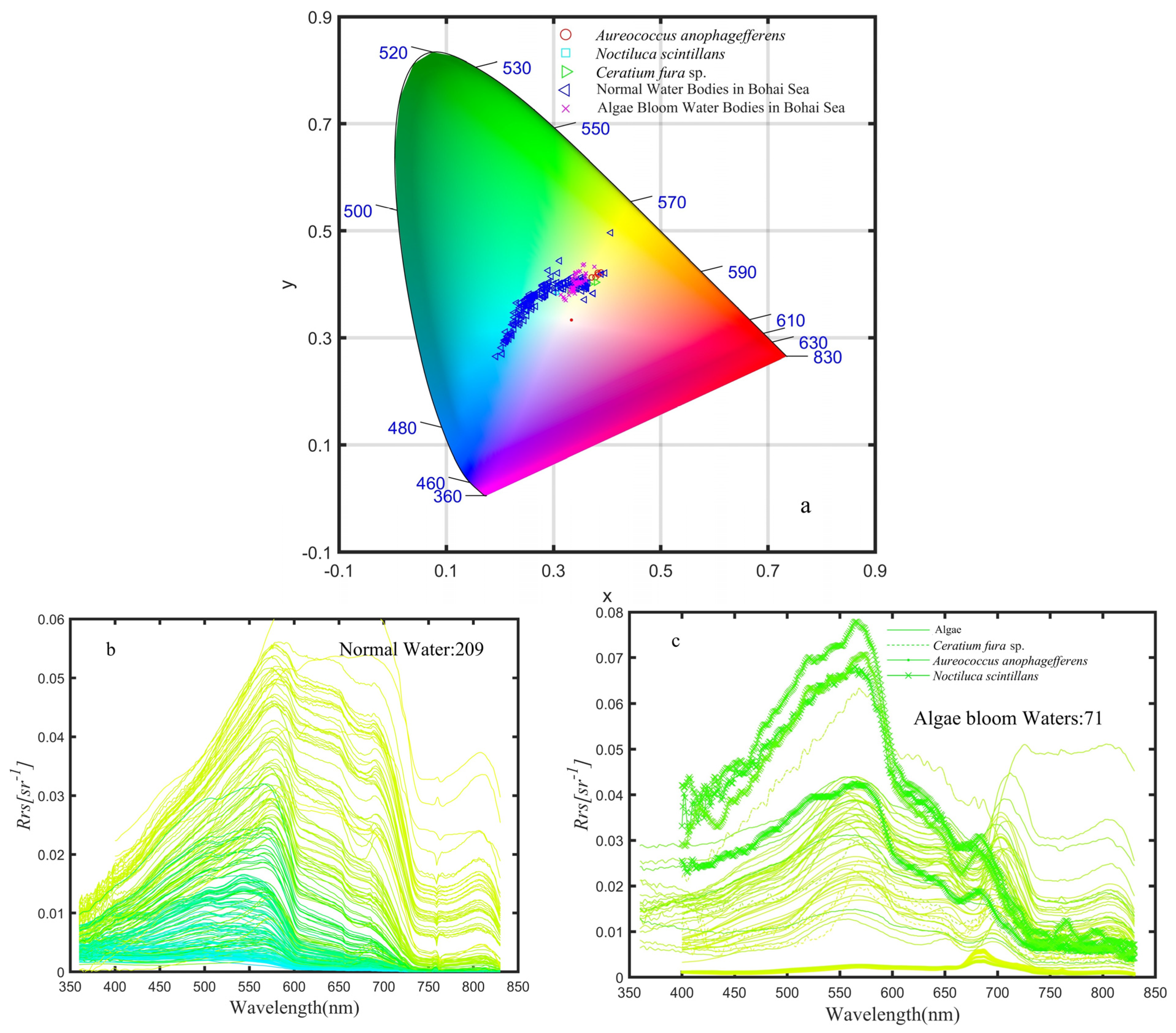
4.2. Case Analysis
4.2.1. Case of Coastal Water in the Bohai Sea
4.2.2. Case of Inland Water in Taihu Lake
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Dominate Wavelength (λd) | X | Y | Z | Hue Angle |
|---|---|---|---|---|
| 360 | 0.0001299 | 3.920 × 10−6 | 0.0006061 | 25.686 |
| 360.1 | 0.0001314 | 3.963 × 10−6 | 0.0006132 | 25.687 |
| 360.2 | 0.0001329 | 4.007 × 10−6 | 0.0006203 | 25.688 |
| 360.3 | 0.0001345 | 4.053 × 10−6 | 0.0006276 | 25.6883 |
| 360.4 | 0.0001361 | 4.098 × 10−6 | 0.0006349 | 25.689 |
| 360.5 | 0.0001376 | 4.1450 × 10−6 | 0.0006424 | 25.690 |
| 360.6 | 0.0001392 | 4.193 × 10−6 | 0.0006499 | 25.691 |
| … | … | … | … | … |
| 829.7 | 1.2747 × 10−6 | 4.618 × 10−7 | 1.801 × 10−109 | 279.546 |
| 829.8 | 1.266 × 10−6 | 4.585 × 10−7 | 1.453 × 10−109 | 279.556 |
| 829.9 | 1.258 × 10−6 | 4.553 × 10−7 | 8.628 × 10−110 | 279.570 |
References
- Zingone, A.; Enevoldsen, H.O. The diversity of harmful algal blooms: A challenge for science and management. Ocean. Coast. Manag. 2000, 43, 725–748. [Google Scholar] [CrossRef]
- Zhao, D.; Wen, S.; Song, L. Red Tide Disaster Risk Assessment Theory and Zoning Method; The Ocean Press: Beijing, China, 2013; Volume 5. [Google Scholar]
- Zhao, D. The Occurrence Law of Red Tide Disasters in Typical Sea Areas of China; Ocean Press: Beijing, China, 2010. [Google Scholar]
- Zhao, D.Z.; Zhao, L.; Zhang, F.S.; Zhang, X.Y. Temporal Occurrence and Spatial Distribution of Red Tide Events in China’s Coastal Waters. Hum. Ecol. Risk Assess. 2004, 10, 945–957. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Zhao, X.H.; Kang, X.Y.; Zhuang, M.M.; Ding, X.W.; Zhao, L.J.; Wen, Q.L.; Zhu, Y.; Gu, K.; Bao, Q.J.; et al. Good news: We can identify Ulva species that erupted in the Yellow Sea more easily and cheaply now. Conserv. Genet. Resour. 2020, 12, 447–449. [Google Scholar] [CrossRef]
- Gobler, C.J.; Sunda, W.G. Brown Tides. In Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006; pp. 111–123. [Google Scholar] [CrossRef]
- Gobler, C.J.; Sunda, W.G. Ecosystem disruptive algal blooms of the brown tide species, Aureococcus anophagefferens and Aureoumbra lagunensis. Harmful Algae 2012, 14, 36–45. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Qiu, L.M.; Yu, R.C.; Kong, F.Z.; Wang, Y.F.; Yan, T.; Gobler, C.J.; Zhou, M.J. Emergence of brown tides caused by Aureococcus anophagefferens Hargraves et Sieburth in China. Harmful Algae 2012, 19, 117–124. [Google Scholar] [CrossRef]
- Zhuang, M.M.; Liu, J.L.; Ding, X.W.; He, J.Z.; Zhao, S.; Wu, L.J.; Gao, S.; Zhao, C.Y.; Liu, D.Y.; Zhang, J.H.; et al. Sargassum blooms in the East China Sea and Yellow Sea: Formation and management. Mar. Pollut. Bull. 2021, 162, 111845. [Google Scholar] [CrossRef]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L.; et al. Marine harmful algal blooms (HABs) in the United States: History, current status and future trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef]
- Pinkerton, M.H.; Richardson, K.M.; Boyd, P.W.; Gall, M.P.; Zeldis, J.; Oliver, M.D.; Murphy, R.J. Intercomparison of ocean colour band-ratio algorithms for chlorophyll concentration in the Subtropical Front east of New Zealand. Remote Sens. Environ. 2005, 97, 382–402. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Wei, X.; Huang, Z.; Xue, K.; Ma, R.; Hu, Z. Uncertainty evaluation of water color parameters inversion in optically complex lakes. Environ. Sci. Technol. 2023, 46, 15–26. [Google Scholar]
- O’Reilly, J.E.; Werdell, P.J. Chlorophyll Algorithms for Ocean Color Sensors—Oc4, Oc5 & Oc6. Remote Sens. Environ. 2019, 229, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Merzlyak, M.N.; Chivkunova, O.B.; Maslova, I.P.; Naqvi, K.R.; Solovchenko, A.E.; Klyachko-Gurvich, G.L. Light absorption and scattering by cell suspensions of some cyanobacteria and microalgae. Russ. J. Plant Physiol. 2008, 55, 420–425. [Google Scholar] [CrossRef]
- Wang, M.J.; Wang, Z.Y.; Huang, C.J. Scattering Characteristics of Marine Mixed Suspended Particles to Blue and Green Lasers. Spectrosc. Spectr. Anal. 2022, 42, 1749–1754. [Google Scholar] [CrossRef]
- Wang, G.; Moisan, J. Remote Sensing of Phytoplankton Pigments. In Plankton Communities; IntechOpen: London, UK, 2022. [Google Scholar]
- Le, C.F.; Li, Y.M.; Zha, Y.; Sun, D.Y.; Huang, C.C.; Lu, H. A four-band semi-analytical model for estimating chlorophyll a in highly turbid lakes: The case of Taihu Lake, China. Remote Sens. Environ. 2009, 113, 1175–1182. [Google Scholar] [CrossRef]
- Luo, J.; Qin, L.; Mao, P.; Xiong, Y.; Zhao, W.; Gao, H.; Qiu, G. Research Progress in the Retrieval Algorithms for Chlorophyll-a, a Key Element of Water Quality Monitoring by Remote Sensing. Remote Sens. Technol. Appl. 2021, 36, 473–488. [Google Scholar]
- Zhao, D.; Zhang, F.; Du, F.; Zhao, L.; Guo, H. Study on Sun-Induced Chlorophyll Fluorescence Peaks (SICF) Characteristics in Water with Different Algal Species. J. Remote Sens. 2005, 9, 265–270. [Google Scholar]
- Gower, J.F.R. A simpler picture of satellite chlorophyll fluorescence. Remote Sens. Lett. 2014, 5, 583–589. [Google Scholar] [CrossRef]
- Lavigne, H.; Ruddick, K.; Vanhellemont, Q. Monitoring of high biomass Phaeocystis globosa blooms in the Southern North Sea by in situ and future spaceborne hyperspectral radiometry. Remote Sens. Environ. 2022, 282, 113270. [Google Scholar] [CrossRef]
- Li, X.; Huang, P.; Sun, W.; Fan, C.; You, L. Study of Chlorophyll-a Inversion Algorithm for Hengshan Reservoir Using Sentinel-2 Images. Hydro Sci. Cold Zone Eng. 2023, 6, 93–96. [Google Scholar]
- Moses, W.J.; Gitelson, A.A.; Berdnikov, S.; Povazhnyy, V. Estimation of chlorophyll-a concentration in case II waters using MODIS and MERIS data-successes and challenges. Environ. Res. Lett. 2009, 4, 045005. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, J.; Zhao, L.; Chen, Y.; Wang, L.; Shen, H. MODIS Bio-optical algorithms in Liaodong Bay(I) Algorithms assessment. Mar. Environ. Sci. 2007, 26, 401–407. [Google Scholar]
- Zhao, D.; Zhang, F.; Yang, J.; Guo, H.; Zhao, L. Band Optimization for Characterizing the Sun-Induced Chlorophyll Fluorescence Height in Red Tide Waters. Acta Oceanol. Sin. 2005, 27, 146–153. [Google Scholar]
- Wynne, T.; Meredith, A.; Briggs, T.; Litaker, W.; Stumpf, R. Harmful Algal Bloom Forecasting Branch Ocean Color Satellite Imagery Processing Guidelines; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2018; pp. 252–296.
- Bukata, R.P.; Pozdnyakov, D.V.; Jerome, J.H.; Tanis, F.J. Validation of a radiometric color model applicable to optically complex water bodies. Remote Sens. Environ. 2001, 77, 165–172. [Google Scholar] [CrossRef]
- Wernand, M.R.; Van Der Woerd, H.J. Spectral analysis of the Forel-Ule ocean colour comparator scale. J. Eur. Opt. Soc. Rapid Publ. 2010, 5, 10014s. [Google Scholar] [CrossRef]
- Novoa, S.; Wernand, M.R.; Van der Woerd, H.J. The Forel-Ule scale revisited spectrally: Preparation protocol, transmission measurements, and chromaticity. J. Eur. Opt. Soc. Rapid Publ. 2013, 8, 13057. [Google Scholar] [CrossRef]
- Vandermeulen, R.A.; Mannino, A.; Craig, S.E.; Werdell, P.J. 150 shades of green: Using the full spectrum of remote sensing reflectance to elucidate color shifts in the ocean. Remote Sens. Environ. 2020, 247, 111900. [Google Scholar] [CrossRef]
- Turner, K.J.; Tzortziou, M.; Grunert, B.K.; Goes, J.; Sherman, J. Optical classification of an urbanized estuary using hyperspectral remote sensing reflectance. Opt. Express 2022, 30, 41590–41612. [Google Scholar] [CrossRef] [PubMed]
- Stuart, V. Remote Sensing of Ocean Colour in Coastal, and Other Optically-Complex, Waters; International Ocean Colour Coordinating Group (IOCCG): Dartmouth, NS, Canada, 2000.
- Lehmann, M.K.; Gurlin, D.; Pahlevan, N.; Alikas, K. GLORIA—A globally representative hyperspectral in situ dataset for optical sensing of water quality. Sci. Data 2023, 10, 100, Erratum in Sci. Data. 2023, 10, 191. [Google Scholar] [CrossRef]
- Lavigne, H.; Dogliotti, A.; Doxaran, D.; Shen, F.; Castagna, A.; Beck, M.; Vanhellemont, Q.; Sun, X.; Gossn, J.I.; Renosh, P.R.; et al. The HYPERMAQ dataset: Bio-optical properties of moderately to extremely turbid waters. Earth Syst. Sci. Data 2022, 14, 4935–4947. [Google Scholar] [CrossRef]
- Knaeps, E.; Doxaran, D.; Dogliotti, A.; Nechad, B.; Ruddick, K.; Raymaekers, D.; Sterckx, S. The SeaSWIR dataset. Earth Syst. Sci. Data 2018, 10, 1439–1449. [Google Scholar] [CrossRef]
- Maier, P.M.; Keller, S. Estimating Chlorophyll a Concentrations of Several Inland Waters with Hyperspectral Data and Machine Learning Models. ISPRS Ann. Photogramm. Remote Sens. Spat. Inf. Sci. 2019, IV-2/W5, 609–614. [Google Scholar] [CrossRef]
- Garaba, S.; Wernand, M.; Zielinski, O. Quality control of automated hyperspectral remote sensing measurements from a seaborne platform. Ocean. Sci. Discuss. 2011, 8, 613–638. [Google Scholar]
- Legleiter, C.J.; King, T.V.; Carpenter, K.D.; Hall, N.C.; Mumford, A.C.; Slonecker, T.; Graham, J.L.; Stengel, V.G.; Simon, N.; Rosen, B.H. Spectral mixture analysis for surveillance of harmful algal blooms (SMASH): A field-, laboratory-, and satellite-based approach to identifying cyanobacteria genera from remotely sensed data. Remote Sens. Environ. 2022, 279, 113089. [Google Scholar] [CrossRef]
- Castagna, A.; Amadei Martínez, L.; Bogorad, M.; Daveloose, I.; Dasseville, R.; Dierssen, H.M.; Beck, M.; Mortelmans, J.; Lavigne, H.; Dogliotti, A.; et al. Optical and biogeochemical properties of diverse Belgian inland and coastal waters. Earth Syst. Sci. Data 2022, 14, 2697–2719. [Google Scholar] [CrossRef]
- Tilstone, G.H.; Pardo, S.; Simis, S.G.; Qin, P.; Selmes, N.; Dessailly, D.; Kwiatkowska, E. Consistency between Satellite Ocean Colour Products under High Coloured Dissolved Organic Matter Absorption in the Baltic Sea. Remote Sens. 2021, 14, 89. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, Y. Chinese Polluted Water Body Spectral Characteristics; The Ocean Press: Beijing, China, 2001. [Google Scholar]
- Xing, Q.; Hu, C. Mapping macroalgal blooms in the Yellow Sea and East China Sea using HJ-1 and Landsat data: Application of a virtual baseline reflectance height technique. Remote Sens. Environ. 2016, 178, 113–126. [Google Scholar] [CrossRef]
- An, D.; Xing, Q.; Wei, Z.; Li, L. Analysis of Spectral Features of Representative Floating Macroalgae in the Yellow Sea. J. Oceanol. Limnol. 2018, 49, 1054–1060. [Google Scholar]
- Zhao, D.; Zhang, F.; Du, F.; Zhao, L.; Guo, H. A Study on relation of chlorophyll-a concentration with the reflectance peak near 700 nm in algae-dominated waters and sensitivity of fluorescence algorithms for detecting algal bloom. Int. J. Remote Sens. 2010, 31, 39–48. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.; Zhao, D.; Wang, X.; Wang, X. Spectral variability of particulate backscattering of nanoplankton and microplankton—Focusing on skeletonema costatum and prorocentrum micans. Mar. Environ. Sci. 2013, 32, 884–888. [Google Scholar]
- Jiang, L.; Wang, L.; Zhao, D.; Wang, X. Study on the Backscattering Characteristics of Amphidinium carterae Hulburt. Spectrosc. Spectr. Anal. 2013, 33, 1892–1896. [Google Scholar]
- Jiang, L.; Wang, L.; Zhang, X.; Chen, Y.; Xiong, D. A Semianalytical Model Using MODIS Data to Estimate Cell Density of Red Tide Algae (Aureococcus anophagefferens). Adv. Meteorol. 2016, 2016, 1780986. [Google Scholar] [CrossRef]
- Slonecker, T.; Bufford, B.; Graham, J.; Carpenter, K.; Opstal, D.; Simon, N.; Hall, N. Hyperspectral reflectance characteristics of cyanobacteria. Adv. Remote Sens. 2021, 10, 66–77. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, K.-Q.; Sun, S.; Chen, K.; Tan, G.-S.; Zhang, J.; Wang, X.-L. Numerical simulation and eutrophication assessment of spatiotemporal distribution of nitrogen, phosphorus, and chlorophyll concentrations in the Bohai Sea. Oceanol. Limnol. Sin. 2023, 55, 118–134. [Google Scholar] [CrossRef]
- Wynne, T.T.; Stumpf, R.P.; Litaker, R.W.; Hood, R.R. Cyanobacterial bloom phenology in Saginaw Bay from MODIS and a comparative look with western Lake Erie. Harmful Algae 2021, 103, 101999. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.J.; Cummins, H.Z. Light and Color in Nature and Art; John Wiley and Sons: Hoboken, NJ, USA, 1985; Volume 10, pp. 123–124. [Google Scholar]
- Bukata, R.P.; Jerome, J.H.; Kondratyev, A.S.; Pozdnyakov, D.V. Optical Properties and Remote Sensing of Inland and Coastal Waters; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Ohta, N.; Robertson, A. Colorimetry: Fundamentals and Applications; John Wiley and Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Woerd, H.J.; Wernand, M.R. True colour classification of natural waters with medium-spectral resolution satellites: SeaWiFS, MODIS, MERIS, and OLCI. Sensors 2015, 15, 25663–25680. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N.; Robertson, A. Colorimetry Fundamentals and Application; Noboru Ohta Rochester Institute of Technology: Rochester, NY, USA, 2005. [Google Scholar]
- Gardner, J.R.; Yang, X.; Topp, S.N.; Ross, M.R.; Altenau, E.H.; Pavelsky, T.M. The Color of Rivers. Geophys. Res. Lett. 2021, 48, e2020GL088946. [Google Scholar] [CrossRef]
- Li, J. MODIS observations of water color of the largest 10 lakes in China between 2000 and 2012. Int. J. Digit. Earth 2016, 9, 788–805. [Google Scholar] [CrossRef]




| No. | The Name of the Spectral Library | Reference | Year | Data Number | Wavelength Range (nm) | Resolution (nm) | Data Source |
|---|---|---|---|---|---|---|---|
| a | GLORIA | [36] | 1990–2022 | 7572 | 350–900 | 1 | https://doi.pangaea.de/10.1594/PANGAEA.948492 accessed on 1 June 2023 |
| b | HYPERMAQ | [37] | 2022 | 111 | 350–900 | 2.5 | https://doi.pangaea.de/10.1594/PANGAEA.944313 accessed on 1 June 2023 |
| c | SeaSWIR | [38] | 2012–2013 | 137,200 | 350–1300, 350–900 | 1, 2.5 | https://doi.pangaea.de/10.1594/PANGAEA.886287 accessed on 1 June 2023 |
| d | SpecWa | [39] | 2018–2019 | 3685 | 389.35–910.32 | 0.74 | https://dataservices.gfz-potsdam.de/panmetaworks/showshort.php?id=6800b0c8-dd51-11ea-9603-497c92695674 accessed on 1 June 2023 |
| e | NORCOHAB II | [40] | 2009 | 44 | 320–950 | 5 | https://doi.pangaea.de/10.1594/PANGAEA.753830?format=html#download accessed on 1 June 2023 |
| f | SMASH | [41] | 2020 | 222 | 325–1075 | 1 | https://www.sciencebase.gov/catalog/item/5fe38f8ed34ea5387deb4923 accessed on 1 June 2023 |
| g | Belgian inland and coastal waters | [42] | 2017–2019 | 14,220 | 380–850, 380–900 | 1 | https://doi.pangaea.de/10.1594/PANGAEA.940240 accessed on 20 October 2022 |
| h | The Baltic Sea dataset | [43] | 2016 | 5805 | 320–953.6 | 3.3 | https://zenodo.org/record/5572537 accessed on 16 October 2023 |
| i | Spectrum of Polluted Water in China | [44] | 2001 | 35 | 393.8–1041.5 398.3–1043.61 | 2.7, 2.69 | accessed on 1 June 2023 |
| j | The Bohai and Huanghai Sea Dataset | in situ bio-optical dataset (2014–2018) measured by Zhongfeng Qiu and Shengqiang Wang | 2014–2018 | 30, 36, 65 | 350–2500 | 1 | in situ accessed on 1 June 2023 |
| k | Ulva prolifera, Sargassum | [45] | 2016, 2018 | 10, 18 | 350.11–999.99 347.07–1040.46 | 0.17 | http://dx.doi.org/10.1016/j.rse.2016.02.065 accessed on 7 August 2024 |
| l | [46] | http://qdhys.ijournal.cn/hyyhz/ch/reader/view_abstract.aspx?doi=10.11693/hyhz20171200331 accessed on 20 June 2023 | |||||
| m | Spectrum of Red Tide | [47] | 2010 | 6, 5, 9, 5, 4, 1, 2, 5, 4, 4, 5, 1 | 396.6–1041.91, 393.8–1041.5, 398.3–1043.61 | 2.69, 2.7, 2.69 | https://www.tandfonline.com/doi/full/10.1080/01431160902882512 accessed on 6 June 2023 |
| n | Bohai Sea 863 Dataset China | Measure by Dongzhi Zhao, 863 Project | 2003–2017 | 31, 54, 15, 45, 22, 26, 51 | 350–1050, 342.5–844.1, 342.5–2509.9, 320–946, 320–950, 325–1072, 400–900, 350–900 | 1, 1.6, 1.2, 1, 1, 1, 1 | in situ accessed on 6 June 2023 |
| o | Taihu Lake Dataset China | Provided by Hongtao Duan et al. | 2021 | 25 | 400–1072 | 1 | in situ accessed on 10 July 2023 |
| p | Chaohu Lake Dataset China | Provided by Hongtao Duan et al. | 2020 | 20 | 400–1072 | 1 | in situ |
| q | Prorocentrum micans | [48] | 2004 | 5 | 400–750 | 1 | https://doi.org/10.3964/j.issn.1000-0593(2013)07-1892-05 accessed on 1 September 2023 |
| r | Amphidiniumcarterae Hulburt | [49] | 2013 | 7 | 400–750 | 1 | https://doi.org/10.3964/j.issn.1000-0593(2013)07-1892-05 accessed on 1 September 2023 |
| s | Skeletonema costatum | [48] | 2014 | 4 | 400–752 | 1 | https://doi.org/10.3964/j.issn.1000-0593(2013)07-1892-05 accessed on 1 September 2023 |
| t | Aureococcus anophagefferens | [50] | 2016 | 6 | 400–899 | 1 | http://dx.doi.org/10.1155/2016/1780986 accessed on 1 September 2023 |
| u | Hyperspectral Reflectance Characteristics of Cyanobacteria | [51] | 2021 | 13 | 400–800 | 1 | https://doi.org/10.4236/ars.2021.103004 accessed on 1 September 2023 |
| Spectral Range (nm) | 360–830 nm | 390–830 nm | 400–830 nm | Total |
|---|---|---|---|---|
| Inland Water | 1767 | 76 | 844 | 2687 |
| Coastal Water | 6885 | 0 | 23 | 6908 |
| Total | 8652 | 76 | 867 | 9595 |
| The Colors of the Tides | Algae Species | Spectral Range (nm) | Data Numbers | Increments (nm) | Measurement Technique |
|---|---|---|---|---|---|
| Red Tides | Ceratium fura sp. | 400–830 | 5 | 1 | in vivo |
| Dinoflagellates | 360–830 | 34 | 1 | in vivo, in situ | |
| 390–830 | 2656 | in situ | |||
| 400–830 | 418 | in situ | |||
| Gymmodinium sp. | 400–830 | 6 | 1 | in vivo | |
| Nitzschia closterium | 400–830 | 4 | 1 | in vivo | |
| Noctiluca scintillans | 400–830 | 5 | 1 | in vivo | |
| Coscinodiscus Concinnus | 400–830 | 1 | 1 | in vivo | |
| Spirulina | 400–830 | 1 | 1 | in vivo | |
| Alexandrium | 400–830 | 10 | 1 | in vivo | |
| Heterosigma akashiwo | 400–830 | 9 | 1 | in vivo | |
| Brown Tides | Aureococcus anophagefferens | 400–830 | 6 | 1 | in situ |
| Dicrateria zhanjiangensis Hu. | 400–830 | 10 | 1 | in vivo | |
| Green Tides | Ulva prolifera | 360–830 | 29 | 1 | in situ |
| 400–830 | 6 | in situ | |||
| Pyramimonas sp. | 400–830 | 5 | 1 | in vivo | |
| Platymonas sp. | 400–830 | 8 | 1 | in vivo | |
| Chlorella sp. | 400–830 | 4 | 1 | in vivo | |
| Green-Blue Tides | Marine Cyanobacteria | 400–830 | 4 | 1 | in vivo |
| Cyanobacteria | 360–830 | 242 | 1 | in situ | |
| 400–830 | 19 | in situ | |||
| Gloden Tides | Sargassum | 360–830 | 5 | 1 | in situ |
| 390–830 | 11 | in situ | |||
| 400–830 | 3 | in situ | |||
| Chaetoceros | 400–830 | 9 | 1 | in vivo | |
| Skeletonema costatum | 400–830 | 4 | 1 | in situ |
| The Colors of the Tides | Algae Species | H | S | λd | IAVW |
|---|---|---|---|---|---|
| Red Tides | Ceratium fura sp. | 176.6–213.3 | 0.055–0.085 | 551.5–573 | 539.0–575.7 |
| Dinoflagellates | 121.5–222.4 | 0.014–0.291 | 499.8–576.9 | 512.3–697.7 | |
| Gymmodinium sp. | 197.0–222.1 | 0.079–0.105 | 565.3–576.8 | 590.3–609.1 | |
| Nitzschia closterium | 64.9–175.0 | 0.021–0.053 | 485.5–550 | 593.2–618.9 | |
| Noctiluca scintillans | 151.6–161.8 | 0.058–0.069 | 518.7–533.8 | 528.7–530.5 | |
| Coscinodiscus Concinnus | 211.5–211.5 | 0.077–0.077 | 572.2–572.2 | 590.5–590.5 | |
| Spirulina | 151.3–151.3 | 0.059–0.059 | 518.2–518.2 | 621.2–621.2 | |
| Alexandrium | 201.6–260.0 | 0.110–0.288 | 567.7–597.6 | 613.7–727.1 | |
| Heterosigma akashiwo | 180.0–225.5 | 0.0287–0.146 | 554.4–578.3 | 539.9–634.6 | |
| Brown Tides | Aureococcus anophagefferens | 200.1–212.0 | 0.075–0.105 | 566.9–572.4 | 576.5–613.3 |
| Dicrateria zhanjiangensis Hu. | 65.4–67.4 | 0.0543–0.068 | 485.6–486.2 | 540.9–571.1 | |
| Green Tides | Ulva prolifera | 167.1–207.0 | 0.070–0.176 | 541.2–570.2 | 600.3–734.6 |
| Platymonas sp. | 71.7–191.7 | 0.025–0.165 | 487.4–562.4 | 554.1–682.7 | |
| Pyramimonas sp. | 64.2–118.9 | 0.0327–0.071 | 485.2–498.9 | 541.6–586.5 | |
| Chlorella sp. | 56.1–169.6 | 0.030–0.072 | 482.5–544.3 | 547.5–652.1 | |
| Green-Blue Tides | Marine Cyanobacteria | 78.4–180.6 | 0.027–0.092 | 489.1–554.9 | 590.8–701.0 |
| Cyanobacteria | 157.0–203.2 | 0.036–0.109 | 526.5–568.4 | 529.16–615.6 | |
| Golden Tides | Skeletonema costatum | 59.7–220.7 | 0.036–0.117 | 483.8–576.2 | 500.5–600.0 |
| Chaetoceros | 217.9–254.2 | 0.0821–0.270 | 575–593.1 | 628.0–709.2 | |
| Sargassum | 187.4–241.1 | 0.029–0.143 | 559.7–585.4 | 617.0–742.9 |
| Type of the Water Bodies | Wavelength Range (nm) | Numbers of the Spectral Data | Total | |
|---|---|---|---|---|
| Normal Water Bodies | 360–830 | 206 | 209 | |
| 400–830 | 3 | |||
| Algal Bloom Water Bodies | Unknown Algae | 360–830 | 22 | 71 |
| 400–830 | 23 | |||
| Noctiluca scintillans | 400–830 | 10 | ||
| Aureococcus anophagefferens | 400–830 | 6 | ||
| Ceratium fura sp. | 400–830 | 10 | ||
| Type of the Water Bodies | Wavelength Range (nm) | Numbers of the Spectral Data | Total |
|---|---|---|---|
| Normal Water Bodies | 360–830 | 215 | 232 |
| 400–830 | 17 | ||
| Algae Bloom Water Bodies | 360–830 | 5 | 22 |
| 400–830 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Luo, Q.; Qiu, Z. Chromaticity-Based Discrimination of Algal Bloom from Inland and Coastal Waters Using In Situ Hyperspectral Remote Sensing Reflectance. Water 2024, 16, 2276. https://doi.org/10.3390/w16162276
Zhao D, Luo Q, Qiu Z. Chromaticity-Based Discrimination of Algal Bloom from Inland and Coastal Waters Using In Situ Hyperspectral Remote Sensing Reflectance. Water. 2024; 16(16):2276. https://doi.org/10.3390/w16162276
Chicago/Turabian StyleZhao, Dongzhi, Qinshun Luo, and Zhongfeng Qiu. 2024. "Chromaticity-Based Discrimination of Algal Bloom from Inland and Coastal Waters Using In Situ Hyperspectral Remote Sensing Reflectance" Water 16, no. 16: 2276. https://doi.org/10.3390/w16162276
APA StyleZhao, D., Luo, Q., & Qiu, Z. (2024). Chromaticity-Based Discrimination of Algal Bloom from Inland and Coastal Waters Using In Situ Hyperspectral Remote Sensing Reflectance. Water, 16(16), 2276. https://doi.org/10.3390/w16162276






