Xylem Formation in Populus euphratica and Its Response to Environmental Factors in the Lower Reaches of Tarim River, China
Abstract
1. Introduction
2. Materials and Methods
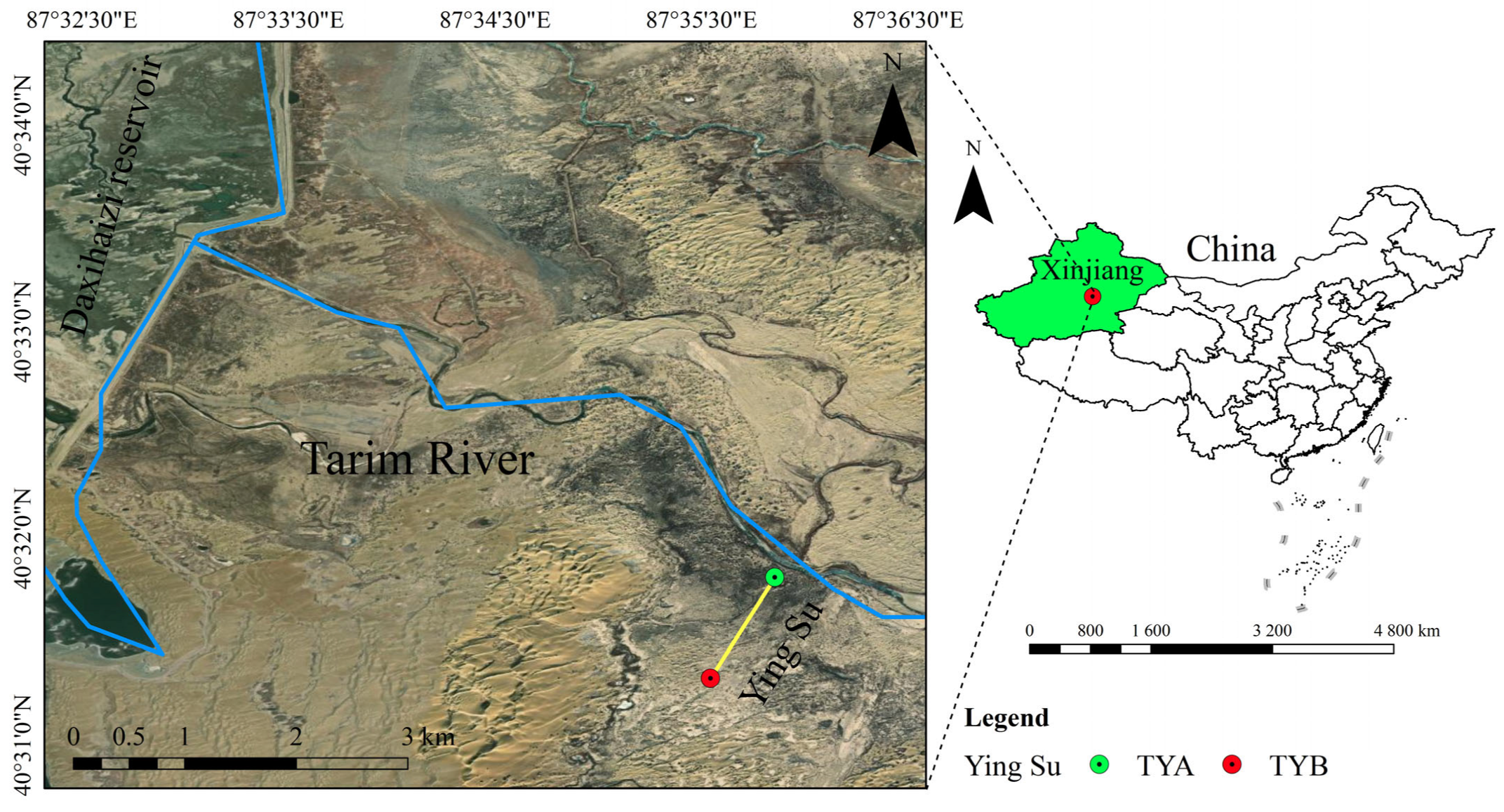
2.1. Overview of the Study Area
2.2. Experimental Design
2.3. Sample Processing
2.4. Data Processing
3. Results
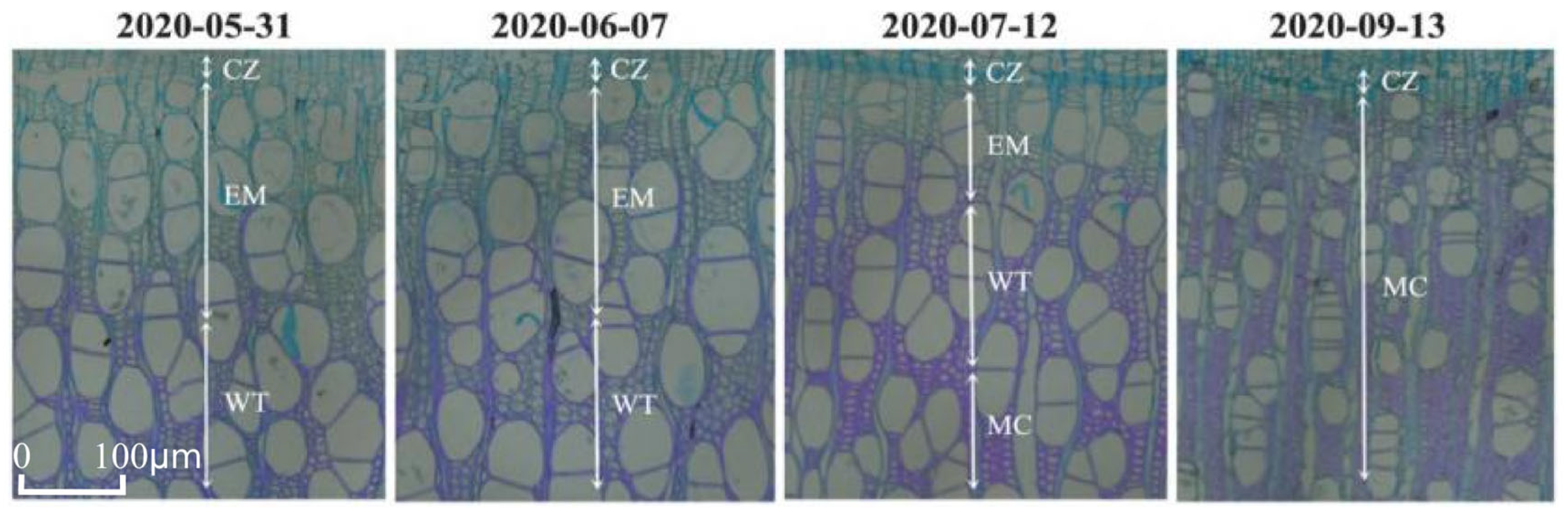
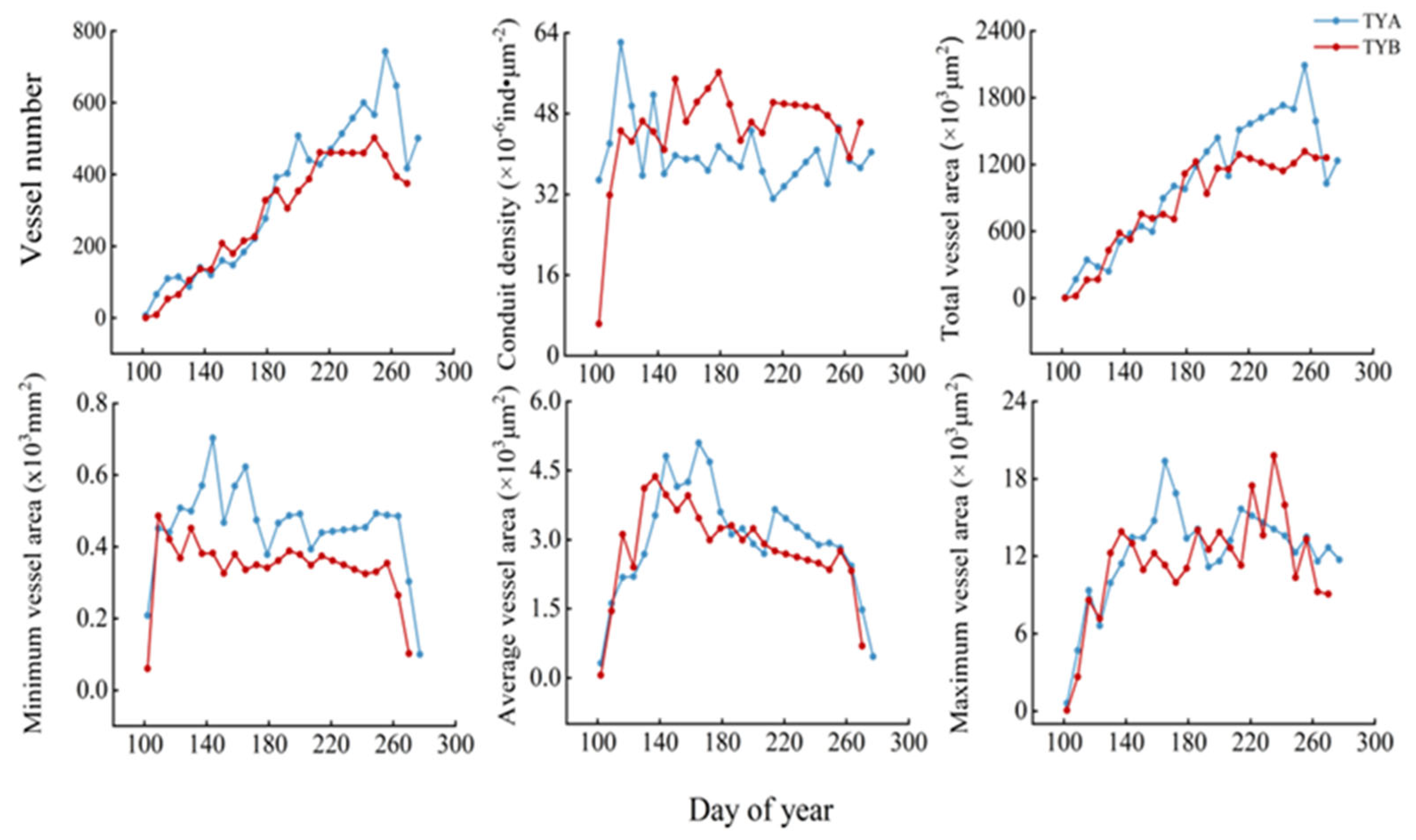
3.1. Intra-Annual Variation in Xylem Formation in Populus euphratica
3.2. Response of Populus euphratica Xylem Anatomical Parameters to Environmental Factors
3.2.1. Principal Component Analysis of Anatomical Parameters of Populus euphratica Xylem
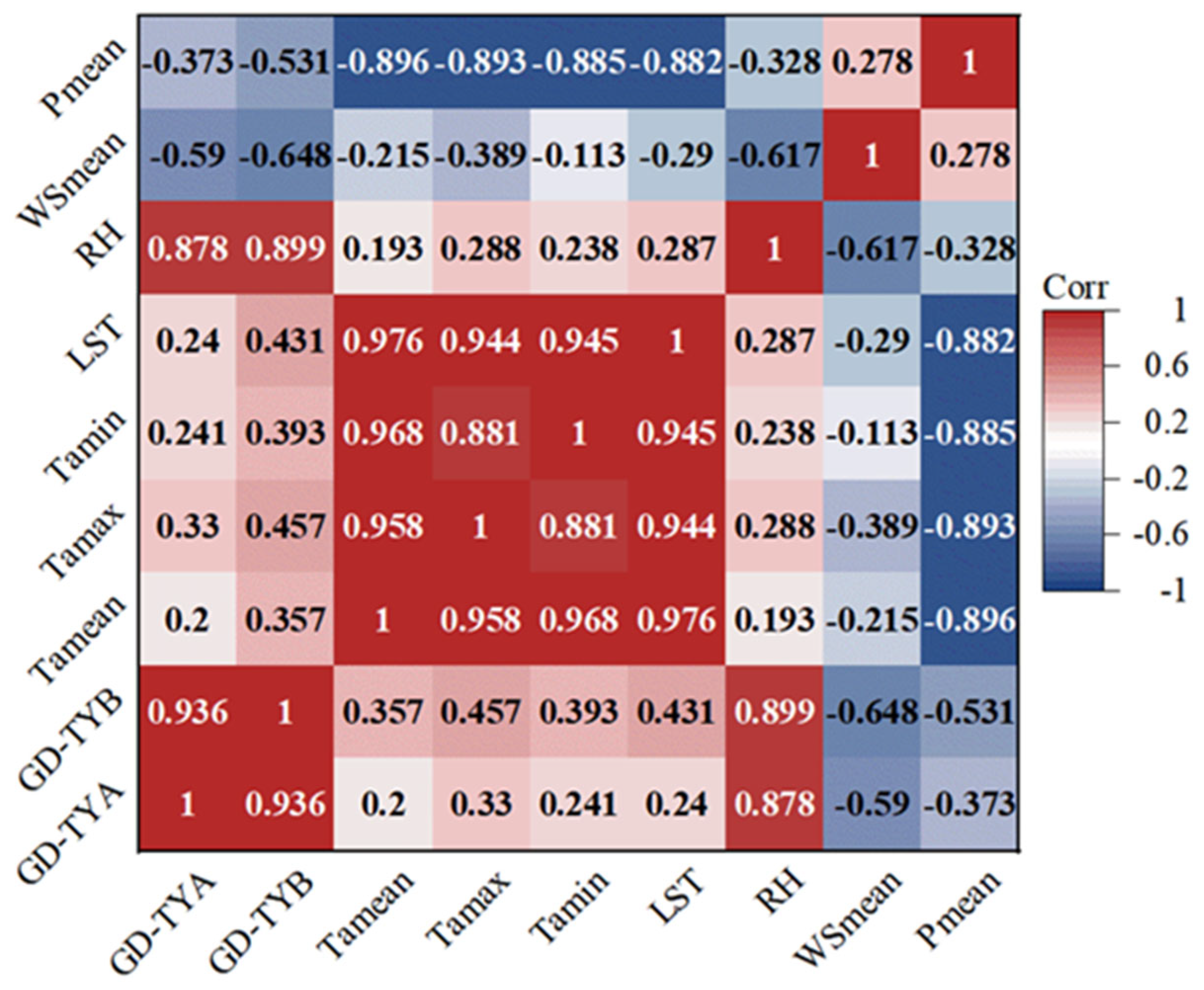
3.2.2. Principal Components of Xylem Parameters of Populus euphratica in Relation to Environmental Factors
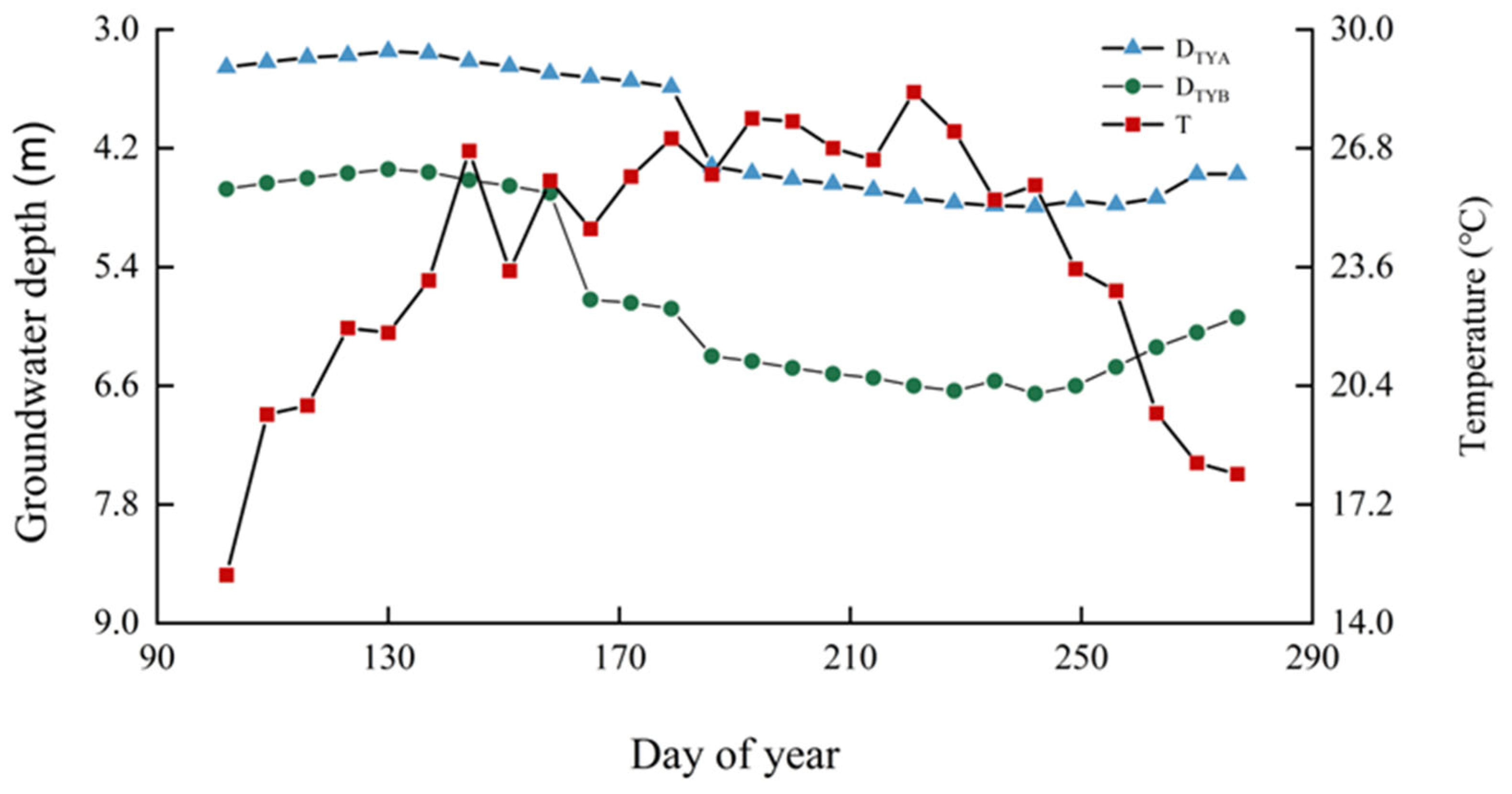
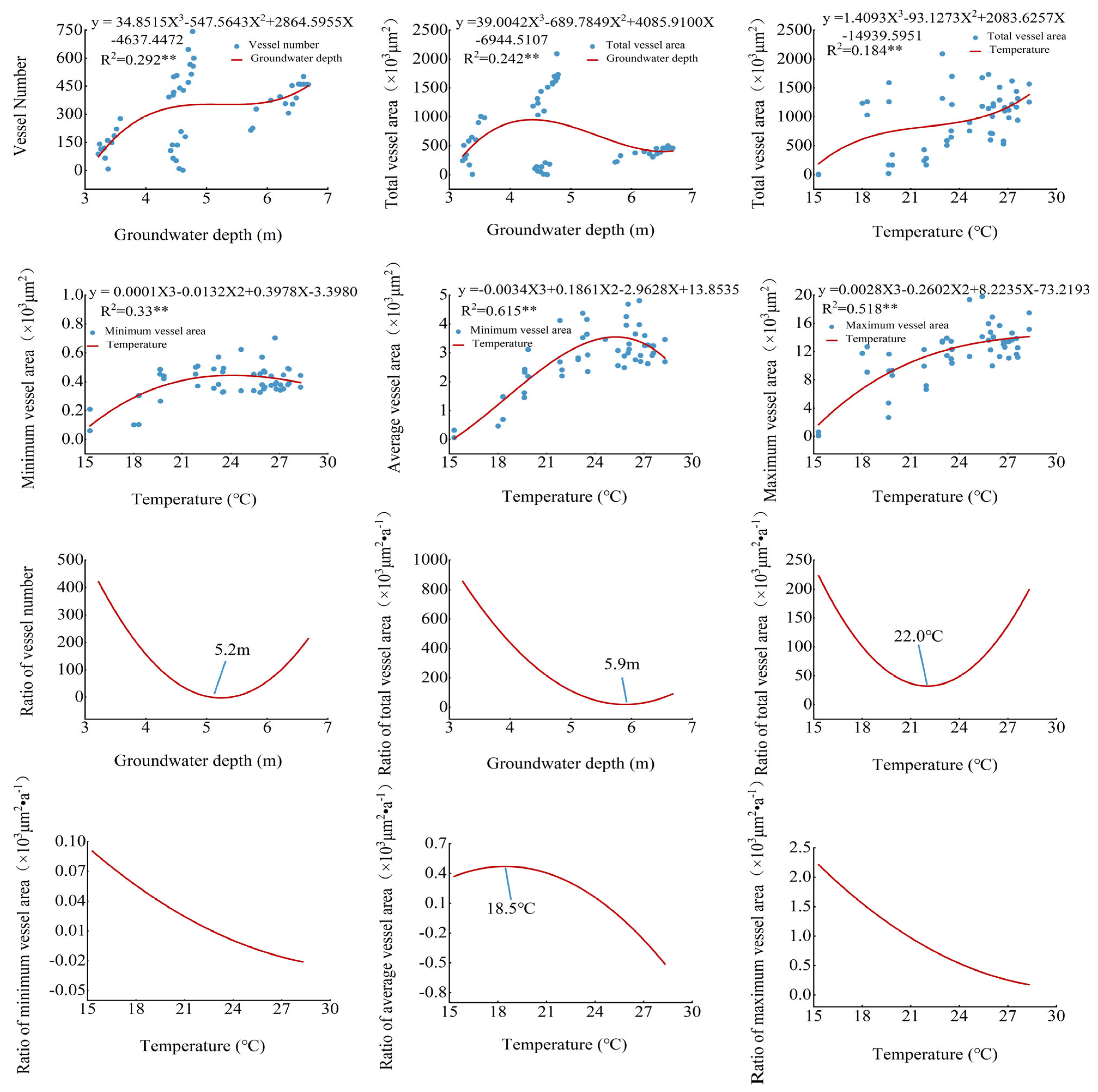
3.3. Populus euphratica Xylem Conduit Parameters in Relation to Temperature and Depth of Groundwater Burial
3.4. Contribution of Hydrothermal Factors to the Effect of Various Parameters of the Xylem Conduit of Populus euphratica
4. Discussion
4.1. Relationship between Anatomical Characteristics of Populus euphratica Xylem and Depth of Groundwater Burial
4.2. Relationship between Anatomical Characteristics of Populus euphratica Xylem and Air Temperature
4.3. Contribution of Air Temperature and Groundwater to Various Parameters of Xylem Anatomy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, R.; Ren, X.L.; Gai, A.H.; He, H.L.; Zhang, L.; Li, P. Use of CERN for estimating the soil conservation capability of typical forest ecosystems in China. Acta Ecol. Sin. 2020, 40, 2310–2320. [Google Scholar]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Nan, H.; Huntingford, C.; Ciais, P.; Friedlingstein, P.; Sitch, S.; Peng, S.; Ahlström, A.; Canadell, J.G.; Cong, N.; et al. Evidence for a weakening relationship between interannual temperature variability and northern vegetation activity. Nat. Commun. 2014, 5, 5018. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.G.; Bergeron, Y.; Zhai, L.H.; Denneler, B. Variation in intra-annual radial growth (xylem formation) of Picea mariana (Pinaceae) along a latitudinal gradient in western Québec, Canada. Am. J. Bot. 2011, 98, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Cuny, H.E.; Rathgeber, C.B.K.; Frank, D.; Fonti, P.; Fournier, M. Kinetics of tracheid development explain conifer tree-ring structure. New Phytol. 2014, 203, 1231–1241. [Google Scholar] [CrossRef]
- Fonti, P.; von Arx, G.; García-González, I.; Eilmann, B.; Sass-Klaassen, U.; Gärtner, H.; Eckstein, D. Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. New Phytol. 2010, 185, 42–53. [Google Scholar] [CrossRef]
- Islam, M.; Rahman, M.; Brauning, A. Xylem anatomical responses of diffuse porous Chukrasia tabularis to climate in a South Asian moist tropical forest. For. Ecol. Manag. 2018, 412, 9–20. [Google Scholar] [CrossRef]
- Zhu, L.J.; Li, Z.S.; Wang, X.C. Anatomical characteristics of xylem in tree rings and its relationship with environments. Chin. J. Plant Ecol. 2017, 41, 238–251. [Google Scholar]
- Ferrenberg, S. Dwarf mistletoe infection interacts with tree growth rate to produce opposing direct and indirect effects on resin duct defenses in lodgepole pine. Forests 2020, 11, 222. [Google Scholar] [CrossRef]
- Vázquez-González, C.; Zas, R.; Erbilgin, N.; Ferrenberg, S.; Rozas, V.; Sampedro, L. Resin ducts as resistance traits in conifers: Linking dendrochronology and resin-based defences. Tree Physiol. 2020, 40, 1313–1326. [Google Scholar] [CrossRef]
- Zhao, P.Q.; Zhao, P.; Niu, J.F.; Zhu, L.W.; Ni, G.Y.; Gao, J.G.; Zhang, Z.Z. Relationship between Vessel Characteristics and Sap Flow of Eight Subtropical Tree Species. J. Trop. Subtrop. Bot. 2014, 22, 537–548. [Google Scholar]
- Wimmer, R.; Grabner, M. Effects of climate on vertical resin duct density and radial growth of Norway spruce [Picea abies (L.) Karst.]. Trees 1997, 11, 271–276. [Google Scholar] [CrossRef]
- Vázquez-González, C.; López-Goldar, X.; Zas, R.; Sampedro, L. Neutral and climate-driven adaptive processes contribute to explain population variation in resin duct traits in a Mediterranean pine species. Front. Plant Sci. 2019, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, J.; Campelo, F.; García-González, I.; Nabais, C. Environmental control of vessel traits in Quercus ilex under Mediterranean climate: Relating xylem anatomy to function. Trees 2013, 27, 655–662. [Google Scholar] [CrossRef]
- Garcia-gonzalez, I.; Fonti, P. Selecting earlywood vessels to maximize their environmental signal. Tree Physiol. 2006, 26, 1289–1296. [Google Scholar] [CrossRef]
- Arbellay, E.; Stoffel, M.; Bollschweiler, M. Wood anatomical analysis of Alnus incana and Betula pendula injured by a debris-flow event. Tree Physiol. 2010, 30, 1290–1298. [Google Scholar] [CrossRef]
- Leal, S.; Sousa, V.B.; Knapic, S.; Louzada, J.L.; Pereira, H. Vessel size and number are contributors to define wood density in cork oak. Eur. J. For. Res. 2011, 130, 1023–1029. [Google Scholar] [CrossRef]
- Gou, X.X.; Ye, M.; Wang, L.L.; Gou, X.H. Sensitivity of Radial growth of populus to temperature in the upper reaches of the Tarim River. Arid Zone Res. 2018, 35, 899–904. [Google Scholar]
- An, H.Y.; Xu, H.L.; Ye, M.; Yu, P.J.; Gong, J.J. The relationship between Populus euphratica’s radial increment and groundwater level at the lower reach of Tarim River. Acta Ecol. Sin. 2011, 31, 2053–2059. [Google Scholar]
- Ren, B.J. Rare desert poplar forests in the world. Xinjiang For. 1980, 2, 2. [Google Scholar]
- Liu, J.Z.; Chen, Y.N.; Chen, Y.J.; Zhang, N.; Li, W.H. Degradation of Populus euphratica community in the lower reaches of the Tarim River, Xinjiang, China. J. Environ. Sci. 2005, 17, 740–747. [Google Scholar]
- Aishan, T.; Halik, Ü.; Betz, F.; Tiyip, T.; Ding, J.; Nuermaimaiti, Y.; Teyip, T. Stand structure and height-diameter relationship of a degraded Populus euphratica forest in the lower reaches of the Tarim River, Northwest China. J. Arid Land 2015, 7, 544–554. [Google Scholar] [CrossRef]
- Huang, Y.; Bao, A.M.; Wang, S.F.; Wang, Y.Q.; Duan, Y.B. Eco-environmental change in the lower Tarim River under the influence of intermittent water transport. Acta Geogr. Sin. 2013, 68, 1251–1262. [Google Scholar]
- Ye, M.; Xu, H.L.; Song, Y.D. Analysis of water resources utilization and its change trend in tarim river basin. Chin. Sci. Bull. 2006, S1, 14–20. [Google Scholar]
- Bai, Y.; Xu, H.L.; Zhang, Q.Q.; Ye, M. Evaluation on ecological water requirement in the lower reaches of Tarim Riner based on groudwater restoration. Acta Ecol. Sin. 2015, 35, 630–640. [Google Scholar]
- Zheng, Z.P. Microcoring Based Monitoring the Intra Annual Growth of Typical Conifer Species in the Humid Subtropics. Ph.D. Thesis, Normal University, Fuzhou, China, 2019. [Google Scholar]
- An, H.Y.; Xu, H.L.; Ye, M.; Yu, P.J.; Gong, Z.Z. Spatial and temporal changes in radial growth of poplar after ecological water transfer in the lower Tarim River. Chin. J. Appl. Ecol. 2011, 22, 29–34. [Google Scholar]
- Chen, L.R.; Li, Y.Y. Response of stem hydraulic traits in Stlix psammophila and Caragana korshinskii to manipulated precipitation variation. Chin. J. Appl. Ecol. 2018, 29, 507–514. [Google Scholar]
- Gou, X.X.; Zhang, T.W.; Yuan, Y.J.; Yu, S.L.; Jiang, S.X.; Guo, Y.L. Radial growth of dominant coniferous species and their responses to climate changes in the Altay Mountains, China. Chin. J. Appl. Ecol. 2021, 32, 3594–3608. [Google Scholar]
- Zheng, Q.Y.; Zhang, G.S.; Zhao, B.Q.; Wang, X.C. Xylem anatomical characteristics of Fraxinus and mandshurica with climate in different slope positions. Chin. J. Appl. Ecol. 2021, 32, 3428–3436. [Google Scholar]
- Zhang, H.; Fu, P.L.; Lin, Y.X.; Ge, S.; Yang, J.Q.; Ge, R.; Qu, Z.; Fan, Z.X. Intra-annual radial growth of Abies georgei and Larix potaninii and its responses to environmental factors in the Baima Mountain, Northwest Yunnan, China. Chin. J. Appl. Ecol. 2022, 33, 2881–2888. [Google Scholar]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Berlyn, G.P. Plant Structures: Xylem Structure and the Ascent of Sap. Science 1983, 222, 500–501. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Liu, H. Precipitation pulses and ecosystem responses in arid and semiarid regions. Chin. J. Appl. Ecol. 2011, 22, 243–249. [Google Scholar]
- Sun, Y.X.; Zhang, J.; Zhou, X.B.; Tao, Y.; Zhan, Y.M. Stem hydraulic architecture of Malus sieversii in degraded wild fruit forest in Ili Valley, China. Chin. J. Appl. Ecol. 2020, 31, 3340–3348. [Google Scholar]
- Campelo, F.; Nabais, C.; Gutierrez, E.; Freitas, H.; García-González, I. Vessel features of Quercus ilex L. growing under Mediterranean climate have a better climatic signal than tree-ring width. Trees 2010, 24, 463–470. [Google Scholar] [CrossRef]
- Denne, M.P. Temperature and tracheid development in Pinus sylvestris seedlings. J. Exp. Bot. 1971, 22, 362–370. [Google Scholar] [CrossRef]
- Xiao, S.C.; Xiao, H.L.; Peng, X.M. Study of Seasonal stem radial growth of Populus euphratica in the lower reaches of the Heihe River. J. Glaciol. Geocryol. 2012, 34, 706–712. [Google Scholar]
- Cheng, R.M.; Liu, Z.B.; Feng, X.H.; Xiao, W.F. Advances in research on the effect of climatic change on xylem growth of trees. Sci. Silvae Sin. 2015, 51, 147–154. [Google Scholar]



| Site | Tree Age (Year) | DBH (m) | Vessel Number | Vessel Density (×10−6 ind·μm−2) | Total Vessel Area (×103 μm2) | Minimum Vessel Area (×103 μm2) | Average Vessel Area (×103 μm2) | Maximum Vessel Area (×103 μm2) |
|---|---|---|---|---|---|---|---|---|
| TYA | 32.87 | 52.84 | 316.4 ± 208.7 | 39.46 ± 7.74 | 964.03 ± 556.13 | 0.46 ± 0.12 | 2.94 ± 1.22 | 11.96 ± 3.85 |
| TYB | 28.45 | 45 | 264.34 ± 153.3 | 44.48 ± 9.83 | 833.78 ± 432.52 | 0.33 ± 0.10 | 2.75 ± 1.10 | 11.50 ± 6.19 |
| Site | Main Component | Trait Root (Math.) | Variance Contribution/% | Cumulative Variance Contribution/% |
|---|---|---|---|---|
| TYA | 1 | 2.68 | 44.65 | 44.65 |
| 2 | 1.20 | 33.20 | 77.85 | |
| TYB | 1 | 3.29 | 54.83 | 54.83 |
| 2 | 1.90 | 31.63 | 86.46 |
| Site | Anatomical Parameters | Principal Component 1 | Principal Component 2 |
|---|---|---|---|
| TYA | Vessel number | 0.89 | 0.196 |
| Vessel density | 0.811 | −0.531 | |
| Total vessel area | −0.41 | 0.241 | |
| Minimum vessel | 0.408 | 0.799 | |
| Average vessel area | 0.671 | 0.717 | |
| Maximum vessel area | 0.666 | −0.678 | |
| TYB | Vessel number | 0.907 | 0.02 |
| Vessel density | 0.872 | 0.188 | |
| Total vessel area | 0.794 | −0.574 | |
| Minimum vessel | 0.756 | −0.616 | |
| Average vessel area | 0.397 | 0.796 | |
| Maximum vessel area | 0.589 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; He, Q.; Ye, M.; Chen, W.; Zeng, G.; Pan, X.; Zhang, X. Xylem Formation in Populus euphratica and Its Response to Environmental Factors in the Lower Reaches of Tarim River, China. Water 2024, 16, 1974. https://doi.org/10.3390/w16141974
Li M, He Q, Ye M, Chen W, Zeng G, Pan X, Zhang X. Xylem Formation in Populus euphratica and Its Response to Environmental Factors in the Lower Reaches of Tarim River, China. Water. 2024; 16(14):1974. https://doi.org/10.3390/w16141974
Chicago/Turabian StyleLi, Miaomiao, Qingzhi He, Mao Ye, Weilong Chen, Guoyan Zeng, Xiaoting Pan, and Xi Zhang. 2024. "Xylem Formation in Populus euphratica and Its Response to Environmental Factors in the Lower Reaches of Tarim River, China" Water 16, no. 14: 1974. https://doi.org/10.3390/w16141974
APA StyleLi, M., He, Q., Ye, M., Chen, W., Zeng, G., Pan, X., & Zhang, X. (2024). Xylem Formation in Populus euphratica and Its Response to Environmental Factors in the Lower Reaches of Tarim River, China. Water, 16(14), 1974. https://doi.org/10.3390/w16141974





