1. Introduction
Chromium is widely used in electroplating, leather tanning, and other industries [
1]. Due to improper disposal and inadvertent leakage, a substantial amount of chromium has been released and entered groundwater systems [
2]. In aquifers, chromium predominantly exists in two stable oxidation states: hexavalent chromium (Cr(VI)) and trivalent chromium (Cr(III)) [
3]. The Cr(VI) species, known for their carcinogenic, mutagenic, and teratogenic properties, manifest high solubility in aqueous solutions and thus are identified as paramount contaminants. In contrast, the Cr(III) species exhibit low solubility and mobility in aquifers and are essential elements for organisms [
4]. Therefore, there is an urgent need to remove Cr(VI) by reducing it to Cr(III) in Cr(VI)-contaminated groundwaters.
The Cr(VI) removal methods in practice include chemical precipitation, photocatalysis, and electrochemical treatment, but they often involve secondary contamination, special reaction conditions, and are energy intensive [
5,
6]. Among various techniques, zero-valent iron (Fe
0) has been widely used as an efficient material in permeable reactive barriers for Cr(VI) removal from groundwater [
7]. In Fe
0-H
2O systems, Cr(VI) is screened and reduced to Cr(III) with Fe
0 being oxidized to Fe(II) and Fe(III). The resultant Fe(III) tends to react with Cr(III) to generate sparingly soluble Fe-Cr hydroxides. Passive Fe-Cr hydroxides formed on the Fe
0 surface serve as a physical barrier limiting electron transfer from the iron core to the surface and ceasing further Fe
0 corrosion (Equations (1)–(3)) [
8].
It is reported that incorporating hydrogen-autotrophic microbes into Fe
0-H
2O systems is an effective strategy to promote Fe
0 corrosion and biogenic iron mineral formation for enhanced Cr(VI) removal [
9]. In biotic Fe
0 systems, H
2 from Fe
0 corrosion can serve as electron donors for autotrophic microbes (e.g., denitrifying, sulfate-reducing, and methanogenic bacteria) to reduce Cr(VI) (Equation (4)) [
10,
11].
However, Fe
0 in groundwater remediation inevitably suffers from interferences by coexisting geochemical constituents such as humic acid (HA) [
12]. Humic acid is known for its strong binding affinity with Fe
3+ to form HA-Fe complexes. These complexes readily turn into macroaggregates by association of hardness ions such as calcium [
13]. Compared with iron oxides, the macroaggregates with low conductivity and thicker dimensions result in greater blockage of the Fe
0 surface and stronger inhibitory effect on Cr(VI) removal [
14]. The depletion of H
2 kinetically promotes Fe
0 corrosion and secondary mineral production for Cr(VI) adsorption, reduction, and sequestration. However, under more complex geochemical conditions, HA preferentially occupies reaction sites on Fe
0 or chelates Fe(II)/Fe(III) with its structural functional groups, such as hydroxyl and carboxyl groups. Therefore, bio-promoted Fe
0 corrosion first requires breaking through the physical barrier formed by HA-Fe complexes, a task that cannot be accomplished by autotrophic microbes alone. Fortunately, it is reported that heterotrophic microbes such as denitrifying and sulfate-reducing bacteria can utilize HA as a carbon and energy source for their metabolic processes [
15,
16]. In addition, those microbes can show a reducing ability from the degradation of HA for Cr(VI) reduction [
17]. Hence, incorporating heterotrophic microbes into a conventional biotic Fe
0 system is expected to resolve the HA inhibition problem with an increase in Cr(VI) removal.
Theoretically, the collaboration between Fe
0 and autotrophic and heterotrophic microbes integrates the inherent unique physicochemical properties of Fe
0 with the complex biochemical characteristics of both microbial types. This integration has a potential to concurrently address inhibition and passivation issues [
18,
19]. However, heterotrophic microbes differ in biological behavior from autotrophic microbes. Further research is needed to determine whether a competitive or synergistic relationship exists between hydrogen-autotrophic and heterotrophic microbes when coexisting.
Therefore, this study aims to establish a ternary system combining Fe0 with heterotrophic and hydrogen-autotrophic microbes to investigate the removal of Cr(VI) from groundwater rich in humic acid (HA), thereby deepening our understanding of these complex chemical and biological processes. The Cr(VI) removal efficiency in abiotic Fe0, HA-abiotic Fe0, biotic Fe0, and HA-biotic Fe0 systems was compared. Solid-phase analysis was used to determine changes on the Fe0 surface and secondary mineral formation. Chromium species on the Fe0 surface were analyzed to describe the mechanism of chromium removal and immobilization. The impact of HA concentration and additional electron acceptors such as nitrate and sulfate on Cr(VI) removal was also investigated to further explore the role of microbes.
2. Materials and Methods
2.1. Materials and Microbes
Iron powder of 0.15 mm was purchased from Guangzhou Reagent Factory (Guangzhou, China) and directly used without any treatment. Other conventional chemicals such as K2Cr2O7, humic acid, and CaCl2 were used as received. They were of analytical purity from Aladdin (Shanghai, China).
Anaerobic sludge was taken from a municipal wastewater treatment plant. To obtain Cr(VI)-resistant mixed heterotrophic and hydrogen-autotrophic microbes, the acclimation of a mixed culture was conducted according to a previous study [
20]. An anaerobic bioreactor with a volume of 5 L was used for acclimation, containing 5 g L
−1 volatile suspended solids (VSS) and 10 g L
−1 of granular iron. Influent Cr(VI) concentration was 25 mg L
−1, along with a nutrient medium containing (mg L
−1) glucose 400, K
2HPO
4 40, NaNO
3 300, Na
2SO
4 300, MgCl
2 35, NaHCO
3 500, and trace metals (CoCl
2·5H
2O, CuCl
2·2H
2O, MnCl
2·4H
2O, NiCl
2·6H
2O, NH
4MO
3, and ZnSO
4, each at 0.5 mg L
−1). Influent retention time was 3 d. After 6 months of acclimation, when the removal rates of Cr(VI), NO
3−-N, and SO
42− reached 90%, this culture could be harvested. The cultivated sludge was collected, rinsed, and mixed with distilled water to obtain a bacterial suspension for batch experiments.
2.2. Solution Preparation
Chromium stock solution was prepared by dissolving chemicals (K
2Cr
2O
7 1 g L
−1, K
2HPO
4 5 g L
−1, NH
4Cl 5 g L
−1, and NaHCO
3 1 g L
−1) into deionized water. Humic acid (HA) stock solution of 1 M was prepared by dissolving HA in deionized water at pH 9 and filtered through a 0.45 µm membrane [
21]. The concentration of HA was expressed as dissolved organic carbon (mg L
−1 as DOC). Calcium ion stock solution was prepared by dissolving CaCl
2 (100 mM) into deionized water.
Synthetic Cr(VI)-contaminated groundwater was prepared by diluting mixed stock solutions of Cr(VI), HA, and Ca2+ to target concentrations with deionized water that was deoxygenated by purging with nitrogen gas for 1 h before usage. The background electrolyte was 5 mM NaCl unless otherwise specified and 0.8 mM Ca2+, equivalent to 80 mg L−1 as CaCO3 hardness, representing moderately hard water. Initial pH was adjusted to 7.0 by 0.1 M NaOH and HCl. No buffer was used.
2.3. Batch Experiment
Batch experiments were conducted in a 300 mL conical flask containing 250 mL of synthetic Cr(VI)-contaminated groundwater and 50 mL headspace, with 10 mg L−1 of Cr(VI) and 20 mg L−1 of HA. The reaction was divided into three systems: (1) abiotic Fe0 (Fe0 only, including abiotic Fe0 and HA-abiotic Fe0), (2) microbes (mixed microbes, including microbe and HA-microbe), and (3) biotic Fe0 (Fe0+ mixed microbes, including biotic Fe0 and HA-biotic Fe0). The reactors were dosed with 1 g L−1 Fe0 and/or 200 mg VSS L−1 mixed microbes under N2 protection. After sealing with rubber plugs, the reactors were set to react at 50 rpm and 25 °C. At certain time intervals (1–5 d), 3 mL of solution was withdrawn via a long needle and filtered through a 0.45 μm membrane for immediate analysis of Cr(VI), HA, and total Fe; 3 mL of nitrogen was injected into the flask to maintain pressure. The values presented in this study were averaged over triplicate experiments for each.
The effects of HA, nitrate, and sulfate were investigated based on the above benchmark experiment, ranging from 0 to 40 mg L−1 (HA), 56 mg L−1 (NO3−-N), and 384 mg L−1 (SO42−), respectively.
2.4. Analytical Methods
The concentration of Cr(VI) in solution was measured by the 1,5-diphenylcarbazide colorimetric method at a wavelength of 540 nm (UV-5200, Metash, Shanghai, China). The concentrations of HA, total Fe, and total Cr in solution were determined by a TOC analyzer (TOC-5000A, Shimadzu, Kyoto, Japan) and an atomic absorbance spectrometer (AAS, GGX-600, Beijing, China), respectively. The pH value was measured with a pH meter (PHS-3C, Leici, Shanghai, China).
After remediation, reacted Fe
0 and unreacted Fe
0 were freeze-dried for 1 d and collected through magnetic dressing. The above process was completed in an anaerobic chamber. All the collected samples were analyzed by scanning electron microscope/energy-dispersive X-ray (SEM/EDX, ZEISS Merlin, Oberkochen, Germany), X-ray diffraction (XRD, Shimadzu XRD-7000, Tokyo, Japan), and X-ray photoelectron spectroscopy (XPS, Shimadzu Axis Supra, Tokyo, Japan). The valence distribution of chromium was analyzed according to the method in the literature [
22]. Detailed methods are provided in
Text S1.
2.5. Data Analysis
The removal rate of Cr(VI) was calculated according to Equation (5). The removal data were fitted with zero-, first-, and second-order kinetic models (Equations (6)–(8)).
where
C0 and
Ct are concentrations of Cr(VI) before and after reaction (mg L
−1);
t is reaction time (d);
k0 −
k2 are zero-, first-, and second-order reaction rate constants (mg (L d)
−1, d
−1, and L (mg d)
−1).
3. Results and Discussion
3.1. Characterization
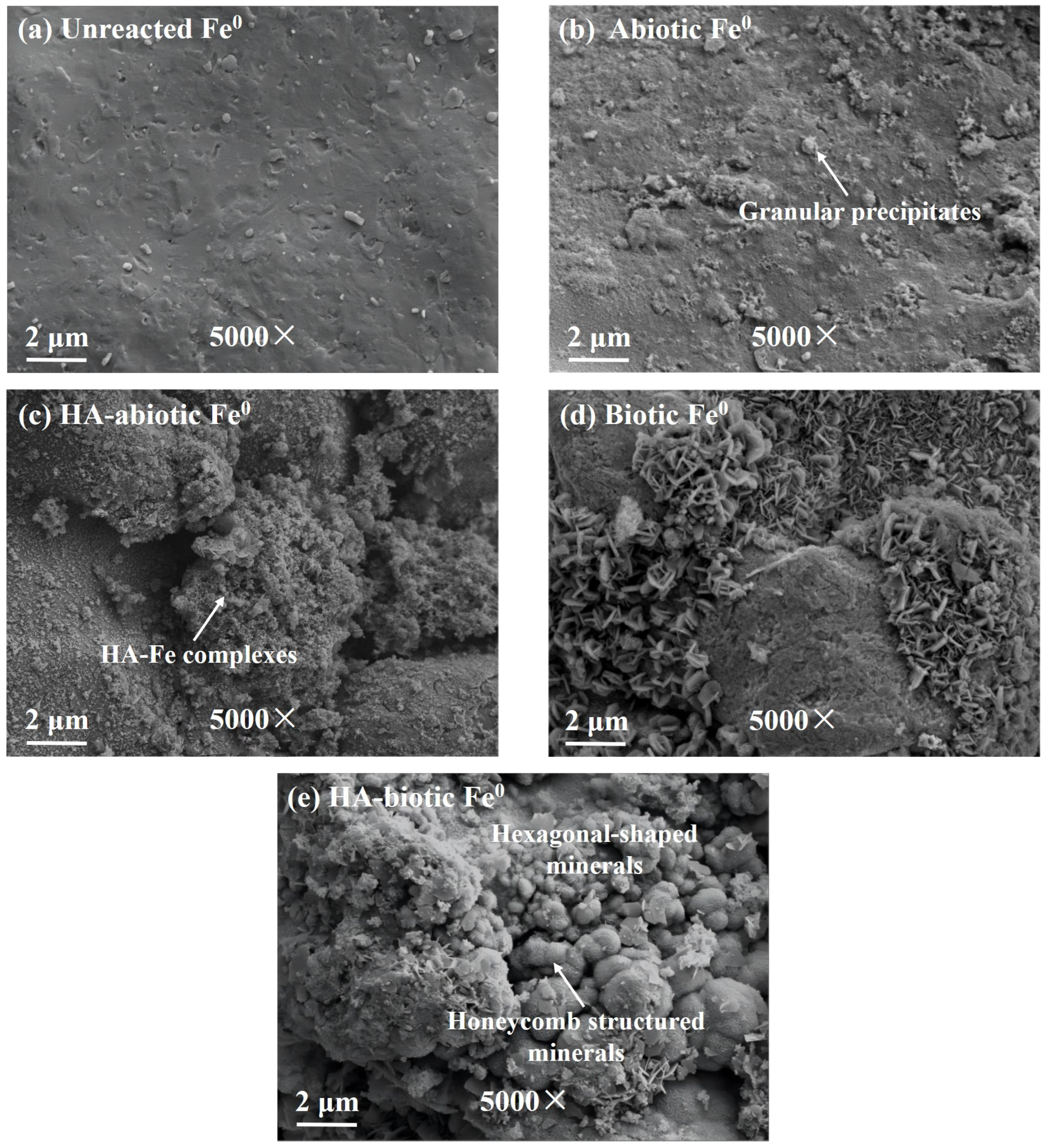
The surface morphology of reacted Fe
0 is shown in
Figure 1. Compared with the smooth surface of unreacted Fe
0 (
Figure 1a), the surface of abiotic Fe
0 became rough with some attached granular precipitates, which could be iron oxides and Fe-Cr precipitates (
Figure 1b). In the presence of HA, a large number of noncrystal aggregates with an apparently greater thickness were observed on the Fe
0 surface in the HA-abiotic Fe
0 system. Based on previous studies, these floccules were assigned to HA-Fe complexes [
12,
23]. They were further confirmed by the observation of higher Fe (86.4%) and Ca (1.6%) than the 82.6% and 1.2% on the Fe
0 surface without HA in EDX analysis (
Table 1). The formation of HA-Fe complexes blocked the Fe
0 surface from further corrosion.
When microbes were introduced, flocculent precipitates disappeared with a sharp decrease in the proportion of Ca from 1.6% to 0.1% on the HA-biotic Fe
0 surface (
Figure 1e). Instead, various secondary minerals began to appear. Additionally, it is evident that the HA-biotic Fe
0 also contained more secondary minerals than the biotic Fe
0 without HA as shown in
Figure 1d. This was attributed to the fact that heterotrophic microbes could effectively degrade HA and clean up HA-Fe complexes. More reaction sites were liberated and available on Fe
0 to interact with autotrophic microbes promote iron corrosion and biogenic mineral production. Petal-like hexagonal plate minerals and honeycomb minerals were observed (
Figure 1e). Their shapes were similar to carbonate green rust, an essential iron mineral with strong adsorption, reduction, and sequestration properties as reported by Legrand et al. [
24]. As shown in
Table 1, due to the formation of these minerals, the content of Fe showed a significant decrease in the HA-biotic Fe
0 system from 72.8% to 45.6% with a sharp increase in O and Cr content from 10.6% and 3.8% to 28.3% and 6.9% as compared to the biotic Fe
0 system. In addition, the Cr on the Fe
0 surface in the HA-biotic Fe
0 system was mainly Cr(III) according to the results presented in
Figure 2b. This finding indicated severe iron corrosion and secondary mineral formation for Cr(VI) removal under the collaboration of heterotrophic and autotrophic microbes.
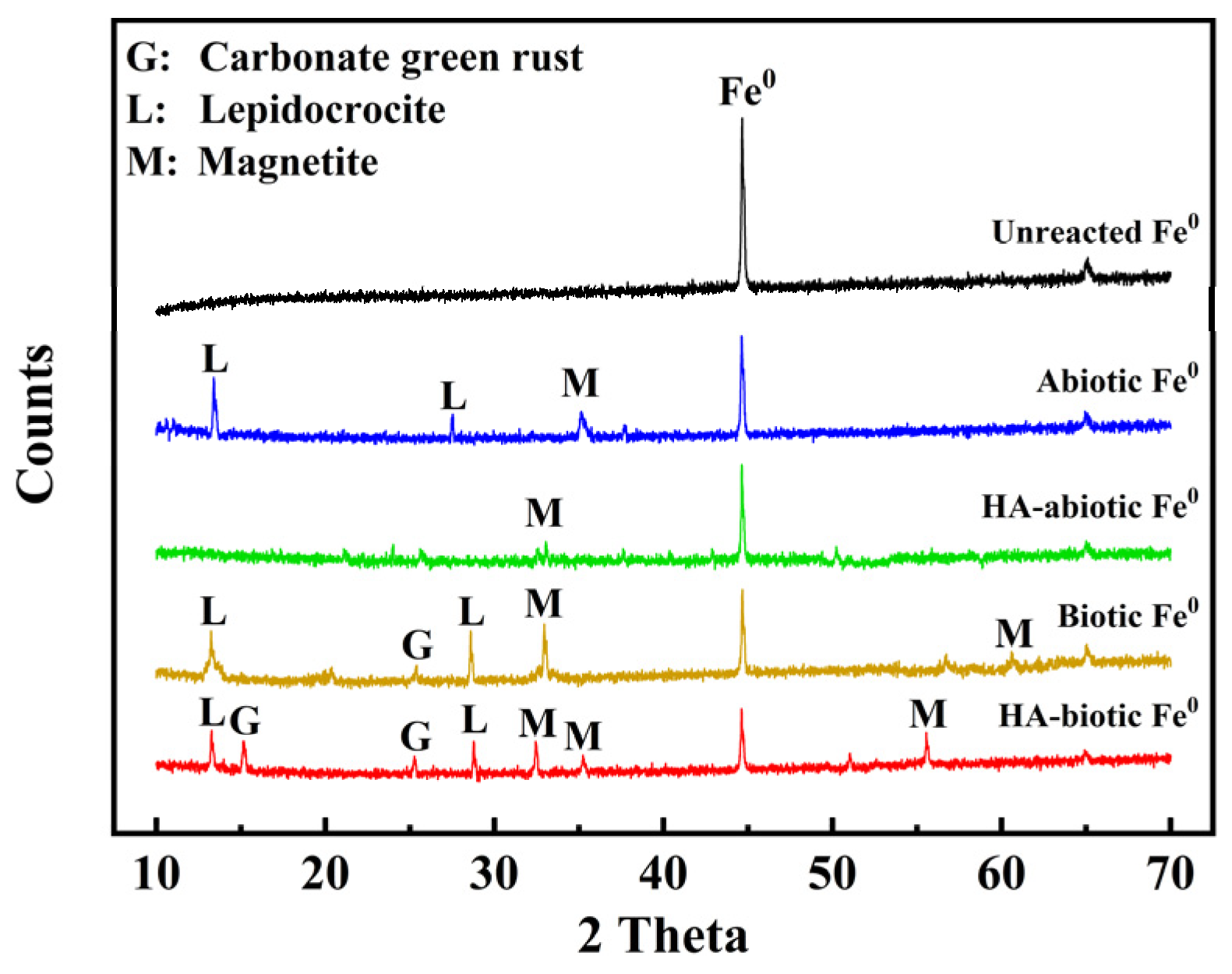
The results of XRD analysis are displayed in
Figure 3. Only one iron diffraction peak was visible on unreacted Fe
0. In addition to the strong iron peak, several weak peaks of lepidocrocite and magnetite were observed on abiotic Fe
0, suggesting slow iron corrosion and mineral generation. Notably, diffraction peaks of iron minerals were hardly seen under the interference of HA due to the generation of HA-Fe complexes. However, the peak intensity of secondary minerals (e.g., lepidolite, magnetite, and green rust) increased on biotic Fe
0 because of microbial activities. With HA, the intensities of those minerals further increased in the HA-biotic Fe
0 system due to the clearance of HA-Fe complexes by heterotrophic microbes. Therefore, the collaboration of autotrophic and heterotrophic microbes alleviated HA inhibition and achieved promoted Fe
0 corrosion for Cr(VI) removal.
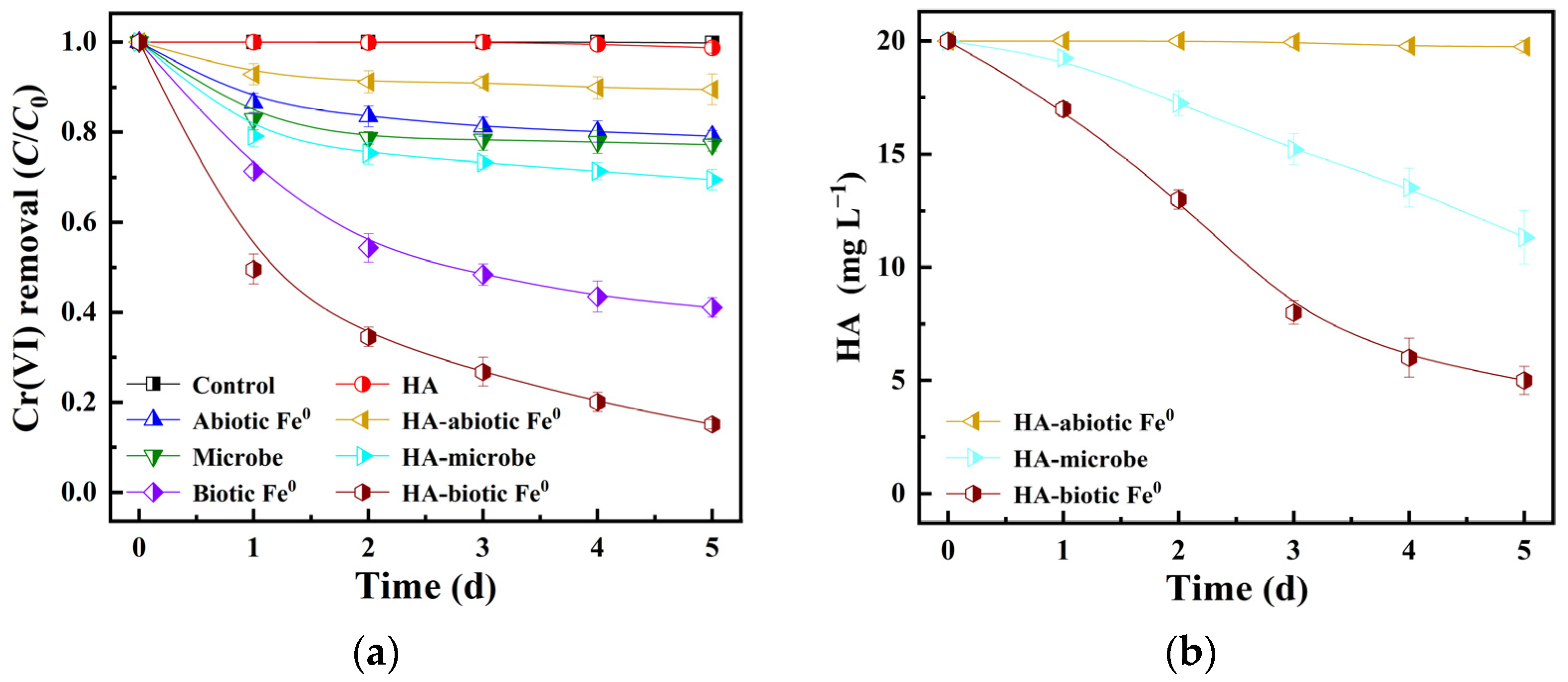
3.2. Batch Experiment
Parallel experiments were carried out to investigate the performance of HA-abiotic Fe
0 and HA-biotic Fe
0 in Cr(VI) removal (
Figure 4). A control system of HA was used to evaluate abiotic Cr(VI) loss with reaction time. The Cr(VI) concentration remained stable and slightly decreased by 1.3% within 5 d, primarily due to the chelation and reduction of Cr(VI) by HA [
25]. For abiotic Fe
0, a low Cr(VI) removal of 20.9% in 5 d was observed, mainly due to passive Fe-Cr hydroxide generation on the Fe
0 surface (
Figure 4a). In the HA-abiotic Fe
0 system, it exhibited an even lower Cr(VI) removal of only 10.5%, which was inhibited by 50% as compared to the absence of HA. It was attributed to the deposition of HA-Fe complexes on the Fe
0 surface. This result was further confirmed by a slight increase in total Fe concentration and HA utilization rate from 0.06 and 0.1 mg L
−1 in 1 d to 0.1 and 0.3 mg L
−1 in 5 d (
Figure 1b,c,
Figure 4b and
Figure S1).
Unlike the abiotic Fe0 system, the microbe system exhibited enhanced behaviors in the presence of HA. Its Cr(VI) removal rate increased from 22.8% to 30.5% within 5 d at 20 mg L−1 HA. The corresponding HA concentration decreased by 8.7 mg L−1 since heterotrophic microbes were capable of degrading HA and obtained reductive ability from this process to reduce Cr(VI). Consequently, the introduction of mixed heterotrophic and autotrophic microbes caused a substantial improvement in Cr(VI) removal of 84.9% in the HA-biotic Fe0 system, 0.4 and 7.1 times higher than those of the biotic Fe0 (59.0%) and HA-abiotic Fe0 (10.5%) systems. Heterotrophic microbes transformed HA from an inhibitor into an ideal carbon source and electron donor. The degradation of HA released reaction sites on the Fe0 surface for autotrophic microbes. By consumption of H2 from iron corrosion and CO2 from HA biodegradation, autotrophic microbes promoted Fe0 corrosion and biogenic mineral production for enhanced Cr(VI) adsorption, reduction, and sequestration. Therefore, a significantly enhanced Cr(VI) removal was achieved in the HA-biotic Fe0 system. It indicated a synergistic interaction between heterotrophic and autotrophic microbes with Fe0 for enhanced Cr(VI) elimination.
As shown in
Figure S1 and
Figure 4b, the HA-biotic Fe
0 system exhibited a significantly higher total Fe of 1.0 mg L
−1 after 2 d than those of the biotic Fe
0 (0.2 mg L
−1) and HA-abiotic Fe
0 (0.1 mg L
−1) systems with the highest HA utilization of 15.2 mg L
−1. The results also suggested a synergistic interaction between two types of microbes for promoted iron corrosion and Cr(VI) removal. Since iron ion was a dominant nutrient to satisfy the growth of heterotrophic microbes [
26] and CO
2 produced from HA degradation was promptly consumed by autotrophic microbes, the HA-biotic Fe
0 system exhibited a higher HA utilization as compared to the pure HA-microbe system. As the reaction progressed, the iron ion concentration dropped to 0.12 mg L
−1 due to the accumulation of secondary minerals on the Fe
0 surface [
27]. Additionally, promoted iron corrosion also led to an increase in pH value from 7.0 to 7.4 in the HA-biotic Fe
0 system (
Table S1), which was conducive to iron hydroxide generation and subsequent Cr(VI) sequestration.
The kinetic analysis of Cr(VI) removal was conducted and the results are presented in
Table S2. Based on these fitting outcomes, the pseudo second-order kinetic model outperformed other models. It indicated that Cr(VI) removal on the HA-biotic Fe
0 surface primarily occurred through more efficient and plausible chemisorption. The corresponding reaction rate constant for the HA-biotic Fe
0 system reached 0.110 L (mg d)
−1, 54 times higher than that of the HA-abiotic Fe
0 system (0.002 L (mg d)
−1). This result demonstrated that Cr(VI) removal could be significantly enhanced by introducing mixed heterotrophic and autotrophic microbes into Fe
0-H
2O systems in the presence of HA.
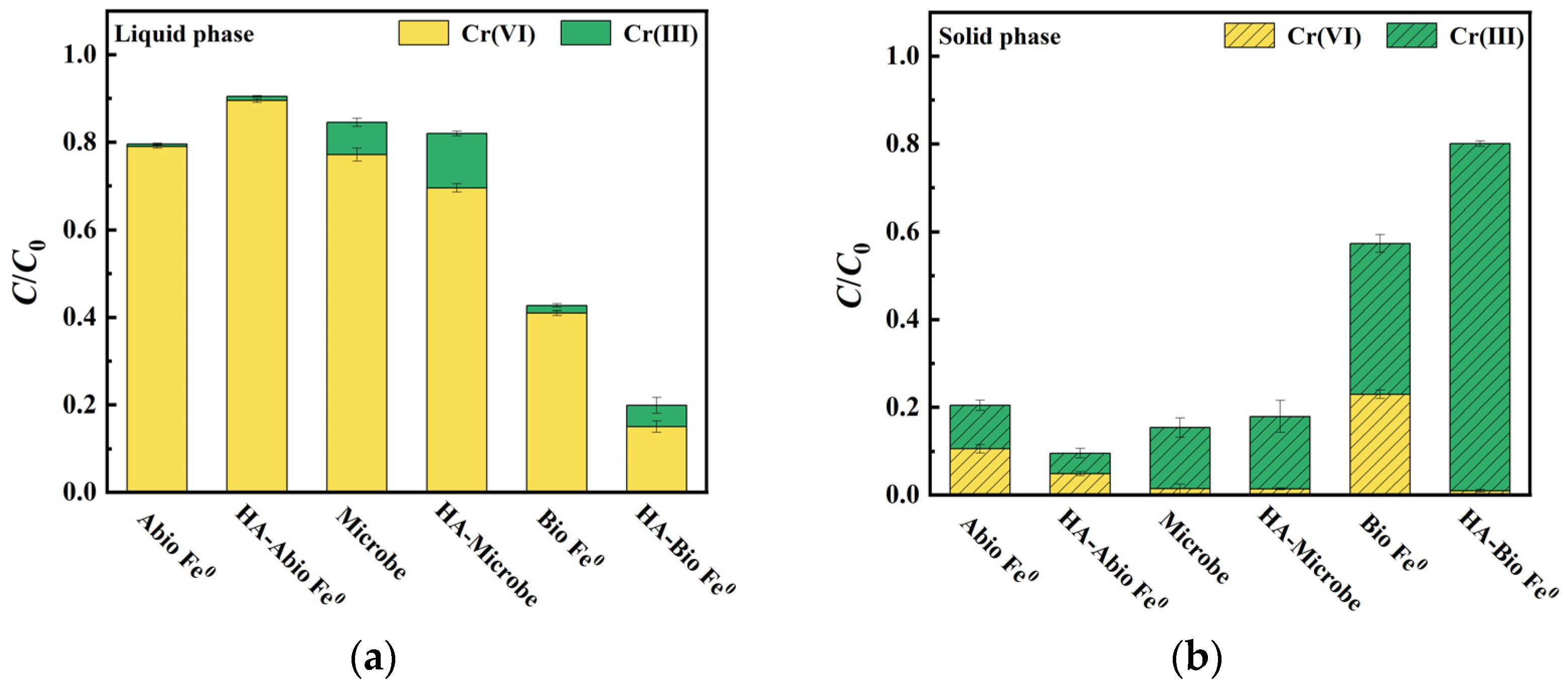
3.3. Valence Distribution of Chromium
The valence distribution of Cr after reaction was determined and the results are presented in
Figure 2. Only 5.6% of Cr(VI) was reduced to Cr(III) on the HA-abiotic Fe
0 surface, half of that in the abiotic Fe
0 system without HA. It was attributed to the inhibitory of HA-Fe complexes that impeded electron transfer from Fe
0 to surface-bonded Cr(VI). In contrast, the HA-microbe system showed an increase in Cr(VI) removal of 29.1% as compared to the microbe system without HA (21.4%). Owing to biological HA degradation by heterotrophic microbes, the reduction of Cr(VI) was improved, resulting in the predominance of Cr(III) in the adsorbed state (16.5% in solid phase and 12.9% in liquid phase) [
28].
Obviously, the removal of Cr(VI) by Fe0 was substantially enhanced by the introduction of microbes in the synergistic system. Both biotic Fe0 and HA-biotic Fe0 systems showed higher Cr(VI) removal as compared to the other systems, which had an overall Cr(VI) reduction rate of 36.1% and 84.0%, respectively. The synergistic system exhibited a high degree of immobilized Cr(III) and low degree of soluble Cr(III), accounting for 34.4% and 1.7% in the biotic Fe0 system and 79.2% and 4.8% in the HA-biotic Fe0 system of the total chromium content, respectively. These results indicated that most of the Cr existed on Fe0 in the form of Cr(III), rather than in solution of the synergistic systems. Interestingly, the Cr(III) on HA-biotic Fe0 showed a much higher portion of 98.8% as compared to that of biotic Fe0 of 60%. It indicated that the HA-biotic Fe0 system not only had an increased ability to reduce Cr(VI) by biodegrading HA but also allowed more iron corrosion for Cr(VI) adsorption and reduction.
In conclusion, the HA-biotic Fe0 system could significantly enhance the immobilization of Cr(VI) by transforming Cr(VI) in solution to Cr(III) on the Fe0 surface and thus decreased the mobility, bioavailability, and toxicity of Cr(VI).
3.4. Effects of Humic Acid Concentration and Common Electron Acceptors
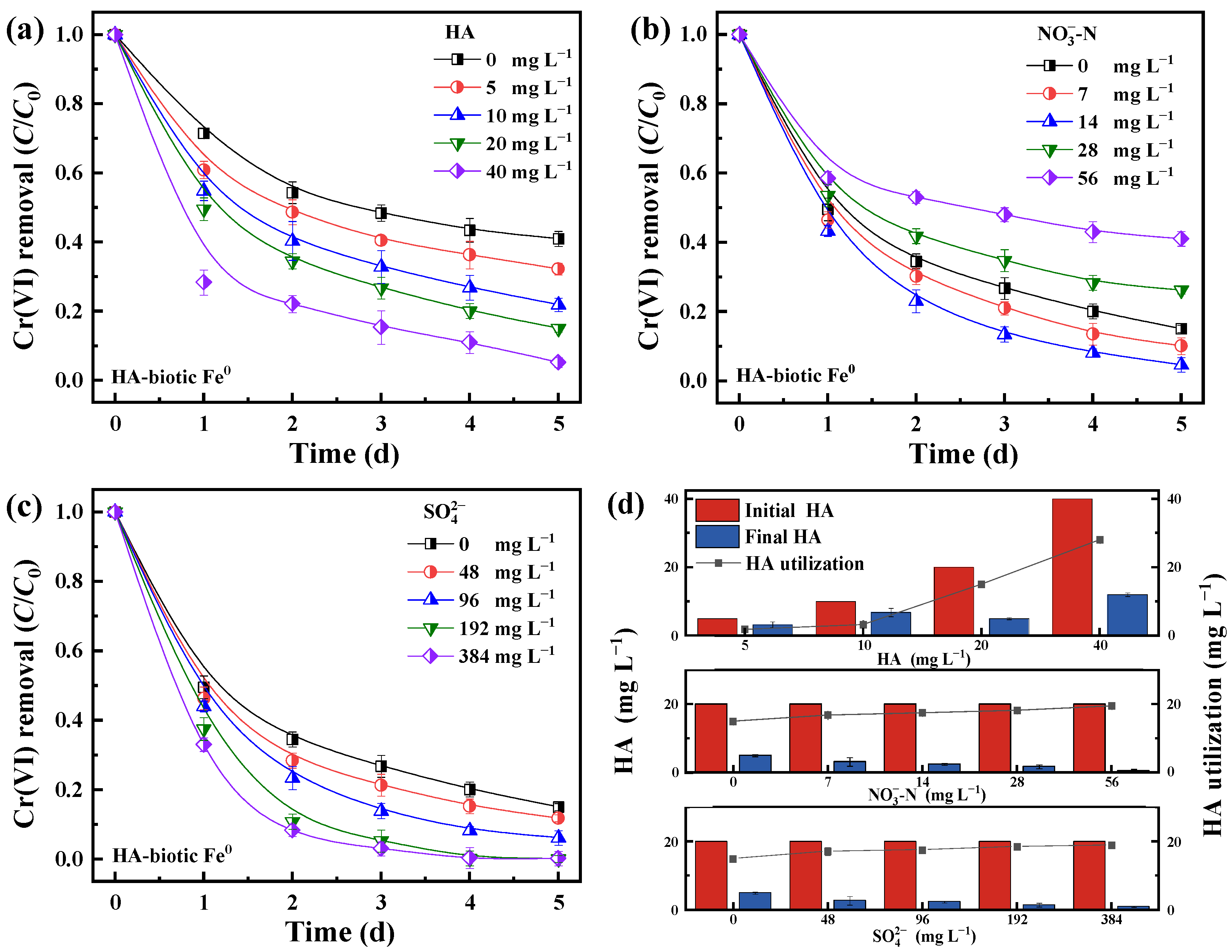
In order to further clarify the contribution of heterotrophic microbes in the HA-biotic Fe
0 system, the effect of HA concentration was investigated (
Figure 5a). As expected, the Cr(VI) removal increased with HA concentration. When the HA concentrations was elevated from 0 to 40 mg L
−1, the Cr(VI) removal rate increased from 59.0% to 94.6%. In contrast, a negative effect of HA was observed without microbes in the HA-abiotic Fe
0 system. It showed a decrease in Cr(VI) removal from 20.9% to 6.9% as the initial HA concentration was increased from 0 to 40 mg L
−1 (
Figure S3). The opposite results emphasized the crucial role of heterotrophic microbes in degrading HA to enhance Cr(VI) removal. In addition, the utilization rate of HA also increased from 1.5 to 28 mg L
−1 with HA concentration from 5 to 40 mg L
−1 (
Figure 5d), probably due to the enhancement of biological activities of heterotrophic microbes by utilizing HA.
The effect of nitrate and sulfate on Cr(VI) removal in the HA-biotic Fe
0 system is predicted in
Figure 5b,c. The Cr(VI) removal rate was 84.9%, 89.9%, 95.3%, 73.8%, and 59.0% within 5 d when initial NO
3−-N concentrations were 0, 7, 14, 28, and 56 mg L
−1, respectively. The NO
3−-N concentration decreased to 0, 1.1, 3.4, 15.6, and 26.1 mg L
−1 after the reaction. Obviously, moderate quantities of NO
3−-N (7–14 mg L
−1) could serve as an efficient electron acceptor for heterotrophic microbes to metabolize HA and reduce Cr(VI). Meanwhile, the HA utilization rate increased steadily from 15 mg L
−1 to 19.5 mg L
−1 with increasing NO
3−-N concentration as shown in
Figure 5b. However, when initial NO
3−-N concentrations were further elevated to 28 and 56 mg L
−1, the Cr(VI) removal decreased from 84.9% to 73.8% and 59.0% in 5 d. The decrease in Cr(VI) removal at this moment was because excessive nitrate competed with Cr(VI) for electron flow from biological HA degradation and Fe
0 corrosion. A similar negative effect was observed in a nitrobenzene reduction by biotic Fe
0. The nitrobenzene reduction rate decreased from 83.6 to 78.1 and 75.2% after a 12 d reaction time with an increase in initial nitrate concentration from 0 to 1 and 5 mmol L
−1.
Different from nitrate, sulfate exhibited a constantly positive effect on Cr(VI) removal in the HA-biotic Fe
0 system. The Cr(VI) removal rate increased from 73.2% to 97% in 3 d as initial SO
42− concentration was increased from 0 to 384 mg L
−1 (
Figure 5c). Meanwhile, the SO
42− concentration decreased from 48, 96, 192, and 384 to 1.5, 3.2, 98.2, and 141.3 mg L
−1, respectively. These results also indicated that heterotrophic microbes could utilize sulfate as electron donors to degrade HA and reduce Cr(VI). Theoretically, sulfate would be similar to nitrate as a common electron acceptor, competing with Cr(VI) for electrons [
29]. However, the presence of sulfate constantly exhibited a significantly enhanced effect on Cr(VI) removal in the HA-biotic Fe
0 system. The Cr(VI) removal rate increased from 84.9% to 99.8% with an increase in sulfate concentration from 0 to 384 mg L
−1. This result was consistent with the finding obtained by Yan et al. that the Cd(II) removal rate increased from 58.6% to 86.1% as initial sulfate concentration was elevated from 0 to 100 mg L
−1 in a biotic Fe
0 system [
30]. It appeared that sulfate promoted the performance of biotic Fe
0 systems due to the formation of iron sulfide minerals, which not only counteracted the inhibitory effect of SO
42− but also substantially improved Cr(VI) removal by generating Cr sulfide precipitation.
In summary, the above results further demonstrated the important role of heterotrophic microbes to eliminate HA inhibition in the HA-biotic Fe0 system, and the presence of sulfate and an appropriate amount of nitrate accelerated the utilization of HA by heterotrophic microbes and enhanced the effectiveness in Cr(VI) removal.
3.5. Immobilization Mechanism
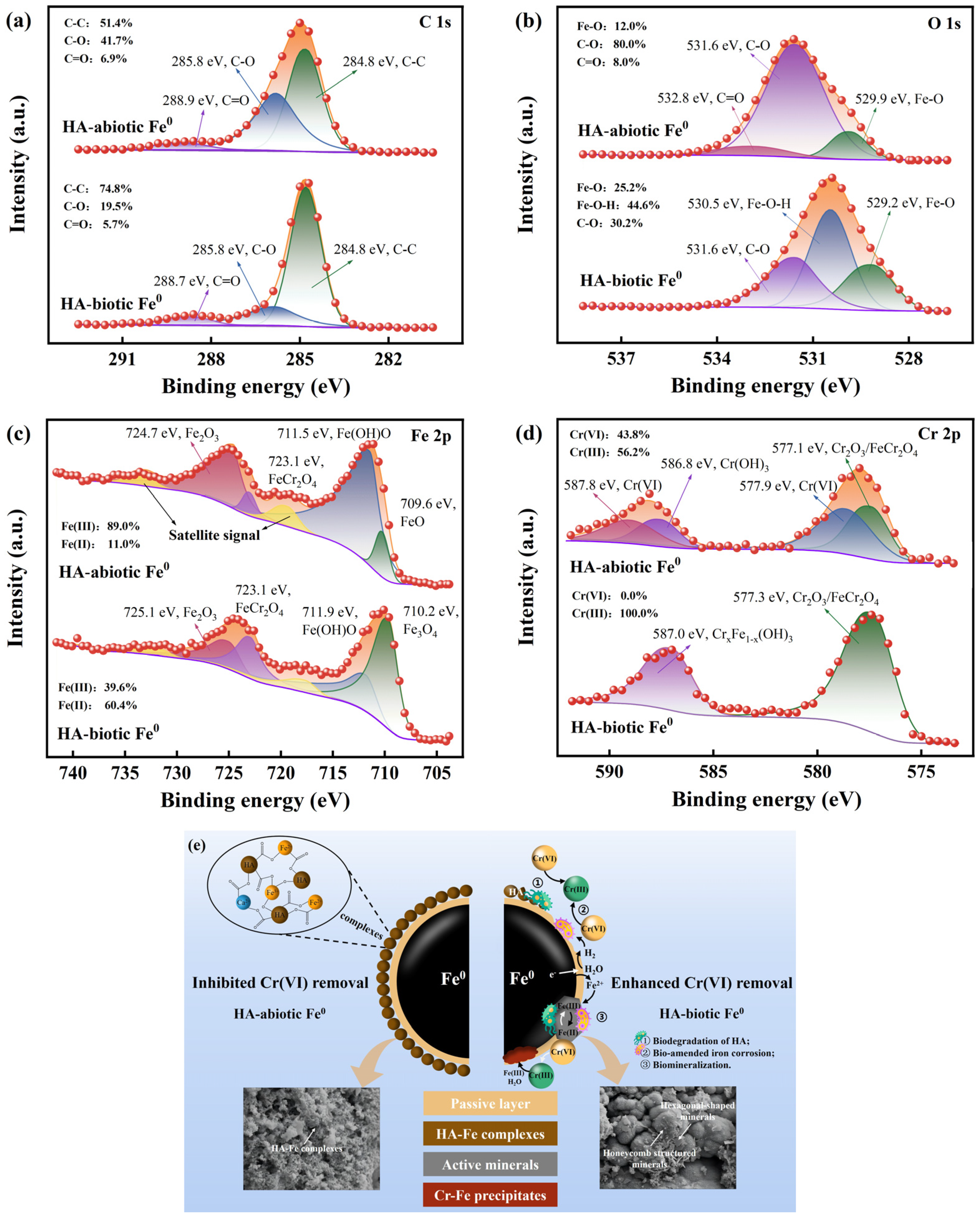
The results of XPS spectra are illustrated in
Figure 6. In the HA-abiotic Fe
0 system, the iron surface exhibited a high proportion of 41.7% C-O and 6.9% C=O in C 1s and O 1s due to the deposition of HA-Fe complexes on the Fe
0 surface via hydroxyl and carboxyl groups (
Figure 1 and
Figure 6a,b) [
31]. After the introduction of microbes, there was a significant decrease in the proportions of C-O (19.5%) and C=O (5.7%) on the HA-biotic Fe
0 surface due to the biodegradation of HA by heterotrophic microbes. Meanwhile, a portion of C-O transformed to 44.6% Fe-O-H, suggesting that ferrous minerals predominantly replaced HA-Fe complexes after their degradation (
Figure 4b). In the Fe 2p spectra, the presence of 89% Fe(III) on HA-abiotic Fe
0 confirmed the formation of HA-Fe(III) species as an insulated barrier hindering electron transfer [
32,
33]. By utilizing HA and accelerating iron corrosion, both heterotrophic and autotrophic microbes significantly stimulated reactive mineral generation and the Fe(II) proportion increased to 60.4% on the HA-biotic Fe
0 surface. This evidence corroborated the generation of Fe(II)-bearing minerals, such as magnetite and green rust, as shown in
Figure 3. These minerals favored the adsorption and reduction of Cr(VI). In the Cr 2p spectra, similar to the results in
Figure 2b, the deconvoluted peaks of HA-abiotic Fe
0 allocated 43.8% to Cr(VI) and 56.2% to Cr(III), whereas only the characteristic peaks of Cr(III) were observed on the HA-biotic Fe
0 surface. The synergistic interaction between Fe
0 and microbes promoted Cr(VI) reduction and Cr(III) distribution into more stable forms on the iron surface, such as Cr
xFe
1-x(OH)
3 and ionic-bonded Cr
2O
3/FeCr
2O
4.
Based on the above discussion, potential pathways to enhance Cr(VI) removal by the combined action of heterotrophic microbes to relieve HA inhibition and hydrogen-autotrophic microbes to promote iron corrosion were hypothesized (
Figure 6e): (1) biodegradation of HA by heterotrophic microbes: as a carbon source and an electron acceptor for heterotrophic microbes, HA biodegradation not only renewed reaction sites by eliminating HA-Fe complexes but also stimulated biological activities to reduce Cr(VI); (2) bio-amended iron corrosion by hydrogenotrophic microbes: after the first step of exposing the iron surface, hydrogenotrophic microbes obtained energy by consuming H
2 from Fe
0 corrosion to reduce Cr(VI) and promoted iron corrosion; (3) biomineralization by microbes: the activities of mixed microbes induced the formation of biominerals, such as green rust. These Fe(II)-containing minerals caused a substantial reduction of adsorbed Cr(VI). Surface-bonded Cr(III) entered the lattice of iron oxides to form Cr-Fe precipitates with low bioavailability and mobility.