Artificial Floating Islands for the Removal of Nutrients and Improvement of the Quality of Urban Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Construction of the AFI
2.3. Selection of Macrophytes
2.4. Operation of Phytoremediation Bioassays
2.5. Physicochemical Characterization of UW
2.6. Analysis of Water Samples
2.7. Phytoaccumulation Evaluation
2.8. Evaluation of Growth Attributes in Macrophytes Exposed to UW
3. Results and Discussion
3.1. Physicochemical Characterization of UW
3.2. Monitoring and Evaluation of Bioassays
3.3. Removal Efficiency of Physicochemical Parameters
3.4. Phytoaccumulation Evaluation
3.5. Effects of UW on Macrophyte Attributes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sousa, S.A.; Esteves, A.F.; Salgado, E.M.; Pires, J.C.M. Enhancing urban wastewater treatment: Chlorella vulgaris performance in tertiary treatment and the impact of anaerobic digestate addition. Environ. Technol. Innov. 2024, 34, 103601. [Google Scholar] [CrossRef]
- Wantzen, K.; Alves, C.; Badiane, S.; Bala, R.; Blettler, M.; Callisto, M.; Cao, Y.; Kolb, M.; Kondolf, G.; Leite, M.; et al. Urban stream and wetland restoration in the global south—A DPSIR analysis. Sustainability 2019, 11, 4975. [Google Scholar] [CrossRef]
- Badr, E.S.A.; Tawfik, R.T.; Alomran, M.S. An Assessment of Irrigation Water Quality with Respect to the Reuse of Treated Wastewater in Al-Ahsa Oasis, Saudi Arabia. Water 2023, 15, 2488. [Google Scholar] [CrossRef]
- Smol, M.; Preisner, M.; Bianchini, A.; Rossi, J.; Hermann, L.; Schaaf, T.; Kruopien Pamakštys, K. Strategies for sustainable and circular management of phosphorus in the Baltic Sea Region: The holistic approach of the InPhos Project. Sustainability 2020, 12, 2567–2588. [Google Scholar] [CrossRef]
- Wang, H.; Bouwman, A.F.; Van Gils, J.; Vilmín, L.; Beusen, A.H.W.; Wang, J.; Liu, X.; Yu, Z.; Ran, X. Hindcasting harmful algal bloom risk due to land-based nutrient pollution in the Eastern Chinese coastal seas. Water Res. 2023, 231, 119669. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M.; Beusen, A.H.W.; Harrison, J.A.; Durr, H.H.; Bouwman, A.F.; Laruelle, G.G. Changing land-, sea-, and airscapes: Sources of nutrient pollution affecting habitat suitability for harmful algae. In Global Ecology and Oceanography of Harmful Algal Blooms; Ecological Studies; Glibert, P., Berdalet, E., Burford, M., Pitcher, G., Zhou, M., Eds.; Springer: Cham, Switzerland, 2023; Volume 232. [Google Scholar] [CrossRef]
- Ansari, A.A.; Singh Gill, S.; Lanza, G.R.; Rast, W. (Eds.) Eutrophication: Causes, Consequences, and Control; Springer: Berlín/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- de Jonge, V.N.; Elliott, M.; Orive, E. Causes, historical development, effects and future challenges of a common environmental problem: Eutrophication. In Nutrients and Eutrophication in Estuaries and Coastal Waters: Proceedings of the 31st Symposium of the Estuarine and Coastal Sciences Association (ECSA), held in Bilbao, Spain, 3–7 July 2000; Orive, E., Elliott, M., de Jonge, V.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 1–19. [Google Scholar] [CrossRef]
- USEPA. National Nutrient Strategy. Available online: https://www.epa.gov/nutrient-policy-data/national-nutrient-strategy (accessed on 15 February 2024).
- USEPA. Algal Toxin Risk Assessment and Management Strategic Plan for DrinkingWater. Available online: https://www.epa.gov/ground-water-and-drinking-water/algal-toxin-risk-assessment-and-management-strategic-plan-drinking (accessed on 15 February 2024).
- USEPA. The Harmful Algal Bloom and Hypoxia Research and Control Amendments Act (HABHRCA). Available online: https://www.epa.gov/cyanohabs/harmful-algal-bloom-and-hypoxia-research-and-control-amendments-act-habhrca (accessed on 20 February 2024).
- Preisner, M.; Neverova-Dziopak, E.; Kowalewski, Z. Mitigation of eutrophication caused by wastewater discharge: A simulation-based approach. Ambio 2021, 50, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Oswald, W. Microalgae and wastewater treatment. In Micro-Algal Biotechnology; Borowitzka, M.A., Borowitzka, L.J., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 305–328. [Google Scholar]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cui, H.; Huang, M.; Él, Y. Artificial Floating Islands for Water Quality Improvement. Environ. Rev. 2017, 25, 350–357. [Google Scholar] [CrossRef]
- Mustafa, H.M.; Hayder, G. Recent studies on applications of aquatic weed plants in phytoremediation of wastewater: A review article. Ain Shams Eng. J. 2020, 12, 355–365. [Google Scholar] [CrossRef]
- Osti, J.A.S.; do Carmo, C.F.; Cerqueira, M.A.S.; Giamas, M.T.D.; Peixoto, A.C.; Vaz-dos-Santos, A.M.; Mercante, C.T.J. Nitrogen and phosphorus removal from fish farming effluents using artificial floating islands colonized by Eichhornia crassipes. Aquac. Rep. 2020, 17, 100324. [Google Scholar] [CrossRef]
- Chen, Z.; Costa, O.S., Jr. Nutrient Sequestration by Two Aquatic Macrophytes on Artificial Floating Islands in a Constructed Wetland. Sustainability 2023, 15, 6553. [Google Scholar] [CrossRef]
- Samal, K.; Kar, S. Ecological Floating Bed (EFB) for Decontamination of Polluted Water Bodies: Design, Mechanism and Performance. J. Environ. Manag. 2019, 251, 109550. [Google Scholar] [CrossRef] [PubMed]
- Pavlineri, N.; Skoulikidis, N.T.; Tsihrintzis, V.A. Constructed Floating Wetlands: A review of research, design, operation and management aspects, and data meta-analysis. Chem. Eng. J. 2017, 308, 1120–1132. [Google Scholar] [CrossRef]
- Afzal, M.; Arslan, M.; Müller, J.A.; Shabir, G.; Islam, E.; Tahseen, R.; Anwar-ul-Haq, M.; Hashmat, A.J.; Iqbal, S.; Khan, Q.M. Floating Treatment Wetlands as a Suitable Option for Large-Scale Wastewater Treatment. Nat. Sustain. 2019, 2, 863–871. [Google Scholar] [CrossRef]
- O’Hare, M.T.; Baattrup-Pedersen, A.; Baumgarte, I.; Freeman, A.; Gunn, I.D.M.; Lázár, A.N.; Sinclair, R.; Wade, A.J.; Bowes, M.J. Responses of aquatic plants to eutrophication in rivers: A revised conceptual model. Front. Plant Sci. 2018, 9, 451. [Google Scholar] [CrossRef]
- Rome, M.; Happel, A.; Dahlenburg, C.; Nicodemus, P.; Schott, E.; Mueller, S.; Lovell, K.; Beighley, R.E. Application of floating wetlands for the improvement of degraded urban waters: Findings from three multi-year pilot-scale installations. Sci. Total Environ. 2023, 877, 162669. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Tondera, K.; Lucke, T. Stormwater treatment evaluation of a constructed floating wetland after two years operation in an urban catchment. Sustainability 2017, 9, 1687. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). The Plant Database, National Plant Data Team 2019; NRCS: Greensboro, NC, USA, 2019. Available online: https://plants.usda.gov (accessed on 19 February 2024).
- Colares, G.S.; Dell’Osbel, N.; Wiesel, P.G.; Oliveira, G.A.; Lemos, P.H.Z.; da Silva, F.P.; Lutterbeck, C.A.; Kist, L.T.; Machado, Ê.L. Floating treatment wetlands: A review and bibliometric analysis. Sci. Total Environ. 2020, 714, 136776. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.; Fox, L.J.; Owen, J.S.; Sample, D.J. Evaluation of commercial floating treatment wetland technologies for nutrient remediation of stormwater. Ecol. Eng. 2015, 75, 61–69. [Google Scholar] [CrossRef]
- Lao, Z.L.; Wu, D.; Li, H.R.; Liu, Y.S.; Zhang, L.W.; Feng, Y.F.; Jiang, X.Y.; Wu, D.W.; Hu, J.J.; Ying, G.G. Uptake mechanism, translocation, and transformation of organophosphate esters in water hyacinth (Eichhornia crassipes): A hydroponic study. Environ. Pollut. 2024, 341, 122933. [Google Scholar] [CrossRef]
- Wang, W.H.; Wang, Y.; Sun, L.Q.; Zheng, Y.C.; Zhao, J.C. Research and application status of ecological floating bed in eutrophic landscape water restoration. Sci. Total Environ. 2020, 704, 135434. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Peña, L.; López-Candela, C. Floating islands as a strategy for the establishment of aquatic plants in the Botanical Garden of Bogotá. Manag. Environ. 2018, 21, 110–120. [Google Scholar] [CrossRef]
- APHA Standard Methods for Examining Waterand Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 1998.
- Goswami, C.; Majumder, A. Potential of Lemna minor in Ni and Cr removal from aqueous solution. Pollution 2015, 1, 3. [Google Scholar] [CrossRef]
- Queiroz, R.D.C.S.D.; Lôbo, I.P.; Ribeiro, V.D.S.; Rodrigues, L.B.; Almeida-Neto, J.A.D. Assessment of autochthonous aquatic macrophytes with phytoremediation potential for dairy wastewater treatment in floating constructed wetlands. Int. J. Phytoremediation 2020, 22, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Haghnazar, H.; Hudson-Edwards, K.A.; Kumar, V.; Pourakbar, M.; Mahdavianpour, M.; Aghayani, E. Potentially toxic elements contamination in surface sediment and indigenous aquatic macrophytes of the Bahmanshir River, Iran: Appraisal of phytoremediation capability. Chemosphere 2021, 285, 131446. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi, M.J.; Keshavarzi, B.; Zaremoaiedi, F.; Rajabzadeh, M.A.; Moore, F. Ecological-health risk assessment and bioavailability of potentially toxic elements (PTEs) in soil and plant around a copper smelter. Environ. Monit. Assess. 2020, 192, 639. [Google Scholar] [CrossRef] [PubMed]
- Parihar, J.K.; Parihar, P.K.; Pakade, Y.B.; Katnoria, J.K. Bioaccumulation potential of indigenous plants for heavy metal phytoremediation in rural areas of Shaheed Bhagat Singh Nagar, Punjab (India). Environ. Sci. Pollut. Control Ser. 2021, 28, 2426–2442. [Google Scholar] [CrossRef] [PubMed]
- Rosell, J.A.; Marcati, C.R.; Olson, M.E.; Lagunes, X.; Vergilio, P.C.; Jiménez-Vera, C.; Campo, J. Inner bark vs sapwood is the main driver of nitrogen and phosphorus allocation in stems and roots across three tropical woody plant communities. New Phytol. 2023, 239, 1665–1678. [Google Scholar] [CrossRef] [PubMed]
- Edegbai, B.O.; Oki, O.C. Growth Response of Abelmoscus esculentus (L.) Monench Planted in Lead Contaminated Soil. Afr. Sci. 2022, 23, 120–132. [Google Scholar]
- Carrasco, G.; Tapia, J.; Urrestarazu, M. Nitrate content in lettuces grown in hydroponic systems. Idesia 2006, 24, 25–30. [Google Scholar] [CrossRef]
- Juárez-Rosete, C.R.; Bugarín-Montoya, R.; Alejo-Santiago, G.; Aguilar-Castillo, J.A.; Peña-Sandoval, G.R.; Palemón-Alberto, F.; Aburto-González, C.A. Nitrate concentration in lettuce (Lactuca sativa L.) in a floating root system. Interscience 2022, 47, 225–231. [Google Scholar]
- Leblebici, Z.; Dalmiş, E.; Andeden, E.E. Determination of the potential of Pistia stratiotes L. in removing nickel from the environment by utilizing its rhizofiltration capacity. Braz. Arch. Biol. Technol. 2019, 62, e19180487. [Google Scholar] [CrossRef]
- Pérez-Patricio, M.; Camas-Anzueto, J.L.; Sanchez-Alegría, A.; Aguilar-González, A.; Gutiérrez-Miceli, F.; Escobar-Gómez, E.; Voisin, Y.; Rios-Rojas, C.; Grajales-Coutiño, R. Optical Method for Estimating the Chlorophyll Contents in Plant Leaves. Sensors 2018, 18, 650. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Thakur, R.K.; Kumar, P. Predicting heavy metals uptake by spinach (Spinacia oleracea) grown in integrated industrial wastewater irrigated soils of Haridwar, India. Environ. Monit. Assess. 2020, 192, 709. [Google Scholar] [CrossRef]
- Mendoza, Y.I.; Pérez, I.J.; Galindo, A.A. Evaluation of the Contribution of the Aquatic Plants Pistia stratiotes and Eichhornia crassipes in the Municipal Wastewater Treatment. Technol. Inf. 2018, 29, 205–214. [Google Scholar] [CrossRef]
- Kobir, M.M.; Ali, M.S.; Ahmed, S.; Sadia, S.I.; Alam, M.A. Assessment of the physicochemical characteristic of wastewater in Kushtia and Jhenaidah Municipal Areas Bangladesh: A Study of DO, BOD, COD, TDS and MPI. Asian J. Geol. Res. 2024, 7, 21–30. [Google Scholar]
- Prasad, R.; Sharma, D.; Yadav, K.D.; Ibrahim, H. Preliminary study on greywater treatment using water hyacinth. Appl. Water Sci. 2021, 11, 88–96. [Google Scholar] [CrossRef]
- Rezania, S.; Din, M.F.M.; Taib, S.M.; Dahalan, F.A.; Songip, A.R.; Singh, L.; Kamyab, H. The efficient role of aquatic plant (water hyacinth) in treating domestic wastewater in continuous system. Int J Phytorem. 2016, 18, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Mera, B.E.D.; García, M.J.L. Phytoremediation with Eichhornia crassipes in wastewater from the Jipijapa canton, Ecuador. Ibero-Am. Mag. Environ. Sustain. 2023, 6, e221. [Google Scholar]
- Cárdenas, E.; Allende, Z.; Ferreira, M.; Velázquez, A.; Vogt, C. Study of the purification capacity of Pistia stratiotes L. in the treatment of wastewater generated in the Effluents Laboratory of FACEN-UNA. FACEN Sci. Rep. 2023, 14, 70–77. [Google Scholar] [CrossRef]
- Haydar, S.; Anis, M.; Afaq, M. Performance evaluation of hybrid constructed wetlands for the treatment of municipal wastewater in developing countries. Chin. J. Chem. Eng. 2020, 28, 1717–1724. [Google Scholar] [CrossRef]
- Mustafa, H.M.; Hayder, G. Cultivation of S. molesta plants for phytoremediation of secondary treated domestic wastewater. Ain Shams Eng. J. 2021, 12, 2585–2592. [Google Scholar] [CrossRef]
- Ng, Y.S.; Chan, D.J.C. Wastewater phytoremediation by Salvinia molesta. J. Water Process Eng. 2017, 15, 107–115. [Google Scholar] [CrossRef]
- Wibowo, Y.G.; Nugraha, A.T.; Rohman, A. Phytoremediation of several wastewater sources using Pistia stratiotes and Eichhornia crassipes in Indonesia. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100781. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lao, Z.; Liu, Y.; Feng, Y.; Song, A.; Hu, J.; Ying, G.G. Uptake, accumulation, and translocation of organophosphate esters and brominated flame retardants in water hyacinth (Eichhornia crassipes): A field study. Sci. Total Environ. 2023, 874, 162435. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, S.; Fujita, M.; Ike, M.; Tateda, M. Growth Dynamics of Pistia stratiotes in Temperate Climate. Wastewater Treatment, Plant Dynamics and Management in Constructed and Natural Wetlands; Springer: Dordrecht, The Netherlands, 2008; pp. 277–287. [Google Scholar] [CrossRef]
- Kumar, S.; Dube, K.K.; Rai, J.P.N. Mathematical model for phytoremediation of pulp and paper industry wastewater. J. Sci. Ind. Res. 2005, 64, 717–721. [Google Scholar]
- Singh, J.; Kumar, V.; Kumar, P.; Kumar, P. Kinetics and prediction modeling of heavy metal phytoremediation from glass industry effluent by water hyacinth (Eichhornia crassipes). Int. J. Environ. Sci. Technol. 2022, 19, 5481–5492. [Google Scholar] [CrossRef]
- González, C.I.; Maine, M.A.; Cazenave, J.; Sanchez, G.C.; Benavides, M.P. Physiological and biochemical responses of Eichhornia crassipes exposed to Cr (III). Environ. Sci. Pollut. Res. Int. 2015, 22, 3739–3747. [Google Scholar] [CrossRef]

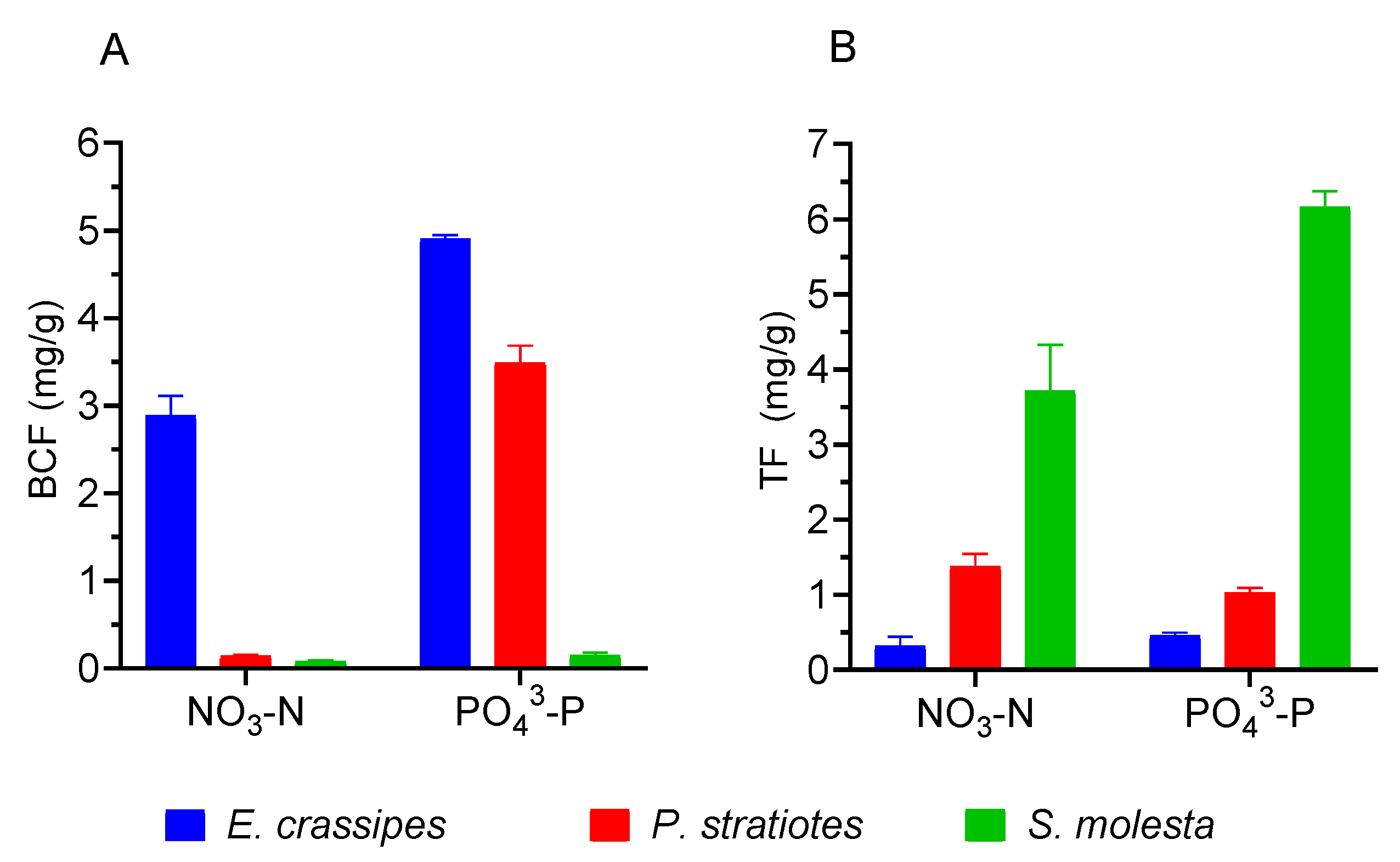
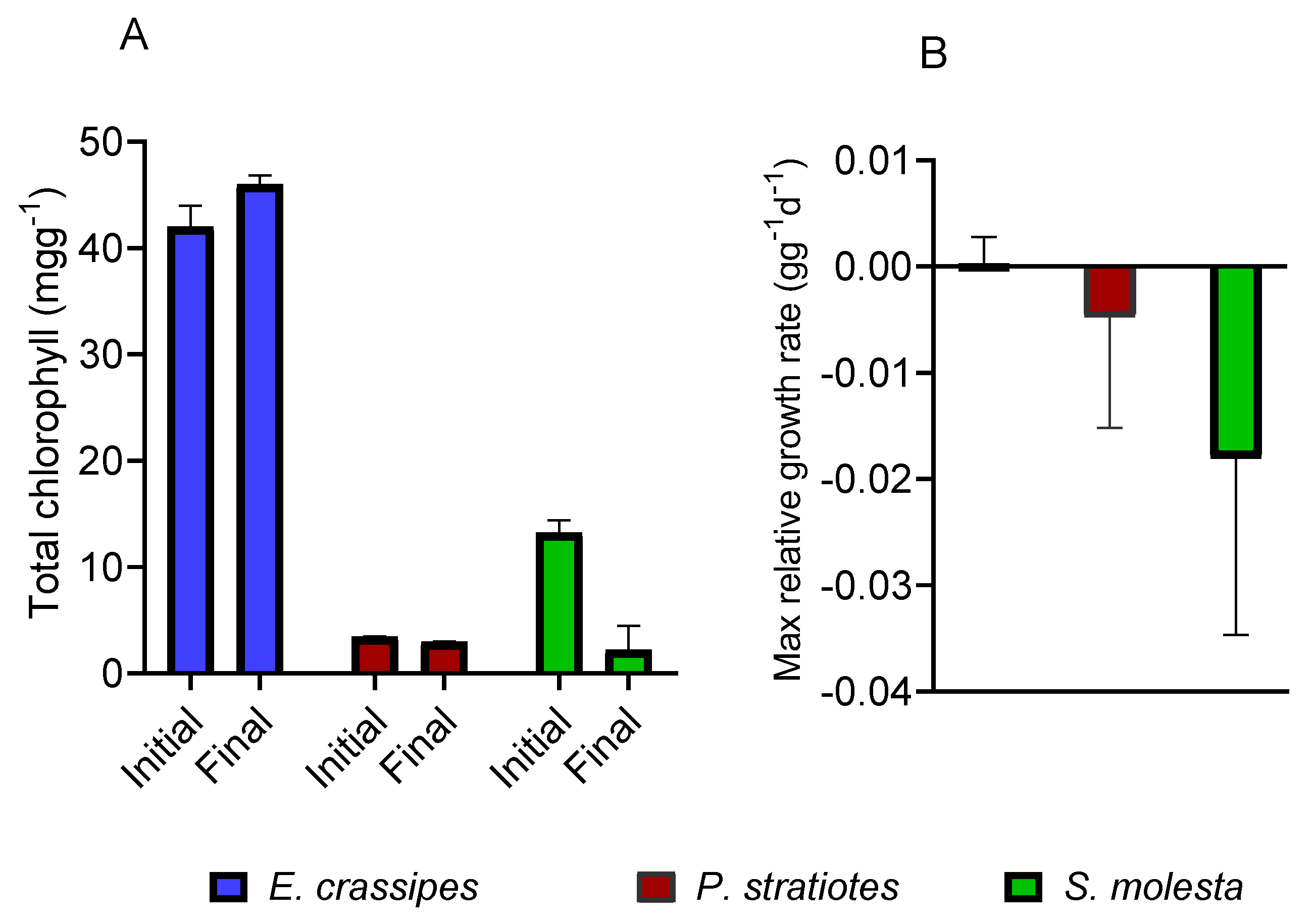
| Parameter | Value |
|---|---|
| Temperature (°C) | 17.4 ± 2.6 |
| pH | 7.81 ± 0.71 |
| EC (µs/cm) | 2639 ± 14.85 |
| TDS (mg/L) | 1319 ± 7.78 |
| DO (mg/L) | 1.63 ± 0.04 |
| Turbidity (FNU) | 248 ± 31.82 |
| COD (mg/L) | 741 ± 4.36 |
| TOC (mg/L) | 170 ± 3.73 |
| PO43-P (mg/L) | 29 ± 0.51 |
| NO3-N (mg/L) | 22 ± 1.06 |
| TN (mg/L) | 117 ± 0.45 |
| Parameter | E. crassipes | P. stratiotes | S. molesta |
|---|---|---|---|
| pH | 8.3 ± 0.28 | 8.44 ± 0.02 | 8.39 ± 0.4 |
| EC (µs/cm) | 2943 ± 9.65 | 2885 ± 15.6 | 3007 ± 21.9 |
| TDS (mg/L) | 1479 ± 14.10 | 1417.5 ± 2.8 | 1463.0 ± 3.5 |
| DO (mg/L) | 1.6 ± 0.3 | 0.5 ± 0.0035 | 0.18 ± 0.008 |
| Turbidity (FNU) | 75.14 ± 24.7 | 94.24 ± 28.3 | 186.0 ± 29 |
| COD (mg/L) | 231.19 ± 26.8 | 285.28 ± 61 | 315.67 ± 26 |
| TOC (mg/L) | 70.21 ± 4.5 | 55.08 ± 2.7 | 84.92 ± 4.5 |
| PO43-P (mg/L) | 8.99 ± 0.51 | 8.99 ± 0.3 | 27.26 ± 0.7 |
| NO3-N (mg/L) | 9.61 ± 0.8 | 16.54 ± 1.5 | 20.28 ± 0.4 |
| TN (mg/L) | 69.14 ± 0.5 | 67.86 ± 1.3 | 81.67 ± 3.5 |
| E (%) | E. crassipes | P. stratiotes | S. molesta |
|---|---|---|---|
| Turbidity | 69.7 ± 1.2 | 62 ± 7.0 | 25 ± 3.1 |
| COD | 68.8 ± 5.0 | 61.5 ± 14.1 | 57.4 ± 9.5 |
| TOC | 58.7 ± 0.1 | 59 ± 1.2 | 50.5 ± 1.6 |
| PO43-P | 69.4 ± 1. 4 | 67.6 ± 0.6 | 6.0 ± 1.8 |
| NO3-N | 56.3 ± 0.10 | 24.8 ± 0.2 | 7.8 ± 0.7 |
| TN | 40.9 ± 0.9 | 42.2 ± 2.4 | 30.2 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Vásquez, L.A.; Romo-Gómez, C.; Alvarado-Lassman, A.; Prieto-García, F.; Camacho-López, C.; Acevedo-Sandoval, O.A. Artificial Floating Islands for the Removal of Nutrients and Improvement of the Quality of Urban Wastewater. Water 2024, 16, 1443. https://doi.org/10.3390/w16101443
Hernández-Vásquez LA, Romo-Gómez C, Alvarado-Lassman A, Prieto-García F, Camacho-López C, Acevedo-Sandoval OA. Artificial Floating Islands for the Removal of Nutrients and Improvement of the Quality of Urban Wastewater. Water. 2024; 16(10):1443. https://doi.org/10.3390/w16101443
Chicago/Turabian StyleHernández-Vásquez, Luis Alfredo, Claudia Romo-Gómez, Alejandro Alvarado-Lassman, Francisco Prieto-García, Cesar Camacho-López, and Otilio Arturo Acevedo-Sandoval. 2024. "Artificial Floating Islands for the Removal of Nutrients and Improvement of the Quality of Urban Wastewater" Water 16, no. 10: 1443. https://doi.org/10.3390/w16101443
APA StyleHernández-Vásquez, L. A., Romo-Gómez, C., Alvarado-Lassman, A., Prieto-García, F., Camacho-López, C., & Acevedo-Sandoval, O. A. (2024). Artificial Floating Islands for the Removal of Nutrients and Improvement of the Quality of Urban Wastewater. Water, 16(10), 1443. https://doi.org/10.3390/w16101443







